MAPK/ERK Pathway as a Central Regulator in Vertebrate Organ Regeneration
Abstract
:1. Introduction
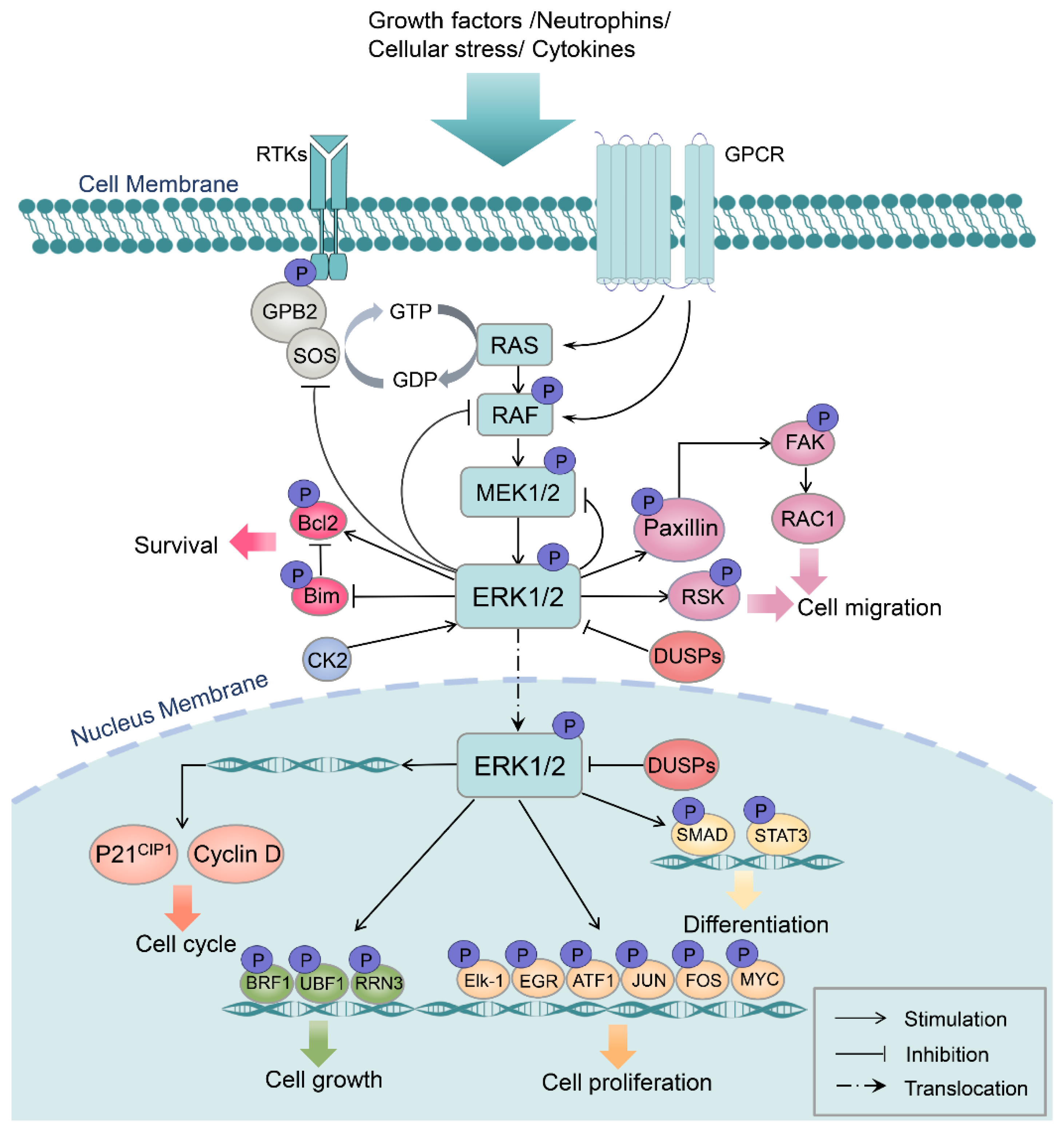
2. MAPK/ERK Structure, Activation, and Function
3. Involvement of the MAPK/ERK Pathway in Tissue/Organ Regeneration Processes
3.1. MAPK/ERK Pathway in Appendage Regeneration
3.2. MAPK/ERK Pathway in Cardiac Regeneration
3.3. MAPK/ERK Pathway in Liver Regeneration
3.4. MAPK/ERK Pathway in Eye Regeneration
3.5. MAPK/ERK Pathway in Central/Peripheral Nerve Regeneration
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FGF2 | Fibroblast growth factor 2 |
| IGF-1 | Insulin-like growth factor type 1 |
| IGF-1R | Insulin-like growth factor type 1 receptor |
| BDNF | Brain-derived neurotrophic factor |
| TGF | Transforming growth factor |
| ROS | Reactive oxygen species |
| MEK | Mitogen-activated protein kinase |
| EGFR | Epidermal growth factor receptor |
| MMP9 | Matrix metallopeptidase 9 |
| FGFR | Fibroblast growth factor receptor |
| PI3K | Phosphoinositide 3-kinase |
| Akt | Protein kinase B |
| IRS-1 | Insulin receptor substrate 1 |
| DUSP6 | Dual specificity phosphatase 6 |
| YAP | Yes-associated protein |
| VEGF | Vascular endothelial growth factor |
| E2F1 | E2F transcription factor 1 |
| ECRAR | Endogenous cardiac regeneration-associated regulator |
| PKA | Protein kinase A |
| NMII | Non-muscle myosin II |
| HB-EGF | Heparin-binding EGF-like growth factor |
| STAT3 | Signal transducer and activator of transcription 3 |
| ascl1a | Achaete-scute complex-like 1a |
| CREB | The cAMP-response element binding protein |
| Rb | Retinoblastoma protein |
| nAG | Newt anterior gradient protein |
| CM | Cardiomyocyte |
| ECM | Extracellular matrix |
| MI | Myocardium infarction |
| RPE | Retinal pigmented epithelium cell |
| MG | Müller glia cell |
| PNS | Peripheral nervous system |
| CNS | Central nervous system |
| SC | Schwann cell |
| OL | Oligodendrocyte |
| OPC | Oligodendrocyte precursor cell |
| Agr | Anterior gradient |
References
- Jazwinska, A.; Sallin, P. Regeneration versus scarring in vertebrate appendages and heart. J. Pathol. 2016, 238, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, E.M. The Molecular and Cellular Choreography of Appendage Regeneration. Cell 2016, 165, 1598–1608. [Google Scholar] [CrossRef] [Green Version]
- Stoick-Cooper, C.L.; Moon, R.T.; Weidinger, G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007, 21, 1292–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maienschein, J. Regenerative medicine’s historical roots in regeneration, transplantation, and translation. Dev. Biol. 2011, 358, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Goldman, J.A.; Poss, K.D. Gene regulatory programmes of tissue regeneration. Nat. Rev. Genet. 2020, 21, 511–525. [Google Scholar] [CrossRef]
- Busca, R.; Pouyssegur, J.; Lenormand, P. ERK1 and ERK2 Map Kinases: Specific Roles or Functional Redundancy? Front. Cell Dev. Biol. 2016, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Tanoue, T.; Adachi, M.; Moriguchi, T.; Nishida, E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2000, 2, 110–116. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.P.; Zhou, B.; Zhang, Z.Y. Structural basis of docking interactions between ERK2 and MAP kinase phosphatase 3. Proc. Natl. Acad. Sci. USA 2006, 103, 5326–5331. [Google Scholar] [CrossRef] [Green Version]
- Robbins, D.J.; Zhen, E.; Owaki, H.; Vanderbilt, C.A.; Ebert, D.; Geppert, T.D.; Cobb, M.H. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J. Biol. Chem. 1993, 268, 5097–5106. [Google Scholar] [CrossRef]
- Lefloch, R.; Pouyssegur, J.; Lenormand, P. Total ERK1/2 activity regulates cell proliferation. Cell Cycle 2009, 8, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Park, O.J.; Kim, H.J.; Woo, K.M.; Baek, J.H.; Ryoo, H.M. FGF2-activated ERK mitogen-activated protein kinase enhances Runx2 acetylation and stabilization. J. Biol. Chem. 2010, 285, 3568–3574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, Y.; Mikelson, J.; Dobrzynski, M.; Ryu, H.; Jacques, M.A.; Jeon, N.L.; Khammash, M.; Pertz, O. Temporal perturbation of ERK dynamics reveals network architecture of FGF2/MAPK signaling. Mol. Syst. Biol. 2019, 15, e8947. [Google Scholar] [CrossRef]
- Lee, C.K.; Lee, H.M.; Kim, H.J.; Park, H.J.; Won, K.J.; Roh, H.Y.; Choi, W.S.; Jeon, B.H.; Park, T.K.; Kim, B. Syk contributes to PDGF-BB-mediated migration of rat aortic smooth muscle cells via MAPK pathways. Cardiovasc. Res. 2007, 74, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Y.; Kim, S.; Izumi, Y.; Izumiya, Y.; Nakao, T.; Miyazaki, H.; Iwao, H. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Tatebe, H.; Nakagawa, T.; Hara, Y.; Weinstein, I.B.; Moriwaki, H. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008, 262, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Glennon, M.S.; Umbarkar, P.; Gupte, M.; Galindo, C.L.; Zhang, Q.; Force, T.; Becker, J.R.; Lal, H. Ponatinib-induced cardiotoxicity: Delineating the signalling mechanisms and potential rescue strategies. Cardiovasc. Res. 2019, 115, 966–977. [Google Scholar] [CrossRef]
- Madhu, L.N.; Kodali, M.; Attaluri, S.; Shuai, B.; Melissari, L.; Rao, X.; Shetty, A.K. Melatonin improves brain function in a model of chronic Gulf War Illness with modulation of oxidative stress, NLRP3 inflammasomes, and BDNF-ERK-CREB pathway in the hippocampus. Redox Biol. 2021, 43, 101973. [Google Scholar] [CrossRef]
- Ivanov, S.V.; Panaccione, A.; Brown, B.; Guo, Y.; Moskaluk, C.A.; Wick, M.J.; Brown, J.L.; Ivanova, A.V.; Issaeva, N.; El-Naggar, A.K.; et al. TrkC signaling is activated in adenoid cystic carcinoma and requires NT-3 to stimulate invasive behavior. Oncogene 2013, 32, 3698–3710. [Google Scholar] [CrossRef] [Green Version]
- Watts, S.W. 5-Hydroxytryptamine-induced potentiation of endothelin-1- and norepinephrine-induced contraction is mitogen-activated protein kinase pathway dependent. Hypertension 2000, 35, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.; Gong, K.; Ali, S.; Ali, N.; Shallwani, S.; Hatanpaa, K.J.; Pan, E.; Mickey, B.; Burma, S.; Wang, D.H.; et al. A TNF-JNK-Axl-ERK signaling axis mediates primary resistance to EGFR inhibition in glioblastoma. Nat. Neurosci. 2017, 20, 1074–1084. [Google Scholar] [CrossRef] [Green Version]
- Eliopoulos, A.G.; Wang, C.C.; Dumitru, C.D.; Tsichlis, P.N. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 2003, 22, 3855–3864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appleton, C.T.; Usmani, S.E.; Mort, J.S.; Beier, F. Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab. Investig. 2010, 90, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Wang, Y.; Zhang, Z.; Wang, J.; Niu, M.; Wu, Y.; Yang, Y.; Dang, Y.; Hui, S.; Ni, M.; et al. Elevated TEFM expression promotes growth and metastasis through activation of ROS/ERK signaling in hepatocellular carcinoma. Cell Death Dis. 2021, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Huang, X.; Feit-Leithman, R.A.; Neve, R.L.; Snider, W.; Dartt, D.A.; Chen, D.F. Bcl-2 enhances Ca2+ signaling to support the intrinsic regenerative capacity of CNS axons. EMBO J. 2005, 24, 1068–1078. [Google Scholar] [CrossRef] [Green Version]
- Doll, M.A.; Soltanmohammadi, N.; Schumacher, B. ALG-2/AGO-Dependent mir-35 Family Regulates DNA Damage-Induced Apoptosis through MPK-1/ERK MAPK Signaling Downstream of the Core Apoptotic Machinery in Caenorhabditis elegans. Genetics 2019, 213, 173–194. [Google Scholar] [CrossRef]
- Ohm, A.M.; Affandi, T.; Reyland, M.E. EGF receptor and PKCdelta kinase activate DNA damage-induced pro-survival and pro-apoptotic signaling via biphasic activation of ERK and MSK1 kinases. J. Biol. Chem. 2019, 294, 4488–4497. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Maik-Rachline, G.; Hacohen-Lev-Ran, A.; Seger, R. Nuclear ERK: Mechanism of Translocation, Substrates, and Role in Cancer. Int. J. Mol. Sci. 2019, 20, 1194. [Google Scholar] [CrossRef] [Green Version]
- Kolch, W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 2005, 6, 827–837. [Google Scholar] [CrossRef]
- Kolch, W. Meaningful relationships: The regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 2000, 351 Pt 2, 289–305. [Google Scholar] [CrossRef]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.B.; Uhlitz, F.; Bluthgen, N. A compendium of ERK targets. FEBS Lett. 2017, 591, 2607–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, M.H.; Gates, P.B.; Brockes, J.P. Sustained ERK activation underlies reprogramming in regeneration-competent salamander cells and distinguishes them from their mammalian counterparts. Stem Cell Rep. 2014, 3, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blassberg, R.A.; Garza-Garcia, A.; Janmohamed, A.; Gates, P.B.; Brockes, J.P. Functional convergence of signalling by GPI-anchored and anchorless forms of a salamander protein implicated in limb regeneration. J. Cell Sci. 2011, 124, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Satoh, A.; Ide, H.; Tamura, K. Transgenic Xenopus with prx1 limb enhancer reveals crucial contribution of MEK/ERK and PI3K/AKT pathways in blastema formation during limb regeneration. Dev. Biol. 2007, 304, 675–686. [Google Scholar] [CrossRef]
- Franklin, B.M.; Voss, S.R.; Osborn, J.L. Ion channel signaling influences cellular proliferation and phagocyte activity during axolotl tail regeneration. Mech. Dev. 2017, 146, 42–54. [Google Scholar] [CrossRef]
- Sato, K.; Umesono, Y.; Mochii, M. A transgenic reporter under control of an es1 promoter/enhancer marks wound epidermis and apical epithelial cap during tail regeneration in Xenopus laevis tadpole. Dev. Biol. 2018, 433, 404–415. [Google Scholar] [CrossRef]
- Yoo, S.K.; Freisinger, C.M.; LeBert, D.C.; Huttenlocher, A. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J. Cell Biol. 2012, 199, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Mathew, L.K.; Sengupta, S.; Franzosa, J.A.; Perry, J.; La Du, J.; Andreasen, E.A.; Tanguay, R.L. Comparative expression profiling reveals an essential role for raldh2 in epimorphic regeneration. J. Biol. Chem. 2009, 284, 33642–33653. [Google Scholar] [CrossRef] [Green Version]
- Owlarn, S.; Klenner, F.; Schmidt, D.; Rabert, F.; Tomasso, A.; Reuter, H.; Mulaw, M.A.; Moritz, S.; Gentile, L.; Weidinger, G.; et al. Generic wound signals initiate regeneration in missing-tissue contexts. Nat. Commun. 2017, 8, 2282. [Google Scholar] [CrossRef]
- De Simone, A.; Evanitsky, M.N.; Hayden, L.; Cox, B.D.; Wang, J.; Tornini, V.A.; Ou, J.; Chao, A.; Poss, K.D.; Di Talia, S. Control of osteoblast regeneration by a train of Erk activity waves. Nature 2021, 590, 129–133. [Google Scholar] [CrossRef]
- Kobayashi-Sun, J.; Suzuki, N.; Hattori, A.; Yamaguchi, M.; Kobayashi, I. Melatonin suppresses both osteoblast and osteoclast differentiation through repression of epidermal Erk signaling in the zebrafish scale. Biochem. Biophys. Res. Commun. 2020, 530, 644–650. [Google Scholar] [CrossRef]
- Yun, C.; Qian, W.; Wu, J.; Yuan, C.; Jiang, S.; Lv, J. Pilose antler peptide promotes osteoblast proliferation, differentiation and mineralization via the insulin signaling pathway. Exp. Ther. Med. 2020, 19, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Harper, A.; Puddick, J.; Wang, W.; McMahon, C. Proteomes and signalling pathways of antler stem cells. PLoS ONE 2012, 7, e30026. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Zhou, X.H.; Chang, N.; Xiao, C.L.; Yan, S.; Ren, H.; Yang, X.Z.; Zhang, M.L.; Wu, Q.; Tang, B.; et al. Hydrogen peroxide primes heart regeneration with a derepression mechanism. Cell Res. 2014, 24, 1091–1107. [Google Scholar] [CrossRef] [Green Version]
- Missinato, M.A.; Saydmohammed, M.; Zuppo, D.A.; Rao, K.S.; Opie, G.W.; Kuhn, B.; Tsang, M. Dusp6 attenuates Ras/MAPK signaling to limit zebrafish heart regeneration. Development 2018, 145, dev157206. [Google Scholar] [CrossRef] [Green Version]
- Aharonov, A.; Shakked, A.; Umansky, K.B.; Savidor, A.; Genzelinakh, A.; Kain, D.; Lendengolts, D.; Revach, O.Y.; Morikawa, Y.; Dong, J.; et al. ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat. Cell Biol. 2020, 22, 1346–1356. [Google Scholar] [CrossRef]
- D’Uva, G.; Aharonov, A.; Lauriola, M.; Kain, D.; Yahalom-Ronen, Y.; Carvalho, S.; Weisinger, K.; Bassat, E.; Rajchman, D.; Yifa, O.; et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 2015, 17, 627–638. [Google Scholar] [CrossRef]
- Bassat, E.; Mutlak, Y.E.; Genzelinakh, A.; Shadrin, I.Y.; Baruch Umansky, K.; Yifa, O.; Kain, D.; Rajchman, D.; Leach, J.; Riabov Bassat, D.; et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 2017, 547, 179–184. [Google Scholar] [CrossRef]
- Lou, X.; Zhao, M.; Fan, C.; Fast, V.G.; Valarmathi, M.T.; Zhu, W.; Zhang, J. N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts. Cardiovasc. Res. 2020, 116, 671–685. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Liu, S.; Pei, J.; Cai, L.; Liu, N.; Liang, T.; Dong, X.; Cong, X.; Chun, J.; Chen, J.; et al. LPA(3)-mediated lysophosphatidic acid signaling promotes postnatal heart regeneration in mice. Theranostics 2020, 10, 10892–10907. [Google Scholar] [CrossRef]
- Strash, N.; DeLuca, S.; Janer Carattini, G.L.; Heo, S.C.; Gorsuch, R.; Bursac, N. Human Erbb2-induced Erk activity robustly stimulates cycling and functional remodeling of rat and human cardiomyocytes. eLife 2021, 10, e65512. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Li, B.; Wang, H.; Li, M.; Huang, S.; Sun, Y.; Chen, G.; Si, X.; Huang, C.; et al. Long Non-coding RNA ECRAR Triggers Post-natal Myocardial Regeneration by Activating ERK1/2 Signaling. Mol. Ther. 2019, 27, 29–45. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, A.; Saito, N.; Kashimoto, R.; Furukawa, S.; Yamamoto, S.; Satoh, A. Axolotl liver regeneration is accomplished via compensatory congestion mechanisms regulated by ERK signaling after partial hepatectomy. Dev. Dyn. 2020, 250, 838–851. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, C.; Shu, B.; Zhai, M.; Deng, C.; He, C.; Luo, M.; Han, T.; Zheng, W.; Zhang, J.; et al. Axis of serotonin-pERK-YAP in liver regeneration. Life Sci. 2018, 209, 490–497. [Google Scholar] [CrossRef]
- Desbois-Mouthon, C.; Wendum, D.; Cadoret, A.; Rey, C.; Leneuve, P.; Blaise, A.; Housset, C.; Tronche, F.; Le Bouc, Y.; Holzenberger, M. Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. FASEB J. 2006, 20, 773–775. [Google Scholar] [CrossRef] [Green Version]
- Abu Rmilah, A.A.; Zhou, W.; Nyberg, S.L. Hormonal Contribution to Liver Regeneration. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 315–338. [Google Scholar] [CrossRef]
- Wang, X.; Ni, C.; Jiang, N.; Wei, J.; Liang, J.; Zhao, B.; Lin, X. Generation of liver bipotential organoids with a small-molecule cocktail. J. Mol. Cell. Biol. 2020, 12, 618–629. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Kang, K.; Lee, S.B.; Seo, D.; Yoon, S.; Kim, S.J.; Jang, K.; Jung, Y.K.; Lee, K.G.; Factor, V.M.; et al. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J. Hepatol. 2019, 70, 97–107. [Google Scholar] [CrossRef]
- Zerrad-Saadi, A.; Lambert-Blot, M.; Mitchell, C.; Bretes, H.; Collin de l’Hortet, A.; Baud, V.; Chereau, F.; Sotiropoulos, A.; Kopchick, J.J.; Liao, L.; et al. GH receptor plays a major role in liver regeneration through the control of EGFR and ERK1/2 activation. Endocrinology 2011, 152, 2731–2741. [Google Scholar] [CrossRef] [Green Version]
- Svegliati-Baroni, G.; Ridolfi, F.; Caradonna, Z.; Alvaro, D.; Marzioni, M.; Saccomanno, S.; Candelaresi, C.; Trozzi, L.; Macarri, G.; Benedetti, A.; et al. Regulation of ERK/JNK/p70S6K in two rat models of liver injury and fibrosis. J. Hepatol. 2003, 39, 528–537. [Google Scholar] [CrossRef]
- Wan, J.; Ramachandran, R.; Goldman, D. HB-EGF is Necessary and Sufficient for Muller Glia Dedifferentiation and Retina Regeneration. Dev. Cell 2012, 22, 334–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Zhao, X.F.; Vojtek, A.; Goldman, D. Retinal Injury, Growth Factors, and Cytokines Converge on beta-Catenin and pStat3 Signaling to Stimulate Retina Regeneration. Cell Rep. 2014, 9, 285–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, A.; Yasumuro, H.; Yoshikawa, T.; Inami, W.; Chiba, C. MEK-ERK signaling in adult newt retinal pigment epithelium cells is strengthened immediately after surgical induction of retinal regeneration. Neurosci. Lett. 2012, 523, 39–44. [Google Scholar] [CrossRef]
- Yasumuro, H.; Sakurai, K.; Toyama, F.; Maruo, F.; Chiba, C. Implications of a Multi-Step Trigger of Retinal Regeneration in the Adult Newt. Biomedicines 2017, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T.; Mizuno, A.; Yasumuro, H.; Inami, W.; Vergara, M.N.; Del Rio-Tsonis, K.; Chiba, C. MEK-ERK and heparin-susceptible signaling pathways are involved in cell-cycle entry of the wound edge retinal pigment epithelium cells in the adult newt. Pigment Cell Melanoma Res. 2012, 25, 66–82. [Google Scholar] [CrossRef]
- Susaki, K.; Chiba, C. MEK mediates in vitro neural transdifferentiation of the adult newt retinal pigment epithelium cells: Is FGF2 an induction factor? Pigment Cell Res. 2007, 20, 364–379. [Google Scholar] [CrossRef]
- Vergara, M.N.; Del Rio-Tsonis, K. Retinal regeneration in the Xenopus laevis tadpole: A new model system. Mol. Vis. 2009, 15, 1000–1013. [Google Scholar]
- Spence, J.R.; Madhavan, M.; Aycinena, J.C.; Del Rio-Tsonis, K. Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requires FGF/FGFR/MEK/Erk dependent upregulation of Pax6. Mol. Vis. 2007, 13, 57–65. [Google Scholar]
- Bao, B.; He, Y.; Tang, D.; Li, W.; Li, H. Inhibition of H3K27me3 Histone Demethylase Activity Prevents the Proliferative Regeneration of Zebrafish Lateral Line Neuromasts. Front. Mol. Neurosci. 2017, 10, 51. [Google Scholar] [CrossRef]
- Harrisingh, M.C.; Perez-Nadales, E.; Parkinson, D.B.; Malcolm, D.S.; Mudge, A.W.; Lloyd, A.C. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004, 23, 3061–3071. [Google Scholar] [CrossRef] [Green Version]
- Napoli, I.; Noon, L.A.; Ribeiro, S.; Kerai, A.P.; Parrinello, S.; Rosenberg, L.H.; Collins, M.J.; Harrisingh, M.C.; White, I.J.; Woodhoo, A.; et al. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 2012, 73, 729–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, A.; Furusho, M.; Bansal, R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J. Neurosci. 2013, 33, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Tong, Z.; Luo, L.; Zhao, Y.; Chen, F.; Li, Y.; Huselstein, C.; Ye, Q.; Ye, Q.; Chen, Y. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair. Bioact. Mater. 2021, 6, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Duprey-Diaz, M.V.; Blagburn, J.M.; Blanco, R.E. Exogenous Modulation of Retinoic Acid Signaling Affects Adult RGC Survival in the Frog Visual System after Optic Nerve Injury. PLoS ONE 2016, 11, e0162626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupack, J.; Xiong, X.P.; Jiang, L.L.; Zhang, T.; Zhou, L.; Campos, A.; Ranscht, B.; Mobley, W.; Pasquale, E.B.; Xu, H.; et al. Soluble SORLA Enhances Neurite Outgrowth and Regeneration through Activation of the EGF Receptor/ERK Signaling Axis. J. Neurosci. 2020, 40, 5908–5921. [Google Scholar] [CrossRef]
- Najm, F.J.; Madhavan, M.; Zaremba, A.; Shick, E.; Karl, R.T.; Factor, D.C.; Miller, T.E.; Nevin, Z.S.; Kantor, C.; Sargent, A.; et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 2015, 522, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Ishii, A.; Fyffe-Maricich, S.L.; Furusho, M.; Miller, R.H.; Bansal, R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J. Neurosci. 2012, 32, 8855–8864. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Zhao, Y.; Xiao, Z.; Wu, X.; Ma, D.; Han, J.; Li, X.; Xue, X.; Yang, Y.; Fang, Y.; et al. Epidermal growth factor receptor-extracellular-regulated kinase blockade upregulates TRIM32 signaling cascade and promotes neurogenesis after spinal cord injury. Stem Cells 2020, 38, 118–133. [Google Scholar] [CrossRef] [Green Version]
- Hollis, E.R., 2nd; Jamshidi, P.; Low, K.; Blesch, A.; Tuszynski, M.H. Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc. Natl. Acad. Sci. USA 2009, 106, 7215–7220. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Sun, H.; Yuan, Q.; Li, N.; Li, H.; Tang, Y.; Leung, G.K.; Wu, W. Targeting proteoglycan receptor PTPsigma restores sensory function after spinal cord dorsal root injury by activation of Erks/CREB signaling pathway. Neuropharmacology 2019, 144, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, H.; Yan, R.; Hu, M. PI3K/Akt and ERK/MAPK Signaling Promote Different Aspects of Neuron Survival and Axonal Regrowth Following Rat Facial Nerve Axotomy. Neurochem. Res. 2017, 42, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.H. Regeneration and Liability to Injury. Science 1901, 14, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Gerber, T.; Taniguchi-Sugiura, Y.; Murawala, P.; Hermann, S.; Grosser, L.; Shibata, E.; Treutlein, B.; Tanaka, E.M. Fibroblast dedifferentiation as a determinant of successful regeneration. Dev. Cell 2021, 56, 1541–1551.e6. [Google Scholar] [CrossRef]
- Kragl, M.; Knapp, D.; Nacu, E.; Khattak, S.; Maden, M.; Epperlein, H.H.; Tanaka, E.M. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 2009, 460, 60–65. [Google Scholar] [CrossRef]
- Tanaka, H.V.; Ng, N.C.Y.; Yang Yu, Z.; Casco-Robles, M.M.; Maruo, F.; Tsonis, P.A.; Chiba, C. A developmentally regulated switch from stem cells to dedifferentiation for limb muscle regeneration in newts. Nat. Commun. 2016, 7, 11069. [Google Scholar] [CrossRef] [Green Version]
- Niethammer, P. The early wound signals. Curr. Opin. Genet. Dev. 2016, 40, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Love, N.R.; Chen, Y.; Ishibashi, S.; Kritsiligkou, P.; Lea, R.; Koh, Y.; Gallop, J.L.; Dorey, K.; Amaya, E. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 2013, 15, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, F.; Raghunathan, V.; Luxardi, G.; Zhu, K.; Zhao, M. Early redox activities modulate Xenopus tail regeneration. Nat. Commun. 2018, 9, 4296. [Google Scholar] [CrossRef]
- Tu, M.K.; Borodinsky, L.N. Spontaneous calcium transients manifest in the regenerating muscle and are necessary for skeletal muscle replenishment. Cell Calcium 2014, 56, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Kujawski, S.; Lin, W.; Kitte, F.; Börmel, M.; Fuchs, S.; Arulmozhivarman, G.; Vogt, S.; Theil, D.; Zhang, Y.; Antos, C.L. Calcineurin regulates coordinated outgrowth of zebrafish regenerating fins. Dev. Cell 2014, 28, 573–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkucur, N.; Epperlein, H.H.; Funk, R.H. Ion imaging during axolotl tail regeneration in vivo. Dev. Dyn. 2010, 239, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.S.; Keyser, M.S.; Gurevich, D.B.; Sturtzel, C.; Mason, E.A.; Paterson, S.; Chen, H.; Scott, M.; Condon, N.D.; Martin, P.; et al. Live-imaging of endothelial Erk activity reveals dynamic and sequential signalling events during regenerative angiogenesis. eLlife 2021, 10, e62196. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lööf, S.; Borg, P.; Nader, G.A.; Blau, H.M.; Simon, A. Turning terminally differentiated skeletal muscle cells into regenerative progenitors. Nat. Commun. 2015, 6, 7916. [Google Scholar] [CrossRef]
- Yun, M.H.; Gates, P.B.; Brockes, J.P. Regulation of p53 is critical for vertebrate limb regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 17392–17397. [Google Scholar] [CrossRef] [Green Version]
- Saera-Vila, A.; Kish, P.E.; Kahana, A. Fgf regulates dedifferentiation during skeletal muscle regeneration in adult zebrafish. Cell. Signal. 2016, 28, 1196–1204. [Google Scholar] [CrossRef] [Green Version]
- Hino, N.; Rossetti, L.; Marin-Llaurado, A.; Aoki, K.; Trepat, X.; Matsuda, M.; Hirashima, T. ERK-Mediated Mechanochemical Waves Direct Collective Cell Polarization. Dev. Cell 2020, 53, 646–660.e8. [Google Scholar] [CrossRef]
- Kierdorf, U.; Li, C.; Price, J.S. Improbable appendages: Deer antler renewal as a unique case of mammalian regeneration. Semin. Cell. Dev. Biol. 2009, 20, 535–542. [Google Scholar] [CrossRef]
- Korotkova, D.D.; Lyubetsky, V.A.; Ivanova, A.S.; Rubanov, L.I.; Seliverstov, A.V.; Zverkov, O.A.; Martynova, N.Y.; Nesterenko, A.M.; Tereshina, M.B.; Peshkin, L.; et al. Bioinformatics Screening of Genes Specific for Well-Regenerating Vertebrates Reveals c-answer, a Regulator of Brain Development and Regeneration. Cell Rep. 2019, 29, 1027–1040.e6. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Godwin, J.W.; Gates, P.B.; Garza-Garcia, A.A.; Brockes, J.P. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 2007, 318, 772–777. [Google Scholar] [CrossRef] [Green Version]
- Grassme, K.S.; Garza-Garcia, A.; Delgado, J.P.; Godwin, J.W.; Kumar, A.; Gates, P.B.; Driscoll, P.C.; Brockes, J.P. Mechanism of Action of Secreted Newt Anterior Gradient Protein. PLoS ONE 2016, 11, e0154176. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Graham, G.M.; Bryant, S.V.; Gardiner, D.M. Neurotrophic regulation of epidermal dedifferentiation during wound healing and limb regeneration in the axolotl (Ambystoma mexicanum). Dev. Biol. 2008, 319, 321–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinarsky, V.; Atkinson, D.L.; Stevenson, T.J.; Keating, M.T.; Odelberg, S.J. Normal newt limb regeneration requires matrix metalloproteinase function. Dev. Biol. 2005, 279, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Jopling, C.; Sleep, E.; Raya, M.; Marti, M.; Raya, A.; Izpisua Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rosa, J.M.; Peralta, M.; Mercader, N. Pan-epicardial lineage tracing reveals that epicardium derived cells give rise to myofibroblasts and perivascular cells during zebrafish heart regeneration. Dev. Biol. 2012, 370, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K.; Gupta, V.; Wang, J.; Holdway, J.E.; Wills, A.A.; Fang, Y.; Poss, K.D. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 2011, 138, 2895–2902. [Google Scholar] [CrossRef] [Green Version]
- Mercer, S.E.; Odelberg, S.J.; Simon, H.G. A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev. Biol. 2013, 382, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [Green Version]
- Vivien, C.J.; Hudson, J.E.; Porrello, E.R. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen. Med. 2016, 1, 16012. [Google Scholar] [CrossRef] [Green Version]
- Uygur, A.; Lee, R.T. Mechanisms of Cardiac Regeneration. Dev. Cell 2016, 36, 362–374. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Zhong, T.P. MAPK/ERK signalling is required for zebrafish cardiac regeneration. Biotechnol. Lett. 2017, 39, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, A.; Tanaka, M.; Itoh, T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell 2014, 14, 561–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preziosi, M.E.; Monga, S.P. Update on the Mechanisms of Liver Regeneration. Semin. Liver Dis. 2017, 37, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Fausto, N. Liver regeneration and repair: Hepatocytes, progenitor cells, and stem cells. Hepatology 2004, 39, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Borowiak, M.; Garratt, A.N.; Wustefeld, T.; Strehle, M.; Trautwein, C.; Birchmeier, C. Met provides essential signals for liver regeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 10608–10613. [Google Scholar] [CrossRef] [Green Version]
- Araujo, T.G.; de Oliveira, A.G.; Tobar, N.; Saad, M.J.; Moreira, L.R.; Reis, E.R.; Nicola, E.M.; de Jorge, G.L.; dos Tartaro, R.R.; Boin, I.F.; et al. Liver regeneration following partial hepatectomy is improved by enhancing the HGF/Met axis and Akt and Erk pathways after low-power laser irradiation in rats. Lasers Med. Sci. 2013, 28, 1511–1517. [Google Scholar] [CrossRef]
- Wilken, M.S.; Reh, T.A. Retinal regeneration in birds and mice. Curr. Opin. Genet. Dev. 2016, 40, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, C.; Ueda, Y.; Okamoto, M.; Araki, M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: Transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev. Biol. 2007, 303, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Goldman, D. Muller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Langhe, R.; Chesneau, A.; Colozza, G.; Hidalgo, M.; Ail, D.; Locker, M.; Perron, M. Müller glial cell reactivation in Xenopus models of retinal degeneration. Glia 2017, 65, 1333–1349. [Google Scholar] [CrossRef]
- Powell, C.; Grant, A.R.; Cornblath, E.; Goldman, D. Analysis of DNA methylation reveals a partial reprogramming of the Muller glia genome during retina regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 19814–19819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kha, C.X.; Guerin, D.J.; Tseng, K.A. Using the Xenopus Developmental Eye Regrowth System to Distinguish the Role of Developmental Versus Regenerative Mechanisms. Front. Physiol. 2019, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Catala, M.; Kubis, N. Gross anatomy and development of the peripheral nervous system. Handb. Clin. Neurol. 2013, 115, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, M.; Negro, S. Signals Orchestrating Peripheral Nerve Repair. Cells 2020, 9, 1768. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell. Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef] [Green Version]
- Negro, S.; Bergamin, E.; Rodella, U.; Duregotti, E.; Scorzeto, M.; Jalink, K.; Montecucco, C.; Rigoni, M. ATP Released by Injured Neurons Activates Schwann Cells. Front. Cell Neurosci. 2016, 10, 134. [Google Scholar] [CrossRef]
- Cervellini, I.; Galino, J.; Zhu, N.; Allen, S.; Birchmeier, C.; Bennett, D.L. Sustained MAPK/ERK Activation in Adult Schwann Cells Impairs Nerve Repair. J. Neurosci. 2018, 38, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Cruz, I.A.; Kappedal, R.; Mackenzie, S.M.; Hailey, D.W.; Hoffman, T.L.; Schilling, T.F.; Raible, D.W. Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance. Dev. Biol. 2015, 402, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Denans, N.; Baek, S.; Piotrowski, T. Comparing Sensory Organs to Define the Path for Hair Cell Regeneration. Annu. Rev. Cell Dev. Biol. 2019, 35, 567–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curcio, M.; Bradke, F. Axon Regeneration in the Central Nervous System: Facing the Challenges from the Inside. Annu. Rev. Cell Dev. Biol. 2018, 34, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Diaz Quiroz, J.F.; Echeverri, K. Spinal cord regeneration: Where fish, frogs and salamanders lead the way, can we follow? Biochem. J. 2013, 451, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Munoz, R.; Edwards-Faret, G.; Moreno, M.; Zuniga, N.; Cline, H.; Larrain, J. Regeneration of Xenopus laevis spinal cord requires Sox2/3 expressing cells. Dev. Biol. 2015, 408, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Alemán, M.M.; Monzón-Mayor, M.; Yanes, C.; Lang, D. Radial glial cells, proliferating periventricular cells, and microglia might contribute to successful structural repair in the cerebral cortex of the lizard Gallotia galloti. Exp. Neurol. 2004, 188, 74–85. [Google Scholar] [CrossRef]
- Zukor, K.A.; Kent, D.T.; Odelberg, S.J. Meningeal cells and glia establish a permissive environment for axon regeneration after spinal cord injury in newts. Neural Dev. 2011, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Joven, A.; Simon, A. Homeostatic and regenerative neurogenesis in salamanders. Prog. Neurobiol. 2018, 170, 81–98. [Google Scholar] [CrossRef]
- Suo, N.; Guo, Y.E.; He, B.; Gu, H.; Xie, X. Inhibition of MAPK/ERK pathway promotes oligodendrocytes generation and recovery of demyelinating diseases. Glia 2019, 67, 1320–1332. [Google Scholar] [CrossRef] [Green Version]
- Keyes, J.; Ganesan, A.; Molinar-Inglis, O.; Hamidzadeh, A.; Zhang, J.; Ling, M.; Trejo, J.; Levchenko, A.; Zhang, J. Signaling diversity enabled by Rap1-regulated plasma membrane ERK with distinct temporal dynamics. eLife 2020, 9, e57410. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Tereshina, M.B.; Ermakova, G.V.; Belousov, V.V.; Zaraisky, A.G. Agr genes, missing in amniotes, are involved in the body appendages regeneration in frog tadpoles. Sci. Rep. 2013, 3, 1279. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, A.S.; Korotkova, D.D.; Ermakova, G.V.; Martynova, N.Y.; Zaraisky, A.G.; Tereshina, M.B. Ras-dva small GTPases lost during evolution of amniotes regulate regeneration in anamniotes. Sci. Rep. 2018, 8, 13035. [Google Scholar] [CrossRef] [PubMed]
- Gerber, T.; Murawala, P.; Knapp, D.; Masselink, W.; Schuez, M.; Hermann, S.; Gac-Santel, M.; Nowoshilow, S.; Kageyama, J.; Khattak, S.; et al. Single-cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science 2018, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aztekin, C.; Hiscock, T.W.; Marioni, J.C.; Gurdon, J.B.; Simons, B.D.; Jullien, J. Identification of a regeneration-organizing cell in the Xenopus tail. Science 2019, 364, 653–658. [Google Scholar] [CrossRef]
- Sanor, L.D.; Flowers, G.P.; Crews, C.M. Multiplex CRISPR/Cas screen in regenerating haploid limbs of chimeric Axolotls. eLife 2020, 9, e48511. [Google Scholar] [CrossRef]
- Wang, W.; Hu, C.K.; Zeng, A.; Alegre, D.; Hu, D.; Gotting, K.; Ortega Granillo, A.; Wang, Y.; Robb, S.; Schnittker, R.; et al. Changes in regeneration-responsive enhancers shape regenerative capacities in vertebrates. Science 2020, 369. [Google Scholar] [CrossRef]


| Organs | (Species) | Signaling Components | Functions | References |
|---|---|---|---|---|
| Limb | (newt) |
|
| [33] |
| (newt) |
|
| [34] | |
| (Xenopus laevis) |
|
| [35] | |
| Tail | (axolotl) |
|
| [36] |
| (Xenopus laevis) |
|
| [37] | |
| (zebrafish) |
|
| [38] | |
| Fin | (zebrafish) |
|
| [39] |
|
| [40] | ||
| ||||
| Scale | (zebrafish) |
|
| [41] |
|
| [42] | ||
| Antler | (deer) |
|
| [43] |
| [44] | |||
| Heart | (zebrafish) |
|
| [45] |
|
| [46] | ||
| (mice) |
|
| [47] | |
|
| [48] | ||
|
| [49] | ||
|
| [50] | ||
|
| [51] | ||
| (rat) |
|
| [52] | |
|
| [53] | ||
| Liver | (axolotl) |
|
| [54] |
| (mice) |
|
| [55] | |
|
| [56,57] | ||
|
| [58] | ||
|
| [59] | ||
|
| [60] | ||
| (rat) |
|
| [61] | |
| Eye | (zebrafish) |
|
| [62] |
|
| [63] | ||
| (newt) |
|
| [64,65,66] | |
|
| [67] | ||
| (Xenopus laevis) |
|
| [68] | |
| (chick) |
|
| [69] | |
| PNS | (zebrafish) |
|
| [70] |
| ||||
| (mice) |
|
| [71,72] | |
| ||||
| ||||
|
| [73] | ||
| (rat) |
|
| [74] | |
| CNS | (frog) |
|
| [75] |
| (mice) |
|
| [76] | |
|
| [24] | ||
|
| [77] | ||
|
| [73,78] | ||
|
| [79] | ||
| (rat) |
|
| [80] | |
|
| [81] | ||
|
| [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Jiao, L.; Tan, H. MAPK/ERK Pathway as a Central Regulator in Vertebrate Organ Regeneration. Int. J. Mol. Sci. 2022, 23, 1464. https://doi.org/10.3390/ijms23031464
Wen X, Jiao L, Tan H. MAPK/ERK Pathway as a Central Regulator in Vertebrate Organ Regeneration. International Journal of Molecular Sciences. 2022; 23(3):1464. https://doi.org/10.3390/ijms23031464
Chicago/Turabian StyleWen, Xiaomin, Lindi Jiao, and Hong Tan. 2022. "MAPK/ERK Pathway as a Central Regulator in Vertebrate Organ Regeneration" International Journal of Molecular Sciences 23, no. 3: 1464. https://doi.org/10.3390/ijms23031464
APA StyleWen, X., Jiao, L., & Tan, H. (2022). MAPK/ERK Pathway as a Central Regulator in Vertebrate Organ Regeneration. International Journal of Molecular Sciences, 23(3), 1464. https://doi.org/10.3390/ijms23031464






