Human Umbilical Cord-Based Therapeutics: Stem Cells and Blood Derivatives for Female Reproductive Medicine
Abstract
:1. Introduction
2. Human Umbilical Cord: Composition and Potential Mechanisms of Action
2.1. The Cellular Components
2.1.1. The Homing Process
2.1.2. Secretion of Paracrine Factors
Growth Factors
Extracellular Vesicles
2.1.3. Immunomodulation
2.2. The Acellular Fractions
2.2.1. Human Umbilical Cord Serum
2.2.2. Human Umbilical Cord Plasma
| Type of Factor | Signaling Pathways | Biological Functions | Therapeutic Applications | References |
|---|---|---|---|---|
| VEGF | PI3K/AKT Ras/MAPKs Src/FAK |
| Reproductive medicine: EA, IUA, POI Other fields: Neurodegenerative diseases, chronic diabetic wounds, neuropathic pain | [54,110,139,140] |
| NGF | PI3K/AKT Ras/MAPKs PLC-ϒ JNK |
| Reproductive medicine: POI Other fields: Brain and nerve injury, myocardial infarction, diabetic cystopathy | [84,141,142,143,144] |
| EGF | PI3K/AKT Ras/MAPKs JAK/STATs |
| Reproductive medicine: IUA, POI Other fields: Dry eye syndrome, atopic dermatitis | [124,145] |
| FGF | PI3K/AKT Ras/MAPKs |
| Reproductive medicine: EA, IUA, vaginal reconstruction Other fields: Autoimmune encephalitis, chronic diabetic wounds, amyotrophic sclerosis, osteoarthritis | [144,146,147,148] |
| HGF | PI3K/AKT Ras/MAPKs JAK/STATs |
| Reproductive medicine: POI Other fields: Parkinson’s disease, cardiopathies, liver fibrosis | [149,150,151,152] |
| G-CSF | PI3K/AKT Ras/MAPKs JAK/STATs |
| Reproductive medicine: POI, recurrent implantation failure Other fields: Neurodegenerative diseases, acute liver failure, brain injury | [54,83,153,154,155] |
| GM-CSF | PI3K/AKT Ras/MAPKs JAK/STATs |
| Reproductive medicine: POI Other fields: Lung injury | [156,157,158] |
| PDGF (*) | PI3K/AKT JAK/STATs Ras/MAPKs PLC-ϒ |
| Reproductive medicine: IUA Other fields: Chronic diabetic wounds, acute kidney injury, liver fibrosis, lung diseases | [159,160,161,162,163] |
| TGFβ | Canonical: SMAD Non-canonical: PI3K/AKT Ras/MAPKs |
| Reproductive medicine: IUA, POI, breast cancer Other fields: Atopic dermatitis, liver fibrosis, renal fibrosis, lung injury, wounds | [87,164,165,166,167,168] |
| ILs | PI3K/AKT Ras/MAPKs JAK/STATs |
| Reproductive medicine: IUA, POI, ovarian carcinoma Other fields: Autoimmune encephalitis, neuropathic pain, spondyloarthritis, brain injury, dermatitis | [139,144,169,170,171,172,173] |
| CKs | PI3K/AKT Ras/MAPKs JAK/STATs PLC-ϒ |
| Reproductive medicine: IUA, POI Other fields: Liver failure, lung injury, brain injury | [94,174,175,176,177,178] |
3. Application of Umbilical Cord Stem Cells and Their Derivatives in the Ovary
3.1. Cellular Therapies Based on hUC-MSCs: Current Applications, Administration, and Fertility Restoration
3.2. Emerging Alternatives: Acellular Therapies
3.2.1. Extracellular Vesicles
3.2.2. Growth Factors
3.2.3. Plasma and Platelet-Rich Plasma
| Treatment | Model | Condition | Administration | Results | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ovarian Morphology | Developing Follicles | Serum Hormone Levels | Estrous Cyclicity | Markers of Regeneration and Function | Fertility Outcomes | |||||
| hUC-MSC | Rat | POI | TV | Improved | Improved | ↑ E2, AMH ↓ FSH | NR | ↑ HGF, VEGF, IGF1 | NR | [180] |
| hUC-MSC | Rat | POI | TV | Improved | Improved | ↑ E2, P4, AMH | Restored | ↑ Cell proliferation ↓ Apoptosis ↑ AMH, Bcl-2, FSHR ↓ Caspase-3 | NR | [186] |
| hUC-MSC | Rat | POI | TV | Improved | Improved | ↑ E2, AMH, GnRH ↓ FSH | Restored | ↑ Cell proliferation ↓ Apoptosis ↑ NGF, TrkA ↓ FSHR, Caspase-3 | NR | [84] |
| hUC-MSC | Rat | POI | TV | Improved | Improved | ↑ E2, LH ↓ FSH | NR | ↓ Apoptosis | NR | [182] |
| hUC-MSC | Mouse | POI | TV | Improved | Improved | ↑ E2 ↓ FSH | Restored | NR | Restored | [181] |
| hUC-MSC | Mouse | POI | TV | Improved | Improved | ↑ E2, AMH ↓ FSH | NR | ↑ Cell proliferation ↑ FSHR, Inhibin α/β | Restored | [187] |
| hUC-MSC (CD146+/−) | Mouse | POI | TV | Improved | Improved | ↑ E2, LH ↓ FSH | NR | ↑ Cell proliferation ↑ IL-2, TNFα | Restored | [185] |
| hUC-MSC | Mouse | POI | TV | Improved | Improved | ↑ E2, P4 ↓ FSH | NR | ↑ IL-4 ↓ IFNγ, NK | NR | [194] |
| hUC-MSC | Mouse | POI | TV | Improved | Improved | ↑ E2 ↓ FSH | Restored | NR | NR | [183] |
| hUC-MSC | Mouse | POI | TV | NR | NR | NR | NR | NR | NR | [184] |
| hUC-MSC | Rat | POI | Local | ↑ | NR | NR | NR | NR | NR | [188] |
| hUC-MSC | Mouse | POI | Local | Improved | Improved | ↑ E2, AMH ↓ FSH | NR | NR | Restored | [37] |
| hUC-MSC | Mouse | POI | Local | Improved | NR | ↑ E2, LH ↓ FSH | NR | ↑ VEGF | Restored | [189] |
| hUC-MSC | Human | POI | Local | Improved | Improved | NR | NR | NR | Restored | [193] |
| hUC-MSC | Rat | POI | IP | NR | Improved | ↑ E2, LH ↓ FSH | NR | ↓ Fibrosis | Restored | [190] |
| hUC-MSC | Rat | POI | TV vs. local | Improved | Improved | ↑ E2, AMH, LH ↓ FSH | Restored | ↓ Apoptosis | Restored | [191] |
| hUC-MSC | Rat | POI | TV vs. local | NR | Improved | NR | NR | ↓ Apoptosis | NR | [36] |
| hUC-MSC | Rat | POI | TV vs. local | Improved | Improved | ↑ E2 ↓ FSH | Restored | NR | Restored | [31] |
| hUC-MSC | Mouse | POI | NR | NR | Improved | ↑ E2 | NR | ↓ Apoptosis | NR | [35] |
| hUC-MSC | Mouse | Aging | TV vs. local | Improved | Improved | ↑ E2, P4 | Restored | ↓ Apoptosis ↓ ROS production | Restored | [192] |
| hUC-MSC + collagen | Mouse | POI | Local | Improved | Improved | ↑ AMH, LH ↓ FSH | Restored | ↑ Cell proliferation ↑ Angiogenesis | NR | [201] |
| hUC-MSC + collagen | Human | POI | Local | Improved | Improved | ↑ E2 ↓ FSH | NR | NR | Restored | [200] |
| hUC-MSC + HA | Mouse | POI | Local | Improved | Improved | NR | NR | ↓ Apoptosis | Restored | [197] |
| hUC-MSC vs. HGF | Mouse | POI | Local | NR | Improved | NR | NR | NR | NR | [198] |
| hUC-MSC + AF | Rat | Abdominal adhesions | IP | Improved | Improved | NR | NR | NR | NR | [196] |
| hUC-MSC EV | Mouse | POI | TV | Improved | Improved | ↑ E2 ↓ FSH | Restored | ↓ Apoptosis | Restored | [204] |
| hUC-MSC exosomes | Mouse | POI | IP | NR | Improved | ↑ E2, AMH ↓ FSH | Restored | ↑ Cell proliferation | Restored | [205] |
| hUC-MSC exosomes | Mouse | POI | Local | Improved | Improved | ↑ E2, AMH ↓ FSH | NR | ↑ Cell proliferation ↓ Apoptosis ↓ ROS production | Restored | [48] |
| hUC-MSC vesicles | Mouse | Aging | Local | NR | Improved | ↑ E2 ↓ FSH | Restored | ↑ Oocyte quality | Restored | [49] |
| hUC-MSC microvesicles | Mouse | POI | Vena caudalis injection | Improved | Improved | ↑ E2 ↓ FSH | Restored | ↑ Angiogenesis ↑ VEGF, IGF1, Ang, AKT, p-AKT | NR | [107] |
| hUC-MSC culture medium | Mouse | POI | IP | Improved | Improved | ↑ AMH | NR | NR | NR | [82] |
| hUC-MSC + hUC-PRP | Rat | POI | Local | Improved | NR | ↑ E2, AMH ↓ FSH | Restored | ↑ Angiogenesis ↓ Apoptosis | NR | [210] |
| G-CSF vs. UC plasma | Mouse | POI | TV | Improved | Improved | NR | NR | ↑ Cell proliferation ↑ Angiogenesis | Restored | [61] |
| GM-CSF | Rat | POI | IP | NR | Improved | NR | NR | ↑ CYP17, CD45 | NR | [158] |
| EGF + Matrigel | Mouse | POI | Local | NR | Improved | NR | NR | NR | Restored | [209] |
4. Application of Umbilical Cord Stem Cells and Their Derivatives in the Endometrium
4.1. Cellular Therapies Based on hUC-MSCs: Current Applications, Administration, and Fertility Restoration
4.2. Emerging Alternatives: Acellular Therapies
4.2.1. Extracellular Vesicles
4.2.2. Growth Factors
4.2.3. Plasma and Platelet-Rich Plasma
| Treatment | Model | Condition | Administration | Results | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Thickness | Gland Number | Fibrosis | Regeneration and Functionality Markers | Fertility Outcomes | |||||
| hUC-MSC | Rat | IUA | TV | Improved | Improved | Reduced | ↑ Cell proliferation ↑ Angiogenesis ↑ Itga1, Thbs, Laminin, collagen ↓ VWF | Restored | [215] |
| hUC-MSC | Rat | IUA | TV | Improved | Improved | Reduced | ↑ Cell proliferation ↑ Angiogenesis ↑ VEGFA, MMP9, CD31 ↓TNFα, IFNγ, IL-2, IL-4, IL-10 | Restored | [40] |
| hUC-MSC | Rat | IUA | Local | Improved | Improved | NR | NR | Restored | [232] |
| hUC-MSC | Rat | Thin endometrium | TV + Local | NR | NR | NR | ↑ FGF ↓ TNFα | NR | [216] |
| hUC-MSC | Rat | IUA | IP | Improved | Improved | Reduced | ↑ Angiogenesis ↓ TGFβ and Smad3 | Restored | [214] |
| hUC-MSC | Human | IUA | Local | Improved | NR | Reduced | Restored menstrual cycle | NR | [68] |
| hUC-MSC + collagen | Rat | IUA | Local | Improved | Improved | Reduced | ↑ Angiogenesis ↑ MMP9 | Restored | [39] |
| hUC-MSC + collagen | Human | IUA | Local | Improved | NR | Reduced | ↑ Cell proliferation ↑ PanCK, ERα, PR ↑ VEGFA, TGFβ, PDGF | Restored | [38] |
| hUC-MSC + collagen | Human | Asherman syndrome | Local | Improved | NR | Reduced | ↑ Cell proliferation ↑ Angiogenesis ↑ ERα, PR | Restored | [67] |
| hUC-MSC + collagen | Human | IUA | Local | Improved | NR | Reduced | ↑ Cell proliferation ↑ Angiogenesis ↑ ERα, PR | Restored | [217] |
| hUC-MSC + AMM | Rat | IUA | Local | Improved | Improved | NR | ↑ Keratin, Vimentin, Integrinβ3, IL-4, IL-10, MMP9, KI67 ↓TNFα, IFNγ, IL-2, VEGF | Non Restored | [218] |
| hUC-MSC + PF-127 | Rat | Thin endometrium | Local | Improved | Improved | NR | ↑ Cell proliferation ↑ Angiogenesis ↑ VEGFA, Nos3 | NR | [219] |
| hUC-MSC + SF-SIS | Mouse | IUA | Local | Improved | Improved | Reduced | NR | NR | [93] |
| hUC-MSC + HA | Monkey | IUA | Local | Improved | Improved | Reduced | ↑ IL-4, IGF1, EGF ↓ IFNγ | NR | [220] |
| hUC-MSCmiR−455−5p | Mouse | IUA | NR | NR | Improved | Reduced | ↑ JAK2, STAT3 ↓ SOCS3 | NR | [90] |
| hUC-MSC EVs | Rat | IUA | IP | NR | Improved | Reduced | ↑ VEGF ↓ TGFβ, TNFα, IL-1, IL-6, RUNX2, COL1A1 | NR | [226] |
| UC plasma | Mouse | IUA | Local | NR | NR | NR | ↑ Cell proliferation ↑ HOXA10, P85, 2aaa, Stat5A, Rhoa | NR | [127] |
| hUC-PRP + EndoECM | Mouse | IUA | Local | Improved | Improved | Reduced | ↑ Cell proliferation ↑ Angiogenesis ↑ AKT1, VEGF, angiogenin | Restored | [231] |
| PDGF-BB + FGF + IGF1 + EndoECM | Mouse | IUA | Local | Improved | Improved | Reduced | ↑ Cell proliferation ↑ Angiogenesis ↓ Col1A1 | Restored | [230] |
| FGF + CBD | Rat | IUA | Local | Improved | NR | Reduced | ↑ Angiogenesis | Restored | [228] |
| FGF + CBD | Human | IUA | Local | Improved | NR | Reduced | ↑ Cell proliferation ↑ Angiogenesis | Restored | [229] |
| FGF + GelMA + Na-alginate scaffold | Rat | IUA | Local | Improved | Improved | Reduced | ↑ Angiogenesis | NR | [227] |
5. Applications of Umbilical Cord Stem Cells and Their Derivatives in Other Female Reproductive Organs
5.1. Vagina
5.2. Oviducts
5.3. Placenta
6. Pros and Cons of Using Human Umbilical Cord Stem Cells and Their Derivatives
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). International Classification of Diseases; 11th Revision (ICD-11); WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Igboeli, P.; El Andaloussi, A.; Sheikh, U.; Takala, H.; Elsharoud, A.; McHugh, A.; Gavrilova-Jordan, L.; Levy, S.; Al-Hendy, A. Intraovarian Injection of Autologous Human Mesenchymal Stem Cells Increases Estrogen Production and Reduces Menopausal Symptoms in Women with Premature Ovarian Failure: Two Case Reports and a Review of the Literature. J. Med. Case Rep. 2020, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.; Dai, Y.; Hu, X.; Zhu, H.; Jiang, Y.; Zhang, S. Endometrial Stem Cells Repair Injured Endometrium and Induce Angiogenesis via AKT and ERK Pathways. Reproduction 2016, 152, 389–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Q.; Wang, L.; Luo, X.; Chen, X. Adult Stem Cells in Endometrial Regeneration: Molecular Insights and Clinical Applications. Mol. Reprod. Dev. 2021, 88, 379. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell—NCBI Bookshelf, 4th ed.; Garland Science, Ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Naji, A.; Rouas-Freiss, N.; Durrbach, A.; Carosella, E.D.; Sensébé, L.; Deschaseaux, F. Concise Review: Combining Human Leukocyte Antigen G and Mesenchymal Stem Cells for Immunosuppressant Biotherapy. Stem Cells 2013, 31, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [Green Version]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Fazeli, Z.; Abedindo, A.; Omrani, M.D.; Ghaderian, S.M.H. Mesenchymal Stem Cells (MSCs) Therapy for Recovery of Fertility: A Systematic Review. Stem Cell Rev. Rep. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Chen, S.R.; Su, P.P.; Huang, F.H.; Shi, Y.C.; Shi, Q.Y.; Lin, S. Using Mesenchymal Stem Cells to Treat Female Infertility: An Update on Female Reproductive Diseases. Stem Cells Int. 2019, 2019, 9071720. [Google Scholar] [CrossRef]
- Esfandyari, S.; Chugh, R.M.; Park, H.S.; Hobeika, E.; Ulin, M.; Al-Hendy, A. Mesenchymal Stem Cells as a Bio Organ for Treatment of Female Infertility. Cells 2020, 9, 2253. [Google Scholar] [CrossRef]
- Rungsiwiwut, R.; Virutamasen, P.; Pruksananonda, K. Mesenchymal Stem Cells for Restoring Endometrial Function: An Infertility Perspective. Reprod. Med. Biol. 2020, 20, 13–19. [Google Scholar] [CrossRef]
- Chang, Z.; Zhu, H.; Zhou, X.; Zhang, Y.; Jiang, B.; Li, S.; Chen, L.; Pan, X.; Feng, X.L. Mesenchymal Stem Cells in Preclinical Infertility Cytotherapy: A Retrospective Review. Stem Cells Int. 2021, 2021, 8882368. [Google Scholar] [CrossRef]
- Lorzadeh, N.; Kazemirad, N. Application of Stem Cells to Infertility Treatment with Emphasis on Mesenchymal Stem Cells and Ovarian Stem Cells. Am. J. Perinatol. 2018, 35, 1142–1147. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Shalaby, S.M.; Abdelaziz, M.; Brakta, S.; Hill, W.D.; Ismail, N.; Al-Hendy, A. Human Mesenchymal Stem Cells Partially Reverse Infertility in Chemotherapy-Induced Ovarian Failure. Reprod. Sci. 2018, 25, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human Mesenchymal Stem Cells—Current Trends and Future Prospective. Biosci. Rep. 2015, 35, 191. [Google Scholar] [CrossRef]
- Spitzhorn, L.S.; Megges, M.; Wruck, W.; Rahman, M.S.; Otte, J.; Degistirici, Ö.; Meisel, R.; Sorg, R.V.; Oreffo, R.O.C.; Adjaye, J. Human IPSC-Derived MSCs (IMSCs) from Aged Individuals Acquire a Rejuvenation Signature. Stem Cell Res. Ther. 2019, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Fong, C.Y.; Chak, L.L.; Biswas, A.; Tan, J.H.; Gauthaman, K.; Chan, W.K.; Bongso, A. Human Wharton’s Jelly Stem Cells Have Unique Transcriptome Profiles Compared to Human Embryonic Stem Cells and Other Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2011, 7, 1–16. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T.; He, H. Umbilical Cord-Derived Mesenchymal Stem Cells: Their Advantages and Potential Clinical Utility. World J. Stem Cells 2014, 6, 195. [Google Scholar] [CrossRef]
- Zhang, C. The Roles of Different Stem Cells in Premature Ovarian Failure. Curr. Stem Cell Res. Ther. 2020, 15, 473–481. [Google Scholar] [CrossRef]
- Yu, Y.B.; Song, Y.; Chen, Y.; Zhang, F.; Qi, F.Z. Differentiation of Umbilical Cord Mesenchymal Stem Cells into Hepatocytes in Comparison with Bone Marrow Mesenchymal Stem Cells. Mol. Med. Rep. 2018, 18, 2009–2016. [Google Scholar] [CrossRef]
- Qiu, Y.; Yun, M.M.; Han, X.; Zhao, R.; Zhou, E.; Yun, S. Human Umbilical Cord Mesenchymal Stromal Cells Suppress MHC Class II Expression on Rat Vascular Endothelium and Prolong Survival Time of Cardiac Allograft. Int. J. Clin. Exp. Med. 2014, 7, 1760. [Google Scholar] [PubMed]
- Alanazi, A.; Alassiri, M.; Jawdat, D.; Almalik, Y. Mesenchymal Stem Cell Therapy: A Review of Clinical Trials for Multiple Sclerosis. Regen. Ther. 2022, 21, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-L.; Liu, Z.; Lu, Z.-J.; Guan, D.-N.; Wang, C.; Chen, Z.-B.; Zhang, J.; Zhang, W.-Y.; Wu, J.-Y.; Xu, Y. Safety and Efficacy of Umbilical Cord Mesenchymal Stem Cell Therapy in Hereditary Spinocerebellar Ataxia. Curr. Neurovasc. Res. 2013, 10, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.X.; Guan, H.; Li, H.B.; Ren, C.A.; Liu, L.; Chu, J.J.; Dai, L.J. Therapeutic Efficacy of Umbilical Cord-Derived Mesenchymal Stem Cells in Patients with Type 2 Diabetes. Exp. Ther. Med. 2015, 9, 1623–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Liu, X.; Hua, R.; Dai, G.; Wang, X.; Gao, J.; An, Y. Clinical Observation of Umbilical Cord Mesenchymal Stem Cell Transplantation in Treatment for Sequelae of Thoracolumbar Spinal Cord Injury. J. Transl. Med. 2014, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.L.; Zeng, W.Z.; Wu, X.L.; Jiang, M.D.; Zheng, S.M.; Zhang, Y.; Li, H.Y. Clinical Therapeutic Effects of Human Umbilical Cord–Derived Mesenchymal Stem Cells Transplantation in the Treatment of End-Stage Liver Disease. Transplant. Proc. 2015, 47, 412–418. [Google Scholar] [CrossRef]
- Ding, D.C.; Chang, Y.H.; Shyu, W.C.; Lin, S.Z. Human Umbilical Cord Mesenchymal Stem Cells: A New Era for Stem Cell Therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef]
- Cao, M.; Chan, R.W.S.; Yeung, W.S.B. Label-Retaining Stromal Cells in Mouse Endometrium Awaken for Expansion and Repair after Parturition. Stem Cells Dev. 2015, 24, 768–780. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.F.; Hu, H.B.; Xu, H.Y.; Fu, X.F.; Peng, D.X.; Su, W.Y.; He, Y.L. Human Umbilical Cord Mesenchymal Stem Cell Transplantation Restores Damaged Ovaries. J. Cell Mol. Med. 2015, 19, 2108–2117. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, M.; Zhang, Y.; Li, W.; Yang, B. Mesenchymal Stem Cells Derived from Wharton Jelly of the Human Umbilical Cord Ameliorate Damage to Human Endometrial Stromal Cells. Fertil. Steril. 2011, 96, 1029–1036. [Google Scholar] [CrossRef]
- Fan, D.; Wu, S.; Ye, S.; Wang, W.; Guo, X.; Liu, Z. Umbilical Cord Mesenchyme Stem Cell Local Intramuscular Injection for Treatment of Uterine Niche: Protocol for a Prospective, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Medicine 2017, 96, e8480. [Google Scholar] [CrossRef]
- Shi, Q.; Gao, J.; Jiang, Y.; Sun, B.; Lu, W.; Su, M.; Xu, Y.; Yang, X.; Zhang, Y. Differentiation of Human Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells into Endometrial Cells. Stem Cell Res. Ther. 2017, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yu, L.; Sun, M.; Mu, S.; Wang, C.; Wang, D.; Yao, Y. The Therapeutic Potential of Umbilical Cord Mesenchymal Stem Cells in Mice Premature Ovarian Failure. Biomed Res. Int. 2013, 2013, 690491. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Song, D.; Zhong, Y.; Qian, C.; Zou, Q.; Ou, J.; Shi, Y.; Gao, L.; Wang, G.; Liu, Z.; et al. Human Umbilical Cord Mesenchymal Stem Cells Therapy in Cyclophosphamide-Induced Premature Ovarian Failure Rat Model. Biomed Res. Int. 2016, 2016, 2517514. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.A.; Shalaby, S.; Brakta, S.; Elam, L.; Elsharoud, A.; Al-Hendy, A. Umbilical Cord Blood Mesenchymal Stem Cells as an Infertility Treatment for Chemotherapy Induced Premature Ovarian Insufficiency. Biomedicines 2019, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Xin, L.; Lin, X.; Pan, Y.; Zheng, X.; Shi, L.; Zhang, Y.; Ma, L.; Gao, C.; Zhang, S. A Collagen Scaffold Loaded with Human Umbilical Cord-Derived Mesenchymal Stem Cells Facilitates Endometrial Regeneration and Restores Fertility. Acta Biomater. 2019, 92, 160–171. [Google Scholar] [CrossRef]
- Xu, L.; Ding, L.; Wang, L.; Cao, Y.; Zhu, H.; Lu, J.; Li, X.; Song, T.; Hu, Y.; Dai, J. Umbilical Cord-Derived Mesenchymal Stem Cells on Scaffolds Facilitate Collagen Degradation via Upregulation of MMP-9 in Rat Uterine Scars. Stem Cell Res. Ther. 2017, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, Y.; Guan, C.Y.; Tian, S.; Lv, X.D.; Li, J.H.; Ma, X.; Xia, H.F. Therapeutic Effect of Human Umbilical Cord-Derived Mesenchymal Stem Cells on Injured Rat Endometrium during Its Chronic Phase. Stem Cell Res. Ther. 2018, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Azizi, R.; Aghebati-Maleki, L.; Nouri, M.; Marofi, F.; Negargar, S.; Yousefi, M. Stem Cell Therapy in Asherman Syndrome and Thin Endometrium: Stem Cell- Based Therapy. Biomed Pharmacother. 2018, 102, 333–343. [Google Scholar] [CrossRef]
- Benor, A.; Gay, S.; DeCherney, A. An Update on Stem Cell Therapy for Asherman Syndrome. J. Assist. Reprod. Genet. 2020, 37, 1511–1529. [Google Scholar] [CrossRef]
- Mei, Q.; Mou, H.; Liu, X.; Xiang, W. Therapeutic Potential of HUMSCs in Female Reproductive Aging. Front. Cell Dev. Biol. 2021, 9, 650003. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [Green Version]
- Bidarimath, M.; Khalaj, K.; Kridli, R.T.; Kan, F.W.K.; Koti, M.; Tayade, C. Extracellular Vesicle Mediated Intercellular Communication at the Porcine Maternal-Fetal Interface: A New Paradigm for Conceptus-Endometrial Cross-Talk. Sci. Rep. 2017, 7, 40476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghadasi, S.; Elveny, M.; Rahman, H.S.; Suksatan, W.; Jalil, A.T.; Abdelbasset, W.K.; Yumashev, A.V.; Shariatzadeh, S.; Motavalli, R.; Behzad, F.; et al. A Paradigm Shift in Cell-Free Approach: The Emerging Role of MSCs-Derived Exosomes in Regenerative Medicine. J. Transl. Med. 2021, 19, 302. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, D.; Song, K.; Wei, J.; Yao, S.; Li, Z.; Su, X.; Ju, X.; Chao, L.; Deng, X.; et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Protect against Cisplatin-Induced Ovarian Granulosa Cell Stress and Apoptosis in Vitro. Sci. Rep. 2017, 7, 2552. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Zhu, L.; Shen, H.; Lu, J.; Zou, Q.; Huang, C.; Li, H.; Huang, B. Exosomal MiRNA-17-5p Derived from Human Umbilical Cord Mesenchymal Stem Cells Improves Ovarian Function in Premature Ovarian Insufficiency by Regulating SIRT7. Stem Cells 2020, 38, 1137–1148. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, J.; Xu, B.; He, Y.; Liu, W.; Li, J.; Zhang, S.; Lin, X.; Su, D.; Wu, T.; et al. HucMSC-Derived Exosomes Mitigate the Age-Related Retardation of Fertility in Female Mice. Mol. Ther. 2020, 28, 1200–1213. [Google Scholar] [CrossRef]
- Roura, S.; Farré, J.; Hove-Madsen, L.; Prat-Vidal, C.; Soler-Botija, C.; Gálvez-Montón, C.; Vilalta, M.; Bayes-Genis, A. Exposure to Cardiomyogenic Stimuli Fails to Transdifferentiate Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Basic Res. Cardiol. 2010, 105, 419–430. [Google Scholar] [CrossRef]
- Roura, S.; Bagó, J.R.; Soler-Botija, C.; Pujal, J.M.; Gálvez-Montón, C.; Prat-Vidal, C.; Llucià-Valldeperas, A.; Blanco, J.; Bayes-Genis, A. Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Promote Vascular Growth in Vivo. PLoS ONE 2012, 7, e49447. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, W.H.; Fu, X.H.; Huang, Q.X.; Guo, X.Y.; Zhang, L.; Li, S.S.; Zhu, J.; Shu, J. Therapeutic Role of Granulocyte Colony-Stimulating Factor (G-CSF) for Infertile Women under in Vitro Fertilization and Embryo Transfer (IVF-ET) Treatment: A Meta-Analysis. Arch. Gynecol. Obstet. 2018, 298, 861–871. [Google Scholar] [CrossRef]
- Castellano, J.M.; Mosher, K.I.; Abbey, R.J.; McBride, A.A.; James, M.L.; Berdnik, D.; Shen, J.C.; Zou, B.; Xie, X.S.; Tingle, M.; et al. Human Umbilical Cord Plasma Proteins Revitalize Hippocampal Function in Aged Mice. Nature 2017, 544, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Ehrhart, J.; Sanberg, P.R.; Garbuzova-Davis, S. Plasma Derived from Human Umbilical Cord Blood: Potential Cell-Additive or Cell-Substitute Therapeutic for Neurodegenerative Diseases. J. Cell Mol. Med. 2018, 22, 6157–6166. [Google Scholar] [CrossRef]
- Murphy, M.B.; Blashki, D.; Buchanan, R.M.; Yazdi, I.K.; Ferrari, M.; Simmons, P.J.; Tasciotti, E. Adult and Umbilical Cord Blood-Derived Platelet-Rich Plasma for Mesenchymal Stem Cell Proliferation, Chemotaxis, and Cryo-Preservation. Biomaterials 2012, 33, 5308–5316. [Google Scholar] [CrossRef]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating Changes in Human Plasma Proteome Profiles across the Lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef]
- Gelmetti, A.; Greppi, N.; Guez, S.; Grassi, F.; Rebulla, P.; Tadini, G. Cord Blood Platelet Gel for the Treatment of Inherited Epidermolysis Bullosa. Transfus. Apher. Sci. 2018, 57, 370–373. [Google Scholar] [CrossRef]
- Tadini, G.; Pezzani, L.; Ghirardello, S.; Rebulla, P.; Esposito, S.; Mosca, F. Case Report: Cord Blood Platelet Gel Treatment of Dystrophic Recessive Epidermolysis Bullosa. BMJ Case Rep. 2015, 2015, bcr2014207364. [Google Scholar] [CrossRef]
- Piccin, A.; Rebulla, P.; Pupella, S.; Tagnin, M.; Marano, G.; Di Pierro, A.M.; Santodirocco, M.; Di Mauro, L.; Beqiri, L.; Kob, M.; et al. Impressive Tissue Regeneration of Severe Oral Mucositis Post Stem Cell Transplantation Using Cord Blood Platelet Gel. Transfusion 2017, 57, 2220–2224. [Google Scholar] [CrossRef]
- Volpe, P.; Marcuccio, D.; Stilo, G.; Alberti, A.; Foti, G.; Volpe, A.; Princi, D.; Surace, R.; Pucci, G.; Massara, M. Efficacy of Cord Blood Platelet Gel Application for Enhancing Diabetic Foot Ulcer Healing after Lower Limb Revascularization. Semin. Vasc. Surg. 2017, 30, 106–112. [Google Scholar] [CrossRef]
- Buigues, A.; Marchante, M.; de Miguel-Gómez, L.; Martinez, J.; Cervelló, I.; Pellicer, A.; Herraiz, S. Stem Cell-Secreted Factor Therapy Regenerates the Ovarian Niche and Rescues Follicles. Am. J. Obstet. Gynecol. 2021, 225, 65.e1–65.e14. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, X.Y.; Liu, J. A New Approach to Cerebral Palsy Treatment: Discussion of the Effective Components of Umbilical Cord Blood and Its Mechanisms of. Cell Transplant. 2019, 28, 497. [Google Scholar] [CrossRef]
- He, X.; Wang, Q.; Zhao, Y.; Zhang, H.; Wang, B.; Pan, J.; Li, J.; Yu, H.; Wang, L.; Dai, J.; et al. Effect of Intramyocardial Grafting Collagen Scaffold with Mesenchymal Stromal Cells in Patients with Chronic Ischemic Heart Disease: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2016236. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients with Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal. Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Özmert, E.; Arslan, U. Management of Retinitis Pigmentosa by Wharton’s Jelly-Derived Mesenchymal Stem Cells: Prospective Analysis of 1-Year Results. Stem Cell Res. Ther. 2020, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Li, Y.; Hao, H.; Liu, J.; Cheng, Y.; Li, B.; Yin, Y.; Zhang, Q.; Gao, F.; Wang, H.; et al. Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stem Cells in Chinese Adults with Type 2 Diabetes: A Single-Center, Double-Blinded, Randomized, Placebo-Controlled Phase II Trial. Stem Cell Res. Ther. 2022, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, L.; Lin, X.; Zhou, F.; Xin, L.; Xu, W.; Yu, H.; Li, J.; Pan, M.; Pan, Y.; et al. Unresponsive Thin Endometrium Caused by Asherman Syndrome Treated with Umbilical Cord Mesenchymal Stem Cells on Collagen Scaffolds: A Pilot Study. Stem Cell Res. Ther. 2021, 12, 420. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Yuan, X.; Liu, Q.; Zhang, W.; Li, P. Intrauterine Infusion of Clinically Graded Human Umbilical Cord-Derived Mesenchymal Stem Cells for the Treatment of Poor Healing after Uterine Injury: A Phase I Clinical Trial. Stem Cell Res. Ther. 2022, 13, 85. [Google Scholar] [CrossRef]
- Benvenuto, F.; Voci, A.; Carminati, E.; Gualandi, F.; Mancardi, G.; Uccelli, A.; Vergani, L. Human Mesenchymal Stem Cells Target Adhesion Molecules and Receptors Involved in T Cell Extravasation. Stem Cell Res. Ther. 2015, 6, 245. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Liang, X.; Brooks, A.; Futrega, K.; Liu, X.; Doran, M.R.; Simpson, M.J.; Roberts, M.S.; Wang, H. Modelling of the SDF-1/CXCR4 Regulated in Vivo Homing of Therapeutic Mesenchymal Stem/Stromal Cells in Mice. PeerJ 2018, 2018, e6072. [Google Scholar] [CrossRef] [Green Version]
- Combadiere, C.; Ahuja, S.K.; van Damme, J.; Tiffany, H.L.; Gao, J.L.; Murphy, P.M. Monocyte Chemoattractant Protein-3 Is a Functional Ligand for CC Chemokine Receptors 1 and 2B. J. Biol. Chem. 1995, 270, 29671–29675. [Google Scholar] [CrossRef] [Green Version]
- Krafts, K.P. Tissue Repair: The Hidden Drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef]
- Zhao, M.; Rotgans, B.; Wang, T.; Cummins, S.F. REGene: A Literature-Based Knowledgebase of Animal Regeneration That Bridge Tissue Regeneration and Cancer. Sci. Rep. 2016, 6, 23167. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Broxmeyer, H.E. Immune Regulatory Cells in Umbilical Cord Blood and Their Potential Roles in Transplantation Tolerance. Crit. Rev. Oncol. Hematol. 2011, 79, 112. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xiang, E.; Li, C.; Han, B.; Zhang, Q.; Rao, W.; Xiao, C.; Wu, D. Umbilical Cord-Derived Mesenchymal Stem Cells Ameliorate Nephrocyte Injury and Proteinuria in a Diabetic Nephropathy Rat Model. J. Diabetes Res. 2020, 2020, 8035853. [Google Scholar] [CrossRef]
- Li, H.; Rong, P.; Ma, X.; Nie, W.; Chen, Y.; Zhang, J.; Dong, Q.; Yang, M.; Wang, W. Mouse Umbilical Cord Mesenchymal Stem Cell Paracrine Alleviates Renal Fibrosis in Diabetic Nephropathy by Reducing Myofibroblast Transdifferentiation and Cell Proliferation and Upregulating MMPs in Mesangial Cells. J. Diabetes Res. 2020, 2020, 3847171. [Google Scholar] [CrossRef]
- Xiang, E.; Han, B.; Zhang, Q.; Rao, W.; Wang, Z.; Chang, C.; Zhang, Y.; Tu, C.; Li, C.; Wu, D. Human Umbilical Cord-Derived Mesenchymal Stem Cells Prevent the Progression of Early Diabetic Nephropathy through Inhibiting Inflammation and Fibrosis. Stem Cell Res. Ther. 2020, 11, 336. [Google Scholar] [CrossRef]
- Barretto, T.A.; Park, E.; Telliyan, T.; Liu, E.; Gallagher, D.; Librach, C.; Baker, A. Vascular Dysfunction after Modeled Traumatic Brain Injury Is Preserved with Administration of Umbilical Cord Derived Mesenchymal Stromal Cells and Is Associated with Modulation of the Angiogenic Response. J. Neurotrauma 2021, 38, 2747–2762. [Google Scholar] [CrossRef]
- Mukai, T.; di Martino, E.; Tsuji, S.; Blomgren, K.; Nagamura-Inoue, T.; Ådén, U. Umbilical Cord-Derived Mesenchymal Stromal Cells Immunomodulate and Restore Actin Dynamics and Phagocytosis of LPS-Activated Microglia via PI3K/Akt/Rho GTPase Pathway. Cell Death Discov. 2021, 7, 46. [Google Scholar] [CrossRef]
- Song, Y.; Wang, B.; Zhu, X.; Hu, J.; Sun, J.; Xuan, J.; Ge, Z. Human Umbilical Cord Blood–Derived MSCs Exosome Attenuate Myocardial Injury by Inhibiting Ferroptosis in Acute Myocardial Infarction Mice. Cell Biol. Toxicol. 2021, 37, 51–64. [Google Scholar] [CrossRef]
- Hong, L.; Yan, L.; Xin, Z.; Hao, J.; Liu, W.; Wang, S.; Liao, S.; Wang, H.; Yang, X. Protective Effects of Human Umbilical Cord Mesenchymal Stem Cell-Derived Conditioned Medium on Ovarian Damage. J. Mol. Cell Biol. 2020, 12, 372–385. [Google Scholar] [CrossRef]
- Shareghi-oskoue, O.; Aghebati-Maleki, L.; Yousefi, M. Transplantation of Human Umbilical Cord Mesenchymal Stem Cells to Treat Premature Ovarian Failure. Stem Cell Res. Ther. 2021, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Fu, X.; Jiang, J.; Zhang, N.; Zou, L.; Wang, W.; Ding, M.; Chen, H. Umbilical Cord Mesenchymal Stem Cell Transplantation Prevents Chemotherapy-Induced Ovarian Failure via the NGF/TrkA Pathway in Rats. Biomed Res. Int. 2019, 2019, 6539294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfayomy, A.K.; Almasry, S.M.; El-Tarhouny, S.A.; Eldomiaty, M.A. Human Umbilical Cord Blood-Mesenchymal Stem Cells Transplantation Renovates the Ovarian Surface Epithelium in a Rat Model of Premature Ovarian Failure: Possible Direct and Indirect Effects. Tissue Cell 2016, 48, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jia, H.; Zhang, B.; Wang, J.; Ji, C.; Zhu, X.; Yan, Y.; Yin, L.; Yu, J.; Qian, H.; et al. Pre-Incubation with HucMSC-Exosomes Prevents Cisplatin-Induced Nephrotoxicity by Activating Autophagy. Stem Cell Res. Ther. 2017, 8, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Liver Fibrosis. Stem Cells Dev. 2013, 22, 845. [Google Scholar] [CrossRef] [Green Version]
- Xin, L.; Lin, X.; Zhou, F.; Li, C.; Wang, X.; Yu, H.; Pan, Y.; Fei, H.; Ma, L.; Zhang, S. A Scaffold Laden with Mesenchymal Stem Cell-Derived Exosomes for Promoting Endometrium Regeneration and Fertility Restoration through Macrophage Immunomodulation. Acta Biomater. 2020, 113, 252–266. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, S.; Wu, X. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Inhibit Ovarian Granulosa Cells Inflammatory Response through Inhibition of NF-ΚB Signaling in Polycystic Ovary Syndrome. J. Reprod. Immunol. 2022, 152, 103638. [Google Scholar] [CrossRef]
- Sun, D.; Jiang, Z.; Chen, Y.; Shang, D.; Miao, P.; Gao, J. MiR-455-5p Upregulation in Umbilical Cord Mesenchymal Stem Cells Attenuates Endometrial Injury and Promotes Repair of Damaged Endometrium via Janus Kinase/Signal Transducer and Activator of Transcription 3 Signaling. Bioengineered 2021, 12, 12891–12904. [Google Scholar] [CrossRef]
- Cai, M.H.; Chen, X.Y.; Fu, L.Q.; Du, W.L.; Yang, X.; Mou, X.Z.; Hu, P.Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 27. [Google Scholar] [CrossRef]
- Cheng, T.; Ding, S.; Liu, S.; Li, Y.; Sun, L. Human Umbilical Cord-Derived Mesenchymal Stem Cell Therapy Ameliorates Lupus through Increasing CD4+ T Cell Senescence via MiR-199a-5p/Sirt1/P53 Axis. Theranostics 2021, 11, 893–905. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, L.; Bi, X.; Xue, R. CircPTP4A2-MiR-330-5p-PDK2 Signaling Facilitates In Vivo Survival of HuMSCs on SF-SIS Scaffolds and Improves the Repair of Damaged Endometrium. Oxid. Med. Cell Longev. 2022, 2022, 2818433. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, Y.Y.; Lin, W.; Chen, C.H.; Wen, Y.C.; Hsiao, T.C.; Chou, H.C.; Chung, K.F.; Chuang, H.C. Therapeutic Potential of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Recovering from Murine Pulmonary Emphysema Under Cigarette Smoke Exposure. Front. Med. 2021, 8, 713824. [Google Scholar] [CrossRef]
- El Omar, R.; Beroud, J.; Stoltz, J.F.; Menu, P.; Velot, E.; Decot, V. Umbilical Cord Mesenchymal Stem Cells: The New Gold Standard for Mesenchymal Stem Cell-Based Therapies? Tissue Eng. Part B Rev. 2014, 20, 523–544. [Google Scholar] [CrossRef]
- Che, N.; Li, X.; Zhou, S.; Liu, R.; Shi, D.; Lu, L.; Sun, L. Umbilical Cord Mesenchymal Stem Cells Suppress B-Cell Proliferation and Differentiation. Cell Immunol. 2012, 274, 46–53. [Google Scholar] [CrossRef]
- Chatterjee, D.; Marquardt, N.; Tufa, D.; Beauclair, G.; Low, H.; Hatlapatka, T.; Hass, R.; Kasper, C.; von Kaisenberg, C.; Schmidt, R.; et al. Role of Gamma-Secretase in Human Umbilical-Cord Derived Mesenchymal Stem Cell Mediated Suppression of NK Cell Cytotoxicity. Cell Commun. Signal. 2014, 12, 63. [Google Scholar] [CrossRef]
- Jerkic, M.; Gagnon, S.; Rabani, R.; Ward-Able, T.; Masterson, C.; Otulakowski, G.; Curley, G.F.; Marshall, J.; Kavanagh, B.P.; Laffey, J.G. Human Umbilical Cord Mesenchymal Stromal Cells Attenuate Systemic Sepsis in Part by Enhancing Peritoneal Macrophage Bacterial Killing via Heme Oxygenase-1 Induction in Rats. Anesthesiology 2020, 132, 140–154. [Google Scholar] [CrossRef]
- Yin, N.; Wu, C.; Qiu, J.; Zhang, Y.; Bo, L.; Xu, Y.; Shi, M.; Zhu, S.; Yang, G.; Mao, C. Protective Properties of Heme Oxygenase-1 Expressed in Umbilical Cord Mesenchymal Stem Cells Help Restore the Ovarian Function of Premature Ovarian Failure Mice through Activating the JNK/Bcl-2 Signal Pathway-Regulated Autophagy and Upregulating the Circulating of CD8+CD28− T cells. Stem Cell Res. Ther. 2020, 11, 49. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.; Kim, S.; Lee, Y.I.; Kim, J.; Lee, J.H. The Effect of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell Media Containing Serum on Recovery after Laser Treatment: A Double-Blinded, Randomized, Split-Face Controlled Study. J. Cosmet. Dermatol. 2020, 19, 651–656. [Google Scholar] [CrossRef]
- Hoss, E.; Kollipara, R.; Alhaddad, M.; Boen, M.; Goldman, M.P. Red Deer Umbilical Cord-Derived Stem Cell Conditioned Media Combined with Ablative Resurfacing of the Face. J. Drugs Dermatol. 2020, 19, 1044–1048. [Google Scholar] [CrossRef]
- Alhaddad, M.; Boen, M.; Wu, D.C.; Goldman, M.P. Red Deer Umbilical Cord Lining Mesenchymal Stem Cell Extract Cream for Rejuvenation of the Face. J. Drugs Dermatol. 2019, 18, 363–366. [Google Scholar]
- Shin, S.; Shin, J.U.; Lee, Y.; Kwon, T.G.; Lee, J.H. The Effects of a Multigrowth Factor-Containing Cream on Recovery after Laser Treatment: A Double-Blinded, Randomized, Split-Face Controlled Study. J. Cosmet. Dermatol. 2017, 16, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. Biomed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Gu, Z.; Zhao, X.; Yang, N.; Wang, F.; Deng, A.; Zhao, S.; Luo, L.; Wei, H.; Guan, L.; et al. Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells Dev. 2016, 25, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Du, X.; Wang, C.; Zhang, J.; Liu, C.; Li, Y.; Jiang, H. Therapeutic Effects of Human Umbilical Cord Mesenchymal Stem Cell-Derived Microvesicles on Premature Ovarian Insufficiency in Mice. Stem Cell Res. Ther. 2019, 10, 250. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes Released by Human Umbilical Cord Mesenchymal Stem Cells Protect against Cisplatin-Induced Renal Oxidative Stress and Apoptosis in Vivo and in Vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Baharlooi, H.; Nouraei, Z.; Azimi, M.; Moghadasi, A.N.; Tavassolifar, M.J.; Moradi, B.; Sahraian, M.A.; Izad, M. Umbilical Cord Mesenchymal Stem Cells as Well as Their Released Exosomes Suppress Proliferation of Activated PBMCs in Multiple Sclerosis. Scand J. Immunol. 2021, 93, e13013. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSc-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-Stem-Cell-Derived Exosomes Accelerate Skeletal Muscle Regeneration. FEBS Lett 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem Cell-1. Burrello, J. et al. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef]
- Maharajan, N.; Cho, G.W.; Choi, J.H.; Jang, C.H. Regenerative Therapy Using Umbilical Cord Serum. In Vivo 2021, 35, 699. [Google Scholar] [CrossRef]
- Romanov, Y.A.; Vtorushina, V.V.; Dugina, T.N.; Romanov, A.Y.; Petrova, N.V. Human Umbilical Cord Blood Serum/Plasma: Cytokine Profile and Prospective Application in Regenerative Medicine. Bull Exp Biol Med. 2019, 168, 173–177. [Google Scholar] [CrossRef]
- Hassan, G.; Kasem, I.; Soukkarieh, C.; Aljamali, M. A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum. Int. J. Stem Cells 2017, 10, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Ang, L.P.K.; Do, T.P.; Thein, Z.M.; Reza, H.M.; Tan, X.W.; Yap, C.; Tan, D.T.H.; Beuerman, R.W. Ex Vivo Expansion of Conjunctival and Limbal Epithelial Cells Using Cord Blood Serum-Supplemented Culture Medium. Invest. Ophthalmol. Vis. Sci. 2011, 52, 6138–6147. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.; Dutta, J.; Das, S.; Datta, H. Effect of Cord Blood Serum on Ex Vivo Human Limbal Epithelial Cell Culture. J. Ocul. Biol. Dis. Informatics 2013, 5, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.G.; Zhao, J.H.; Wei, Z.L.; Cong, L.; Zhou, P.; Cao, Y.X. Human Umbilical Cord Blood Serum in Culture Medium on Oocyte Maturation In Vitro. Arch. Androl. 2007, 53, 303–307. [Google Scholar] [CrossRef] [Green Version]
- Giannaccare, G.; Carnevali, A.; Senni, C.; Logozzo, L.; Scorcia, V. Umbilical Cord Blood and Serum for the Treatment of Ocular Diseases: A Comprehensive Review. Ophthalmol. Ther. 2020, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Tovar, A.A.; White, I.A.; Sabater, A.L. Use of Acellular Umbilical Cord-Derived Tissues in Corneal and Ocular Surface Diseases. Medicines 2021, 8, 12. [Google Scholar] [CrossRef]
- Versura, P.; Profazio, V.; Buzzi, M.; Stancari, A.; Arpinati, M.; Malavolta, N.; Campos, E.C. Efficacy of Standardized and Quality-Controlled Cord Blood Serum Eye Drop Therapy in the Healing of Severe Corneal Epithelial Damage in Dry Eye. Cornea 2013, 32, 412–418. [Google Scholar] [CrossRef]
- Vajpayee, R.B.; Mukerji, N.; Tandon, R.; Sharma, N.; Pandey, R.M.; Biswas, N.R.; Malhotra, N.; Melki, S.A. Evaluation of Umbilical Cord Serum Therapy for Persistent Corneal Epithelial Defects. Br. J. Ophthalmol. 2003, 87, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Im, S.K.; Park, Y.G.; Jung, Y.D.; Yang, S.Y.; Choi, J. Application of Umbilical Cord Serum Eyedrops for the Treatment of Dry Eye Syndrome. Cornea 2006, 25, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Jang, J.Y.; Li, Z.; Park, S.H.; Yoon, K.C. Effects of Umbilical Cord Serum Eye Drops in a Mouse Model of Ocular Chemical Burn. Curr. Eye Res. 2012, 37, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Subiran, C.; Kristensen, S.G.; Andersen, C.Y. Umbilical Cord Blood–Derived Platelet-Rich Plasma: A Clinically Acceptable Substitute for Fetal Bovine Serum? Fertil. Steril. 2021, 115, 336–337. [Google Scholar] [CrossRef] [PubMed]
- de Miguel–Gómez, L.; López-Martínez, S.; Campo, H.; Francés-Herrero, E.; Faus, A.; Díaz, A.; Pellicer, A.; Domínguez, F.; Cervelló, I. Comparison of Different Sources of Platelet-Rich Plasma as Treatment Option for Infertility-Causing Endometrial Pathologies. Fertil. Steril. 2021, 115, 490–500. [Google Scholar] [CrossRef]
- Jain, N.K.; Gulati, M. Platelet-Rich Plasma: A Healing Virtuoso. Blood Res. 2016, 51, 3. [Google Scholar] [CrossRef] [Green Version]
- Tibial Fracture—Platelet-Rich Plasma and Bone Marrow Concentrate—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03100695 (accessed on 25 August 2022).
- Gentile, P.; Garcovich, S. Autologous Activated Platelet-Rich Plasma (AA-PRP) and Non-Activated (A-PRP) in Hair Growth: A Retrospective, Blinded, Randomized Evaluation in Androgenetic Alopecia. Expert. Opin. Biol. Ther. 2020, 20, 327–337. [Google Scholar] [CrossRef]
- Use of Platelet-Rich Plasma (PRP) and Platelet-Poor Plasma (PPP) to Prevent Infection and Delayed Wound Healing—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01639144 (accessed on 26 August 2022).
- Dório, M.; Pereira, R.M.R.; Luz, A.G.B.; Deveza, L.A.; de Oliveira, R.M.; Fuller, R. Efficacy of Platelet-Rich Plasma and Plasma for Symptomatic Treatment of Knee Osteoarthritis: A Double-Blinded Placebo-Controlled Randomized Clinical Trial. BMC Musculoskelet. Disord. 2021, 22, 822. [Google Scholar] [CrossRef]
- Sirithanabadeekul, P.; Dannarongchai, A.; Suwanchinda, A. Platelet-Rich Plasma Treatment for Melasma: A Pilot Study. J. Cosmet. Dermatol. 2020, 19, 1321–1327. [Google Scholar] [CrossRef]
- Ahmed, M.; Reffat, S.A.; Hassan, A.; Eskander, F. Platelet-Rich Plasma for the Treatment of Clean Diabetic Foot Ulcers. Ann. Vasc. Surg. 2017, 38, 206–211. [Google Scholar] [CrossRef]
- Schlüssel, M.M.; Keene, D.J.; Wagland, S.; Alsousou, J.; Lamb, S.E.; Willett, K.; Dutton, S.J.; Willett, K.; Alsousou, J.; Lamb, S.E.; et al. Platelet-Rich Plasma in Achilles Tendon Healing 2 (PATH-2) Trial: Statistical Analysis Plan for a Multicentre, Double-Blinded, Parallel-Group, Placebo-Controlled Randomised Clinical Trial. Trials 2018, 19, 464. [Google Scholar] [CrossRef]
- Alam, M.; Hughart, R.; Champlain, A.; Geisler, A.; Paghdal, K.; Whiting, D.; Hammel, J.A.; Maisel, A.; Rapcan, M.J.; West, D.P.; et al. Effect of Platelet-Rich Plasma Injection for Rejuvenation of Photoaged Facial Skin: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 1447–1452. [Google Scholar] [CrossRef]
- Caiaffa, V.; Ippolito, F.; Abate, A.; Nappi, V.; Santodirocco, M.; Visceglie, D. Allogenic Platelet Concentrates from Umbilical Cord Blood for Knee Osteoarthritis: Preliminary Results. Med. Glas. (Zenica) 2021, 18, 260–266. [Google Scholar] [CrossRef]
- Umbilical Cord Plasma for Treating Endometrial Pathologies (Thin Endometrium/Asherman’s Syndrome/Endometria Atrophy)—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05095597 (accessed on 25 August 2022).
- Behroozi, Z.; Ramezani, F.; Janzadeh, A.; Rahimi, B.; Nasirinezhad, F. Platelet-Rich Plasma in Umbilical Cord Blood Reduces Neuropathic Pain in Spinal Cord Injury by Altering the Expression of ATP Receptors: PRP, a New Window for Pain Reduction Post Spinal Cord Injury. Physiol. Behav. 2021, 228, 113186. [Google Scholar] [CrossRef]
- Guo, D.; Murdoch, C.E.; Liu, T.; Qu, J.; Jiao, S.; Wang, Y.; Wang, W.; Chen, X. Therapeutic Angiogenesis of Chinese Herbal Medicines in Ischemic Heart Disease: A Review. Front. Pharmacol. 2018, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Gong, Y.; Qiu, F.; Yuan, Y.; Jia, W.; Liu, Z.; Gao, L. NGF Nanoparticles Enhance the Potency of Transplanted Human Umbilical Cord Mesenchymal Stem Cells for Myocardial Repair. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1959–H1974. [Google Scholar] [CrossRef]
- WenBo, W.; Fei, Z.; YiHeng, D.; Wei, W.; TingMang, Y.; WenHao, Z.; QianRu, L.; HaiTao, L. Human Umbilical Cord Mesenchymal Stem Cells Overexpressing Nerve Growth Factor Ameliorate Diabetic Cystopathy in Rats. Neurochem. Res. 2017, 42, 3537–3547. [Google Scholar] [CrossRef]
- Pan, Y.; Jiao, G.; Yang, J.; Guo, R.; Li, J.; Wang, C. Insights into the Therapeutic Potential of Heparinized Collagen Scaffolds Loading Human Umbilical Cord Mesenchymal Stem Cells and Nerve Growth Factor for the Repair of Recurrent Laryngeal Nerve Injury. Tissue Eng. Regen. Med. 2017, 14, 317–326. [Google Scholar] [CrossRef]
- Rafieemehr, H.; KheIrandish, M.; Soleimani, M. Neuroprotective Effects of Transplanted Mesenchymal Stromal Cells-Derived Human Umbilical Cord Blood Neural Progenitor Cells in EAE. Iran J. Allergy Asthma Immunol. 2015, 14, 596–604. [Google Scholar]
- Jung, N.; Kong, T.H.; Yu, Y.; Park, H.; Lee, E.; Yoo, S.M.; Baek, S.Y.; Lee, S.; Kang, K.S. Immunomodulatory Effect of Epidermal Growth Factor Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells on Atopic Dermatitis. Int. J. Stem Cells 2022, 15, 311–323. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, L.; Yan, B.; Zhang, L.; Du, W.; Liu, F.; Yuan, Q.; Tong, P.; Shan, L.; Efferth, T. Growth Factors-Based Beneficial Effects of Platelet Lysate on Umbilical Cord-Derived Stem Cells and Their Synergistic Use in Osteoarthritis Treatment. Cell Death Dis 2020, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Çil, N.; Oğuz, E.O.; Mete, E.; Çetinkaya, A.; Mete, G. Effects of Umbilical Cord Blood Stem Cells on Healing Factors for Diabetic Foot Injuries. Biotech. Histochem. 2017, 92, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Rizvanov, A.A.; Guseva, D.S.; Salafutdinov, I.I.; Kudryashova, N.V.; Bashirov, F.V.; Kiyasov, A.P.; Yalvaç, M.E.; Gazizov, I.M.; Kaligin, M.S.; Sahin, F.; et al. Genetically Modified Human Umbilical Cord Blood Cells Expressing Vascular Endothelial Growth Factor and Fibroblast Growth Factor 2 Differentiate into Glial Cells after Transplantation into Amyotrophic Lateral Sclerosis Transgenic Mice. Exp. Biol. Med. (Maywood) 2011, 236, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Hou, X.; Wang, B.; Chi, J.; Jiang, Y.; Zhang, C.; Li, Z. Intramuscular Injection of Human Umbilical Cord-Derived Mesenchymal Stem Cells Improves Cardiac Function in Dilated Cardiomyopathy Rats. Stem Cell Res. Ther. 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, F.; Wang, W.Y.; Mao, L.C.; Cai, Q.Q.; Jiang, W.H. Effect of Human Umbilical Cord Mesenchymal Stem Cells Transfected with HGF on TGF-Β1/Smad Signaling Pathway in Carbon Tetrachloride-Induced Liver Fibrosis Rats. Stem Cells Dev. 2020, 29, 1395–1406. [Google Scholar] [CrossRef]
- He, Y.; Guo, X.; Lan, T.; Xia, J.; Wang, J.; Li, B.; Peng, C.; Chen, Y.; Hu, X.; Meng, Z. Human Umbilical Cord-Derived Mesenchymal Stem Cells Improve the Function of Liver in Rats with Acute-on-Chronic Liver Failure via Downregulating Notch and Stat1/Stat3 Signaling. Stem Cell Res. Ther. 2021, 12, 396. [Google Scholar] [CrossRef]
- Liu, X.S.; Li, J.F.; Wang, S.S.; Wang, Y.T.; Zhang, Y.Z.; Yin, H.L.; Geng, S.; Gong, H.C.; Han, B.; Wang, Y.L. Human Umbilical Cord Mesenchymal Stem Cells Infected with Adenovirus Expressing HGF Promote Regeneration of Damaged Neuron Cells in a Parkinson’s Disease Model. Biomed Res. Int. 2014, 2014, 909657. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Tang, S.; Liao, J.; Liu, M.; Lin, Y. Therapeutic Effect of Human Umbilical Cord Blood Mesenchymal Stem Cells Combined with G-CSF on Rats with Acute Liver Failure. Biochem. Biophys. Res. Commun. 2019, 517, 670–676. [Google Scholar] [CrossRef]
- Acosta, S.A.; Tajiri, N.; Shinozuka, K.; Ishikawa, H.; Sanberg, P.R.; Sanchez-Ramos, J.; Song, S.; Kaneko, Y.; Borlongan, C.V. Combination Therapy of Human Umbilical Cord Blood Cells and Granulocyte Colony Stimulating Factor Reduces Histopathological and Motor Impairments in an Experimental Model of Chronic Traumatic Brain Injury. PLoS ONE 2014, 9, e90953. [Google Scholar] [CrossRef]
- Eftekhar, M.; Naghshineh, E.; Khani, P. Role of Granulocyte Colony-Stimulating Factor in Human Reproduction. J. Res. Med. Sci. 2018, 23, 7. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Achuthan, A.; Hamilton, J.A. Colony Stimulating Factors (CSFs). Encycl. Immunobiol. 2016, 2, 586–596. [Google Scholar] [CrossRef]
- Zhu, H.; Xiong, Y.; Xia, Y.; Zhang, R.; Tian, D.; Wang, T.; Dai, J.; Wang, L.; Yao, H.; Jiang, H.; et al. Therapeutic Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Acute Lung Injury Mice. Sci. Rep. 2017, 7, 39889. [Google Scholar] [CrossRef]
- Wang, H.; Wen, Y.; Polan, M.L.; Boostanfar, R.; Feinman, M.; Behr, B. Exogenous Granulocyte–Macrophage Colony-Stimulating Factor Promotes Follicular Development in the Newborn Rat in Vivo. Hum. Reprod. 2005, 20, 2749–2756. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Yin, L.; Zhang, B.; Shi, H.; Sun, Y.; Ji, C.; Chen, J.; Wu, P.; Zhang, L.; Xu, W.; et al. Resveratrol Improves Human Umbilical Cord-Derived Mesenchymal Stem Cells Repair for Cisplatin-Induced Acute Kidney Injury. Cell Death Dis. 2018, 9, 965. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gao, F.; Yan, Y.; Ruan, Z.; Liu, Z. Combination Therapy with Human Umbilical Cord Mesenchymal Stem Cells and Angiotensin-Converting Enzyme 2 Is Superior for the Treatment of Acute Lung Ischemia-Reperfusion Injury in Rats. Cell Biochem. Funct. 2015, 33, 113–120. [Google Scholar] [CrossRef]
- Shrestha, C.; Zhao, L.; Chen, K.; He, H.; Mo, Z. Enhanced Healing of Diabetic Wounds by Subcutaneous Administration of Human Umbilical Cord Derived Stem Cells and Their Conditioned Media. Int. J. Endocrinol. 2013, 2013, 592454. [Google Scholar] [CrossRef] [Green Version]
- Sungkar, T.; Putra, A.; Lindarto, D.; Juwita Sembiring, R. Anti-Fibrotic Effect of Intravenous Umbilical Cord-Derived Mesenchymal Stem Cells (UC-MSCs) Injection in Experimental Rats Induced Liver Fibrosis. Med. Glas. (Zenica) 2021, 18, 62–69. [Google Scholar] [CrossRef]
- Guérit, E.; Arts, F.; Dachy, G.; Boulouadnine, B.; Demoulin, J.B. PDGF receptor mutations in human diseases. Cell. Mol. Life Sci. 2021, 78, 3867–3881. [Google Scholar] [CrossRef]
- Park, H.H.; Lee, S.; Yu, Y.; Yoo, S.M.; Baek, S.Y.; Jung, N.; Seo, K.W.; Kang, K.S. TGF-β Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Ameliorates Atopic Dermatitis by Inhibiting Secretion of TNF-α and IgE. Stem Cells 2020, 38, 904–916. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Liu, Y.; Li, X.; Tang, L.; Duan, M.; Li, J.; Zhang, G. Exosomes Derived from Human Umbilical Cord Blood Mesenchymal Stem Cells Stimulate Regenerative Wound Healing via Transforming Growth Factor-β Receptor Inhibition. Stem Cell Res. Ther. 2021, 12, 434. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, D.; Zhou, Y.; Xiang, H.; Liu, B.; Shen, L.; Long, C.; Liu, X.; Lin, T.; He, D.; et al. Human Umbilical Cord Mesenchymal Stem Cell Attenuates Renal Fibrosis via TGF-β/Smad Signaling Pathways in Vivo and in Vitro. Eur. J. Pharmacol. 2020, 883, 173343. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Qi, L.; Dai, X.; Liu, H.; Wang, Y. Low Levels of TGF-Β1 Enhance Human Umbilical Cord-Derived Mesenchymal Stem Cell Fibronectin Production and Extend Survival Time in a Rat Model of Lipopolysaccharide-Induced Acute Lung Injury. Mol. Med. Rep. 2016, 14, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Khoshakhlagh, M.; Soleimani, A.; Binabaj, M.M.; Avan, A.; Ferns, G.A.; Khazaei, M.; Hassanian, S.M. Therapeutic Potential of Pharmacological TGF-β Signaling Pathway Inhibitors in the Pathogenesis of Breast Cancer. Biochem. Pharmacol. 2019, 164, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gu, J.; Gu, Y.; He, M.; Bi, Y.; Chen, J.; Li, T. Human Umbilical Cord-Derived Mesenchymal Stem Cells Improve Learning and Memory Function in Hypoxic-Ischemic Brain-Damaged Rats via an IL-8-Mediated Secretion Mechanism Rather than Differentiation Pattern Induction. Cell Physiol. Biochem. 2015, 35, 2383–2401. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.F.; Zhu, J.; Lu, S.H.; Zhang, J.L.; Chen, X.D.; Du, L.X.; Yang, Z.G.; Song, Y.K.; Wu, D.Y.; Liu, B.; et al. Inhibitory Effect of Human Umbilical Cord-Derived Mesenchymal Stem Cells on Interleukin-17 Production in Peripheral Blood T Cells from Spondyloarthritis Patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2013, 21, 455–459. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ahn, H.J.; Lee, S.H.; Lee, M.H.; Kang, K.S. Effects of Conditioned Media from Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells in the Skin Immune Response. Biomed Pharmacother. 2020, 131, 110789. [Google Scholar] [CrossRef]
- Zhao, W.H.; Cheng, J.X.; Shi, P.F.; Huang, J.Y. Human Umbilical Cord Mesenchymal Stem Cells with Adenovirus-Mediated Interleukin 12 Gene Transduction Inhibits the Growth of Ovarian Carcinoma Cells Both in Vitro and in Vivo. Nan Fang Yi Ke Da Xue Xue Bao 2011, 31, 903–907. [Google Scholar]
- Niu, X.; Xu, X.; Luo, Z.; Wu, D.; Tang, J. The Expression of Th9 and Th22 Cells in Rats with Cerebral Palsy after HUC-MSC Transplantation. J. Chin. Med. Assoc. 2020, 83, 60–66. [Google Scholar] [CrossRef]
- Liu, M.; He, J.; Zheng, S.; Zhang, K.; Ouyang, Y.; Zhang, Y.; Li, C.; Wu, D. Human Umbilical Cord Mesenchymal Stem Cells Ameliorate Acute Liver Failure by Inhibiting Apoptosis, Inflammation and Pyroptosis. Ann. Transl. Med. 2021, 9, 1615. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Y.; Wang, J.; Hou, L.; Li, W.; An, H. CXCR4-Overexpressing Umbilical Cord Mesenchymal Stem Cells Enhance Protection against Radiation-Induced Lung Injury. Stem Cells Int. 2019, 2019, 2457082. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Liu, N.; Yi, B.; Zhang, X.; Gao, B.B.; Zhang, Y.; Xu, R.; Li, X.; Dai, Y. Transplanted HUCB-MSCs Migrated to the Damaged Area by SDF-1/CXCR4 Signaling to Promote Functional Recovery after Traumatic Brain Injury in Rats. Neurol Res. 2015, 37, 50–56. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, J.; Luo, X.; Wen, H.; Luo, Y. Collagen Scaffold with Human Umbilical Cord Mesenchymal Stem Cells Remarkably Improves Intrauterine Adhesions in a Rat Model. Gynecol. Obstet. Invest. 2020, 85, 267–276. [Google Scholar] [CrossRef]
- Borish, L.C.; Steinke, J.W. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003, 111 (Suppl. 2), S460–S475. [Google Scholar] [CrossRef]
- Na, J.; Kim, G.J. Recent Trends in Stem Cell Therapy for Premature Ovarian Insufficiency and Its Therapeutic Potential: A Review. J. Ovarian Res. 2020, 13, 74. [Google Scholar] [CrossRef]
- Li, J.; Mao, Q.X.; He, J.J.; She, H.Q.; Zhang, Z.; Yin, C.Y. Human Umbilical Cord Mesenchymal Stem Cells Improve the Reserve Function of Perimenopausal Ovary via a Paracrine Mechanism. Stem Cell Res. Ther. 2017, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ma, J.; Yi, P.; Wu, J.; Zhao, F.; Tu, W.; Liu, W.; Li, T.; Deng, Y.; Hao, J.; et al. Human Umbilical Cord Mesenchymal Stem Cells Restore the Ovarian Metabolome and Rescue Premature Ovarian Insufficiency in Mice. Stem Cell Res. Ther. 2020, 11, 466. [Google Scholar] [CrossRef]
- Lu, X.; Bao, H.; Cui, L.; Zhu, W.; Zhang, L.; Xu, Z.; Man, X.; Chu, Y.; Fu, Q.; Zhang, H. HUMSC Transplantation Restores Ovarian Function in POI Rats by Inhibiting Autophagy of Theca-Interstitial Cells via the AMPK/MTOR Signaling Pathway. Stem Cell Res. Ther. 2020, 11, 268. [Google Scholar] [CrossRef]
- Shen, J.; Cao, D.; Sun, J.-L. Ability of Human Umbilical Cord Mesenchymal Stem Cells to Repair Chemotherapy-Induced Premature Ovarian Failure. World J. Stem Cells 2020, 12, 277–287. [Google Scholar] [CrossRef]
- Jalalie, L.; Rezaie, M.J.; Jalili, A.; Rezaee, M.A.; Vahabzadeh, Z.; Rahmani, M.R.; Karimipoor, M.; Hakhamaneshi, M.S. Distribution of the CM-Dil-Labeled Human Umbilical Cord Vein Mesenchymal Stem Cells Migrated to the Cyclophosphamide-Injured Ovaries in C57BL/6 Mice. Iran Biomed J. 2019, 23, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sun, Y.; Zhang, X.-X.; Liu, Y.-B.; Sun, H.-Y.; Wu, C.-T.; Xiao, F.-J.; Wang, L.-S. Comparison of CD146 +/− Mesenchymal Stem Cells in Improving Premature Ovarian Failure. Stem Cell Res. Ther. 2022, 13, 267. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Q.; Wang, H.; Han, L.; Dai, H.; Qian, X.; Yu, H.; Yin, M.; Shi, F.; Qi, N. Mesenchymal Stem Cell Therapy Using Human Umbilical Cord in a Rat Model of Autoimmune-Induced Premature Ovarian Failure. Stem Cells Int. 2020, 2020, 3249495. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Guan, C.; Li, Y.; Su, X.; Zhang, L.; Wang, X.; Xia, H.F.; Ma, X. Effects of Single and Multiple Transplantations of Human Umbilical Cord Mesenchymal Stem Cells on the Recovery of Ovarian Function in the Treatment of Premature Ovarian Failure in Mice. J. Ovarian Res. 2021, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, Y.; Dong, X.; Pan, Y.; Ying, H.; Chen, J.; Yang, W.; Zhang, Y.; Fei, H.; Liu, X.; et al. Toxicity from a Single Injection of Human Umbilical Cord Mesenchymal Stem Cells into Rat Ovaries. Reprod. Toxicol. 2022, 110, 9–18. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, L.; Zhang, X.; Hu, C.; Liu, R. Biological and Biomechanical Analysis of Two Types of Mesenchymal Stem Cells for Intervention in Chemotherapy-Induced Ovarian Dysfunction. Arch. Gynecol. Obstet. 2016, 295, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Bao, H.; Liu, Z.; Man, X.; Liu, H.; Hou, Y.; Luo, Q.; Wang, S.; Fu, Q.; Zhang, H. HUMSCs Regulate the Differentiation of Ovarian Stromal Cells via TGF-Β1/Smad3 Signaling Pathway to Inhibit Ovarian Fibrosis to Repair Ovarian Function in POI Rats. Stem Cell Res. Ther. 2020, 11, 386. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, T.; Dai, W.; Zhao, B.; Zheng, Y.; Hu, J.; Pan, R.; Wang, L. Umbilical Cord Mesenchymal Stem Cells Ameliorate Premature Ovarian Insufficiency in Rats. Evid.-Based Complementary Altern. Med. 2022, 2022, 9228456. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, J.; Fang, L.; Lu, Z.; Wu, M.; Shi, L.; Qin, X.; Luo, A.; Wang, S. The Protective Effects of Human Umbilical Cord Mesenchymal Stem Cells on Damaged Ovarian Function: A Comparative Study. Biosci. Trends 2016, 10, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Wu, Y.; Li, L.; Wu, J.; Zhao, F.; Gao, Z.; Liu, W.; Li, T.; Fan, Y.; Hao, J.; et al. Clinical Analysis of Human Umbilical Cord Mesenchymal Stem Cell Allotransplantation in Patients with Premature Ovarian Insufficiency. Cell Prolif. 2020, 53, e12938. [Google Scholar] [CrossRef]
- Lu, X.; Cui, J.; Cui, L.; Luo, Q.; Cao, Q.; Yuan, W.; Zhang, H. The Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation on Endometrial Receptivity Are Associated with Th1/Th2 Balance Change and UNK Cell Expression of Uterine in Autoimmune Premature Ovarian Failure Mice. Stem Cell Res. Ther. 2019, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Ramathal, C.Y.; Bagchi, I.C.; Taylor, R.N.; Bagchi, M.K. Endometrial Decidualization: Of Mice and Men. Semin. Reprod. Med. 2010, 28, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Aygün, E.G.; Tümentemur, G. Effects of Stem Cells and Amniotic Fluid on Uterus and Ovaries on a Rat Model with Abdominal Adhesions: A Controlled Study. J. Turk. Ger. Gynecol. Assoc. 2022, 23, 154–166. [Google Scholar] [CrossRef]
- Jiao, W.; Mi, X.; Yang, Y.; Liu, R.; Liu, Q.; Yan, T.; Chen, Z.J.; Qin, Y.; Zhao, S. Mesenchymal Stem Cells Combined with Autocrosslinked Hyaluronic Acid Improve Mouse Ovarian Function by Activating the PI3K-AKT Pathway in a Paracrine Manner. Stem Cell Res. Ther. 2022, 13, 49. [Google Scholar] [CrossRef]
- Mi, X.; Jiao, W.; Yang, Y.; Qin, Y.; Chen, Z.J.; Zhao, S. HGF Secreted by Mesenchymal Stromal Cells Promotes Primordial Follicle Activation by Increasing the Activity of the PI3K-AKT Signaling Pathway. Stem Cell Rev. Rep. 2022, 18, 1834–1850. [Google Scholar] [CrossRef]
- Francés-Herrero, E.; Lopez, R.; Hellström, M.; de Miguel-Gómez, L.; Herraiz, S.; Brännström, M.; Pellicer, A.; Cervelló, I. Bioengineering Trends in Female Reproduction: A Systematic Review. Hum. Reprod. Update 2022, 28, 798–837. [Google Scholar] [CrossRef]
- Ding, L.; Yan, G.; Wang, B.; Xu, L.; Gu, Y.; Ru, T.; Cui, X.; Lei, L.; Liu, J.; Sheng, X.; et al. Transplantation of UC-MSCs on Collagen Scaffold Activates Follicles in Dormant Ovaries of POF Patients with Long History of Infertility. Sci. China Life Sci. 2018, 61, 1554–1565. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, L.; Wang, S.; Sheng, X.; Yan, G.; Xu, L.; Liu, J.; Liu, M.; Zhen, X.; Ding, L.; et al. Transplantation of Umbilical Cord–Derived Mesenchymal Stem Cells on a Collagen Scaffold Improves Ovarian Function in a Premature Ovarian Failure Model of Mice. In Vitro Cell Dev. Biol. Anim. 2019, 55, 302–311. [Google Scholar] [CrossRef]
- Liu, F.; Hu, S.; Yang, H.; Li, Z.; Huang, K.; Su, T.; Wang, S. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv. Healthc. Mater. 2019, 8, 1900411. [Google Scholar] [CrossRef]
- Yaghoubi, Y.; Movassaghpour, A.A.; Zamani, M.; Talebi, M.; Mehdizadeh, A.; Yousefi, M. Human Umbilical Cord Mesenchymal Stem Cells Derived-Exosomes in Diseases Treatment. Life Sci. 2019, 233, 116733. [Google Scholar] [CrossRef]
- Liu, C.; Yin, H.; Jiang, H.; Du, X.; Wang, C.; Liu, Y.; Li, Y.; Yang, Z. Extracellular Vesicles Derived from Mesenchymal Stem Cells Recover Fertility of Premature Ovarian Insufficiency Mice and the Effects on Their Offspring. Cell Transplant. 2020, 29, 1–11. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M.; Zheng, J.; Tian, Y.; Zhang, H.; Tan, Y.; Li, Q.; Zhang, J.; Huang, X. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Improve Ovarian Function and Proliferation of Premature Ovarian Insufficiency by Regulating the Hippo Signaling Pathway. Front. Endocrinol. (Lausanne) 2021, 12, 996. [Google Scholar] [CrossRef]
- Gershon, E.; Dekel, N. Newly Identified Regulators of Ovarian Folliculogenesis and Ovulation. Int. J. Mol. Sci. 2020, 21, 4565. [Google Scholar] [CrossRef] [PubMed]
- Velarde, F.; Castañeda, V.; Morales, E.; Ortega, M.; Ocaña, E.; Álvarez-Barreto, J.; Grunauer, M.; Eguiguren, L.; Caicedo, A. Use of Human Umbilical Cord and Its Byproducts in Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, S.; Kaplan, G.; Gottlieb, A.B.; Schwartz, M.; Walshs, G.; Abalos, R.M.; Fajardo, T.T.; Guido, L.S.; Krueger, J.G. GM-CSF Activates Regenerative Epidermal Growth and Stimulates Keratinocyte Proliferation in Human Skin in Vivo. J. Investig. Dermatol. 1994, 103, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, L.; Wang, Y.; Zhang, S.; Xu, X.; Dai, Y.; Zhao, S.; Li, Z.; Zhang, Y.; Xia, G.; et al. In Vivo and in Vitro Activation of Dormant Primordial Follicles by EGF Treatment in Mouse and Human. Clin. Transl. Med. 2020, 10, e182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Zheng, F.; Ma, N.; Qin, R.; Qin, W.; Liu, B.; Qin, A. Activated Human Umbilical Cord Blood Platelet-Rich Plasma Enhances the Beneficial Effects of Human Umbilical Cord Mesenchymal Stem Cells in Chemotherapy-Induced POF Rats. Stem Cells Int. 2021, 2021, 8293699. [Google Scholar] [CrossRef]
- de Miguel-Gómez, L.; López-Martínez, S.; Francés-Herrero, E.; Rodríguez-Eguren, A.; Pellicer, A.; Cervelló, I. Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models. Cells 2021, 10, 595. [Google Scholar] [CrossRef]
- Santamaria, X.; Cabanillas, S.; Cervelló, I.; Arbona, C.; Raga, F.; Ferro, J.; Palmero, J.; Remohí, J.; Pellicer, A.; Simón, C. Autologous Cell Therapy with CD133+ Bone Marrow-Derived Stem Cells for Refractory Asherman’s Syndrome and Endometrial Atrophy: A Pilot Cohort Study. Hum. Reprod. 2016, 31, 1087–1096. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Li, P.; Wang, Q.; Li, Y.; Li, X.; Zhao, D.; Xu, X.; Kong, L. Autologous Menstrual Blood-Derived Stromal Cells Transplantation for Severe Asherman’s Syndrome. Hum. Reprod. 2016, 31, 2723–2729. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.H.; Zhang, J.K.; Kong, D.S.; Song, Y.B.; Zhao, S.D.; Qi, W.B.; Li, Y.N.; Zhang, M.L.; Huang, X.H. Quantification of the CM-Dil-Labeled Human Umbilical Cord Mesenchymal Stem Cells Migrated to the Dual Injured Uterus in SD Rat. Stem Cell Res. Ther. 2020, 11, 280. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Dong, Y.C.; Guan, C.Y.; Tian, S.; Lv, X.D.; Li, J.H.; Su, X.; Xia, H.F.; Ma, X. Transplantation of Umbilical Cord-Derived Mesenchymal Stem Cells Promotes the Recovery of Thin Endometrium in Rats. Sci. Rep. 2022, 12, 412. [Google Scholar] [CrossRef]
- Zhuang, M.; Zhang, W.; Cheng, N.; Zhou, L.; Liu, D.; Yan, H.; Fang, G.; Heng, B.C.; Sun, Y.; Tong, G. Human Umbilical Cord Mesenchymal Stromal Cells Promote the Regeneration of Severe Endometrial Damage in a Rat Model. Acta Biochim. Biophys. Sin. (Shanghai) 2022, 54, 148–151. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, H.; Zhu, H.; Zhu, X.; Tang, X.; Yan, G.; Wang, J.; Bai, D.; Wang, J.; Wang, L.; et al. Allogeneic Cell Therapy Using Umbilical Cord MSCs on Collagen Scaffolds for Patients with Recurrent Uterine Adhesion: A Phase i Clinical Trial. Stem Cell Res. Ther. 2018, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Shi, C.; Cai, X.; Wang, Y.; Chen, X.; Han, H.; Shen, H. Human Acellular Amniotic Matrix with Previously Seeded Umbilical Cord Mesenchymal Stem Cells Restores Endometrial Function in a Rat Model of Injury. Mediators Inflamm. 2021, 2021, 5573594. [Google Scholar] [CrossRef]
- Zhou, S.; Lei, Y.; Wang, P.; Chen, J.; Zeng, L.; Qu, T.; Maldonado, M.; Huang, J.; Han, T.; Wen, Z.; et al. Human Umbilical Cord Mesenchymal Stem Cells Encapsulated with Pluronic F-127 Enhance the Regeneration and Angiogenesis of Thin Endometrium in Rat via Local IL-1β Stimulation. Stem Cells Int. 2022, 2022, 7819234. [Google Scholar] [CrossRef]
- Wang, L.; Yu, C.; Chang, T.; Zhang, M.; Song, S.; Xiong, C.; Su, P.; Xiang, W. In Situ Repair Abilities of Human Umbilical Cord-Derived Mesenchymal Stem Cells and Autocrosslinked Hyaluronic Acid Gel Complex in Rhesus Monkeys with Intrauterine Adhesion. Sci. Adv. 2020, 6, 6357–6379. [Google Scholar] [CrossRef]
- Krenning, G.; Harmsen, M.C. MicroRNAs in Tissue Engineering and Regenerative Medicine. In MicroRNA in Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2015; pp. 1159–1200. [Google Scholar] [CrossRef]
- Singh, R.; Kaundal, R.K.; Zhao, B.; Bouchareb, R.; Lebeche, D. Resistin Induces Cardiac Fibroblast-Myofibroblast Differentiation through JAK/STAT3 and JNK/c-Jun Signaling. Pharmacol. Res. 2021, 167, 105414. [Google Scholar] [CrossRef]
- Liu, J.; Shang, B.; Bai, J. IL-22/IL-22R1 Promotes Proliferation and Collagen Synthesis of MRC-5 Cells via the JAK/STAT3 Signaling Pathway and Regulates Airway Subepithelial Fibrosis. Exp. Ther. Med. 2020, 20, 2148–2156. [Google Scholar] [CrossRef]
- Yang, L.; Han, B.; Zhang, M.; Wang, Y.H.; Tao, K.; Zhu, M.X.; He, K.; Zhang, Z.G.; Hou, S. Activation of BK Channels Prevents Hepatic Stellate Cell Activation and Liver Fibrosis Through the Suppression of TGFβ1/SMAD3 and JAK/STAT3 Profibrotic Signaling Pathways. Front. Pharmacol. 2020, 11, 165. [Google Scholar] [CrossRef]
- Zhu, D.; Cheng, K. Cardiac Cell Therapy for Heart Repair: Should the Cells Be Left Out? Cells 2021, 10, 641. [Google Scholar] [CrossRef]
- Ebrahim, N.; Mostafa, O.; el Dosoky, R.E.; Ahmed, I.A.; Saad, A.S.; Mostafa, A.; Sabry, D.; Ibrahim, K.A.; Farid, A.S. Human Mesenchymal Stem Cell-Derived Extracellular Vesicles/Estrogen Combined Therapy Safely Ameliorates Experimentally Induced Intrauterine Adhesions in a Female Rat Model. Stem Cell Res. Ther. 2018, 9, 175. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, F.; Yu, Y.; Liu, Y.; Shao, C.; Gu, H.; Li, M.; Zhao, Y. Porous Scaffolds from Droplet Microfluidics for Prevention of Intrauterine Adhesion. Acta Biomater. 2019, 84, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, H.; Lin, N.; Hou, X.; Wang, J.; Zhou, B.; Xu, P.; Xiao, Z.; Chen, B.; Dai, J.; et al. Regeneration of Uterine Horns in Rats by Collagen Scaffolds Loaded with Collagen-Binding Human Basic Fibroblast Growth Factor. Biomaterials 2011, 32, 8172–8181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Tang, X.; Wang, H.; Dai, C.; Su, J.; Zhu, H.; Song, M.; Liu, J.; Nan, Z.; Ru, T.; et al. Collagen-Binding Basic Fibroblast Growth Factor Improves Functional Remodeling of Scarred Endometrium in Uterine Infertile Women: A Pilot Study. Sci. China Life Sci. 2019, 62, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, S.; Rodríguez-Eguren, A.; de Miguel-Gómez, L.; Francés-Herrero, E.; Faus, A.; Díaz, A.; Pellicer, A.; Ferrero, H.; Cervelló, I. Bioengineered Endometrial Hydrogels with Growth Factors Promote Tissue Regeneration and Restore Fertility in Murine Models. Acta Biomater. 2021, 135, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Eguren, A.; de Miguel-Gómez, L.; Francés-Herrero, E.; Gómez-Álvarez, M.; Faus, A.; Gómez-Cerdá, M.; Moret-Tatay, I.; Díaz, A.; Pellicer, A.; Cervelló, I. Human Umbilical Cord Platelet-Rich Plasma to Treat Endometrial Pathologies: Methodology, Composition and Pre-Clinical Models. Hum. Reprod. Open 2022, 2023, 1–14. [Google Scholar] [CrossRef]
- Zheng, S.; Gao, Y.; Chen, K.; Liu, Y.; Xia, N.; Fang, F. A Robust and Highly Efficient Approach for Isolation of Mesenchymal Stem Cells from Wharton’s Jelly for Tissue Repair. Cell Transplant. 2022, 31, 1–16. [Google Scholar] [CrossRef]
- Hamahata, Y.; Akagi, K.; Maeda, T.; Nemoto, K.; Koike, J. Management of Pelvic Organ Prolapse (POP) and Rectal Prolapse. J. Anus. Rectum. Colon. 2022, 6, 83. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, Z.W.; Wen, J.R.; Wang, L.; Huang, L.W.; Yang, Y.L.; Zhao, F.N.; Xiao, J.Y.; Fang, F.; Wu, J.; et al. Status, Challenges, and Future Prospects of Stem Cell Therapy in Pelvic Floor Disorders. World J. Clin. Cases 2020, 8, 1400. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Chen, J.; Li, L.; Liu, X.; Zhang, L.; Ma, C.; Wang, Y.; Tian, W.; Song, X.; et al. Mesenchymal Stem Cell-Based Bioengineered Constructs Enhance Vaginal Repair in Ovariectomized Rhesus Monkeys. Biomaterials 2021, 275, 120863. [Google Scholar] [CrossRef]
- Mao, M.; Li, Y.; Zhang, Y.; Kang, J.; Zhu, L. Human Umbilical Cord Mesenchymal Stem Cells Reconstruct the Vaginal Wall of Ovariectomized Sprague–Dawley Rats: Implications for Pelvic Floor Reconstruction. Cell Tissue Res. 2021, 386, 571–583. [Google Scholar] [CrossRef]
- Deng, M.; Ding, J.; Ai, F.; Mao, M.; Zhu, L. Impact of Human Umbilical Cord–Derived Stem Cells (HUMSCs) on Host Responses to a Synthetic Polypropylene Mesh for Pelvic Floor Reconstruction in a Rat Model. Cell Tissue Res. 2020, 382, 519–527. [Google Scholar] [CrossRef]
- Norton, P.; Brubaker, L. Urinary Incontinence in Women. Lancet 2006, 367, 57–67. [Google Scholar] [CrossRef]
- Hannestad, Y.S.; Rortveit, G.; Sandvik, H.; Hunskaar, S. A Community-Based Epidemiological Survey of Female Urinary Incontinence: The Norwegian EPINCONT Study. J. Clin. Epidemiol. 2000, 53, 1150–1157. [Google Scholar] [CrossRef]
- Lee, C.N.; Jang, J.B.; Kim, J.Y.; Koh, C.; Baek, J.Y.; Lee, K.J. Human Cord Blood Stem Cell Therapy for Treatment of Stress Urinary Incontinence. J. Korean Med. Sci. 2010, 25, 813. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Tang, X.; Li, X.; Li, T. Therapeutic Effect of Human Umbilical Cord Mesenchymal Stem Cells on Tubal Factor Infertility Using a Chronic Salpingitis Murine Model. Arch. Gynecol. Obstet. 2019, 300, 421–429. [Google Scholar] [CrossRef]
- Detels, R.; Green, A.M.; Klausner, J.D.; Katzenstein, D.; Gaydos, C.; Handsfield, H.H.; Pequegnat, W.; Mayer, K.; Hartwell, T.D.; Quinn, T.C. The Incidence and Correlates of Symptomatic and Asymptomatic Chlamydia Trachomatis and Neisseria Gonorrhoeae Infections in Selected Populations in Five Countries. Sex Transm. Dis. 2011, 38, 503. [Google Scholar] [CrossRef] [Green Version]
- Griebel, C.P.; Halvorsen, J.; Golemon, T.B.; Day, A.A. Management of Spontaneous Abortion. Am. Fam. Physician 2005, 72, 1243–1250. [Google Scholar]
- Chen, X.; Yang, X.; Wu, R.; Chen, W.; Xie, H.; Qian, X.; Zhang, Y. Therapeutic Effects of Wharton Jelly-Derived Mesenchymal Stem Cells on Rat Abortion Models. J. Obstet. Gynaecol. Res. 2016, 42, 972–982. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, R.; Jiang, J.; Peng, J.; Yang, C.; Zhang, W.; Wang, S.; Song, J. What Is the Impact of Human Umbilical Cord Mesenchymal Stem Cell Transplantation on Clinical Treatment? Stem Cell Res. Ther. 2020, 11, 519. [Google Scholar] [CrossRef]
- Baba, K.; Yamazaki, Y.; Sone, Y.; Sugimoto, Y.; Moriyama, K.; Sugimoto, T.; Kumazawa, K.; Shimakura, Y.; Takeda, A. An in Vitro Long-Term Study of Cryopreserved Umbilical Cord Blood-Derived Platelet-Rich Plasma Containing Growth Factors—PDGF-BB, TGF-β and VEGF. J. Cranio-Maxillofac. Surg. 2019, 47, 668–675. [Google Scholar] [CrossRef]
- Francés-Herrero, E.; Rodríguez-Eguren, A.; Gómez-Álvarez, M.; Miguel-Gómez, L.d.; Ferrero, H.; Cervelló, I. Future Challenges and Opportunities of Extracellular Matrix Hydrogels in Female Reproductive Medicine. Int. J. Mol. Sci. 2022, 23, 3765. [Google Scholar] [CrossRef] [PubMed]
- Center for Biologics Evaluation and Research BLA for Minimally Manipulated, Unrelated Allogeneic Placental/Umbilical Cord Blood Intended for Hematopoietic and Immunologic Reconstitution in Patients with Disorders Affecting the Hematopoietic System | FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bla-minimally-manipulated-unrelated-allogeneic-placentalumbilical-cord-blood-intended-hematopoietic (accessed on 22 November 2022).
- The Efficacy and Safety of Collagen Scaffold Loaded with Umbilical Cord Derived Mesenchymal Stem Cells in Infertile Women with Thin Endometrium or Endometrial Scarring—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03592849?term=endometrium%2C+umbilical+cord&draw=2&rank=2 (accessed on 29 August 2022).
- HUC Mesenchymal Stem Cells (19#iSCLife®-UT) Therapy for Patients with Thin Endometrial Infertility—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05495711?term=endometrium%2C+umbilical+cord&draw=2&rank=5 (accessed on 29 August 2022).
- Clinical Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Premature Ovarian Insufficiency—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05308342?term=ovary%2C+umbilical+cord&draw=2&rank=1 (accessed on 30 August 2022).
- Stem Cell Therapy Combined Hormone Replacement Therapy in Patients with Premature Ovarian Failure—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01742533?term=ovary%2C+umbilical+cord&draw=2&rank=3 (accessed on 30 August 2022).
- Stem Cells and Secretomes for Infertility Therapy in Polycystic Ovary Syndrome (PCOS) Patients with Insulin Resistance.—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05279768?term=umbilical+cord+stem+cell&cond=ovary&draw=2&rank=1 (accessed on 27 November 2022).
- Chen, H.; Tang, Q.L.; Wu, X.Y.; Xie, L.C.; Lin, L.M.; Ho, G.Y.; Ma, L. Differentiation of Human Umbilical Cord Mesenchymal Stem Cells into Germ-like Cells in Mouse Seminiferous Tubules. Mol. Med. Rep. 2015, 12, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Allah, S.H.; Pasha, H.F.; Abdelrahman, A.A.; Mazen, N.F. Molecular Effect of Human Umbilical Cord Blood CD34-Positive and CD34-Negative Stem Cells and Their Conjugate in Azoospermic Mice. Mol. Cell Biochem. 2017, 428, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.I.; Alam, S.S. Evaluation of Mesenchymal Stem Cells in Treatment of Infertility in Male Rats. Stem Cell Res. Ther. 2014, 5, 131. [Google Scholar] [CrossRef] [Green Version]
- Tamadon, A.; Zhan-byrbekuly, U.; Kairgaliyev, I.; Khoradmehr, A. Mesenchymal Stem Cell Therapy of Male Infertility. In Male Reproductive Health; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
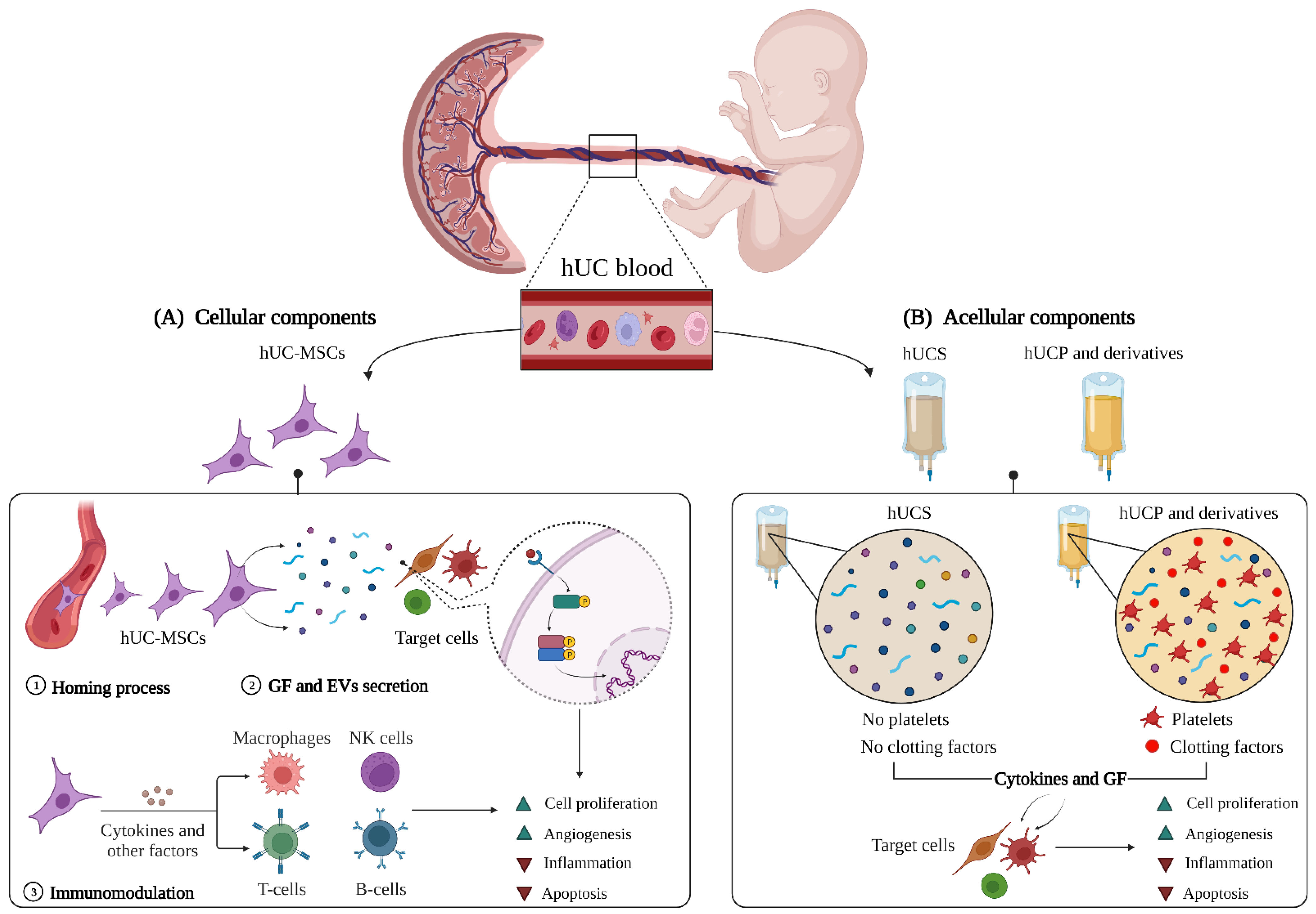

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Eguren, A.; Gómez-Álvarez, M.; Francés-Herrero, E.; Romeu, M.; Ferrero, H.; Seli, E.; Cervelló, I. Human Umbilical Cord-Based Therapeutics: Stem Cells and Blood Derivatives for Female Reproductive Medicine. Int. J. Mol. Sci. 2022, 23, 15942. https://doi.org/10.3390/ijms232415942
Rodríguez-Eguren A, Gómez-Álvarez M, Francés-Herrero E, Romeu M, Ferrero H, Seli E, Cervelló I. Human Umbilical Cord-Based Therapeutics: Stem Cells and Blood Derivatives for Female Reproductive Medicine. International Journal of Molecular Sciences. 2022; 23(24):15942. https://doi.org/10.3390/ijms232415942
Chicago/Turabian StyleRodríguez-Eguren, Adolfo, María Gómez-Álvarez, Emilio Francés-Herrero, Mónica Romeu, Hortensia Ferrero, Emre Seli, and Irene Cervelló. 2022. "Human Umbilical Cord-Based Therapeutics: Stem Cells and Blood Derivatives for Female Reproductive Medicine" International Journal of Molecular Sciences 23, no. 24: 15942. https://doi.org/10.3390/ijms232415942





