Real-Time Fluorescence Microscopy on Living E. coli Sheds New Light on the Antibacterial Effects of the King Penguin β-Defensin AvBD103b
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of the Minimal Inhibitory Concentrations
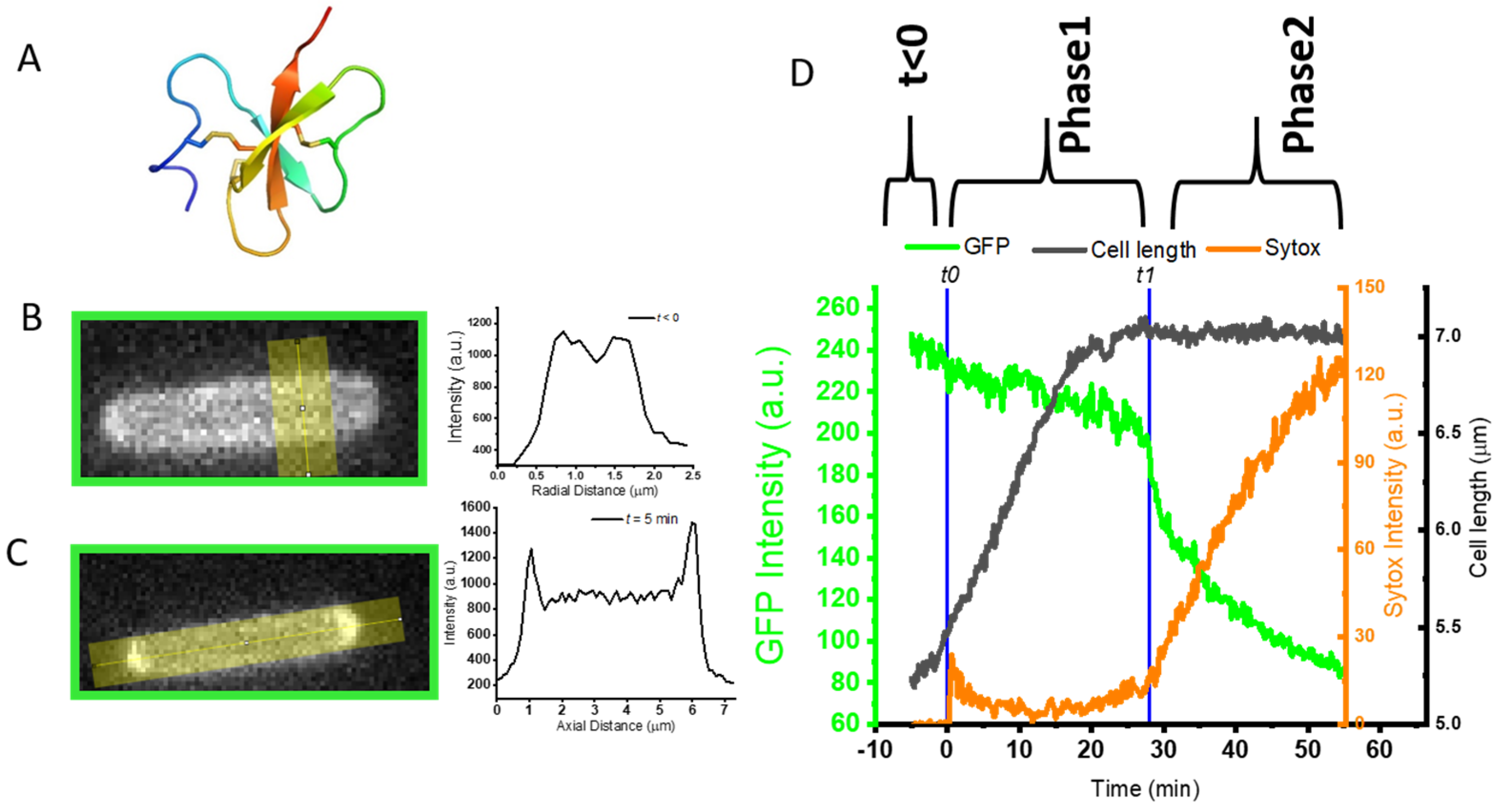
2.2. Delayed and Transient Effect of L-AvBD103b Defensin on E. coli Membrane Permeability
2.2.1. Phase 1: Apparent Osmotic Effects and the Halting of Growth as Defensin Accesses Periplasm
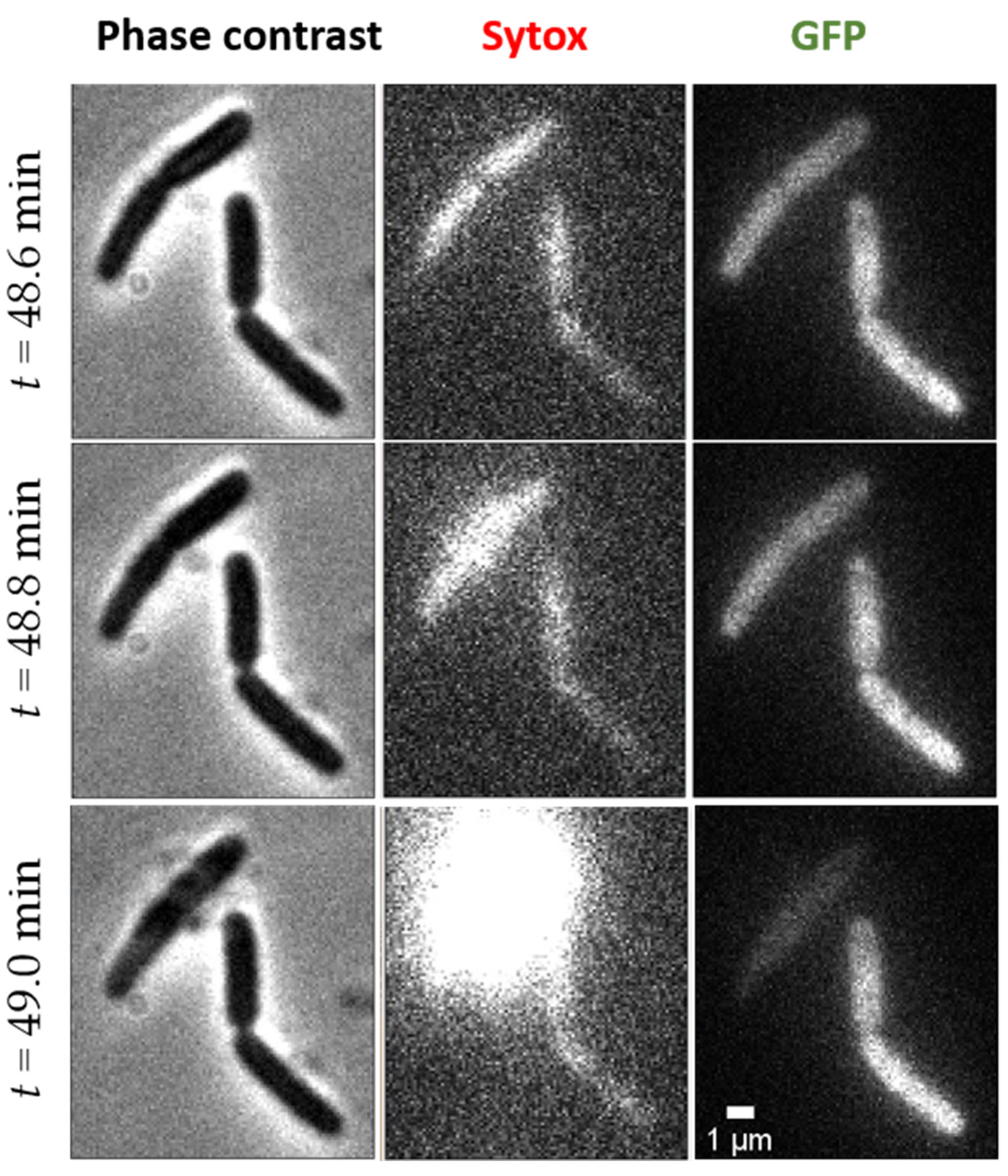
2.2.2. Abrupt Transition Time t1: Strong, Transient Permeabilization of OM and CM
2.2.3. Phase 2: Slow Leakage of GFP and Sytox across the Cell Envelope
2.3. Final Indirect Effect of L-AvBD103b on E. coli
2.4. Antibacterial Mechanism of AvBD103b Does Not Involve Significant Stereo-Selective Interactions with Any Partner
2.5. The Permeabilization Profile of AvBD103b Is Atypical
3. Materials and Methods
3.1. Defensins Synthesis
3.2. Bacterial Strain, Cultures Conditions
3.3. Evaluation of the Minimal Inhibitory Concentration
3.4. Preparation of PDMS Microfluidic Devices
3.5. Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hamoen, L.W.; Wenzel, M. Editorial: Antimicrobial peptides—Interaction with membrane lipids and proteins. Front. Cell Dev. Biol. 2017, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Bastos, P.; Trindade, F.; da Costa, J.; Ferreira, R.; Vitorino, R. Human antimicrobial peptides in bodily fluids: Current knowledge and therapeutic perspectives in the postantibiotic era. Med. Res. Rev. 2018, 38, 101–146. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.N.; Nizet, V. Bacterial evasion of host antimicrobial peptide defenses. Microbiol. Spectr. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski Lda, S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4, 353. [Google Scholar] [CrossRef] [Green Version]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehbach, J.; Craik, D.J. The vast structural diversity of antimicrobial peptides. Trends Pharmacol. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Chan, S.Y.; Song, Q.; Li, P.; Huang, W. The strategies of pathogen-oriented therapy on circumventing antimicrobial resistance. Research 2020, 2020, 2016201. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Bjorn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Upert, G.; Luther, A.; Obrecht, D.; Ermert, P. Emerging peptide antibiotics with therapeutic potential. Med. Drug Discov. 2021, 9, 100078. [Google Scholar] [CrossRef] [PubMed]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B. Review: Lessons learned from clinical trials using antimicrobial peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 40, 488–505. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Gheorghe, I.; Czobor, I.B.; Chifiriuc, M.C. Antibiotic resistance profiles, molecular mechanisms and innovative treatment strategies of Acinetobacter baumannii. Microorganisms 2020, 8, 935. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Uddin, S.J.; Shilpi, J.A.; Nahar, L.; Sarker, S.D.; Goransson, U. Editorial: Natural antimicrobial peptides: Hope for new antibiotic lead molecules. Front. Pharmacol. 2021, 12, 640938. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- WHO. 2020 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. Available online: https://www.who.int/publications/i/item/9789240021303 (accessed on 15 April 2021).
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Scocchi, M.; Tossi, A.; Gennaro, R. Proline-rich antimicrobial peptides: Converging to a non-lytic mechanism of action. Cell. Mol. Life Sci. CMLS 2011, 68, 2317–2330. [Google Scholar] [CrossRef]
- Sugiarto, H.; Yu, P.L. Mechanisms of action of ostrich beta-defensins against Escherichia coli. FEMS Microbiol. Lett. 2007, 270, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafer, A.B.; Wenzel, M. A how-to guide for mode of action analysis of antimicrobial peptides. Front. Cell. Infect. Microbiol. 2020, 10, 540898. [Google Scholar] [CrossRef]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial peptides: Primeval molecules or future drugs? PLoS Pathog. 2010, 6, e1001067. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.T.; Gellatly, S.L.; Hancock, R.E. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. CMLS 2011, 68, 2161–2176. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.E.; Bjornestad, V.A.; Pipich, V.; Jenssen, H.; Lund, R. Beyond structural models for the mode of action: How natural antimicrobial peptides affect lipid transport. J. Colloid Interface Sci. 2021, 582, 793–802. [Google Scholar] [CrossRef]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-membrane permeabilizing modes of action of antimicrobial peptides on bacteria. Curr. Top. Med. Chem. 2016, 16, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Weisshaar, J.C.; Mustafi, M. Long-term effects of the proline-rich antimicrobial peptide Oncocin112 on the Escherichia coli translation machinery. J. Biol. Chem. 2020, 295, 13314–13325. [Google Scholar] [CrossRef]
- Shafee, T.M.; Lay, F.T.; Phan, T.K.; Anderson, M.A.; Hulett, M.D. Convergent evolution of defensin sequence, structure and function. Cell. Mol. Life Sci. CMLS 2017, 74, 663–682. [Google Scholar] [CrossRef] [PubMed]
- Landon, C.; Thouzeau, C.; Labbe, H.; Bulet, P.; Vovelle, F. Solution structure of spheniscin, a beta-defensin from the penguin stomach. J. Biol. Chem. 2004, 279, 30433–30439. [Google Scholar] [CrossRef]
- Lapierre, S.G.; Phelippeau, M.; Hakimi, C.; Didier, Q.; Reynaud-Gaubert, M.; Dubus, J.-C.; Drancourt, M. Cystic fibrosis respiratory tract salt concentration. An exploratory cohort study. Medicine 2017, 96, e8423. [Google Scholar] [CrossRef]
- Langton Hewer, S.C.; Smyth, A.R. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst. Rev. 2017, 4, CD004197. [Google Scholar] [CrossRef]
- Gauthier-Clerc, M.; Le Maho, Y.; Clerquin, Y.; Drault, S.; Handrich, Y. Penguin fathers preserve food for their chicks. Nature 2000, 408, 928–929. [Google Scholar] [CrossRef]
- Thouzeau, C.; Le Maho, Y.; Froget, G.; Sabatier, L.; Le Bohec, C.; Hoffmann, J.A.; Bulet, P. Spheniscins, avian beta-defensins in preserved stomach contents of the king penguin, Aptenodytes patagonicus. J. Biol. Chem. 2003, 278, 51053–51058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.Y.; Andalibi, A.; Webster, P.; Moon, S.K.; Teufert, K.; Kang, S.H.; Li, J.D.; Nagura, M.; Ganz, T.; Lim, D.J. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect. Dis. 2004, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, B.; Nagaraj, R. Variations in the interaction of human defensins with Escherichia coli: Possible implications in bacterial killing. PLoS ONE 2017, 12, e0175858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgera, F.; Antcheva, N.; Pacor, S.; Quaroni, L.; Berti, F.; Vaccari, L.; Tossi, A. Structuring and interactions of human beta-defensins 2 and 3 with model membranes. J. Pept. Sci. 2008, 14, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.S.; Ruan, L.S.; Tu, J.; Qi, K.Z.; Jiang, L.H. Tissue distribution, expression, and antimicrobial activity of Anas platyrhynchos avian beta-defensin 6. Poult. Sci. 2013, 92, 97–104. [Google Scholar] [CrossRef]
- Sahl, H.G.; Pag, U.; Bonness, S.; Wagner, S.; Antcheva, N.; Tossi, A. Mammalian defensins: Structures and mechanism of antibiotic activity. J. Leukoc. Biol. 2005, 77, 466–475. [Google Scholar] [CrossRef]
- Sharma, H.; Nagaraj, R. Antimicrobial activity of human beta-defensin 4 analogs: Insights into the role of disulfide linkages in modulating activity. Peptides 2012, 38, 255–265. [Google Scholar] [CrossRef]
- Teng, D.; Wang, X.; Xi, D.; Mao, R.; Zhang, Y.; Guan, Q.; Zhang, J.; Wang, J. A dual mechanism involved in membrane and nucleic acid disruption of AvBD103b, a new avian defensin from the king penguin, against Salmonella enteritidis CVCC3377. Appl. Microbiol. Biotechnol. 2014, 98, 8313–8325. [Google Scholar] [CrossRef]
- van Dijk, A.; Veldhuizen, E.J.; Kalkhove, S.I.; Tjeerdsma-van Bokhoven, J.L.; Romijn, R.A.; Haagsman, H.P. The beta-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrob. Agents Chemother. 2007, 51, 912–922. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Zhang, C.; Zhang, X.; Zhang, M.Z.; Rottinghaus, G.E.; Zhang, S. Structure-function analysis of Avian beta-defensin-6 and beta-defensin-12: Role of charge and disulfide bridges. BMC Microbiol. 2016, 16, 210. [Google Scholar] [CrossRef] [Green Version]
- Sass, V.; Schneider, T.; Wilmes, M.; Korner, C.; Tossi, A.; Novikova, N.; Shamova, O.; Sahl, H.G. Human beta-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect. Immun. 2010, 78, 2793–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sochacki, K.A.; Barns, K.J.; Bucki, R.; Weisshaar, J.C. Real-time attack on single Escherichia coli cells by the human antimicrobial peptide LL-37. Proc. Natl. Acad. Sci. USA 2011, 108, E77–E81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Rangarajan, N.; Weisshaar, J.C. Lights, camera, action! antimicrobial peptide mechanisms imaged in space and time. Trends Microbiol. 2016, 24, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Choi, H.; Weisshaar, J.C. Melittin-induced permeabilization, re-sealing, and re-permeabilization of E. coli membranes. Biophys. J. 2018, 114, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Yang, Z.; Weisshaar, J.C. Oxidative stress induced in E. coli by the human antimicrobial peptide LL-37. PLoS Pathog. 2017, 13, e1006481. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Mohapatra, S.; Weisshaar, J.C. Rigidification of the Escherichia coli cytoplasm by the human antimicrobial peptide LL-37 revealed by superresolution fluorescence microscopy. Proc. Natl. Acad. Sci. USA 2019, 116, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Chakraborty, S.; Liu, R.; Gellman, S.H.; Weisshaar, J.C. Single-cell, time-resolved antimicrobial effects of a highly cationic, random nylon-3 copolymer on live Escherichia coli. ACS Chem. Biol. 2016, 11, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Cayley, D.S.; Guttman, H.J.; Record, M.T., Jr. Biophysical characterization of changes in amounts and activity of Escherichia coli cell and compartment water and turgor pressure in response to osmotic stress. Biophys. J. 2000, 78, 1748–1764. [Google Scholar] [CrossRef] [Green Version]
- Guha, S.; Ghimire, J.; Wu, E.; Wimley, W.C. Mechanistic landscape of membrane-permeabilizing peptides. Chem. Rev. 2019, 119, 6040–6085. [Google Scholar] [CrossRef]
- Ulmschneider, J.P. Charged antimicrobial peptides can translocate across membranes without forming channel-like pores. Biophys. J. 2017, 113, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.; Weisshaar, J.C. Effects of alterations of the E. coli lipopolysaccharide layer on membrane permeabilization events induced by Cecropin A. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1860, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Yeaman, M.R. Peptide antimicrobials: Cell wall as a bacterial target. Ann. N. Y. Acad. Sci. 2013, 1277, 127–138. [Google Scholar] [CrossRef]
- Irazazabal, L.N.; Porto, W.F.; Fensterseifer, I.C.M.; Alves, E.S.F.; Matos, C.O.; Menezes, A.C.S.; Felicio, M.R.; Goncalves, S.; Santos, N.C.; Ribeiro, S.M.; et al. Fast and potent bactericidal membrane lytic activity of PaDBS1R1, a novel cationic antimicrobial peptide. Biochim. Biophys. Acta (BBA) Biomembr. 2019, 1861, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Schneider, V.A.; Coorens, M.; Ordonez, S.R.; Tjeerdsma-van Bokhoven, J.L.; Posthuma, G.; van Dijk, A.; Haagsman, H.P.; Veldhuizen, E.J. Imaging the antimicrobial mechanism(s) of cathelicidin-2. Sci. Rep. 2016, 6, 32948. [Google Scholar] [CrossRef] [Green Version]
- van den Berg, J.; Boersma, A.J.; Poolman, B. Microorganisms maintain crowding homeostasis. Nat. Rev. Microbiol. 2017, 15, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Schavemaker, P.E.; Smigiel, W.M.; Poolman, B. Ribosome surface properties may impose limits on the nature of the cytoplasmic proteome. eLife 2017, 6, e30084. [Google Scholar] [CrossRef]
- Schmitt, P.; Wilmes, M.; Pugniere, M.; Aumelas, A.; Bachere, E.; Sahl, H.G.; Schneider, T.; Destoumieux-Garzon, D. Insight into invertebrate defensin mechanism of action: Oyster defensins inhibit peptidoglycan biosynthesis by binding to lipid II. J. Biol. Chem. 2010, 285, 29208–29216. [Google Scholar] [CrossRef] [Green Version]
- Schneider, T.; Kruse, T.; Wimmer, R.; Wiedemann, I.; Sass, V.; Pag, U.; Jansen, A.; Nielsen, A.K.; Mygind, P.H.; Raventos, D.S.; et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science 2010, 328, 1168–1172. [Google Scholar] [CrossRef] [Green Version]
- Essig, A.; Hofmann, D.; Munch, D.; Gayathri, S.; Kunzler, M.; Kallio, P.T.; Sahl, H.G.; Wider, G.; Schneider, T.; Aebi, M. Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J. Biol. Chem. 2014, 289, 34953–34964. [Google Scholar] [CrossRef] [Green Version]
- Medeiros-Silva, J.; Jekhmane, S.; Breukink, E.; Weingarth, M. Towards the native binding modes of antibiotics that target Lipid II. ChemBioChem 2019, 20, 1731–1738. [Google Scholar] [CrossRef] [Green Version]
- Scheffers, D.J.; Tol, M.B. LipidII: Just another brick in the wall? PLoS Pathog. 2015, 11, e1005213. [Google Scholar] [CrossRef]
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Kramer, U.; et al. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E1409–E1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.V.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, E7077–E7086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Lu, W. Mirror image proteins. Curr. Opin. Chem. Biol. 2014, 22, 56–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, D.; Boman, A.; Wahlin, B.; Drain, C.M.; Andreu, D.; Boman, H.G.; Merrifield, R.B. All-D amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. USA 1990, 87, 4761–4765. [Google Scholar] [CrossRef] [Green Version]
- Mandal, K.; Pentelute, B.L.; Tereshko, V.; Thammavongsa, V.; Schneewind, O.; Kossiakoff, A.A.; Kent, S.B. Racemic crystallography of synthetic protein enantiomers used to determine the X-ray structure of plectasin by direct methods. Protein Sci. 2009, 18, 1146–1154. [Google Scholar] [CrossRef] [Green Version]
- Krizsan, A.; Volke, D.; Weinert, S.; Strater, N.; Knappe, D.; Hoffmann, R. Insect-derived proline-rich antimicrobial peptides kill bacteria by inhibiting bacterial protein translation at the 70S ribosome. Angew. Chem. 2014, 53, 12236–12239. [Google Scholar] [CrossRef]
- Podda, E.; Benincasa, M.; Pacor, S.; Micali, F.; Mattiuzzo, M.; Gennaro, R.; Scocchi, M. Dual mode of action of Bac7, a proline-rich antibacterial peptide. Biochim. Biophys. Acta 2006, 1760, 1732–1740. [Google Scholar] [CrossRef]
- Fehlbaum, P.; Bulet, P.; Chernysh, S.; Briand, J.P.; Roussel, J.P.; Letellier, L.; Hetru, C.; Hoffmann, J.A. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc. Natl. Acad. Sci. USA 1996, 93, 1221–1225. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, M.G.; Roy, R.N.; Lomakin, I.B.; Florin, T.; Mankin, A.S.; Steitz, T.A. Structures of proline-rich peptides bound to the ribosome reveal a common mechanism of protein synthesis inhibition. Nucleic Acids Res. 2016, 44, 2439–2450. [Google Scholar] [CrossRef] [Green Version]
- Mardirossian, M.; Grzela, R.; Giglione, C.; Meinnel, T.; Gennaro, R.; Mergaert, P.; Scocchi, M. The host antimicrobial peptide Bac71-35 binds to bacterial ribosomal proteins and inhibits protein synthesis. Chem. Biol. 2014, 21, 1639–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seefeldt, A.C.; Nguyen, F.; Antunes, S.; Perebaskine, N.; Graf, M.; Arenz, S.; Inampudi, K.K.; Douat, C.; Guichard, G.; Wilson, D.N.; et al. The proline-rich antimicrobial peptide Onc112 inhibits translation by blocking and destabilizing the initiation complex. Nat. Struct. Mol. Biol. 2015, 22, 470–475. [Google Scholar] [CrossRef]
- Sinha, S.; Zheng, L.; Mu, Y.; Ng, W.J.; Bhattacharjya, S. Structure and interactions of a host defense antimicrobial peptide thanatin in lipopolysaccharide micelles reveal mechanism of bacterial cell agglutination. Sci. Rep. 2017, 7, 17795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, E.; Baeta, T.; Romanelli, A.; Laguri, C.; Martorana, A.M.; Erba, E.; Simorre, J.P.; Sperandeo, P.; Polissi, A. Thanatin impairs lipopolysaccharide transport complex assembly by targeting LptC-LptA interaction and decreasing LptA stability. Front. Microbiol. 2020, 11, 909. [Google Scholar] [CrossRef]
- Vetterli, S.U.; Zerbe, K.; Muller, M.; Urfer, M.; Mondal, M.; Wang, S.Y.; Moehle, K.; Zerbe, O.; Vitale, A.; Pessi, G.; et al. Thanatin targets the intermembrane protein complex required for lipopolysaccharide transport in Escherichia coli. Sci. Adv. 2018, 4, eaau2634. [Google Scholar] [CrossRef] [Green Version]
- Henriques, S.T.; Peacock, H.; Benfield, A.H.; Wang, C.K.; Craik, D.J. Is the mirror image a true reflection? Intrinsic membrane chirality modulates peptide binding. J. Am. Chem. Soc. 2019, 141, 20460–20469. [Google Scholar] [CrossRef] [PubMed]
- Sando, L.; Henriques, S.T.; Foley, F.; Simonsen, S.M.; Daly, N.L.; Hall, K.N.; Gustafson, K.R.; Aguilar, M.I.; Craik, D.J. A Synthetic mirror image of kalata B1 reveals that cyclotide activity is independent of a protein receptor. ChemBioChem 2011, 12, 2456–2462. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Findlay, B.; Bakhtiary, A.; Acedo, J.Z.; Rodriguez-Lopez, E.M.; Mercier, P.; Vederas, J.C. Antimicrobial lipopeptide tridecaptin A1 selectively binds to Gram-negative lipid II. Proc. Natl. Acad. Sci. USA 2016, 113, 11561–11566. [Google Scholar] [CrossRef] [Green Version]
- Bucki, R.; Janmey, P.A. Interaction of the gelsolin-derived antibacterial PBP 10 peptide with lipid bilayers and cell membranes. Antimicrob. Agents Chemother. 2006, 50, 2932–2940. [Google Scholar] [CrossRef] [Green Version]
- Macleod, T.; Ward, J.; Alase, A.A.; Bridgewood, C.; Wittmann, M.; Stonehouse, N.J. Antimicrobial peptide LL-37 facilitates intracellular uptake of RNA aptamer Apt 21-2 without inducing an inflammatory or interferon response. Front. Immunol. 2019, 10, 857. [Google Scholar] [CrossRef]
- Wei, G.; de Leeuw, E.; Pazgier, M.; Yuan, W.; Zou, G.; Wang, J.; Ericksen, B.; Lu, W.Y.; Lehrer, R.I.; Lu, W. Through the looking glass, mechanistic insights from enantiomeric human defensins. J. Biol. Chem. 2009, 284, 29180–29192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derache, C.; Meudal, H.; Aucagne, V.; Mark, K.J.; Cadene, M.; Delmas, A.F.; Lalmanach, A.C.; Landon, C. Initial insights into structure-activity relationships of avian defensins. J. Biol. Chem. 2012, 287, 7746–7755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangarajan, N.; Bakshi, S.; Weisshaar, J.C. Localized permeabilization of E. coli membranes by the antimicrobial peptide Cecropin A. Biochemistry 2013, 52, 6584–6594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Weisshaar, J.C. HaloTag assay suggests common mechanism of E. coli membrane permeabilization induced by cationic peptides. ACS Chem. Biol. 2018, 13, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Sochacki, K.A.; Shkel, I.A.; Record, M.T.; Weisshaar, J.C. Protein diffusion in the periplasm of E. coli under osmotic stress. Biophys. J. 2011, 100, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.D.; Daniel, R.A.; Errington, J.; Robinson, C. Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli. Mol. Microbiol. 2001, 39, 47–53. [Google Scholar] [CrossRef]
- Choi, H.; Yang, Z.; Weisshaar, J.C. Single-cell, real-time detection of oxidative stress induced in Escherichia coli by the antimicrobial peptide CM15. Proc. Natl. Acad. Sci. USA 2015, 112, E303–E310. [Google Scholar] [CrossRef] [Green Version]
- Edelstein, A.D.; Tsuchida, M.A.; Amodaj, N.; Pinkard, H.; Vale, R.D.; Stuurman, N. Advanced methods of microscope control using muManager software. J. Biol. Methods 2014, 1, e10. [Google Scholar] [CrossRef] [Green Version]


| Antimicrobial Compound (Charge, Hydrophobicity) | Observation of Periplasmic Bubbles (Duration) | Event1: OM Permeabilization (GFP Leak to Surroundings) | Event2: CM Permeabilization (Sytox Increase) | Concomitance of Event 1 and Event 2 | Halt of Growth or Cell Shrinkage and Correlation with Event 1 | Refs |
|---|---|---|---|---|---|---|
| LL-37 (+6, 35%) | No | Very High | High | No | Cell shrinkage correlates with event 1 | [43] |
| Cecropin A (+6, 48%) | No | Very High | Weak | No | Cell shrinkage correlates with event 1 | [52,84] |
| Melittin (+6, 46%) | Yes (<24 s) | High | High | Yes | Cell shrinkage correlates with event 1 | [45] |
| MM63:CHx37 (mean charge +22) | Yes | Weak | High | No | Cell shrinkage precedes event 1 | [48,85] |
| L-AvBD103b (+10, 47%) | Yes (minutes) | Weak | Weak | Yes | Halt of growth precedes event 1 | This work |
| D-AvBD103b (+10, 47%) | Yes (minutes) | Weak | Weak | Yes | Halt of growth precedes event 1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landon, C.; Zhu, Y.; Mustafi, M.; Madinier, J.-B.; Lelièvre, D.; Aucagne, V.; Delmas, A.F.; Weisshaar, J.C. Real-Time Fluorescence Microscopy on Living E. coli Sheds New Light on the Antibacterial Effects of the King Penguin β-Defensin AvBD103b. Int. J. Mol. Sci. 2022, 23, 2057. https://doi.org/10.3390/ijms23042057
Landon C, Zhu Y, Mustafi M, Madinier J-B, Lelièvre D, Aucagne V, Delmas AF, Weisshaar JC. Real-Time Fluorescence Microscopy on Living E. coli Sheds New Light on the Antibacterial Effects of the King Penguin β-Defensin AvBD103b. International Journal of Molecular Sciences. 2022; 23(4):2057. https://doi.org/10.3390/ijms23042057
Chicago/Turabian StyleLandon, Céline, Yanyu Zhu, Mainak Mustafi, Jean-Baptiste Madinier, Dominique Lelièvre, Vincent Aucagne, Agnes F. Delmas, and James C. Weisshaar. 2022. "Real-Time Fluorescence Microscopy on Living E. coli Sheds New Light on the Antibacterial Effects of the King Penguin β-Defensin AvBD103b" International Journal of Molecular Sciences 23, no. 4: 2057. https://doi.org/10.3390/ijms23042057
APA StyleLandon, C., Zhu, Y., Mustafi, M., Madinier, J.-B., Lelièvre, D., Aucagne, V., Delmas, A. F., & Weisshaar, J. C. (2022). Real-Time Fluorescence Microscopy on Living E. coli Sheds New Light on the Antibacterial Effects of the King Penguin β-Defensin AvBD103b. International Journal of Molecular Sciences, 23(4), 2057. https://doi.org/10.3390/ijms23042057






