Molecular Markers of Adult Neurogenesis in the Telencephalon and Tectum of Rainbow Trout, Oncorhynchus mykiss
Abstract
:1. Introduction
2. Results
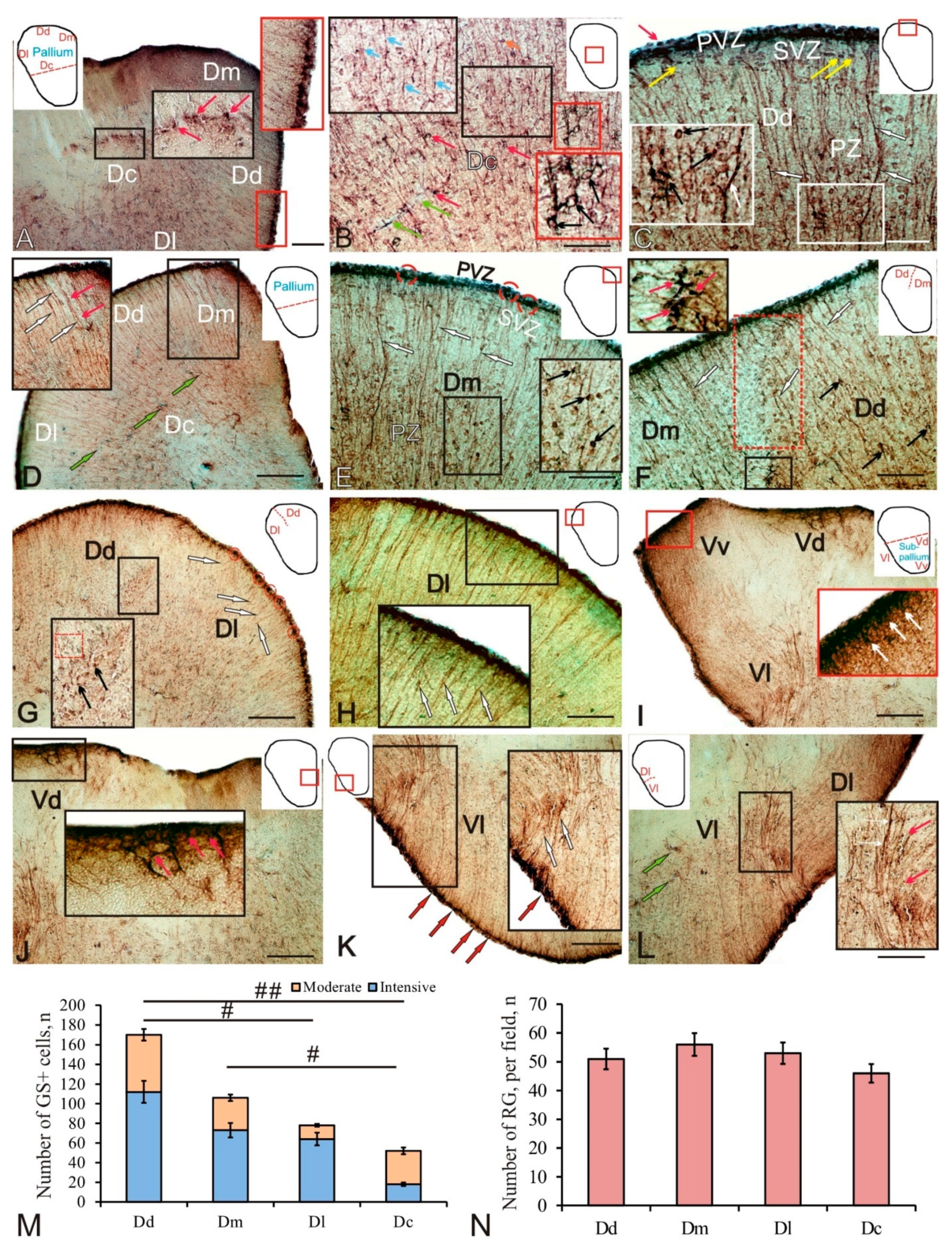
2.1. Glutamine Synthetase in the Telencephalon of Trout
2.2. Glutamine Synthetase in the Trout Tectum Opticum
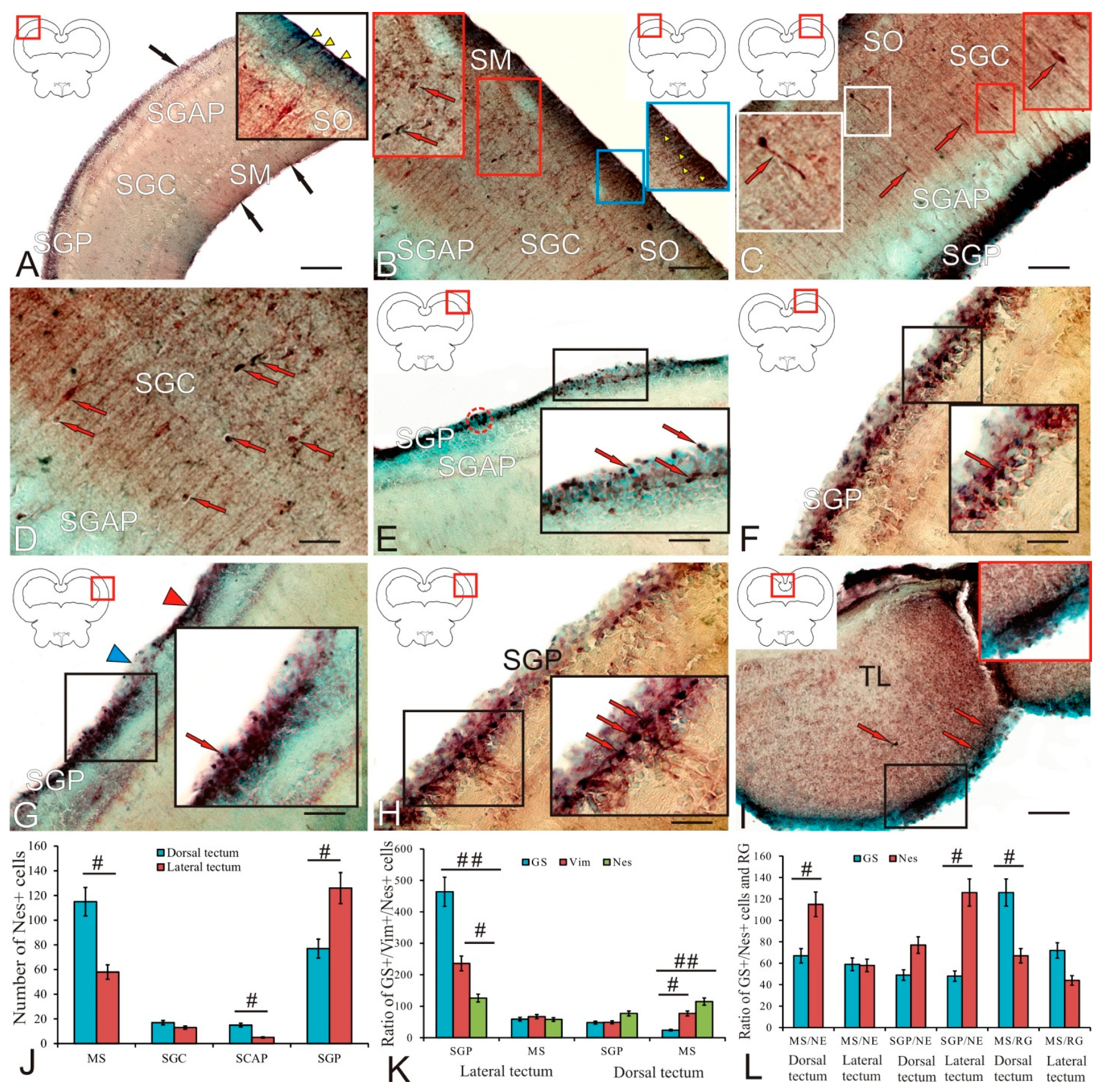
2.3. Doublecortin in the Trout Telencephalon
2.4. Doublecortin in the Trout Tectum Opticum
2.5. Vimentin in the Trout Telencephalon
2.6. Vimentin in the Trout Tectum
2.7. Nestin in the Trout Telencephalon
2.8. Nestin in the Tectum
3. Discussion
3.1. Telencephalon
3.1.1. Expression of Glutamine Synthetase in the Telencephalon
3.1.2. Expression of Doublecortin and Cell Migration in the Telencephalon
3.1.3. Expression of Vimentin and Glial Cell Specialization in the Telencephalon
3.1.4. Expression of Nestin in Telencephalon Progenitor Cells and Radial Glia
3.2. Tectum Opticum
3.2.1. Expression of Glutamine Synthetase in the Tectum
3.2.2. Expression of Doublecortin in the Tectum
3.2.3. Expression of Vimentin and Neuroregenerative Properties of Tectum
3.2.4. Expression of Nestin in the Tectum
4. Material and Methods
4.1. Experimental Animals
4.2. Preparation of Material for Immunohistochemical Studies
4.3. Immunohistochemistry
4.4. Microscopy
4.5. Densitometry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
References
- Ganz, J.; Kaslin, J.; Freudenreich, D.; Machate, A.; Geffarth, M.; Brand, M. Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J. Comp. Neurol. 2012, 520, 633–655. [Google Scholar] [CrossRef] [PubMed]
- Gorsuch, R.A.; Lahne, M.; Yarka, C.E.; Petravick, M.E.; Li, J.; Hyde, D.R. Sox2 regulates Muller glia reprogramming and proliferation in the regenerating zebrafish retina via Lin28 and Ascl1a. Exp. Eye Res. 2017, 161, 174–192. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.F.; Malva, J.O.; Crespo-Lopez, M.E. Adult hippocampal neurogenesis in different taxonomic groups: Possible functional similarities and striking controversies. Cells 2019, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Grandel, H.; Brand, M. Comparative aspects of adult neural stem cell activity in vertebrates. Dev. Genes Evol. 2013, 223, 131–147. [Google Scholar] [CrossRef]
- Zupanc, G.K.; Hinsch, K.; Gage, F.H. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J. Comp. Neurol. 2005, 488, 290–319. [Google Scholar] [CrossRef]
- Maruska, K.P.; Carpenter, R.E.; Fernald, R.D. Characterization of cell proliferation throughout the brain of the African cichlid fish Astatotilapia burtoni and its regulation by social status. J. Comp. Neurol. 2012, 520, 3471–3491. [Google Scholar] [CrossRef]
- Ganz, J.; Brand, M. Adult neurogenesis in fish. Cold Spring Harb. Perspect. Biol. 2016, 8, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Pushchina, E.V.; Zharikova, E.I.; Varaksin, A.A.; Prudnikov, I.M.; Tsyvkin, V.N. Proliferation, adult neuronal stem cells and cells migration in pallium during constitutive neurogenesis and after traumatic injury of telencephalon of juvenile masu salmon, Oncorhynchus masou. Brain Sci. 2020, 10, 222. [Google Scholar] [CrossRef] [Green Version]
- Von Krogh, K.; Sørensen, C.; Nilsson, G.E.; Øverli, Ø. Forebrain cell proliferation, behavior, and physiology of zebrafish, Danio rerio, kept in enriched or barren environments. Physiol. Behav. 2010, 101, 32–39. [Google Scholar] [CrossRef]
- Akle, V.; Stankiewicz, A.J.; Kharchenko, V.Y.; Kharchenko, P.V.; Zhdanova, I.V. Circadian kinetics of cell cycle progression in adult neurogenic niches of a diurnal vertebrate. J. Neurosci. 2017, 37, 1900–1909. [Google Scholar] [CrossRef] [Green Version]
- Ampatzis, K.; Makantasi, P.; Dermon, C.R. Cell proliferation pattern in adult zebrafish forebrain is sexually dimorphic. Neuroscience 2012, 226, 367–381. [Google Scholar] [CrossRef]
- Grupp, L.; Wolburg, H.; Mack, A.F. Astroglial structures in the zebrafish brain. J. Comp. Neurol. 2010, 518, 4277–4287. [Google Scholar] [CrossRef]
- Olivera-Pasilio, V.; Lasserre, M.; Castelló, M.E. Cell Proliferation, Migration, and Neurogenesis in the Adult Brain of the Pulse Type Weakly Electric Fish, Gymnotus omarorum. Front. Neurosci. 2017, 11, 437. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.S.; Marz, M.; Strahle, U. Gfap and nestin reporter lines reveal characteristics of neural progenitors in the adult zebrafish brain. Dev. Dyn. 2009, 238, 475–486. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Kapustyanov, I.A.; Varaksin, A.A. Proliferation and neuro- and gliogenesis in normal and mechanically damaged mesencephalic tegmentum in juvenile chum salmon Oncorhynchus keta. Russ. J. Dev. Biol. 2019, 50, 59–76. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Zharikova, E.I.; Varaksin, A.A. Persistent and reparative neurogenesis in the juvenile masu salmon Oncorhynchus masou telencephalon after mechanical injury. Russ. J. Dev. Biol. 2017, 48, 307–320. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Kapustyanov, I.A.; Varaksin, A.A. Neural stem cells/neuronal precursor cells and postmitotic neuroblasts in constitutive neurogenesis and after, traumatic injury to the mesencephalic tegmentum of juvenile chum salmon, Oncorhynchus keta. Brain Sci. 2020, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [Green Version]
- Pushchina, E.V.; Varaksin, A.A.; Shukla, S.; Obukhov, D.K. The Neurochemical Organization and Adult Neurogenesis in the Masu Salmon Brain; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; p. 267. [Google Scholar]
- Dambroise, E.; Simion, M.; Bourquard, T.; Rizzi, B.; Jaszczyszyn, Y.; Bourge, M.; Affaticati, P.; Heuzé, A.; Jouralet, J. Postembryonic fish brain proliferation zones exhibit neuroepithelial-type gene expression profile. Stem Cells 2017, 35, 1505–1518. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Kawasaki, T. Differential regenerative capacity of the optic tectum of adult medaka and zebrafish. Front. Cell Dev. Biol. 2021, 9, 686755. [Google Scholar] [CrossRef]
- Alunni, A.; Hermel, J.-M.; Heuze, A.; Bourrat, F.; Jamen, F.; Joly, J.S. Evidence for neural stem cells in the medaka optic tectum proliferation zones. Dev. Neurobiol. 2010, 70, 693–713. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Zharikova, E.I.; Varaksin, A.A. Mechanical brain injury increases cells’ production of cystathionine β-synthase and glutamine synthetase, but reduces Pax2 expression in the telencephalon of juvenile chum salmon, Oncorhynchus keta. Int. J. Mol. Sci. 2021, 22, 1279. [Google Scholar] [CrossRef]
- Dirian, L.; Galant, S.; Coolen, M.; Chen, W.; Bedu, S.; Houart, C.; Bally-Cuif, L.; Foucher, I. Spatial regionalization and heterochrony in the formation of adult pallial neural stem cells. Dev. Cell. 2014, 30, 123–136. [Google Scholar] [CrossRef] [Green Version]
- März, M.; Chapouton, P.; Diotel, N.; Vaillant, C.; Hesl, B.; Takamiya, M.; Lam, C.S.; Kah, O.; Bally-Cuif, L.; Strähle, U. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 2010, 58, 870–888. [Google Scholar] [CrossRef]
- Mack, A.F.; DeOliveira-Mello, L.; Mattheus, U.; Neckel, P.H. Organization of radial glia reveals growth pattern in the telencephalon of a percomorph fish Astatotilapia burtoni. J. Comp. Neurol. 2021, 529, 2813–2823. [Google Scholar] [CrossRef]
- März, M.; Schmidt, R.; Rastegar, S.; Strähle, U. Expression of the transcription factor Olig2 in proliferating cells in the adult zebrafish telencephalon. Dev. Dyn. 2010, 239, 3336–3349. [Google Scholar] [CrossRef]
- Kishimoto, N.; Shimizu, K.; Sawamoto, K. Neuronal regeneration in a zebrafish model of adult brain injury. Dis. Model. Mech. 2012, 5, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Kizil, C.; Kaslin, J.; Kroehne, V.; Brand, M. Adult neurogenesis and brain regeneration in zebrafish. Dev. Neurobiol. 2012, 72, 429–461. [Google Scholar] [CrossRef]
- Alunni, A.; Bally-Cuif, L. A comparative view of regenerative neurogenesis in vertebrates. Development 2016, 143, 741–753. [Google Scholar] [CrossRef] [Green Version]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- White, B.C.; Sullivan, J.M.; DeGracia, D.J.; O’Neil, B.J.; Neumar, R.W.; Grossman, L.I.; Rafols, J.A.; Krause, G.S. Brain ischemia and reperfusion: Molecular mechanisms of neuronal injury. J. Neurol. Sci. 2000, 179, 1–33. [Google Scholar] [CrossRef]
- Mergenthaler, P.; Dirnagl, U.; Meisel, A. Pathophysiology of stroke: Lessons from animal models. Metab. Brain Dis. 2004, 19, 151–167. [Google Scholar] [CrossRef]
- Zupanc, G.K.; Sîrbulescu, R.F. Teleost fish as a model system to study successful regeneration of the central nervous system. Curr. Top. Microbiol. Immunol. 2013, 367, 193–233. [Google Scholar] [PubMed]
- Brazel, C.Y.; Nunez, J.L.; Yang, Z.; Levison, S.W. Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience 2005, 131, 55–65. [Google Scholar] [CrossRef]
- Nakamichi, N.; Takarada, T.; Yoneda, Y. Neurogenesis mediated by gamma-aminobutyric acid and glutamate signaling. J. Pharmacol. Sci. 2009, 110, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, T.; Vernier, P.; Wullimann, M.F. A phylotypic stage in vertebrate brain development: GABA cell patterns in zebrafish compared with mouse. J. Comp. Neurol. 2006, 494, 620–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, T.; Wullimann, M.F.; Guo, S. Early teleostean basal ganglia development visualized by Zebrafish Dlx2a, Lhx6, Lhx7, Tbr2 (eomesa), and GAD67 gene expression. J. Comp. Neurol. 2008, 507, 1245–1257. [Google Scholar] [CrossRef]
- Mueller, T.; Wullimann, M.F. Chapter 2—Atlas of cellular markers in zebrafish neurogenesis. In Atlas of Early Zebrafish Brain Development—A Tool for Molecular Neurogenetics, 2nd ed.; Mueller, T., Wullimann, M.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–157. [Google Scholar]
- Maruska, K.P.; Butler, J.M.; Field, K.E.; Porter, D.T. Localization of glutamatergic, GABAergic, and cholinergic neurons in the brain of the African cichlid fish, Astatotilapia burtoni. J. Comp. Neurol. 2017, 525, 610–638. [Google Scholar] [CrossRef]
- Czapski, G.A.; Strosznajder, J.B. Glutamate and GABA in microglia-neuron cross-talk in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 11677. [Google Scholar] [CrossRef]
- Burmeister, S.S.; Munshi, R.G.; Fernald, R.D. Cytoarchitecture of a cichlid fish telencephalon. Brain Behav. Evol. 2009, 74, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Grandel, H.; Kaslin, J.; Ganz, J.; Wenzel, I.; Brand, M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Dev. Biol. 2006, 295, 263–277. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.P.; Couillard-Després, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ambrogini, P.; Lattanzi, D.; Ciuffoli, S.; Agostini, D.; Bertini, L.; Stocchi, V.; Santi, S.; Cuppini, R. Morphofunctional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004, 1017, 21–31. [Google Scholar] [CrossRef]
- Tozzini, E.T.; Baumgart, M.; Battistoni, G.; Cellerino, A. Adult neurogenesis in the short-lived teleost Nothobranchius furzeri: Localization of neurogenic niches, molecular characterization and effects of aging. Aging Cell 2012, 11, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Kroehne, V.; Freudenreich, D.; Hans, S.; Kaslin, J.; Brand, M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 2011, 138, 4831–4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothenaigner, I.; Krecsmarik, M.; Hayes, J.A.; Bahn, B.; Lepier, A.; Fortin, G.; Götz, M.; Jagasia, R.; Bally-Cuif, L. Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development 2011, 138, 1459–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapouton, P.; Skupien, P.; Hesl, B.; Coolen, M.; Moore, J.C.; Madelaine, R.; Kremmer, E.; Faus-Kessler, T.; Blader, P.; Lawson, N.D.; et al. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J. Neurosci. 2010, 30, 7961–7974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, J.; Kaslin, J.; Hochmann, S.; Freudenreich, D.; Brand, M. Heterogeneity and Fgf dependence of adult neural progenitors in the zebrafish telencephalon. Glia 2010, 58, 1345–1363. [Google Scholar] [CrossRef]
- Doetsch, F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and threeimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [CrossRef] [PubMed]
- Cerdà, J.; Conrad, M.; Markl, J.; Brand, M.; Herrmann, H. Zebrafish vimentin: Molecular characterization, assembly properties and developmental expression. Eur. J. Cell Biol. 1998, 77, 175–187. [Google Scholar] [CrossRef]
- Arochena, M.; Anadon, R.; Diaz-Regueira, S.M. Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of gray mullet (Chelon labrosus), an advanced teleost. J. Comp. Neurol. 2004, 469, 413–436. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.-M.; Cheng, Y.-H.; Huang, Y.-F.; Wang, C.-S. Isolation and characterization of a neural progenitor cell line from tilapia brain. Comp. Bioch. Physiol. A 2008, 149, 167–180. [Google Scholar] [CrossRef]
- Schaffeld, M.; Herrmann, H.; Schultess, J.; Markl, J. Vimentin and desmin of a cartilaginous fish, the shark Scyliorhinus stellaris: Sequence, expression patterns and in vitro assembly. Eur. J. Cell Biol. 2001, 80, 692–702. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Kim, M.; Kim, H.S.; Huh, T.L.; Chitnis, A.B. Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev. Biol. 2007, 301, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Yang, N.; Yeo, S.Y.; Chitnis, A.; Guo, S. Intralineage directional notch signaling regulates self-renewal and differentiation of asymmetrically dividing radial glia. Neuron 2012, 74, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Kaslin, J.; Ganz, J.; Geffarth, M.; Grandel, H.; Hans, S.; Brand, M. Stem cells in the adult zebrafish cerebellum: Initiation and maintenance of a novel stem cell niche. J. Neurosci. 2009, 29, 6142–6153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalczyk, K.; Ziman, M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol. Histopathol. 2005, 20, 665–671. [Google Scholar]
- Mahler, J.; Driever, W. Expression of the zebrafish intermediate neurofilament nestin in the developing nervous system and in neural proliferation zones at postembryonic stages. BMC Dev. Biol. 2007, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Carmona, I.C.; Luesma-Bartolomé, M.J.; Lavoie-Gagnon, C.; Junquera-Escribano, C. Distribution of nestin protein: Immunohistochemical study in enteric plexus of rat duodenum. Microsc. Res. Tech. 2011, 74, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Venables, M.J.; Navarro-Martína, L.; Basak, A.; Bauma, B.R.; Zhangc, D.; Trudeau, V.L. Characterization of multiple nestin isoforms in the goldfish brain. Comp. Biochem. Physiol. Part D 2016, 19, 8–17. [Google Scholar] [CrossRef]
- Wiese, C.; Rolletschek, A.; Kania, G.; Blyszczuk, P.; Tarasov, K.V.; Tarasova, Y.; Wersto, R.P.; Boheler, K.R.; Wobus, A.M. Nestin expression—A property of muti-lineage progenitor cells. Cell. Mol. Life Sci. 2004, 61, 2510–2522. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Darabie, A.; Tropepe, V. The cellular composition of neurogenic periventricular zones in the adult zebrafish forebrain. J. Comp. Neurol. 2012, 520, 2275–2316. [Google Scholar] [CrossRef] [PubMed]
- Merkle, F.T.; Mirzadeh, Z.; Alvarez-Buylla, A. Mosaic organization of neural stem cells in the adult brain. Science 2007, 317, 381–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsey, B.W.; Aitken, G.E.; Tang, J.K.; Khabooshan, M.; Douek, A.M.; Vandestadt, C. Midbrain tectal stem cells display diverse regenerative capacities in zebrafish. Sci. Rep. 2019, 9, 4420. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.M. Tanycytes in the sunfish brain: NADPH-diaphorase histochemistry and regional distribution. J. Comp. Neurol. 1993, 336, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.A.; Yoon, M.G. Morphology of radial glia, ependymal cells, and periventricular neurons in the optic tectum of goldfish (Carassius auratus). J. Comp. Neurol. 1982, 205, 128–138. [Google Scholar] [CrossRef]
- Kruger, L.; Maxwell, D.S. Comparative fine structure of vertebrate neuroglia: Teleosts and reptiles. J. Comp. Neurol. 1967, 129, 115–142. [Google Scholar] [CrossRef]
- Ito, Y.; Tanaka, H.; Okamoto, H.; Ohshima, T. Characterization of neural stem cells and their progeny in the adult zebrafish optic tectum. Dev. Biol. 2010, 342, 26–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond, P.A.; Easter, S.S., Jr. Postembryonic growth of the optic tectum in goldfish. I. Location of germinal cells and numbers of neurons produced. J. Neurosci. 1983, 3, 1077–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.; Deschet, K.; Henrich, T.; Godet, E.; Joly, J.S.; Wittbrodt, J.; Chourrout, D.; Bourrat, F. Morphogenesis of the optic tectum in the medaka Oryzias latipes: A morphological and molecular study, with special emphasis on cell proliferation. J. Comp. Neurol. 1999, 413, 385–404. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A. Neurolin expression in the optic nerve and mmunoreactivity of Pax6-positive niches in the brain of rainbow trout (Oncorhynchus mykiss) after unilateral eye injury. Neural. Regen. Res. 2019, 14, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Hinsch, K.; Zupanc, G.K.H. Generation and long-term persistence of new neurons in the adult zebrafish brain: A quantitative analysis. Neuroscience 2007, 146, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Cuoghi, B.; Mola, L. Macroglial cells of the teleost central nervous system: A survey of the main types. Cell Tissue Res. 2009, 338, 319–332. [Google Scholar] [CrossRef]
- Kàlmàn, M. Astroglial architecture of the carp (Cyprinus carpio) brain as revealed by immunohistochemical staining against glial fibrillary acidic protein (GFAP). Anat. Embryol. 1998, 198, 409–433. [Google Scholar]
- Bodega, G.; Suàrez, I.; Fernàndez, B. Radial astrocytes and ependymocytes in the spinal cord of the adult toad (Bufo bufo L.). An immunohistochemical and ultrastructural study. Cell Tissue Res. 1990, 260, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Bodega, G.; Suàrez, L.; Rubio, M.; Villalba, R.M.; Fernàndez, B. Astroglial pattern in the spinal cord of the adult barbel (Barbus comiza). Anat. Embryol. 1993, 187, 385–395. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Obukhov, D.K.; Varaksin, A.A. Features of adult neurogenesis and neurochemical signaling in the Cherry salmon Oncorhynchus masou brain. Neural Regen Res. 2013, 8, 13–23. [Google Scholar]
- Nelson, C.M.; Gorsuch, R.A.; Bailey, T.J.; Ackerman, K.M.; Kassen, S.C.; Hyde, D.R. Stat3 defines three populations of Muller glia and is required for initiating maximal muller glia proliferation in the regenerating zebrafish retina. J. Comp. Neurol. 2012, 520, 4294–4311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Gupta, S.; Chaudhary, M.; Mitra, S.; Chawla, B.; Khursheed, M.A.; Ramachandran, R. Oct4 mediates Muller glia reprogramming and cell cycle exit during retina regeneration in zebrafish. Life Sci. Alliance 2019, 2, e201900548. [Google Scholar] [CrossRef] [Green Version]
- März, M.; Schmidt, R.; Rastegar, S.; Strähle, U. Regenerative response following stab injury in the adult zebrafish telencephalon. Dev. Dyn. 2011, 240, 2221–2231. [Google Scholar] [CrossRef]
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016, 275, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Zupanc, G.K.; Sîrbulescu, R.F. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Eur. J. Neurosci. 2011, 34, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Yuh, C.-H.; Wu, K.K. Nestin is essential for zebrafish brain and eye development through control of progenitor cell apoptosis. PLoS ONE 2010, 5, e9318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.C.S.; Matesic, D.F.; Marvin, M.; McKay, R.D.G.; Brüstle, O. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol. Dis. 1995, 2, 69–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, Y.; Ueda, Y.; Ohshima, T. Wnt signaling regulates proliferation and differentiation of radial glia in regenerative processes after stab injury in the optic tectum of adult zebrafish. Glia 2018, 66, 1382–1394. [Google Scholar] [CrossRef]
- Raymond, P.A.; Barthel, L.K.; Bernardos, R.L.; Perkowski, J.J. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev. Biol. 2006, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Pushchina, E.V.; Stukaneva, M.E.; Varaksin, A.A. Hydrogen Sulfide Modulates Adult and Reparative Neurogenesis in the Cerebellum of Juvenile Masu Salmon, Oncorhynchus masou. Int. J. Mol. Sci. 2020, 21, 9638. [Google Scholar] [CrossRef]






| Brain Area | Type of Cells, Brain Localization | Size of Cells, μm | Intensity of Labeling |
|---|---|---|---|
| Telencephalon | |||
| Pallium Dorsal Dd | Undiffer. (PVZ) Elong. (PVZ, SVZ) Radial glia (PVZ) | 5.5 ± 0.3/4.2 ± 0.7 7.0 ± 0.6/4.7 ± 0.7 9.4 ± 0.7/6.0 ± 0.6 | +++ +++/++ +++ |
| Medial Dm | Undiffer. (PVZ) Elongated (PVZ, PZ) Radial glia (PVZ) | 4.9 ± 0.6/3.6 ± 0.8 6.7 ± 0.5/4.6 ± 0.8 8.8 ± 0.7/5.3 ± 0.9 | +++ +++/++ +++ |
| Lateral Dl | Undiffer. (PVZ) Elong. (PVZ, SVZ) Radial glia (PVZ) | 5.2 ± 0.4/3.7 ± 0.5 7.2 ± 0.6/4.7 ± 0.8 8.4 ± 0.7/6.9 ± 1.5 | +++ +++/++ +++ |
| Central Dc | Undiffer. (PZ) Elongated (PZ) | 5.2 ± 0.6/3.6 ± 0.4 7.0 ± 0.6/4.7 ± 0.7 | +++/++ ++ |
| Subpallium Dorsal Vd | Undiffer. (PVZ) Elongated (PVZ) | 6.0 ± 0.6/5.3 ± 0.3 7.8 ± 0.4/5.4 ± 0.8 | +++ +++/++ |
| Ventral Vv | Elongated 1 (PVZ) Elongated 2 (PVZ) | 7.5 ± 0.3/5.6 ± 0.6 8.9 ± 0.5/5.3 ± 0.4 | +++ +++/++ |
| Lateral Vl | Undiffer. (PVZ) Elongated (PVZ) | 4.9 ± 0.5/3.6 ± 0.5 6.1 ± 0.3/4.0 ± 0.8 | +++ +++/++ |
| Tectum opticum | |||
| Dorsal part Stratum marginale SM | Undifferentiated Elongated Radial glia 1 Radial glia 2 | 4.9 ± 0.1/3.1 ± 0.5 7.2 ± 0.7/5.7 ± 1.6 9.0 ± 0.4/5.5 ± 0.4 10.7 ± 0.4/6.0 ± 0.2 | +++ +++ +++ +++ |
| Stratum griseum periventriculare SGP | Elongated Radial glia 1 | 7.1 ± 0.5/4.6 ± 0.8 8.8 ± 0.5/5.2 ± 0.7 | +++ +++ |
| Lateral part Stratum marginale SM | Undifferentiated Elongated Radial glia 1 | 5.6 ± 0.3/3.9 ± 0.6 6.7 ± 0.6/4.3 ± 0.6 8.9 ± 0.4/5.3 ± 0.4 | +++ +++ +++ |
| Stratum griseum periventriculare SGP | Undifferentiated Elongated Radial glia 1 Radial glia 2 | 5.4 ± 0.2/4.3 ± 0.6 6.8 ± 0.5/4.6 ± 0.8 8.9 ± 0.6/5.7 ± 0.7 11.9 ± 0.3/5.1 ± 0.2 | +++ +++ +++ +++ |
| Brain Area | Type of Cells, Brain Localization | Size of Cells, µm | Intensity of Labeling |
|---|---|---|---|
| Telencephalon | |||
| Pallium Dorsal Dd | Undifferentiated (PVZ) Elongated (PVZ, SVZ) Oval (SVZ, PZ) Polygonal 1(PZ) Polygonal 2(PZ) | 4.2 ± 0.4/3.1 ± 0.3 5.6 ± 0.8/4.0 ± 0.6 9.2 ± 0.9/8.2 ± 0.6 10.9 ± 0.9/8.6 ± 1.4 12.6 ± 0.4/9.4 ± 1.1 | +++ +++/++ +++/++ ++ ++ |
| Medial Dm | Undifferentiated (PVZ) Elongated (PVZ, SVZ) Oval (SVZ, PZ) Polygonal 1(PZ) | 4.2 ± 0.2/3.1 ± 0.2 5.8 ± 0.6/4.0 ± 0.7 8.2 ± 0.4/5.8 ± 0.4 9.7 ± 0.3/5.1 ± 0.2 | +++ +++/++ ++ ++ |
| Lateral Dl | Elongated (PVZ, SVZ) Oval (SVZ, PZ) Polygonal 1(PZ) Polygonal 2(PZ) | 5.3 ± 0.4/3.7 ± 0.4 9.3 ± 0.5/6.1 ± 0.7 11.1 ± 0.6/8.0 ± 1.6 12.7 ± 0.6/8.9 ± 0.8 | +++ +++/++ +++ +++/++ |
| Central Dc | Oval (SVZ, PZ) Polygonal 1(PZ) Polygonal 2(PZ) | 10.1 ± 0.6/7.7 ± 1.2 11.8 ± 0.4/8.2 ± 0.6 13.7 ± 0.6/8.8 ± 1.4 | +++/++ ++ ++ |
| Subpallium Dorsal Vd | Undifferentiated (PVZ) Elongated (PVZ) Oval (SVZ, PZ) | 4.6 ± 0.3/3.2 ± 0.4 5.6 ± 0.5/4.4 ± 0.7 7.5 ± 0.4/5.5 ± 0.9 | +++ +++/++ +++/++ |
| Ventral Vv | Oval (SVZ, PZ) Elongated 1 (SVZ, PZ) Elongated 2 (PZ) | 5.6 ± 0.5/4.4 ± 0.7 7.5 ± 0.3/5.6 ± 0.6 8.9 ± 0.5/5.3 ± 0.4 | +++ +++/++ ++ |
| Central Vc | Elongated (PZ) Elongated 1 (PZ) Oval (PZ) | 4.6 ± 0.5/3.4 ± 03 5.6 ± 0.5/4.4 ± 0.7 7.5 ± 0.4/5.5 ± 0.9 | +++/++ ++ +++/++ |
| Lateral Vl | Undifferentiated (PVZ) Elongated 1(SVZ, PVZ) Oval (SVZ, PZ) Polygonal 1(PZ) | 4.5 ± 0.2/3.8 ± 0.3 5.9 ± 0.6/4.7 ± 0.8 7.7 ± 0.5/5.7 ± 1.4 10.2 ± 0.6/6.0 ± 0.2 | +++ +++/++ ++ ++ |
| Tectum Opticum | |||
| Stratum marginale SM | Undifferentiated (PVZ) Elongated (PVZ) Radial glia 1(PVZ) | 4.8 ± 0.5/3.5 ± 0.4 7.0 ± 0.7/4.4 ± 0.5 9.2 ± 0.8/5.5 ± 0.2 | +++ +++ +++ |
| Stratum opticum SO | Elongated (SVZ, PZ) Polygonal (PZ) | 9.0 ± 0.7/5.7 ± 0.4 11.8 ± 2.0/6.4 ± 2.4 | +++ +++ |
| Stratum griseumcentrale SGC | Undifferentiated (PZ) Elongated (PZ) Oval (PZ) Polygonal 1(PZ) Polygonal 2(PZ) | 5.6 ± 0.3/4.2 ± 1.0 9.4 ± 0.9/6.4 ± 0.4 11.4 ± 0.4/9.0 ± 2.0 13.2 ± 0.6/7.7 ± 0.4 15.3 ± 0.3/6.3 ± 1.2 | +++ +++/++ ++ ++ ++ |
| Stratum griseum et al. bum periventriculare SGAP | Undifferentiated (SVZ) Elongated (SVZ, PZ) Oval (SVZ, PZ) | 6.4 ± 0.4/4.6 ± 0.4 9.3 ± 0.4/5.2 ± 0.5 10.8 ± 0.2/6.4 ± 0.6 | +++ ++ ++ |
| Stratum griseum periventriculare SGP | Undifferentiated (PVZ) Elongated (PVZ) Oval (PVZ) | 5.3 ± 0.4/4.0 ± 0.7 6.7 ± 0.6/4.5 ± 0.8 8.7 ± 0.6/6.3 ± 0.3 | +++ +++ +++ |
| Torus longitudinalis TL | Undifferentiated (PVZ) Elongated 1(PVZ) Elongated 2 (PVZ) Polygonal (PZ) | 4.7 ± 0.6/3.0 ± 0.5 7.0 ± 0.6/5.5 ± 1.4 8.7 ± 0.6/7.0 ± 1.6 11.2 ± 0.4/6.7 ± 1.3 | +++ +++/++ ++ ++ |
| Brain Area | Type of Cells, Brain Localization | Size of Cells, µm | Intensity of Labeling |
|---|---|---|---|
| Telencephalon | |||
| Pallium Dorsal Dd | Undiffer. (PVZ) Elongated (PVZ) Radial glia (PVZ) | 4.0 ± 0.4/2.8 ± 0.6 5.7 ± 0.6/4.1 ± 1.1 8.4 ± 0.4/6.9 ± 0.9 | +++ +++/++ +++ |
| Lateral Dl | Undiffer. (PVZ) Elongated (PVZ, PZ) Radial glia (PVZ) | 3.9 ± 0.5/2.8 ± 0.5 6.2 ± 0.7/4.0 ± 0.6 8.3 ± 0.5/5.3 ± 0.5 | +++ +++/++ +++ |
| Medial Dm | Undiffer. (PVZ) Elongated (PVZ) | 3.5 ± 0.3/2.5 ± 0.3 4.9 ± 0.4/3.4 ± 0.6 | +++ +++ |
| Central Dc | - | - | - |
Subpallium Dorsal Vd | Undiffer. (PVZ) Elongated 1(SVZ) Elongated 2 (PZ) | 6.0 ± 0.6/5.3 ± 0.3 7.8 ± 0.4/5.4 ± 0.8 | +++ + + |
| Ventral Vv | - | - | - |
| Dl/Vl | Undiffer. (PVZ) Elongated (PVZ) | 3.4 ± 0.3/2.6 ± 0.6 4.7 ± 0.5/3.3 ± 0.5 | +++ +++ |
Tectum Opticum | |||
| Stratum marginale SM | Undiffer. 1 (SM) Undiffer. 2 (SM) Elongated (SM) Elongated 1 (SM) Elongated 2 (SM) | 3.3 ± 0.5/2.3 ± 0.3 5.8 ± 1.0/4.0 ± 1.1 8.0 ± 0.6/6.2 ± 1.1 9.9 ± 0.3/5.6 ± 0.3 13.6 ± 0.4/8.4 ± 2.9 | +++ +++ +++ +++/++ +++/++ |
| Stratum marginale Neurogenic nishes NN | ependyma ependyma ependyma ependyma ependyma | 12.2 ± 0.8/8.7 ± 2.0 16.5 ± 0.7/‘11.3 ± 0.7 20.8 ± 0.6/19.0 ± 9.1 24.8 ± 1.4/15.3 ± 5.3 28.5 ± 1.6/20.3 ± 8.9 | +++ +++ +++ +++ +++ |
| Stratum griseum periventriculare SGP | Undiffer. 1(SGP) Undiffer. 2 (SGP) Elongated (SGP) Elongated 1 (SGP) Elongated 2 (SGP) | 4.3 ± 1.2/2.7 ± 0.5 7.0 ± 0.3/5.7 ± 0.9 9.0 ± 0.3/6.7 ± 0.7 10.8 ± 0.5/7.5 ± 0.9 13.3 ± 1.4/9.4 ± 1.9 | +++ +++ +++ +++/++ +++/++ |
| Brain Area | Type of Cells, Brain Localization | Size of Cells, µm | Intensity of Labeling |
|---|---|---|---|
| Telencephalon | |||
| Pallium Dorsal Dd | Undiffer. (PVZ) Elong. (PVZ, SVZ) Oval (SVZ, PZ) | 5.0 ± 0.5/3.3 ± 0.2 6.7 ± 0.5/4.1 ± 0.6 9.0 ± 0.7/4.2 ± 0.6 | +++ +++/++ +++/++ |
| Medial Dm | Undiffer. (PVZ) Elong. (PVZ, SVZ) Oval (SVZ, PZ) | 5.7 ± 0.3/4.6 ± 0.9 7.2 ± 0.4/4.0 ± 0.8 8.8 ± 0.5/6.2 ± 1.3 | +++ +++/++ +++/++ |
| Dm/Dc | Elong. (PZ) Oval (PZ) Polygonal 1 (PZ) Polygonal 2 (PZ) | 7.7 ± 0.4/6.2 ± 0.5 10.2 ± 0.9/5.9 ± 0.3 11.9 ± 0.8/7.7 ± 1.6 17.8 ± 1.6/10.2 ± 0.8 | +++ +++/++ +++/++ +++/++ |
| Central Dc | Oval (SVZ, PZ) Polygonal (PZ) | 11.1 ± 0.4/8.9 ± 1.0 12.8 ± 0.3/8.8 ± 0.9 | +++/++ ++ |
| Lateral Dl | Undiffer. (PVZ) Elong. (PVZ, SVZ) Oval (SVZ, PZ) Polygonal (SVZ) | 5.2 ± 0.5/3.9 ± 0.5 7.0 ± 0.5/4.4 ± 0.7 9.3 ± 0.7/5.4 ± 0.4 11.3 ± 0.6/3.7 ± 0.8 | +++ +++/++ +++ +++/++ |
| Lateral posterior zone Dlp | Elong. (PVZ, SVZ) Oval (SVZ, PZ) Polygonal 1 (PZ) Polygonal 2 (PZ) | 7.3 ± 0.4/6.5 ± 1.3 9.8 ± 0.5/7.0 ± 0.3 11.9 ± 0.6/6.6 ± 0.9 11.6 ± 0.4/7.8 ± 1.0 | +++ +++/++ +++ +++/++ |
| Subpallium Dorsal Vd | Undiffer. (PVZ) Elongated (PVZ) | 5.0 ± 0.4/3.6 ± 0.4 7.0 ± 0.4/4.5 ± 0.3 | +++ +++/++ |
| Ventral Vv | Oval (SVZ, PZ) Elong. 1 (SVZ, PZ) Elongated 2 (PZ) | 9.0 ± 0.6/8.0 ± 1.2 11.1 ± 0.6/8.3 ± 1.6 13.4 ± 0.4/8.7 ± 2.3 | +++ +++/++ ++ |
| Lateral Vl | Undiffer. (PVZ) Elongated 1(PVZ, PZ) Oval (PVZ) Polygonal (PZ) | 4.5 ± 0.4/3.8 ± 0.5 5.9 ± 0.5/4.5 ± 0.7 7.8 ± 0.6/5.5 ± 1.4 10.2 ± 0.6/6.0 ± 0.2 | +++ +++/++ ++ ++ |
| Tectum opticum | |||
| Stratum marginale SM | Undiffer. (PVZ) Elongated (PVZ) Radial glia 1(PVZ) | 4.3 ± 0.5/3.2 ± 0.4 5.8 ± 0.5/3.8 ± 0.7 8.0 ± 1.4/3.5 ± 0.7 | +++ +++ +++ |
| Stratum griseum centrale SGC | Elongated (PZ) Oval (PZ) Polygonal (PZ) | 8.5 ± 0.5/5.3 ± 1.1 11.0 ± 1.0/6.3 ± 1.3 26.2 ± 2.5/7.3 ± 1.3 | +++/++ ++ ++ |
| Stratum griseum periventriculare SGP | Undiffer. (PVZ) Elongated (PVZ) | 4.8 ± 0.6/3.5 ± 0.6 6.7 ± 0.6/4.5 ± 0.9 | +++ +++ |
| Torus longitudinalis TL | Undiffer. (PVZ) Elongated 1(PVZ) Elongated 2 (PVZ) | 5.5 ± 0.4/3.4 ± 0.6 6.3 ± 0.2/3.9 ± 0.8 8.3 ± 0.6/3.3 ± 0.4 | +++ +++/++ ++ |
| Parenchymal cells PC | Undiffer. (PVZ) Elongated (PVZ) | 4.8 ± 0.5/3.3 ± 0.3 6.3 ± 0.4/3.7 ± 0.4 | +++ +++/++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushchina, E.V.; Varaksin, A.A.; Obukhov, D.K. Molecular Markers of Adult Neurogenesis in the Telencephalon and Tectum of Rainbow Trout, Oncorhynchus mykiss. Int. J. Mol. Sci. 2022, 23, 1188. https://doi.org/10.3390/ijms23031188
Pushchina EV, Varaksin AA, Obukhov DK. Molecular Markers of Adult Neurogenesis in the Telencephalon and Tectum of Rainbow Trout, Oncorhynchus mykiss. International Journal of Molecular Sciences. 2022; 23(3):1188. https://doi.org/10.3390/ijms23031188
Chicago/Turabian StylePushchina, Evgeniya V., Anatoly A. Varaksin, and Dmitry K. Obukhov. 2022. "Molecular Markers of Adult Neurogenesis in the Telencephalon and Tectum of Rainbow Trout, Oncorhynchus mykiss" International Journal of Molecular Sciences 23, no. 3: 1188. https://doi.org/10.3390/ijms23031188






