Efficient and Reusable Sorbents Based on Nanostructured BN Coatings for Water Treatment from Antibiotics
Abstract
1. Introduction
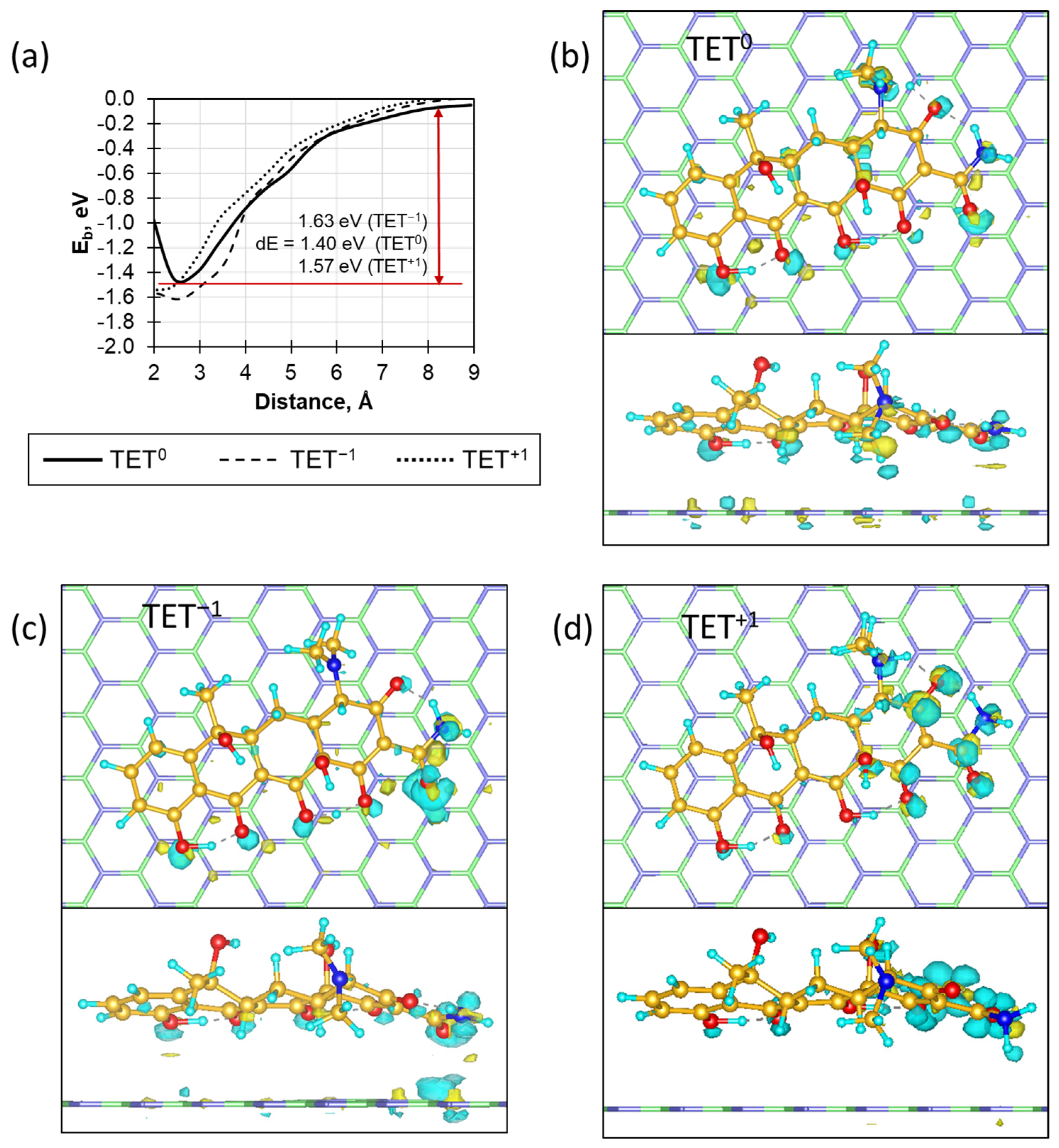
2. Results and Discussion
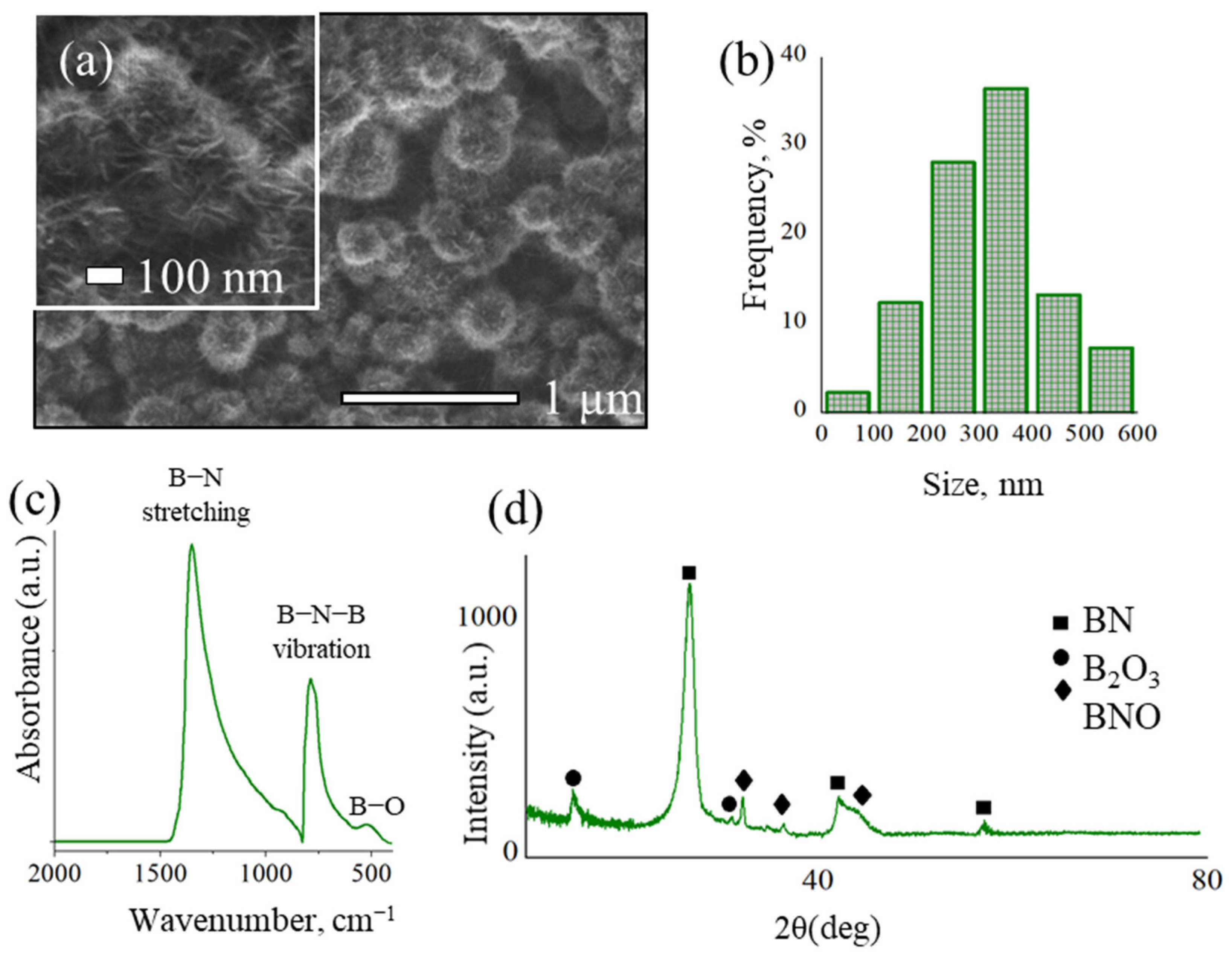
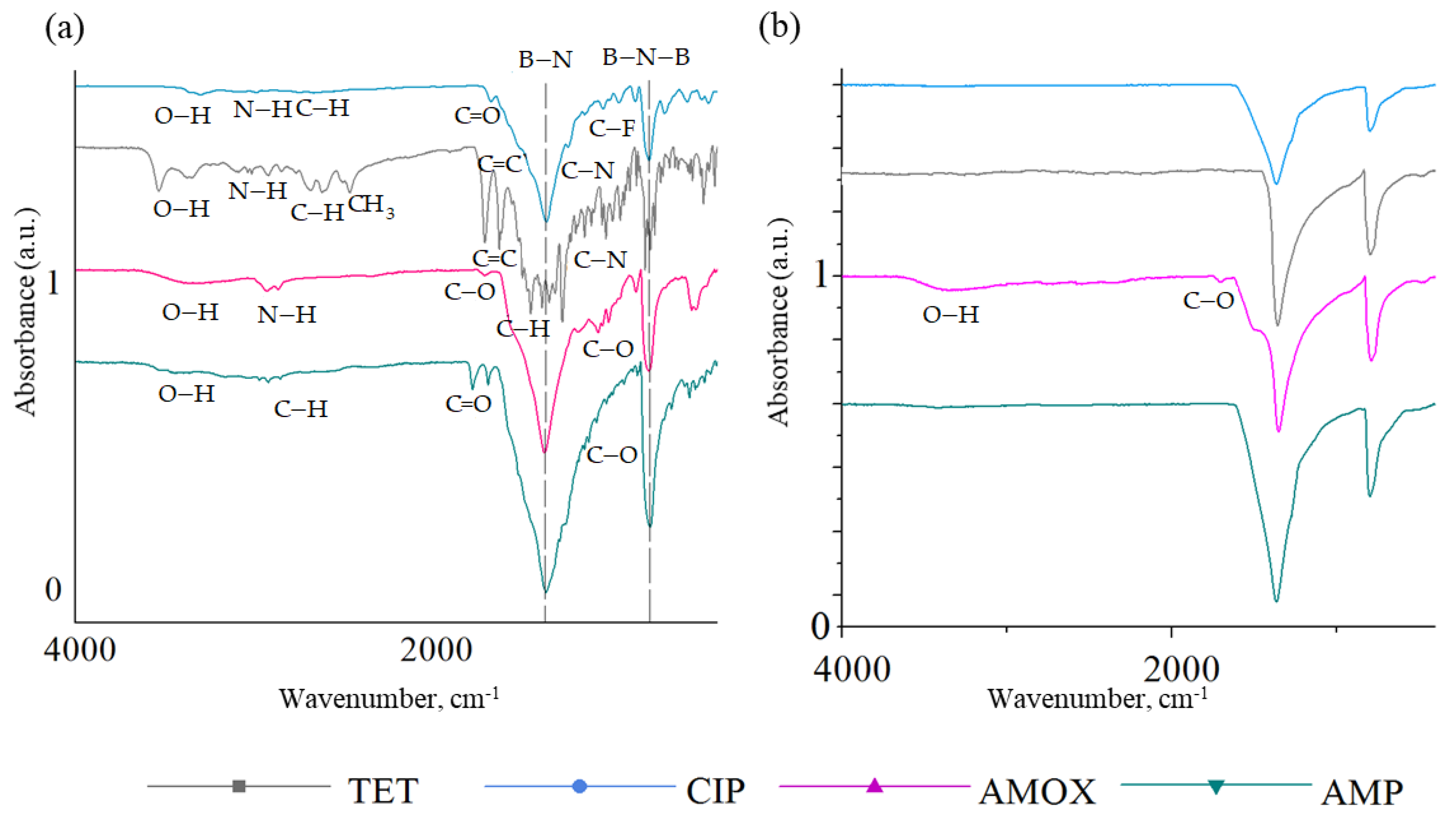
2.1. Characterization of BN Coatings
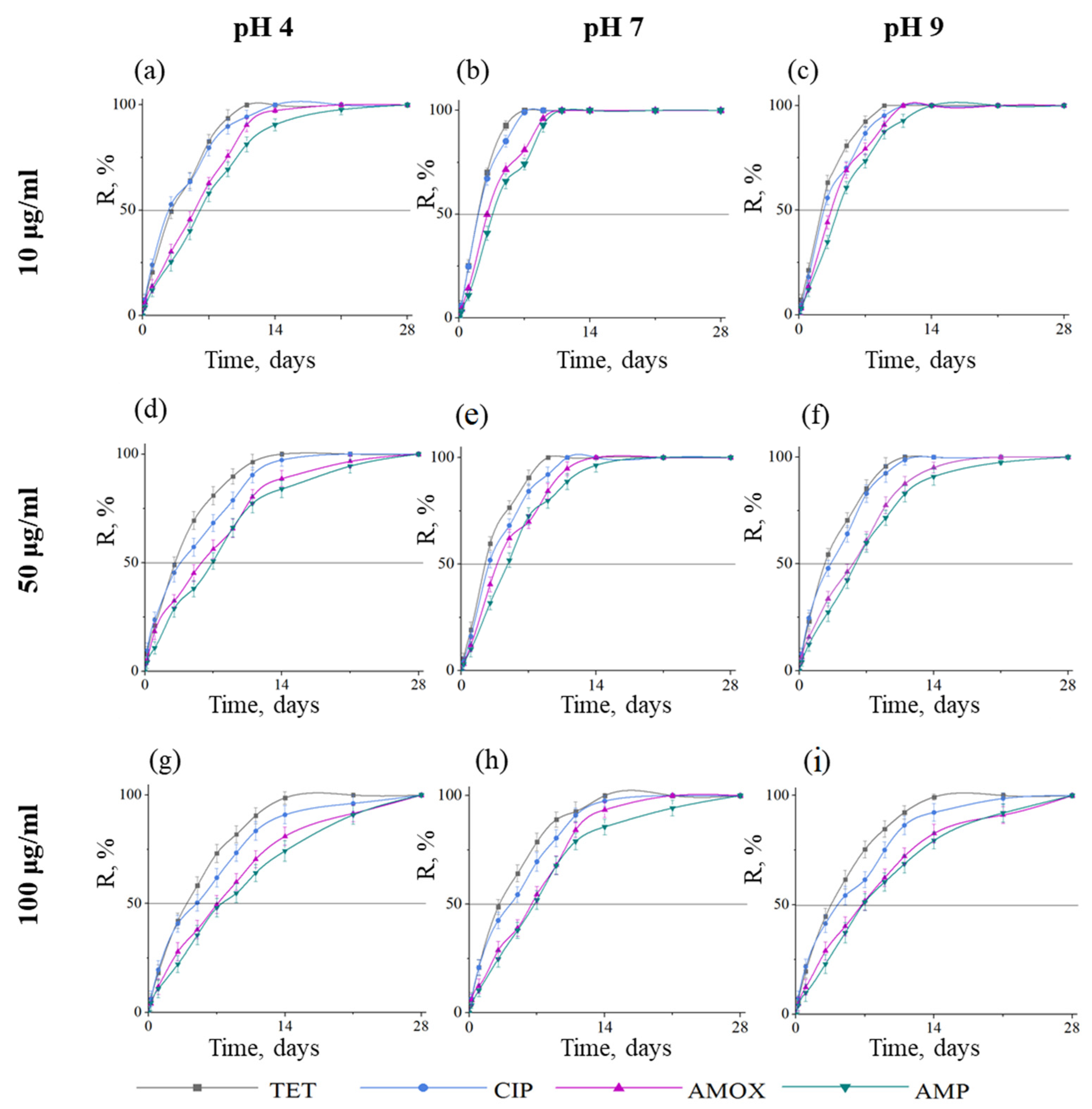
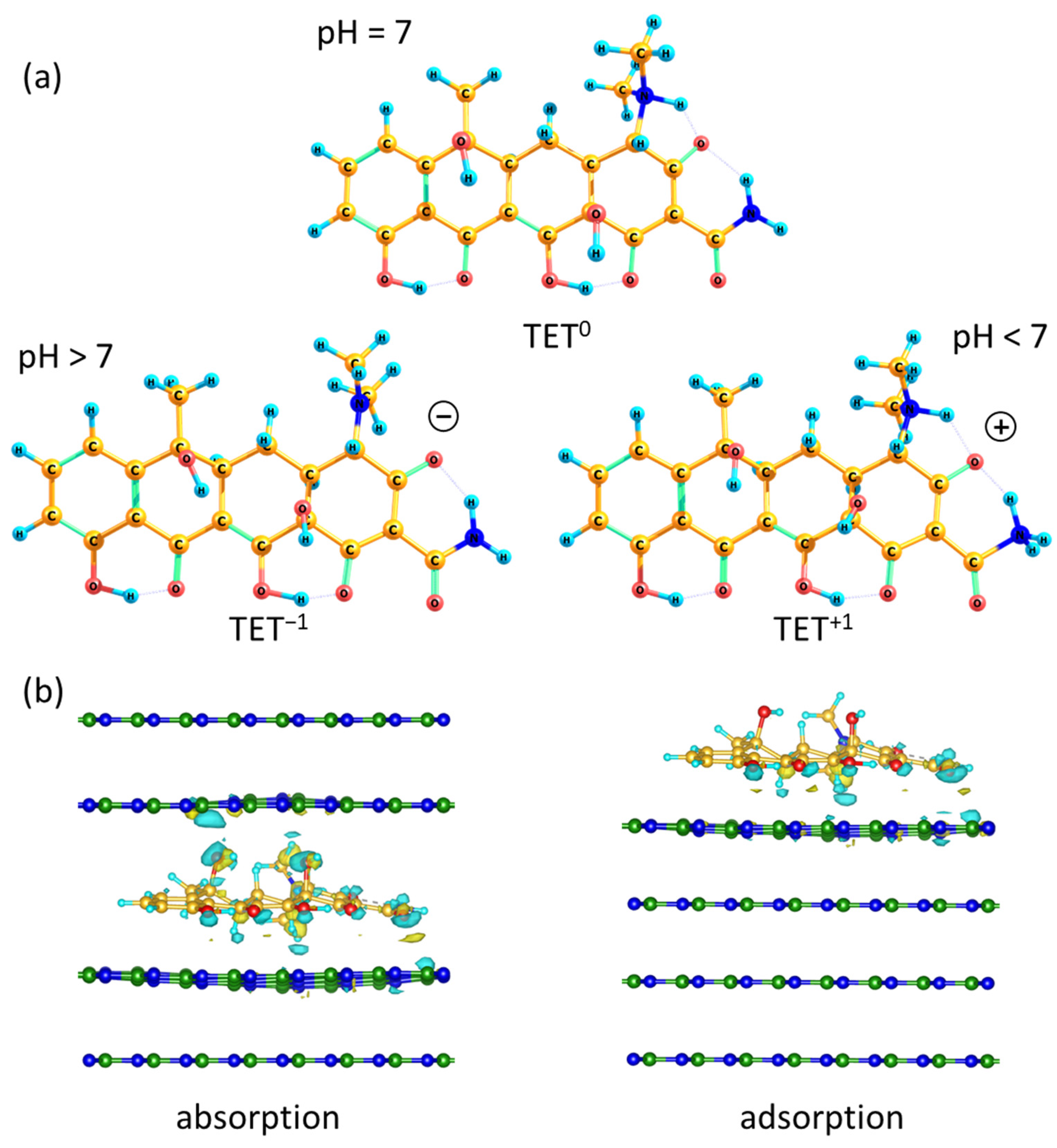
2.2. Kinetics of Antibiotic Adsorption on BN Coatings
2.3. Cleaning BN Coatings from Adsorbed Antibiotics
3. Materials and Methods
3.1. Preparation of BN Coatings
3.2. Structural Characterization of BN Coatings
3.3. Adsorption Studies
3.4. BN Coating Purification from Adsorbed Antibiotics
3.5. DFT Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MEDBOX. The State of the World’s Antibiotics Report 2021. Available online: https://www.medbox.org/document/the-state-of-the-worlds-antibiotics-report-2021#GO (accessed on 1 November 2022).
- Antibiotics Market Size, Growth & Trends|Forecast, 2021–2028. Available online: https://www.fortunebusinessinsights.com/antibiotics-market-104583 (accessed on 1 November 2022).
- Yadav, A.; Indurkar, P.D. Gas Sensor Applications in Water Quality Monitoring and Maintenance. Water Conserv. Sci. Eng. 2021, 6, 175–190. [Google Scholar] [CrossRef]
- Alnajrani, M.N.; Alsager, O.A. Removal of Antibiotics from Water by Polymer of Intrinsic Microporosity: Isotherms, Kinetics, Thermodynamics, and Adsorption Mechanism. Sci. Rep. 2020, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and Antibiotic Resistant Genes (ARGs) in Groundwater: A Global Review on Dissemination, Sources, Interactions, Environmental and Human Health Risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.; Pan, X.; Qiang, Z. Occurrence and Partition of Antibiotics in the Liquid and Solid Phases of Swine Wastewater from Concentrated Animal Feeding Operations in Shandong Province, China. Environ. Sci. Process. Impacts 2013, 15, 870–875. [Google Scholar] [CrossRef]
- Daouk, S.; Chèvre, N.; Vernaz, N.; Widmer, C.; Daali, Y.; Fleury-Souverain, S. Dynamics of Active Pharmaceutical Ingredients Loads in a Swiss University Hospital Wastewaters and Prediction of the Related Environmental Risk for the Aquatic Ecosystems. Sci. Total Environ. 2016, 547, 244–253. [Google Scholar] [CrossRef]
- Hou, J.; Wang, C.; Mao, D.; Luo, Y. The Occurrence and Fate of Tetracyclines in Two Pharmaceutical Wastewater Treatment Plants of Northern China. Environ. Sci. Pollut. Res. 2016, 23, 1722–1731. [Google Scholar] [CrossRef]
- Mohapatra, S.; Huang, C.-H.; Mukherji, S.; Padhye, L.P. Occurrence and Fate of Pharmaceuticals in WWTPs in India and Comparison with a Similar Study in the United States. Chemosphere 2016, 159, 526–535. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental Risk Assessment of Pharmaceutical Residues in Wastewater Effluents, Surface Waters and Sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Hartmann, A.; Alder, A.C.; Koller, T.; Widmer, R.M. Identification of Fluoroquinolone Antibiotics as the Main Source of UmuC Genotoxicity in Native Hospital Wastewater. Environ. Toxicol. Chem. 1998, 17, 377–382. [Google Scholar] [CrossRef]
- Reinthaler, F.F.; Posch, J.; Feierl, G.; Wüst, G.; Haas, D.; Ruckenbauer, G.; Mascher, F.; Marth, E. Antibiotic Resistance of E. Coli in Sewage and Sludge. Water Res. 2003, 37, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- GLASS Report: Early Implementation 2020. Available online: https://www.who.int/publications-detail-redirect/9789240005587 (accessed on 1 November 2022).
- McConnell, M.M.; Truelstrup Hansen, L.; Jamieson, R.C.; Neudorf, K.D.; Yost, C.K.; Tong, A. Removal of Antibiotic Resistance Genes in Two Tertiary Level Municipal Wastewater Treatment Plants. Sci. Total Environ. 2018, 643, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Tototzintle, M.; Ferreira, I.J.; da Silva Duque, S.; Guimarães Barrocas, P.R.; Saggioro, E.M. Removal of Contaminants of Emerging Concern (CECs) and Antibiotic Resistant Bacteria in Urban Wastewater Using UVA/TiO2/H2O2 Photocatalysis. Chemosphere 2018, 210, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, B. Occurrence, Transformation, and Fate of Antibiotics in Municipal Wastewater Treatment Plants. Crit. Rev. Environ. Sci. Technol. 2011, 41, 951–998. [Google Scholar] [CrossRef]
- Boeckel, T.P.V.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global Antibiotic Consumption 2000 to 2010: An Analysis of National Pharmaceutical Sales Data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Fate of Antibiotics in Soil and Their Uptake by Edible Crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Hatzisymeon, M.; Tataraki, D.; Rassias, G. Remediation of Ciprofloxacin-Contaminated Soil by Nanosecond Pulsed Dielectric Barrier Discharge Plasma: Influencing Factors and Degradation Mechanisms. Chem. Eng. J. 2020, 393, 124768. [Google Scholar] [CrossRef]
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.-H.; Oeurng, C.; et al. Antibiotics in Surface Water of East and Southeast Asian Countries: A Focused Review on Contamination Status, Pollution Sources, Potential Risks, and Future Perspectives. Sci. Total Environ. 2021, 764, 142865. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nam, S.-N.; Son, J.; Oh, J. Tungsten Trioxide (WO3)-Assisted Photocatalytic Degradation of Amoxicillin by Simulated Solar Irradiation. Sci. Rep. 2019, 9, 9349. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic Residues in Final Effluents of European Wastewater Treatment Plants and Their Impact on the Aquatic Environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Riva, F.; Zuccato, E.; Davoli, E.; Fattore, E.; Castiglioni, S. Risk Assessment of a Mixture of Emerging Contaminants in Surface Water in a Highly Urbanized Area in Italy. J. Hazard. Mater. 2019, 361, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Fülöp, Z.; Ritthidej, G.C. Amphotericin B Loaded Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carrier (NLCs): Physicochemical and Solid-Solution State Characterizations. Drug Dev. Ind. Pharm. 2019, 45, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Krsmanovic, A.; Ballhausen, M.-B.; Rillig, M.C. Fungal Response to Abruptly or Gradually Delivered Antifungal Agent Amphotericin B Is Growth Stage Dependent. Environ. Microbiol. 2021, 23, 7701–7709. [Google Scholar] [CrossRef]
- Gallis, H.A.; Drew, R.H.; Pickard, W.W. Amphotericin B: 30 Years of Clinical Experience. Rev. Infect. Dis. 1990, 12, 308–329. [Google Scholar] [CrossRef]
- Chang, C.; Gupta, P. In-Situ Degradation of Amphotericin B in a Microbial Electrochemical Cell Containing Wastewater. Chemosphere 2022, 309, 136726. [Google Scholar] [CrossRef] [PubMed]
- Blum, G.; Perkhofer, S.; Haas, H.; Schrettl, M.; Würzner, R.; Dierich, M.P.; Lass-Flörl, C. Potential Basis for Amphotericin B Resistance in Aspergillus Terreus. Antimicrob. Agents Chemother. 2008, 52, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Balarak, D.; Mostafapour, F.K. Photocatalytic Degradation of Amoxicillin Using UV/Synthesized NiO from Pharmaceutical Wastewater. Indones. J. Chem. 2019, 19, 211–218. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Liu, S.; Shu, D.; Zeng, G.; Hu, X.; Tan, X.; Jiang, L.; Yan, Z.; Cai, X. Cu(II)-Influenced Adsorption of Ciprofloxacin from Aqueous Solutions by Magnetic Graphene Oxide/Nitrilotriacetic Acid Nanocomposite: Competition and Enhancement Mechanisms. Chem. Eng. J. 2017, 319, 219–228. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Ng, Y.H.; Ok, Y.S. Recent Advances in Photodegradation of Antibiotic Residues in Water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef]
- Zhong, Y.; Han, L.; Yin, X.; Li, H.; Fang, D.; Hong, G. Three Dimensional Functionalized Carbon/Tin(IV) Sulfide Biofoam for Photocatalytical Purification of Chromium(VI)-Containing Wastewater. ACS Sustain. Chem. Eng. 2018, 6, 10660–10667. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Y.-J.; He, G.; Li, H.; Tao, J.-C. Multifunctional Photocatalytic Filter Paper Based on Ultralong Nanowires of the Calcium-Alendronate Complex for High-Performance Water Purification. ACS Appl. Mater. Interfaces 2022, 14, 9464–9479. [Google Scholar] [CrossRef] [PubMed]
- Alagha, O.; Ouerfelli, N.; Kochkar, H.; Almessiere, M.A.; Slimani, Y.; Manikandan, A.; Baykal, A.; Mostafa, A.; Zubair, M.; Barghouthi, M.H. Kinetic Modeling for Photo-Assisted Penicillin G Degradation of (Mn0.5Zn0.5)[CdxFe2−x]O4 (x ≤ 0.05) Nanospinel Ferrites. Nanomaterials 2021, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Antipina, L.Y.; Kotyakova, K.Y.; Tregubenko, M.V.; Shtansky, D.V. Experimental and Theoretical Study of Sorption Capacity of Hexagonal Boron Nitride Nanoparticles: Implication for Wastewater Purification from Antibiotics. Nanomaterials 2022, 12, 3157. [Google Scholar] [CrossRef] [PubMed]
- Yaghmaeian, K.; Moussavi, G.; Alahabadi, A. Removal of Amoxicillin from Contaminated Water Using NH4Cl-Activated Carbon: Continuous Flow Fixed-Bed Adsorption and Catalytic Ozonation Regeneration. Chem. Eng. J. 2014, 236, 538–544. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, P.; Seredych, M.; Bandosz, T.J. Removal of Antibiotics from Water Using Sewage Sludge- and Waste Oil Sludge-Derived Adsorbents. Water Res. 2012, 46, 4081–4090. [Google Scholar] [CrossRef] [PubMed]
- Guiza, S. Biosorption of Heavy Metal from Aqueous Solution Using Cellulosic Waste Orange Peel. Ecol. Eng. 2017, 99, 134–140. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Kareem, S.L. Adsorption of Tetracycline Fom Wastewater by Using Pistachio Shell Coated with ZnO Nanoparticles: Equilibrium, Kinetic and Isotherm Studies. Alex. Eng. J. 2019, 58, 917–928. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Singh, A.; Carabineiro, S.A.C. Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics. Nanomaterials 2021, 11, 568. [Google Scholar] [CrossRef]
- Liu, W.-X.; Song, S.; Ye, M.-L.; Zhu, Y.; Zhao, Y.-G.; Lu, Y. Nanomaterials with Excellent Adsorption Characteristics for Sample Pretreatment: A Review. Nanomaterials 2022, 12, 1845. [Google Scholar] [CrossRef]
- Patiño, Y.; Díaz, E.; Ordóñez, S.; Gallegos-Suarez, E.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Adsorption of Emerging Pollutants on Functionalized Multiwall Carbon Nanotubes. Chemosphere 2015, 136, 174–180. [Google Scholar] [CrossRef]
- Wang, X.; Yin, R.; Zeng, L.; Zhu, M. A Review of Graphene-Based Nanomaterials for Removal of Antibiotics from Aqueous Environments. Environ. Pollut. 2019, 253, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, W.; Jiang, H.; Lin, R.; Wang, Z.; Wu, J.; He, G.; Shearing, P.R.; Brett, D.J.L. ZIF-8-Derived Hollow Carbon for Efficient Adsorption of Antibiotics. Nanomaterials 2019, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhu, Y.; Yuan, S.; Wang, F.; Tang, H.; Bao, Z.; Zhou, H.; Chen, Y. Adsorption of Tetracycline with Reduced Graphene Oxide Decorated with MnFe2O4 Nanoparticles. Nanoscale Res. Lett. 2018, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and Removal of Tetracycline Antibiotics from Aqueous Solution by Graphene Oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Al-Musawi, T.J.; Saloot, M.K.; Khatibi, A.D.; Baniasadi, M.; Balarak, D. Synthesis of Activated Carbon from Lemna Minor Plant and Magnetized with Iron (III) Oxide Magnetic Nanoparticles and Its Application in Removal of Ciprofloxacin. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Sun, T.; Fan, R.; Zhang, J.; Qin, M.; Chen, W.; Jiang, X.; Zhu, K.; Ji, C.; Hao, S.; Yang, Y. Stimuli-Responsive Metal–Organic Framework on a Metal–Organic Framework Heterostructure for Efficient Antibiotic Detection and Anticounterfeiting. ACS Appl. Mater. Interfaces 2021, 13, 35689–35699. [Google Scholar] [CrossRef]
- Azhar, M.R.; Abid, H.R.; Sun, H.; Periasamy, V.; Tadé, M.O.; Wang, S. Excellent Performance of Copper Based Metal Organic Framework in Adsorptive Removal of Toxic Sulfonamide Antibiotics from Wastewater. J. Colloid Interface Sci. 2016, 478, 344–352. [Google Scholar] [CrossRef]
- Dehghan, A.; Mohammadi, A.A.; Yousefi, M.; Najafpoor, A.A.; Shams, M.; Rezania, S. Enhanced Kinetic Removal of Ciprofloxacin onto Metal-Organic Frameworks by Sonication, Process Optimization and Metal Leaching Study. Nanomaterials 2019, 9, 1422. [Google Scholar] [CrossRef]
- Yadav, A.; Dindorkar, S.S.; Ramisetti, S.B. Adsorption Behaviour of Boron Nitride Nanosheets towards the Positive, Negative and the Neutral Antibiotics: Insights from First Principle Studies. J. Water Process Eng. 2022, 46, 102555. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Shen, G.; Zhi, C.; Golberg, D. Single-Source Precursor for Chemical Vapour Deposition of Collapsed Boron Nitride Nanotubes. Nanotechnology 2006, 17, 5882–5888. [Google Scholar] [CrossRef]
- Dai, P.; Xue, Y.; Wang, X.; Weng, Q.; Zhang, C.; Jiang, X.; Tang, D.; Wang, X.; Kawamoto, N.; Ide, Y.; et al. Pollutant Capturing SERS Substrate: Porous Boron Nitride Microfibers with Uniform Silver Nanoparticle Decoration. Nanoscale 2015, 7, 18992–18997. [Google Scholar] [CrossRef] [PubMed]
- Shtansky, D.V.; Firestein, K.L.; Golberg, D. Fabrication and Application of BN Nanoparticles, Nanosheets, and Their Nanohybrids. Nanoscale 2018, 10, 17477. [Google Scholar] [CrossRef]
- Zhang, X.; Lian, G.; Zhang, S.; Cui, D.; Wang, Q. Boron Nitride Nanocarpets: Controllable Synthesis and Their Adsorption Performance to Organic Pollutants. CrystEngComm 2012, 14, 4670–4676. [Google Scholar] [CrossRef]
- Bangari, R.S.; Yadav, A.; Bharadwaj, J.; Sinha, N. Boron Nitride Nanosheets Incorporated Polyvinylidene Fluoride Mixed Matrix Membranes for Removal of Methylene Blue from Aqueous Stream. J. Environ. Chem. Eng. 2022, 10, 107052. [Google Scholar] [CrossRef]
- Bangari, R.S.; Sinha, N. Adsorption of Tetracycline, Ofloxacin and Cephalexin Antibiotics on Boron Nitride Nanosheets from Aqueous Solution. J. Mol. Liq. 2019, 293, 111376. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, K.; Li, D.; Tang, Y. Boron Nitride Adsorbents with Sea Urchin-like Structures for Enhanced Adsorption Performance. J. Am. Ceram. Soc. 2021, 104, 1601–1610. [Google Scholar] [CrossRef]
- Song, Q.; Liang, J.; Fang, Y.; Guo, Z.; Du, Z.; Zhang, L.; Liu, Z.; Huang, Y.; Lin, J.; Tang, C. Nickel (II) Modified Porous Boron Nitride: An Effective Adsorbent for Tetracycline Removal from Aqueous Solution. Chem. Eng. J. 2020, 394, 124985. [Google Scholar] [CrossRef]
- Liu, D.; Lei, W.; Qin, S.; Klika, K.D.; Chen, Y. Superior Adsorption of Pharmaceutical Molecules by Highly Porous BN Nanosheets. Phys. Chem. Chem. Phys. 2015, 18, 84–88. [Google Scholar] [CrossRef]
- Moon, O.M.; Kang, B.C.; Lee, S.B.; Boo, J.H. Temperature Effect on Structural Properties of Boron Oxide Thin Films Deposited by MOCVD Method. Thin Solid Films 2004, 464–465, 164. [Google Scholar] [CrossRef]
- Konopatsky, A.S.; Firestein, K.L.; Leybo, D.V.; Popov, Z.I.; Larionov, K.V.; Steinman, A.E.; Kovalskii, A.M.; Matveev, A.T.; Manakhov, A.M.; Sorokin, P.B.; et al. BN Nanoparticle/Ag Hybrids with Enhanced Catalytic Activity: Theory and Experiments. Catal. Sci. Technol. 2018, 8, 1652–1662. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Liu, Z.; Zhang, J.; Liu, X.; Luo, H.; Ma, Y.; Xu, X.; Lu, Y.; Lin, J.; et al. Chemical Activation of Boron Nitride Fibers for Improved Cationic Dye Removal Performance. J. Mater. Chem. A 2015, 3, 8185–8193. [Google Scholar] [CrossRef]
- Lincopan, N.; Carmona-Ribeiro, A.M. Lipid-Covered Drug Particles: Combined Action of Dioctadecyldimethylammonium Bromide and Amphotericin B or Miconazole. J. Antimicrob. Chemother. 2006, 58, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Chakraborti, C.K.; Mishra, S.C. Qualitative Analysis of Controlled Release Ciprofloxacin/Carbopol 934 Mucoadhesive Suspension. J. Adv. Pharm. Technol. Res. 2011, 2, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Patil, S.; Shettigar, H.; Bairwa, K.; Jana, S. Spectroscopic Characterization of Chloramphenicol and Tetracycline: An Impact of Biofield Treatment. Pharm. Anal. Acta 2015, 6, 395. [Google Scholar] [CrossRef]
- Junejo, Y.; Güner, A.; Baykal, A. Synthesis and Characterization of Amoxicillin Derived Silver Nanoparticles: Its Catalytic Effect on Degradation of Some Pharmaceutical Antibiotics. Appl. Surf. Sci. 2014, 317, 914–922. [Google Scholar] [CrossRef]
- Chhonker, Y.S.; Prasad, Y.D.; Chandasana, H.; Vishvkarma, A.; Mitra, K.; Shukla, P.K.; Bhatta, R.S. Amphotericin-B Entrapped Lecithin/Chitosan Nanoparticles for Prolonged Ocular Application. Int. J. Biol. Macromol. 2015, 72, 1451–1458. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, T.; Liu, J.; Li, D. Adsorption of Tetracycline Antibiotics from an Aqueous Solution onto Graphene Oxide/Calcium Alginate Composite Fibers. RSC Adv. 2018, 8, 2616–2621. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mahvi, A.H.; Khatibi, A.D.; Balarak, D. Effective Adsorption of Ciprofloxacin Antibiotic Using Powdered Activated Carbon Magnetized by Iron(III) Oxide Magnetic Nanoparticles. J. Porous Mater. 2021, 28, 835–852. [Google Scholar] [CrossRef]
- Chaba, J.M.; Nomngongo, P.N. Effective Adsorptive Removal of Amoxicillin from Aqueous Solutions and Wastewater Samples Using Zinc Oxide Coated Carbon Nanofiber Composite. Emerg. Contam. 2019, 5, 143–149. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Liu, H.; Xie, H.; Lu, S.; Wang, Q.; Zhang, J. Adsorption of Amoxicillin by Mn-Impregnated Activated Carbons: Performance and Mechanisms. RSC Adv. 2016, 6, 11454–11460. [Google Scholar] [CrossRef]
- Jannat Abadi, M.H.; Nouri, S.M.M.; Zhiani, R.; Heydarzadeh, H.D.; Motavalizadehkakhky, A. Removal of Tetracycline from Aqueous Solution Using Fe-Doped Zeolite. Int. J. Ind. Chem. 2019, 10, 291–300. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Gupta, K.; Li, J.-R.; Yuan, B.; Yang, J.-C.E.; Fu, M.-L. Novel Chalcogenide Based Magnetic Adsorbent KMS-1/L-Cystein/Fe3O4 for the Facile Removal of Ciprofloxacin from Aqueous Solution. Colloids Surf. Physicochem. Eng. Asp. 2018, 538, 378–386. [Google Scholar] [CrossRef]
- Fazelirad, H.; Ranjbar, M.; Taher, M.A.; Sargazi, G. Preparation of Magnetic Multi-Walled Carbon Nanotubes for an Efficient Adsorption and Spectrophotometric Determination of Amoxicillin. J. Ind. Eng. Chem. 2015, 21, 889–892. [Google Scholar] [CrossRef]
- Chang, J.; Shen, Z.; Hu, X.; Schulman, E.; Cui, C.; Guo, Q.; Tian, H. Adsorption of Tetracycline by Shrimp Shell Waste from Aqueous Solutions: Adsorption Isotherm, Kinetics Modeling, and Mechanism. ACS Omega 2020, 5, 3467–3477. [Google Scholar] [CrossRef]
- Stylianou, M.; Christou, A.; Michael, C.; Agapiou, A.; Papanastasiou, P.; Fatta-Kassinos, D. Adsorption and Removal of Seven Antibiotic Compounds Present in Water with the Use of Biochar Derived from the Pyrolysis of Organic Waste Feedstocks. J. Environ. Chem. Eng. 2021, 9, 105868. [Google Scholar] [CrossRef]
- Hu, D.; Wang, L. Adsorption of Amoxicillin onto Quaternized Cellulose from Flax Noil: Kinetic, Equilibrium and Thermodynamic Study. J. Taiwan Inst. Chem. Eng. 2016, 64, 227–234. [Google Scholar] [CrossRef]
- Lu, L.; Liu, M.; Chen, Y.; Luo, Y. Effective Removal of Tetracycline Antibiotics from Wastewater Using Practically Applicable Iron(III)-Loaded Cellulose Nanofibres. R. Soc. Open Sci. 2021, 8, 210336. [Google Scholar] [CrossRef]
- Abu Rumman, G.; Al-Musawi, T.J.; Sillanpaa, M.; Balarak, D. Adsorption Performance of an Amine-Functionalized MCM–41 Mesoporous Silica Nanoparticle System for Ciprofloxacin Removal. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100536. [Google Scholar] [CrossRef]
- Jafari, K.; Heidari, M.; Rahmanian, O. Wastewater Treatment for Amoxicillin Removal Using Magnetic Adsorbent Synthesized by Ultrasound Process. Ultrason. Sonochem. 2018, 45, 248–256. [Google Scholar] [CrossRef]
- Gudz, K.Y.; Permyakova, E.S.; Matveev, A.T.; Bondarev, A.V.; Manakhov, A.M.; Sidorenko, D.A.; Filippovich, S.Y.; Brouchkov, A.V.; Golberg, D.V.; Ignatov, S.G.; et al. Pristine and Antibiotic-Loaded Nanosheets/Nanoneedles-Based Boron Nitride Films as a Promising Platform to Suppress Bacterial and Fungal Infections. ACS Appl. Mater. Interfaces 2020, 12, 42485–42498. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, 1133–1138. [Google Scholar] [CrossRef]
- Troullier, N.; Martins, J.L. Efficient Pseudopotentials for Plane-Wave Calculations. Phys. Rev. B Condens. Matter 1991, 43, 1993–2006. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]

| Antibiotic | Purification Efficiency, % | Initial Antibiotic Concentration, µg/mL | Time, Days | ||
|---|---|---|---|---|---|
| pH 4 | pH 7 | pH 9 | |||
| TET | R50 | 10 | 2 | 2 | 2 |
| 50 | 3 | 2 | 2 | ||
| 100 | 4 | 2 | 3 | ||
| R100 | 10 | 12 | 7 | 11 | |
| 50 | 15 | 9 | 12 | ||
| 100 | 18 | 10 | 14 | ||
| CIP | R50 | 10 | 3 | 2 | 3 |
| 50 | 4 | 3 | 3 | ||
| 100 | 4 | 3 | 4 | ||
| R100 | 10 | 14 | 8 | 12 | |
| 50 | 21 | 10 | 14 | ||
| 100 | 23 | 11 | 14 | ||
| AMOX | R50 | 10 | 5 | 3 | 4 |
| 50 | 5 | 3 | 5 | ||
| 100 | 8 | 4 | 6 | ||
| R100 | 10 | 17 | 10 | 14 | |
| 50 | 28 | 11 | 14 | ||
| 100 | 26 | 12 | 15 | ||
| AMP | R50 | 10 | 5 | 3 | 5 |
| 50 | 6 | 4 | 7 | ||
| 100 | 8 | 5 | 6 | ||
| R100 | 10 | 23 | 11 | 18 | |
| 50 | 28 | 12 | 18 | ||
| 100 | 28 | 14 | 21 | ||
| Time, Days | TET | CIP | AMOX | AMP | ||||
|---|---|---|---|---|---|---|---|---|
| I-R,% | II-R,% | I-R,% | II-R,% | I-R,% | II-R,% | I-R,% | II-R,% | |
| Initial concentration of antibiotic 10 µg/mL | ||||||||
| 1 | 26.5 | 25.0 | 24.7 | 23.1 | 17.9 | 14.3 | 13.3 | 10.9 |
| 7 | 100 | 100 | 98.7 | 97.2 | 86.0 | 80.9 | 83.9 | 74.2 |
| 14 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 28 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Initial concentration of antibiotic 50 µg/mL | ||||||||
| 1 | 23.9 | 21.0 | 19.2 | 16.1 | 15.5 | 12.0 | 12.7 | 10.1 |
| 7 | 94.7 | 90.5 | 90.7 | 84.1 | 82.1 | 69.9 | 80.1 | 72.5 |
| 14 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 96.3 |
| 28 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Initial concentration of antibiotic 100 µg/mL | ||||||||
| 1 | 20.4 | 19.3 | 17.9 | 20.9 | 14.1 | 12.4 | 13.9 | 10.3 |
| 7 | 89.5 | 78.6 | 83.4 | 69.5 | 73.8 | 54.4 | 77.6 | 51.9 |
| 14 | 100 | 100 | 100 | 97.6 | 100 | 93.5 | 100 | 85.6 |
| 28 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Material | Adsorption Capacity (qe), mg/g | Material | Adsorption Capacity (qe), mg/g | Material | Adsorption Capacity (qe), mg/g |
|---|---|---|---|---|---|
| Tetracycline | Ciprofloxacin | Amoxicillin | |||
| Graphene oxide/calcium alginate composite fibers [70] | 131.6 | Powdered activated carbon magnetized by iron(III) oxide NPs [71] | 109.6 | Zinc oxide coated carbon nanofiber composite [72] | 156.0 |
| Graphene oxide [47] | 313 | Activated carbon magnetized with iron (III) oxide NPs [48] | 178.7 | Mn-impregnated activated carbons [73] | 122.0–132.0 |
| Fe-doped zeolite [74] | 200.0 | Chalcogenide based magnetic adsorbent [75] | 181.3 | Magnetic multi-walled carbon nanotubes [76] | 50.0 |
| Shrimp shell waste [77] | 230.0 | Hydrogel derived from agrowaste [78] | 106.9 | Quaternized cellulose from flax noil [79] | 183.1 |
| Iron(III)-loaded cellulose nanofibers [80] | 294.1 | Amine-functionalized MCM-41 mesoporous silica NPs [81] | 164.3 | Magnetic adsorbent [82] | 238.1 |
| BN coatings * | 502.8 | BN coatings * | 315.4 | BN coatings * | 400.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotyakova, K.Y.; Antipina, L.Y.; Sorokin, P.B.; Shtansky, D.V. Efficient and Reusable Sorbents Based on Nanostructured BN Coatings for Water Treatment from Antibiotics. Int. J. Mol. Sci. 2022, 23, 16097. https://doi.org/10.3390/ijms232416097
Kotyakova KY, Antipina LY, Sorokin PB, Shtansky DV. Efficient and Reusable Sorbents Based on Nanostructured BN Coatings for Water Treatment from Antibiotics. International Journal of Molecular Sciences. 2022; 23(24):16097. https://doi.org/10.3390/ijms232416097
Chicago/Turabian StyleKotyakova, Kristina Yu., Liubov Yu. Antipina, Pavel B. Sorokin, and Dmitry V. Shtansky. 2022. "Efficient and Reusable Sorbents Based on Nanostructured BN Coatings for Water Treatment from Antibiotics" International Journal of Molecular Sciences 23, no. 24: 16097. https://doi.org/10.3390/ijms232416097
APA StyleKotyakova, K. Y., Antipina, L. Y., Sorokin, P. B., & Shtansky, D. V. (2022). Efficient and Reusable Sorbents Based on Nanostructured BN Coatings for Water Treatment from Antibiotics. International Journal of Molecular Sciences, 23(24), 16097. https://doi.org/10.3390/ijms232416097








