Dorsolateral Prefrontal Cortex Glutamate/Gamma-Aminobutyric Acid (GABA) Alterations in Clinical High Risk and First-Episode Schizophrenia: A Preliminary 7-T Magnetic Resonance Spectroscopy Imaging Study
Abstract
:1. Introduction
2. Results
2.1. Clinical and Demographic Measures
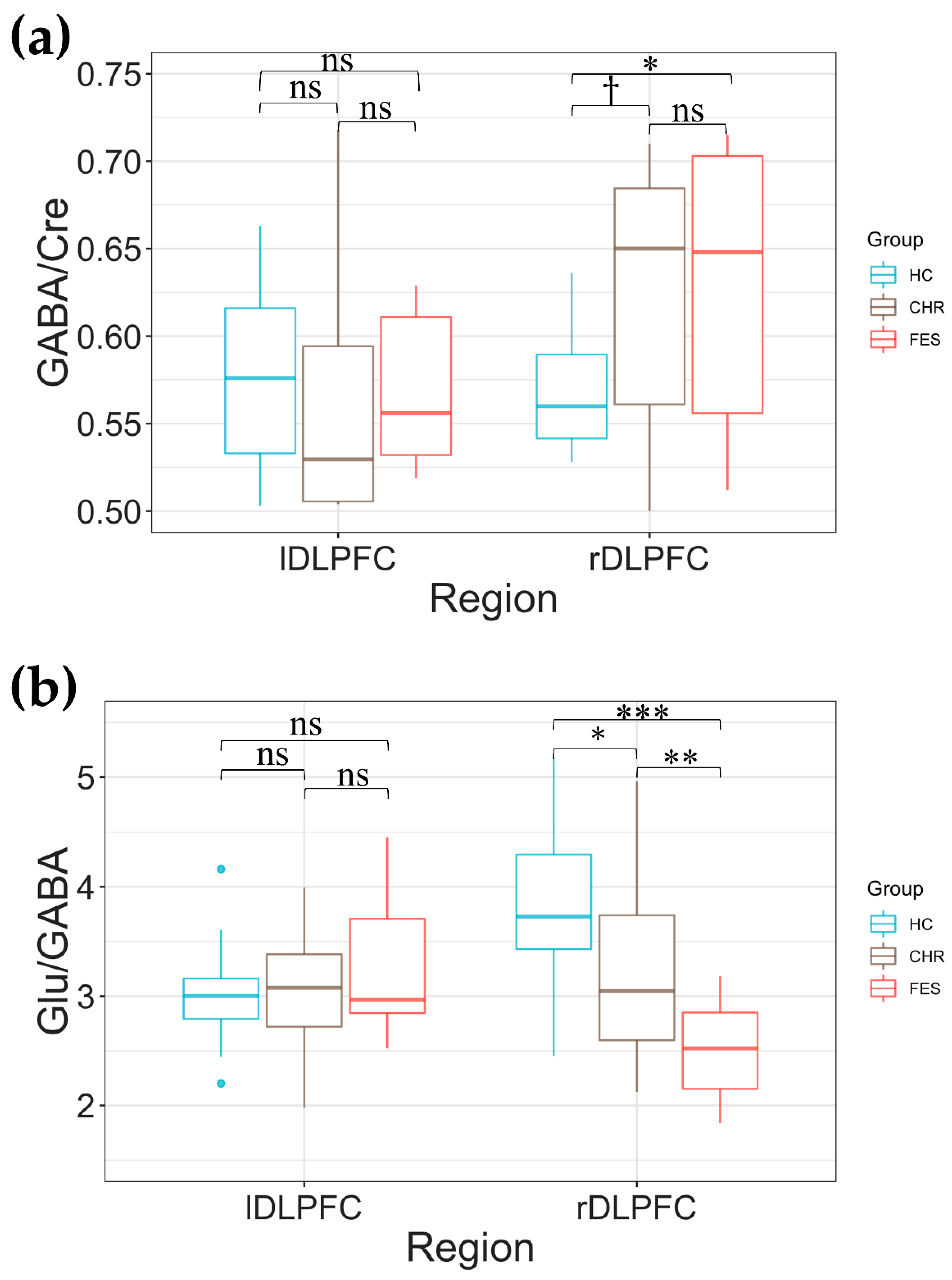
2.2. Group Differences in DLPFC Glu/Creatine (Cre), GABA/Cre, and Glu/GABA
2.3. Glu/GABA Asymmetry in DLPFC across the Three Groups
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Recruitment, Eligibility Criteria, and Clinical and Cognition Measurements
4.3. 1H MRSI Data Acquisition and Processing
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weickert, C.; Hyde, T.; Lipska, B.; Herman, M.; Weinberger, D.; Kleinman, J. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol. Psychiatry 2003, 8, 592–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smucny, J.; Dienel, S.J.; Lewis, D.A.; Carter, C.S. Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia. Neuropsychopharmacology 2022, 47, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Hoftman, G.D.; Datta, D.; Lewis, D.A. Layer 3 excitatory and inhibitory circuitry in the prefrontal cortex: Developmental trajectories and alterations in schizophrenia. Biol. Psychiatry 2017, 81, 862–873. [Google Scholar] [CrossRef] [Green Version]
- Minzenberg, M.J.; Laird, A.R.; Thelen, S.; Carter, C.S.; Glahn, D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatry 2009, 66, 811–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dienel, S.J.; Lewis, D.A. Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiol. Dis. 2019, 131, 104208. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Arion, D.; Unger, T.; Maldonado-Aviles, J.; Morris, H.; Volk, D.; Mirnics, K.; Lewis, D. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 2008, 13, 147–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Aviles, J.G.; Curley, A.A.; Hashimoto, T.; Morrow, A.L.; Ramsey, A.J.; O’Donnell, P.; Volk, D.W.; Lewis, D.A. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am. J. Psychiatry 2009, 166, 450–459. [Google Scholar] [CrossRef] [Green Version]
- Mirnics, K.; Middleton, F.A.; Marquez, A.; Lewis, D.A.; Levitt, P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron 2000, 28, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, M.; Mizukami, K.; Iwakiri, M.; Asada, T. Immunohistochemical and immunoblot analysis of γ-aminobutyric acid B receptor in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Neurosci. Lett. 2005, 383, 272–277. [Google Scholar] [CrossRef]
- Sokolov, B.P. Expression of NMDAR1, GluR1, GluR7, and KA1 glutamate receptor mRNAs is decreased in frontal cortex of “neuroleptic-free” schizophrenics: Evidence on reversible up-regulation by typical neuroleptics. J. Neurochem. 1998, 71, 2454–2464. [Google Scholar] [CrossRef]
- Weickert, C.S.; Fung, S.; Catts, V.; Schofield, P.; Allen, K.; Moore, L.; Newell, K.A.; Pellen, D.; Huang, X.-F.; Catts, S. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol. Psychiatry 2013, 18, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminski, J.; Gleich, T.; Fukuda, Y.; Katthagen, T.; Gallinat, J.; Heinz, A.; Schlagenhauf, F. Association of cortical glutamate and working memory activation in patients with schizophrenia: A multimodal proton magnetic resonance spectroscopy and functional magnetic resonance imaging study. Biol. Psychiatry 2020, 87, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Nakajima, S.; Plitman, E.; Caravaggio, F.; Kim, J.; Shah, P.; Mar, W.; Chavez, S.; De Luca, V.; Mimura, M. Glutamatergic neurometabolite levels in patients with ultra-treatment-resistant schizophrenia: A cross-sectional 3T proton magnetic resonance spectroscopy study. Biol. Psychiatry 2019, 85, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Y.; Zhang, J.; Wang, Z.; Xu, J.; Li, Y.; Yang, Z.; Liu, D. Abnormal concentration of GABA and glutamate in the prefrontal cortex in schizophrenia.-an in vivo 1H-MRS study. Shanghai Arch. Psychiatry 2017, 29, 277. [Google Scholar] [PubMed]
- Kumar, V.; Vajawat, B.; Rao, N.P. Frontal GABA in schizophrenia: A meta-analysis of 1H-MRS studies. World J. Biol. Psychiatry 2020, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; McGorry, P.D.; Kane, J.M. Improving outcomes of first-episode psychosis: An overview. World Psychiatry 2017, 16, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.M.; Pradhan, S.; Coughlin, J.M.; Trivedi, A.; DuBois, S.L.; Crawford, J.L.; Sedlak, T.W.; Nucifora, F.C.; Nestadt, G.; Nucifora, L.G. Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiatry 2019, 76, 314–323. [Google Scholar] [CrossRef]
- Huang, M.-L.; Khoh, T.-T.; Lu, S.-J.; Pan, F.; Chen, J.-K.; Hu, J.-B.; Hu, S.-H.; Xu, W.-J.; Zhou, W.-H.; Wei, N. Relationships between dorsolateral prefrontal cortex metabolic change and cognitive impairment in first-episode neuroleptic-naive schizophrenia patients. Medicine 2017, 96, e7228. [Google Scholar] [CrossRef]
- Paterson, C.; Law, A.J. Toward better strategies for understanding disrupted cortical excitatory/inhibitory balance in schizophrenia. Biol. Psychiatry 2018, 83, 632–634. [Google Scholar] [CrossRef]
- Qian, N.; Lipkin, R.M.; Kaszowska, A.; Silipo, G.; Dias, E.C.; Butler, P.D.; Javitt, D.C. Computational modeling of excitatory/inhibitory balance impairments in schizophrenia. Schizophr. Res. 2020, 249, 49–55. [Google Scholar] [CrossRef]
- Javitt, D.C.; Sweet, R.A. Auditory dysfunction in schizophrenia: Integrating clinical and basic features. Nat. Rev. Neurosci. 2015, 16, 535–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.D.; Anticevic, A. Toward understanding thalamocortical dysfunction in schizophrenia through computational models of neural circuit dynamics. Schizophr. Res. 2017, 180, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waagepetersen, H.S.; Sonnerwald, U.; Schousboe, A. Glutamine, glutamate and GABA: Metabolic aspects. In Handbook of Neurochemistry and Molecular Neurobiology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–21. [Google Scholar]
- Glausier, J.R.; Lewis, D.A. Mapping pathologic circuitry in schizophrenia. Handb. Clin. Neurol. 2018, 150, 389–417. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, G.M.; Mayeli, A.; Yushmanov, V.E.; Hetherington, H.P.; Ferrarelli, F. Reduced GABA/glutamate in the thalamus of individuals at clinical high risk for psychosis. Neuropsychopharmacology 2021, 46, 1133–1139. [Google Scholar] [CrossRef]
- Pan, J.; Avdievich, N.; Hetherington, H. J-refocused coherence transfer spectroscopic imaging at 7 T in human brain. Magn. Reson. Med. 2010, 64, 1237–1246. [Google Scholar] [CrossRef] [Green Version]
- Sonnenschein, S.F.; Mayeli, A.; Yushmanov, V.E.; Blazer, A.; Calabro, F.J.; Perica, M.; Foran, W.; Luna, B.; Hetherington, H.P.; Ferrarelli, F. A longitudinal investigation of GABA, glutamate, and glutamine across the insula during antipsychotic treatment of first-episode schizophrenia. Schizophr. Res. 2022, 248, 98–106. [Google Scholar] [CrossRef]
- Pan, J.W.; Duckrow, R.B.; Gerrard, J.; Ong, C.; Hirsch, L.J.; Resor, S.R., Jr.; Zhang, Y.; Petroff, O.; Spencer, S.; Hetherington, H.P. 7 T MR spectroscopic imaging in the localization of surgical epilepsy. Epilepsia 2013, 54, 1668–1678. [Google Scholar] [CrossRef] [Green Version]
- de Jonge, J.C.; Vinkers, C.H.; Hulshoff Pol, H.E.; Marsman, A. GABAergic mechanisms in schizophrenia: Linking postmortem and in vivo studies. Front. Psychiatry 2017, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Benes, F.M.; Sorensen, I.; Vincent, S.L.; Bird, E.D.; Sathi, M. Increased density of glutamate-immunoreactive vertical processes in superficial laminae in cingulate cortex of schizophrenic brain. Cereb. Cortex 1992, 2, 503–512. [Google Scholar] [CrossRef]
- Menzies, L.; Ooi, C.; Kamath, S.; Suckling, J.; McKenna, P.; Fletcher, P.; Bullmore, E.; Stephenson, C. Effects of γ-aminobutyric acid–modulating drugs on working memory and brain function in patients with schizophrenia. Arch. Gen. Psychiatry 2007, 64, 156–167. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.A.; Stanford, A.D.; Mao, X.; Abi-Dargham, A.; Shungu, D.C.; Lisanby, S.H.; Schroeder, C.E.; Kegeles, L.S. GABA level, gamma oscillation, and working memory performance in schizophrenia. NeuroImage Clin. 2014, 4, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragland, J.; Maddock, R.; Hurtado, M.; Tanase, C.; Lesh, T.; Niendam, T.; Carter, C.; Ranganath, C. Disrupted GABAergic facilitation of working memory performance in people with schizophrenia. NeuroImage Clin. 2020, 25, 102127. [Google Scholar] [CrossRef] [PubMed]
- Kegeles, L.S.; Mao, X.; Stanford, A.D.; Girgis, R.; Ojeil, N.; Xu, X.; Gil, R.; Slifstein, M.; Abi-Dargham, A.; Lisanby, S.H. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 2012, 69, 449–459. [Google Scholar] [PubMed] [Green Version]
- Wijtenburg, S.A.; Wang, M.; Korenic, S.A.; Chen, S.; Barker, P.B.; Rowland, L.M. Metabolite alterations in adults with schizophrenia, first degree relatives, and healthy controls: A multi-region 7T MRS study. Front. Psychiatry 2021, 12, 656459. [Google Scholar] [CrossRef]
- Hugdahl, K.; Craven, A.R.; Nygård, M.; Løberg, E.-M.; Berle, J.Ø.; Johnsen, E.; Kroken, R.; Specht, K.; Andreassen, O.A.; Ersland, L. Glutamate as a mediating transmitter for auditory hallucinations in schizophrenia: A 1H MRS study. Schizophr. Res. 2015, 161, 252–260. [Google Scholar] [CrossRef]
- Smesny, S.; Gussew, A.; Biesel, N.J.; Schack, S.; Walther, M.; Rzanny, R.; Milleit, B.; Gaser, C.; Sobanski, T.; Schultz, C.C.; et al. Glutamatergic dysfunction linked to energy and membrane lipid metabolism in frontal and anterior cingulate cortices of never treated first-episode schizophrenia patients. Schizophr. Res. 2015, 168, 322–329. [Google Scholar] [CrossRef]
- Kaminski, J.; Mascarell-Maricic, L.; Fukuda, Y.; Katthagen, T.; Heinz, A.; Schlagenhauf, F. Glutamate in the dorsolateral prefrontal cortex in patients with schizophrenia: A meta-analysis of 1H-magnetic resonance spectroscopy studies. Biol. Psychiatry 2021, 89, 270–277. [Google Scholar] [CrossRef]
- Byun, M.S.; Choi, J.-S.; Yoo, S.Y.; Kang, D.-H.; Choi, C.-H.; Jang, D.P.; Jung, W.H.; Jung, M.H.; Jang, J.H.; Lee, J.-M. Depressive symptoms and brain metabolite alterations in subjects at ultra-high risk for psychosis: A preliminary study. Psychiatry Investig. 2009, 6, 264. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Yeon, S.; Choi, C.-H.; Kang, D.-H.; Lee, J.-M.; Shin, N.Y.; Jung, W.H.; Choi, J.-S.; Jang, D.-P.; Kwon, J.S. Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: Investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr. Res. 2009, 111, 86–93. [Google Scholar] [CrossRef]
- Tian, G.; Li, S.; Huang, T.; Wu, S. Excitation-Inhibition Balanced Neural Networks for Fast Signal Detection. Front. Comput. Neurosci. 2020, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Duncan, N.W.; Wiebking, C.; Northoff, G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—A review of multimodal imaging studies. Neurosci. Biobehav. Rev. 2014, 47, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.E. Neurotransmitters. Curr. Biol. 2005, 15, R154–R158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, G.; Jeon, S.J.; Ko, I.O.; Park, J.H.; Lee, K.C.; Kim, M.-S.; Shin, C.Y.; Kim, H.; Lee, Y.-S. Decreased in vivo glutamate/GABA ratio correlates with the social behavior deficit in a mouse model of autism spectrum disorder. Mol. Brain 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, N.; Lin, Y.; Chen, R.; Zhang, J. Hemispheric Differences in Functional Interactions Between the Dorsal Lateral Prefrontal Cortex and Ipsilateral Motor Cortex. Front. Hum. Neurosci. 2020, 14, 202. [Google Scholar] [CrossRef]
- Moser, E.; Stahlberg, F.; Ladd, M.E.; Trattnig, S. 7-T MR--from research to clinical applications? NMR Biomed. 2012, 25, 695–716. [Google Scholar] [CrossRef]
- Aleman, A.; van’t Wout, M. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex disrupts digit span task performance. Neuropsychobiology 2008, 57, 44–48. [Google Scholar] [CrossRef]
- Olesen, P.J.; Westerberg, H.; Klingberg, T. Increased prefrontal and parietal activity after training of working memory. Nat. Neurosci. 2004, 7, 75–79. [Google Scholar] [CrossRef]
- Curtis, C.E.; Rao, V.Y.; D’Esposito, M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J. Neurosci. 2004, 24, 3944–3952. [Google Scholar] [CrossRef] [Green Version]
- Giglia, G.; Brighina, F.; Rizzo, S.; Puma, A.; Indovino, S.; Maccora, S.; Baschi, R.; Cosentino, G.; Fierro, B. Anodal transcranial direct current stimulation of the right dorsolateral prefrontal cortex enhances memory-guided responses in a visuospatial working memory task. Funct. Neurol. 2014, 29, 189. [Google Scholar]
- Tandon, R.; Gaebel, W.; Barch, D.M.; Bustillo, J.; Gur, R.E.; Heckers, S.; Malaspina, D.; Owen, M.J.; Schultz, S.; Tsuang, M. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 2013, 150, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.W.; Lo, K.M.; Hetherington, H.P. Role of very high order and degree B0 shimming for spectroscopic imaging of the human brain at 7 tesla. Magn. Reson. Med. 2012, 68, 1007–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavassila, S.; Deval, S.; Huegen, C.; van Ormondt, D.; Graveron-Demilly, D. Cramer-Rao bound expressions for parametric estimation of overlapping peaks: Influence of prior knowledge. J. Magn. Reson. 2000, 143, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Schirda, C.V.; Zhao, T.; Yushmanov, V.E.; Lee, Y.; Ghearing, G.R.; Lieberman, F.S.; Panigrahy, A.; Hetherington, H.P.; Pan, J.W. Fast 3D rosette spectroscopic imaging of neocortical abnormalities at 3 T: Assessment of spectral quality. Magn. Reson. Med. 2017, 79, 2470–2480. [Google Scholar] [CrossRef]
- Hetherington, H.P.; Avdievich, N.I.; Kuznetsov, A.M.; Pan, J.W. RF shimming for spectroscopic localization in the human brain at 7 T. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2010, 63, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Bonekamp, S.; Gillen, J.S.; Rowland, L.M.; Wijtenburg, S.A.; Edden, R.A.; Barker, P.B. Comparison of single voxel brain MRS AT 3 T and 7 T using 32-channel head coils. Magn. Reson. Imaging 2015, 33, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Marques, J.P.; Kober, T.; Krueger, G.; van der Zwaag, W.; Van de Moortele, P.-F.; Gruetter, R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010, 49, 1271–1281. [Google Scholar] [CrossRef] [Green Version]
- Provencher, S.W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. Vivo 2001, 14, 260–264. [Google Scholar]
- Smith, S.; Levante, T.; Meier, B.H.; Ernst, R.R. Computer simulations in magnetic resonance. An object-oriented programming approach. J. Magn. Reson. Ser. A 1994, 106, 75–105. [Google Scholar] [CrossRef]
- Hetherington, H.; Kuzniecky, R.; Vives, K.; Devinsky, O.; Pacia, S.; Luciano, D.; Vasquez, B.; Haut, S.; Spencer, D.; Pan, J. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology 2007, 69, 2256–2265. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Stephens, M. False discovery rates: A new deal. Biostatistics 2017, 18, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]

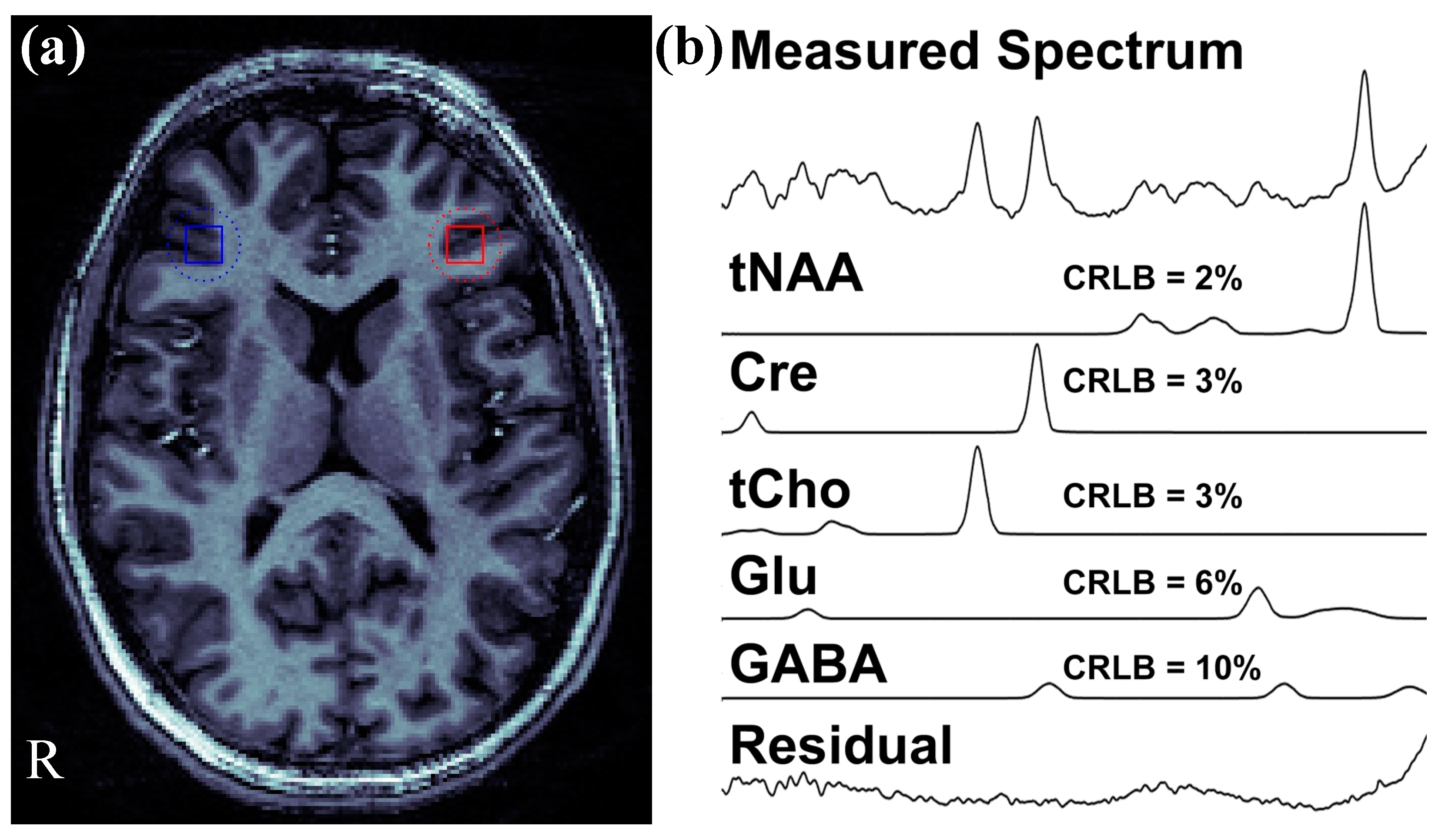
| Measures | Healthy Control | Clinical High Risk | First Episode Schizophrenia | p-Value |
|---|---|---|---|---|
| Number of subjects | 26 | 17 | 16 | - |
| Sex (# female) | 15 | 9 | 4 | - |
| Average Age ± SD in years (range) | 21.97 ± 4.57 (14–33) | 19.98 ± 3.10 (15–26) | 23.25 ± 4.88 (18–33) | 0.095 1 |
| SOPS_PS (average ± SD) | - | 12.875 ± 2.83 | - | - |
| SOPS_NS (average ± SD) | - | 12.56 ± 3.81 | - | - |
| SOPS_DS (average ± SD) | - | 5.19 ± 1.64 | - | - |
| SOPS_GS (average ± SD) | - | 7.00 ± 2.87 | - | - |
| BPRS (Total) | - | - | 53.56 ± 9.79 | - |
| BPRS (Negative Symptoms) | - | - | 8.62 ± 3.09 | - |
| BPRS (Hallucination Symptoms) | - | - | 13.56 ± 3.58 | - |
| BPRS (Positive Symptoms) | - | - | 16.50 ± 3.76 | - |
| DLPFC | ||||
|---|---|---|---|---|
| Glu/Cre | GABA/Cre | Glu/GABA | ||
| Group | DF | 2 | 2 | 2 |
| F-Value | 1.151 | 0.948 | 0.167 | |
| p-Value | 0.321 | 0.392 | 0.846 | |
| Hemisphere | DF | 1 | 1 | 1 |
| F-Value | 1.383 | 0.083 | 1.128 | |
| p-Value | 0.243 | 0.773 | 0.291 | |
| Group × Hemisphere | DF | 2 | 2 | 2 |
| F-Value | 0.96 | 4.723 | 10.26 | |
| p-Value | 0.387 | 0.011 | <0.001 | |
| Age | DF | 1 | 1 | 1 |
| F-Value | 0.0004 | 0.14 | 0.544 | |
| p-Value | 0.984 | 0.709 | 0.463 | |
| Sex | DF | 1 | 1 | 1 |
| F-Value | 0.141 | 5.266 | 3.993 | |
| p-Value | 0.708 | 0.024 | 0.049 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayeli, A.; Sonnenschein, S.F.; Yushmanov, V.E.; Wilson, J.D.; Blazer, A.; Foran, W.; Perica, M.; Calabro, F.J.; Luna, B.; Hetherington, H.P.; et al. Dorsolateral Prefrontal Cortex Glutamate/Gamma-Aminobutyric Acid (GABA) Alterations in Clinical High Risk and First-Episode Schizophrenia: A Preliminary 7-T Magnetic Resonance Spectroscopy Imaging Study. Int. J. Mol. Sci. 2022, 23, 15846. https://doi.org/10.3390/ijms232415846
Mayeli A, Sonnenschein SF, Yushmanov VE, Wilson JD, Blazer A, Foran W, Perica M, Calabro FJ, Luna B, Hetherington HP, et al. Dorsolateral Prefrontal Cortex Glutamate/Gamma-Aminobutyric Acid (GABA) Alterations in Clinical High Risk and First-Episode Schizophrenia: A Preliminary 7-T Magnetic Resonance Spectroscopy Imaging Study. International Journal of Molecular Sciences. 2022; 23(24):15846. https://doi.org/10.3390/ijms232415846
Chicago/Turabian StyleMayeli, Ahmad, Susan F. Sonnenschein, Victor E. Yushmanov, James D. Wilson, Annie Blazer, William Foran, Maria Perica, Finnegan J. Calabro, Beatriz Luna, Hoby P. Hetherington, and et al. 2022. "Dorsolateral Prefrontal Cortex Glutamate/Gamma-Aminobutyric Acid (GABA) Alterations in Clinical High Risk and First-Episode Schizophrenia: A Preliminary 7-T Magnetic Resonance Spectroscopy Imaging Study" International Journal of Molecular Sciences 23, no. 24: 15846. https://doi.org/10.3390/ijms232415846





