Abstract
Obesity is a risk factor that leads to the development of other diseases such as dyslipidemia and diabetes. These three metabolic disorders can occur simultaneously, hence, the treatment requires many drugs. Antioxidant compounds have been reported to have activities against obesity, dyslipidemia and diabetes via several mechanisms. This review aims to discuss the antioxidant compounds that have activity against obesity, dyslipidemia and diabetes together with their molecular signaling mechanism. The literature discussed in this review was obtained from the PUBMED database. Based on the collection of literature obtained, antioxidant compounds having activity against the three disorders (obesity, dyslipidemia and diabetes) were identified. The activity is supported by various molecular signaling pathways that are influenced by these antioxidant compounds, further study of which would be useful in predicting drug targets for a more optimal effect. This review provides insights on utilizing one of these antioxidant compounds as opposed to several drugs. It is hoped that in the future, the number of drugs in treating obesity, dyslipidemia and diabetes altogether can be minimized consequently reducing the risk of side effects.
1. Introduction
Obesity is a pathological condition of excessive fat accumulating in the tissues under the skin and spreading to the organs and tissues around the body. From a health perspective, obesity is malnutrition caused by long-term excessive consumption of unhealthy food. Obese patients have health problems, one of which is an increase in total cholesterol levels > 200 mg/dL. The World Health Organization (WHO) points out that obesity is a chronic disease and is one of the risk factors for degenerative diseases such as diabetes and dyslipidemia, as well as acute coronary disease, hypertension, hyperuricemia and polycystic ovary syndrome [1]. Obesity can trigger oxidative stress through various mechanisms such as oxidative phosphorylation, glyceraldehyde autoxidation and superoxide formation [2]. Oxidative stress is a condition when there is an increase in the number of free radicals and/or a decrease in antioxidant activity [3]. Oxidative stress plays a role in comorbid obesity such as diabetes, dyslipidemia, endothelial dysfunction and mitochondrial dysfunction [2].
Patients with obesity are often associated with lipid abnormalities [4]. Approximately 60–70% of obese patients have dyslipidemia. In addition, insulin resistance disorders also contribute to the development of dyslipidemia. In recent years, dyslipidemia caused by the combined action of insulin resistance and obesity has been referred to as “metabolic dyslipidemia” with the main characteristics of increasing levels of triglycerides (TG) and decreasing levels of high-density lipoprotein (HDL). Under this condition, there can also be an increase in low-density lipoprotein (LDL) levels [5].
Diabetes mellitus (DM) is one of the comorbidities of obesity caused by oxidative stress. DM is a chronic disease affecting the body’s metabolism, characterized by increased blood sugar levels exceeding normal limits. In Southeast Asia, the number of diabetes cases in 2019 reached 88 million people and 90% of them were type 2 diabetes mellitus, half of which have complications that lead to death. The International Diabetes Federation (IDF) listed Indonesia as the 7th highest diabetic country with a prevalence of 8.5 million, and the number is predicted to increase to 14.1 million by 2035 [6]. The study by Mohieldein et al. (2015) reported that prediabetes was associated with obesity, development of dyslipidemia and decreasing total antioxidant status. Lifestyle changes such as weight loss, regular physical activity and a healthy diet should be encouraged to prevent progression to type 2 diabetes and its complications from prediabetes [7].
The hyperglycemia condition in DM has a significant impact on the vascular endothelium, which is caused by the auto-oxidation of glucose during the formation of free radicals, which in turn leads to macro- and microvascular dysfunction due to oxidative stress [3]. Oxidative stress conditions in DM are usually associated with an increase in endothelial cell apoptosis, which shows an increase in the free radical formation and a decrease in antioxidant capacity [8].
Metabolic disorders such as obesity, dyslipidemia and diabetes are the main causes of life-threatening ischemic heart disease [9]. Based on research by Vona et al. (2019) and Pechánová et al. (2015), it is reported that this metabolic disorder is accompanied by chronic inflammation mediated by oxidative stress. Increased oxidative stress in metabolic disorders plays a role in causing mitochondrial dysfunction, accumulation of protein and lipid oxidation products and disruption of the antioxidant system [10,11]. Clinical studies show that obesity co-occurring with metabolic disorders such as dyslipidemia and diabetes will increase the risk of death compared to obesity without metabolic disorders. However, when compared with lean individuals, obesity may increase the risk of death from various complications that accompany this condition [12].
At present, many people have adopted a healthy lifestyle, such as eating foods or taking medications derived from natural ingredients, especially those containing antioxidant compounds that can prevent and treat various diseases [13]. Compounds with antioxidant activity such as kahweol have been reported to have antidiabetic properties by suppressing pancreatic cell apoptosis and increasing insulin secretion in streptozotocin (STZ)-induced mice [14]. Another study by Pan et al. (2018) reported that flavonoids such as resveratrol (3,4′,5-trihydroxy-stilbene, RES) are widely present in vegetables and fruits with biological and pharmacological effects such as antiobesity, antioxidation activity and antidiabetic [15]. The combination of resveratrol and quercetin has also been reported to reduce hyperglycemia, serum glucose dysfunction and dyslipidemia in streptozotocin (STZ)-induced diabetic rats [16].
Of the many studies that discuss metabolic disorders and antioxidants from the literature, only two studies are obtained, which are closest to the discussion in this review. The two studies are conducted by Dal et al. (2016) and Shabbir et al. (2021). In a review article by Dal et al. (2016), the effect of consuming antioxidants from various sources such as functional foods, plants, fruits, vegetables, vitamins, supplements and other natural sources rich in polyphenols on diabetes and vascular complications based on in vivo, in vitro and clinical trials in humans were discussed [17]. Another review article by Shabbir et al. (2021) discusses the activity of polyphenol antioxidant compounds, namely, curcumin, quercetin and catechins, against metabolic disorders that focus on the role of the gut microbiota, which is affected by these antioxidant compounds to improve metabolic disorders [18].
In the two aforementioned reviews, there has been no detailed discussion on the effect of antioxidant compounds and their molecular signaling mechanisms against obesity, dyslipidemia and diabetes. The novelty of this review is the summary of information on antioxidant compounds derived from natural products based on the results of in vivo, in vitro and clinical trials that can treat obesity, dyslipidemia and diabetes. This could provide an insight on the antioxidant compounds that can simultaneously act as an antiobesity, antidyslipidemia and antidiabetic as compared to the current practices that require several drugs for the treatment of the three metabolic disorders, hence minimizing the use of multiple drugs and the risk of side effects and drug interactions. Identifying the plants containing these valuable compounds could also potentially yield a cheaper alternative treatment in the form of a herbal preparation to treat all three illnesses in the future.
In addition, this review also provides information on the molecular signaling pathways influenced by the antioxidant compounds from natural products that play a role in the development of obesity, dyslipidemia and diabetes. This would be useful for researchers to further investigate the activity of these antioxidant compounds to determine therapeutic targets.
2. Method
This review was made based on the results of the collection and review of journals obtained from the PUBMED database with several related keywords such as “antioxidant AND natural product AND obesity”, “antioxidant AND natural product AND antidiabetes”, “antioxidant AND natural product AND antidyslipidemia”, “signaling pathways AND natural product”, “obesity AND dyslipidemia AND natural product”, “antioxidant AND oxidative stress AND obesity AND dyslipidemia AND diabetes”, “ resveratrol AND metabolis disorders AND clinical study”, “quercetin AND clinical study AND antiobesity”, “curcumin AND antiobesity AND clinical study”, ”anthocyanins AND clinical study AND anti obesity”, “antioxidant AND metabolic disorders AND clinical study”, “antioxidant AND antiobesity AND antidiabetes”, “antioxidant AND antidislipidemia AND antidiabetes”.
The inclusion criteria for the main article are articles published in ≥2016 and research articles that discuss pharmacological antioxidant activity against obesity, dyslipidemia and diabetes as well as the signaling pathways of these antioxidant activities. Inclusion criteria for supporting articles are articles that discuss the metabolic disorders of obesity, dyslipidemia, diabetes and oxidative stress including the mechanism of metabolic disorders and the relationship between these metabolic disorders. This supporting article is taken from articles published between 2000 and 2021 with most of the articles included being published after 2016. Exclusion criteria for the main articles were duplicate articles, review articles, research with crude extracts and unrelated articles/irrelevant articles that do not discuss in detail the activity of the chemical compounds contained.
Based on the search results using related keywords, 782 journals were obtained, which were then reduced after removing 60 duplicate articles, 155 review articles, 152 research articles with crude extracts and 270 unrelated/not specific articles. The results are 145 selected articles consisting of 49 supporting articles, 68 articles discussing antioxidant activity against obesity, dyslipidemia and diabetes and 28 articles discussing signaling pathways of antioxidant activity, which were used to be studied in this review. The article search flow can be seen in Figure 1.
Figure 1.
Article literature search flow chart.
3. Oxidative Stress and Its Relation to Metabolic Disorders (Obesity, Dyslipidemia and Diabetes)
Oxidative stress is a condition of an imbalance of production and accumulation of reactive oxygen species (ROS) in cells and tissues with the ability of biological systems to detoxify these reactive products [19]. Reactive oxygen species (ROS) in normal amounts contribute to various physiological processes such as hormone biosynthesis, defense systems, cellular signaling and fertilization. However, increased ROS production results in a condition known as oxidative stress, which has implications for various diseases such as diabetes, dyslipidemia, hypertension, atherosclerosis, heart failure, stroke and other chronic diseases [20,21]. Oxidative stress contributes to the development of obesity’s comorbidities [2]. Possible contributors to oxidative stress in obesity include increased hyperglycemia in tissue, lipids, vitamin and mineral deficiencies, chronic inflammation, endothelial dysfunction and impaired mitochondrial function [19].
3.1. Obesity
Obesity is one of the metabolic disorders resulting from an imbalance between energy intake and consumption. Obesity is marked by the inflammation of many cells, including macrophages [22] and adipose tissue [23]. Macrophages produce cytokines such as IL-6 and tumor necrosis factor alpha (TNFα), which also play a role in causing insulin resistance. In addition, obesity can cause the body’s resistance to insulin, mediated in part by free fatty acids (FFA) and adipokines such as retinol binding protein-4 (RBP4) and resistin, which can reduce insulin sensitivity [24]. Obesity is the main cause of metabolic syndromes such as type 2 diabetes mellitus, insulin resistance, dyslipidemia, hypertension and non-alcoholic fatty liver diseases (NAFLD) [1,25].
Obesity is known to have a relationship with the incidence of oxidative stress. Based on research done on several obese patients, it was reported that abdominal obesity may affect the occurrence of inflammation that triggers an increase in oxidative stress [26]. Studies show that obesity in visceral adipose tissue contributes to a state of oxidative stress that may lead to insulin resistance [27,28].
3.2. Dyslipidemia
Dyslipidemia is a serious problem because it is a major risk for coronary heart disease. Dyslipidemia is caused by several factors such as genes, diet, lifestyle, obesity, and many more [29]. Dyslipidemia in obesity consists of elevated levels of triglycerides (TG) and free fatty acids (FFA), decreased and dysfunctional high-density lipoprotein (HDL) and a slight increase of low-density lipoprotein-cholesterol (LDL) levels. In addition, the concentration of apolipoprotein B (apo B) may also increase due to excessive production of apo B in the liver containing lipoproteins [30].
Dyslipidemia in obesity is characterized by the occurrence of hypertriglyceridemia due to the accumulation of triglycerides in the liver. This leads to the inhibition of chylomicron lipolysis caused by increased synthesis of very low-density lipoprotein (VLDL) in the liver due to the competition for lipoprotein lipase (LPL). Hypertriglyceridemia will induce an increase in the exchange of cholesterol esters (CE) and triglycerides between VLDL, LDL and HDL via cholesteryl ester transfer protein (CETP) [25].
Based on previous research, hypercholesterolemia may induce apoptosis and autophagy caused by ROS activation [31]. Increased production of ROS affects the development of dyslipidemia and other cardiovascular diseases. Free radicals function physiologically as signal transducers and maintain homeostasis in cellular signaling, but when these pathways are disrupted, they may cause risk factors for atherosclerosis, one of which is dyslipidemia [32,33].
3.3. Diabetes Mellitus
Diabetes mellitus (DM) is a metabolic disorder characterized by increased blood sugar levels. DM can be divided into two types: DM type 1 and DM type 2. DM type 1 is caused by the unavailability of insulin produced by pancreatic beta cells, which can be caused by genetic or autoimmune disorders, while type 2 DM occurs due to lack of insulin secretion or insulin resistance, or both. DM type 2 can occur due to the influence of genetic, epigenetic or lifestyle factors [34].
Various inflammatory cytokines such as IL-1β produced by M1 macrophages can cause local and systemic inflammation, pancreatic cell dysfunction and insulin resistance in the liver, adipose and musculoskeletal tissues [35]. M1 macrophages are also associated with diabetes complications, such as kidney disease, neurological diseases, retinopathy, and cardiovascular diseases. However, to date, the underlying mechanism of M1 macrophage accumulation in diabetic patients is not fully known [22].
Hyperglycemia in prediabetes may trigger oxidative stress and increase inflammatory factors that affect vascular dysfunction. This oxidative stress may also cause interference with glucose uptake from muscle cells and fat cells and may reduce insulin sensitivity [27]. Another study also reported an increase in ROS concomitantly with suppression of the antioxidant enzyme superoxide dismutase (SOD) in rats induced by hyperglycemia [36].
4. Antioxidant Activities from Natural Products to Treat Obesity, Dyslipidemia and Diabetes Mellitus
Antioxidants are substances that can counteract free radicals and prevent free radicals from damaging cells. Free radicals are the root cause of health problems, such as cancer, premature aging, cardiovascular disease and digestive diseases. The body naturally produces antioxidants, but when free radicals are abundant, this process will not be efficient and its effectiveness also decreases with age. Increasing the intake of antioxidants can prevent various diseases and reduce health problems. Food such as fruits and vegetables contain important antioxidants such as vitamins A, C, E and beta-carotene, as well as essential minerals such as selenium and zinc [37]. Several antioxidant compounds from natural ingredients that have been widely studied and have antiobesity, antidyslipidemia and antidiabetic activity are resveratrol, curcumin, quercetin and anthocyanin as well as other antioxidants.
4.1. Resveratrol
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a natural antioxidant compound that can be found in various plants, such as Polygonum cuspidatum, peanuts and fruits, such as grapes and berries. The use of resveratrol as a nutraceutical has also been widely reported based on testing in animal and human models as a treatment for obesity, metabolic disorders and cardiovascular disorders [38]. Resveratrol is a polyphenolic antioxidant compound that has various biological activities and has been used as a dietary supplement [38].
A clinical study was conducted on 13 patients with type 1 diabetes who were given resveratrol in 500 mg capsules for 60 days. From the results of this study, it is known that the administration of resveratrol can reduce fasting blood sugar (FBS) significantly (p < 0.05) compared to the initial value with FBS levels of 253.69 ± 49.67 vs. 174.38 ± 45.19 [39]. Another clinical study by Jorge et al. (2020) was conducted on 25 obese individuals (BMI 30 kg/m2) aged 30–60 years who were randomly assigned to a placebo group and a group given resveratrol at a dose of 250 mg/day accompanied by the same program of physical activity and diet carried out for three months. After 3 months, it was reported that in the resveratrol group there was a significant decrease (p < 0.05) in body weight, BMI, waist circumference, total cholesterol (TC), VLDL and a significant increase in HDL levels, while in the placebo group significant reduction in body weight, BMI and waist circumference was also reported but no significant reduction in lipid profile [40].
Another study by Rabbani et al. (2021) reported that administration of oral capsules containing a combination of trans-resveratrol and hesperetin (90 mg tRES: 120 mg HESP) for 8 weeks tested on obese patients showed a decrease in inflammation which was characterized by a decrease in the expression of IL-8 and receptor for the advanced glycation end product (RAGE). In addition, this combination also improves insulin resistance and hyperglycemia [41].
In vivo testing has been carried out on rats induced with a high-fat diet for 12 weeks which were then given resveratrol at a dose of 20 mg/kg/day for 4 weeks, and the results showed a reduction in total cholesterol by 8.4% and LDL by 6.6%. compared to the HFD group (hyperlipidemia group) [42]. Another study was conducted by Campbell et al. (2019) on male C57BL/6J rats which were given a high-fat diet for 16 weeks accompanied by the administration of resveratrol doses of 50, 75 and 100 mg/kg body weight via drinking water. Based on the results, the administration of resveratrol doses of 75 and 100 mg/kgbw could significantly (p < 0.05) prevent weight gain in rats compared to the HFD group. In addition, the administration of resveratrol doses of 75 and 100 mg/kgbw was also reported to prevent chronic inflammation, which was characterized by a decrease in serum IL-1 and TNFα (p < 0.05), as well as oxidative stress in the liver and brain as indicated by an increase in activity of superoxide dismutase, catalase and glutathione peroxidase (p < 0.05) [43].
Research on the antiobesity activity of resveratrol compounds has also been carried out by Chang et al. (2016) in vivo and in vitro. In vivo testing activity of resveratrol was carried out on male C57BL/6C rats induced with high fat diet (HFD) accompanied by the administration of resveratrol at doses of 1, 10 and 30 mg/kgbw for 10 weeks with the results showing that the administration of resveratrol with these three doses may significantly attenuate dose-dependent HFD-induced weight gain compared to the HFD group without any treatment. Furthermore, in vitro testing on 3T3-L1 cells by administering resveratrol at a concentration of 0.03 to 100 μM for 24 h significantly inhibited dose-dependent adipose lipolysis [44]. Based on in vivo, in vitro and clinical trials discussed previously, resveratrol is a potent antioxidant compound in overcoming metabolic disorders of obesity, dyslipidemia and diabetes. However, there is very little information about the dosage and safety for long-term use of this compound, hence, further research is needed to provide an optimal effect of resveratrol and reduce the risk of side effects.
4.2. Curcumin
Curcumin found in turmeric root (Curcuma longa L.) has been reported to have physiological effects such as antioxidant, antiobesity, anti-inflammatory, antidyslipidemic and antidiabetic. Turmeric is widely consumed in Asian countries and used as a cooking spice with no reported toxicity [45]. In vitro assays were carried out by Zhao et al. (2021) on 3T3-L1 preadipocytes and the results showed that incubation of curcumin at doses of 10, 20 and 35 μM for 8 days could induce adipogenic differentiation and accumulation of intracellular fat droplets. These results also showed that there was a 55.0% and 74.7% decrease in preadipocyte viability compared to the control group on incubation with 50 μM and 75 μM curcumin, respectively (p < 0.01). Administration of curcumin has also been reported to enhance mitochondrial respiratory function, induce adipogenic differentiation and regulate peroxisome proliferation activated receptor γ (PPARγ) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) expression [46,47].
In vivo testing was carried out on male albino wistar rats induced with HFD for 4 weeks followed by administration of curcumin at a dose of 80 mg/kg body weight/day for the next 6 weeks. The results showed that there was a significant decrease in BMI (p < 0.05) in rats given curcumin, a BMI value of 0.78 g/cm2 compared to the obese group with a BMI of 0.86 g/cm2 [48].
Clinical trials have been carried out by Thota et al. (2019) on several individuals with a high risk of developing diabetes, having a BMI ranging from 25–45 kg/m2, with fasting glucose levels of 6.1–6.9 mmol/L and HbA1c levels between 5.7–6.4% were given curcumin tablets at a dose of 2 × 500 mg curcumin/day taken every morning and night for 12 weeks. The results of this study indicate that curcumin can increase insulin sensitivity by 32.7 ± 10.3%, reduce serum triglycerides by 0.79%, compared to the placebo group, which had an increase of 26.89%, and significantly reduce insulin resistance (−0.3 ± 0.1 vs. 0.01 ± 0.05, p = 0.0142), as compared to the placebo group [49,50].
In vivo testing was carried out on male wistar rats by inducing rats with an intraperitoneal injection of nicotinamide (110 mg/kg) and streptozotocin (45 mg/kg) in a fasting state. The results of this study by Goushki et al. (2020) reported that the administration of curcumin (100 and 200 mg/kg/day) and nano curcumin (100 and 200 mg/kg/day) could significantly (p < 0.001) reduce fasting blood sugar (FBS) with FBS levels respectively also 158.13, 163.75, 173.38 and 158 mg/dl, compared to the FBS value in the diabetes group of 518.5 mg/dl [51]. In addition, a study by Roxo et al. (2019) was also carried out on male wistar rats induced with STZ at a dose of 40 mg/kg IV so that diabetic rats with blood sugar levels in the range of 380–510 mg/dl were then given a dose of curcumin, 30 mg/kg, 60 mg/kg and 90 mg/kg, and the results showed that none of these doses caused toxicity in rats. Furthermore, the study reported that administration of curcumin at a dose of 90 mg/kg could improve the lipid profile, which was indicated by a significant decrease (p < 0.05) in plasma triacylglycerol and cholesterol levels compared to diabetes controls, and inhibit the advanced glycation end products (AGE)/RAGE signaling pathway [52,53].
Tests in streptozotocin-induced diabetic mice show that tetrahydrocurcumin (THC) at a dose of 120 mg/kg/day for 12 weeks can relieve diabetic cardiomyopathy by attenuating oxidative stress due to hyperglycemia and activating the SIRT1 pathway [54]. Other tests by Lima et al. (2020) reported that administration of curcumin at a dose of 90 mg/kg for 45 days can significantly increase the activity of antioxidant enzymes such as superoxide dismutase, paraoxonase 1 and catalase in 40 mg/kg STZ-induced rats compared to negative controls [55].
Based on a study by Li et al. (2019), the administration of curcumin 20 μM for 24 h at 37 °C to INS-1 cells induced with high glucose/palmitate could effectively inhibit oxidative stress, cell proliferation, increase insulin levels and reduce nicotinamide adenine dinucleotide phosphate (NADPH) oxidase expression as compared to high palmitate (PH) [56], while an in vivo study conducted on C57BL/6J rats induced with a high-fat diet for 3 months and then given curcumin at a dose of 1.5 g/kg/day for 8 weeks showed that curcumin can protect islet cells of Langerhans from apoptosis by modulating the NADPH pathway [57].
Based on the results of curcumin testing in vivo, in vitro and based on clinical trials, it is known that curcumin compounds have good potential in the treatment of metabolic disorders of obesity, dyslipidemia and diabetes. However, further research is needed to obtain the optimal dose, the appropriate dosage form, safety testing for long-term use and the possible side effects so that it can provide optimal effects on metabolic disorders of obesity, dyslipidemia and diabetes in humans.
4.3. Quercetin
Quercetin belongs to the class of flavonols found in many fruits and vegetables such as apples, berries, cauliflower, cabbage and beans. Quercetin has also been widely studied as having antioxidant, antidyslipidemic, antidiabetic, anti-inflammatory and other activities [58]. In vitro tests were carried out on 3T3-L1 adipocytes, and it was reported that administration of pentamethylquercetin (PMQ) at concentrations of 1 and 10 M increased glucose consumption by 24.6% and 66.4% (p < 0.05 and p < 0.01 vs. vehicle). This suggests that PMQ can increase insulin activity in 3T3L1 adiposity. Furthermore, in vivo testing was carried out on wistar rats induced with HFD and given PMQ at a dose of 0.04% g/g for 17 weeks. The test results showed a significant decrease (p < 0.05) in serum glucose, TC, TG and LDL levels in rats given PMQ compared to the HFD group of rats [59]. Tests on rats induced using STZ reported that there was a significant decrease (p < 0.05) in body weight in diabetic rats, and administration of 75 mg/kg bw of quercetin for 28 days showed an increase in body weight of 17.83%, a decrease in blood glucose of 66.80%, triglycerides of 24%, VLDL of 48%, LDL of 31.75% and total cholesterol 26.43% and an increase in HDL of 78.88%, compared to the group of HFD mice [60].
In vivo testing was carried out on wistar rats with STZ induction of 55 mg/kg bw, and an excision wound of 2 cm × 2 cm (400 mm2) was made. Then, after being declared diabetic on blood sugar measurements after 72 h, quercetin was given orally at a dose of 100 mg/kg body weight + quercetin ointment (1%) for 21 days. The results showed that quercetin can normalize changes in blood glucose levels comparable to the normal control group with blood sugar levels ranging from ±150 mg/dl, while the blood sugar levels of the HFD group were ±350 mg/dl and could heal wound areas in diabetic rats significantly greater than the diabetes group [58]. A study by Zhuang et al. (2018) reported that administration of quercetin isolated from Edgeworthia gardneri at a dose of 0.5 g/kg quercetin per day for 4 weeks in db/db mice with type 2 diabetes mellitus (T2DM) could induce insulin secretion at a concentration of 0.10 mol/L, inhibit palmitate-induced pancreatic cell apoptosis and ameliorate mitochondrial dysfunction [61].
Clinical testing was conducted by Lee et al. (2016) on obese male and female Korean individuals who were given quercetin-rich onion peel extract (OPE) capsules at a dose of 100 mg for 12 weeks. The results obtained were that quercetin-rich OPE supplementation significantly reduced body weight and BMI from 70.0 ± 11.4 to 69.2 ± 11.4 kg (p = 0.02) and BMI from 26.6 ± 3.3 to 26.3 ± 3.2 kg/m2 (p = 0.03), whereas in the placebo group there was no significant change. Waist and hip circumference showed significant changes in both groups. The waist circumference of the control group decreased from 90.2 ± 6.5 to 89.5 ± 6.4 cm, while the OPE group decreased by 2 cm from 91.9 ± 7.6 to 89.9 ± 7.7 cm. The hip circumference of the control group decreased from 100.7 ± 5.2 to 99.9 ± 4.6 cm, while the OPE group decreased by 1.3 cm from 101.1 ± 5.9 cm before the experiment to 99.9 ± 6.3 cm after the experiment. In addition, skinfold thickness in the control group decreased significantly by 2.2 mm from 33.2 ± 5.5 to 31.1 ± 5.6 mm (p < 0.001), whereas in the OPE group it decreased significantly by 3.2 mm from 34.1 ± 7.1 to 30.9 ± 6.4 mm (p < 0.001). OPE also showed a significant reduction in arm fat percentage by 0.7% from 36.1% ± 8.8% to 35.5% ± 5.5% (p = 0.03) and total body fat by 0.6% from 38.2% ± 6.5% to 37.6% ± 6.4% (p = 0.02) [62].
4.4. Anthocyanin
Anthocyanins are polyphenolic compounds found in pigmented fruits and vegetables. It is reported that this compound has pharmacological activities such as antioxidant, anti-inflammatory and anti-obesity [63]. In vitro assays were carried out on 3T3-L1 cells by administering 5, 10, 15, 20, 25, 30, 50, 100 and 200 g/mL anthocyanin fraction (AnT Fr) for 24 h, and the results showed the inhibition of lipid accumulation via regulation of adipogenesis and lipogenesis-related genes and signaling proteins [64]. An in vitro study by Han et al. (2018) reported that administration of anthocyanins at a dose of 200 g/mL showed a lipid reduction of 60% [65] and the administration of 10 g/mL can decrease ROS and increase catalase (CAT) and superoxide dismutase (SOD) enzymes significantly [66]. A study by Suantawee et al. (2017) reported that administration of cyanidin, an anthocyanin, at a dose of 1–300 μM can increase insulin release from INS-1 cells and stimulate insulin secretion [67], whereas administration at 60, 100 and 300 μM increased insulin secretion six times higher than the control [68]. In addition, in vivo studies on obese rats reported that administration of blackberry anthocyanins (BLA) and blueberry anthocyanins (BBA) at a dose of 200 mg/kg food for 12 weeks could inhibit body weight gain by 40.5% and 55.4%, respectively [63].
A clinical trial was conducted by Zhang et al. (2020) on dyslipidemic patients who were given anthocyanins in the form of supplements to see the dose-response relationship of oxidative stress and inflammation in dyslipidemic patients. Based on these tests, it was found that anthocyanin supplementation (320 mg/day) for 6 weeks significantly increased total-SOD compared to the placebo (p < 0.05). Anthocyanins (80 mg/day) significantly reduced serum IL-6 (−20%), TNF-α (−11%) and urinary 8-iso-PGF2α (−27%) versus the placebo (p < 0.05). A dosage of 320 mg/day anthocyanin supplementation can significantly reduce serum IL-6 (−40%), TNF-α (−21%) and malondialdehyde (MDA) (−20%) [69].
4.5. Other Antioxidants
Obesity may cause a decrease in total antioxidant capacity (TAC) in obese compared to normal individuals. This condition may also lead to a decrease in HDL levels [70]. Various in vivo and in vitro studies have been carried out to investigate the activity of antioxidant compounds from natural ingredients towards obesity. Lemon is known to have antioxidant activity, and lemon fermented product (LFP) at 0.75 and 1 mg/mL for 10 days was reported to inhibit the accumulation of lipids by 8.3% in 3T3L1 adipocytes. In addition, based on in vivo studies using mice, LFP at a dose of 2.89 g/kg for 9 weeks can reduce the body weight of obese mice by 9.7%, decrease triglyceride levels (17.0%), glucose (29.3%) and free fatty acids (17.9%) and can increase serum HDL (17.6%) [71].
In other studies, by Liao et al. (2019), antioxidant polysaccharides okra (OP) derived from okra (Abelmoschus esculentus L.) at doses of 200 and 400 mg/kgbw showed a significant reduction of dyslipidemia in rats induced by a high-fat diet and streptozotocin 100 mg/kg. The lipid profiles such as total cholesterol, triglycerides and LDL were significantly reduced as compared to the negative control group. These studies also reported that there was an increase in antioxidant enzymes at a dose of 400 mg/kg of OP such as superoxide dismutase (sod), catalase (cat) and glutathione peroxidase (gsh-px) by 274.18 ± 24.1, 57.09 ± 6.91 and 530.08 ± 45.1 u/mg prot respectively [72].
Clinical trials were conducted on healthy individuals aged 30–75 years who consumed green tea in combination with glucosyl hesperidin (GT gH), which contained 178 mg glucosyl hesperidin and 146 mg epigallocatechin gallate (EGCG), for 12 weeks. The results showed that GT gH prevented the addition of body weight, and the antiobesity effect of GT gH is more pronounced in people < 50 years old [73].
In vivo testing was carried out on wistar rats fed with strawberry ellagitannins (ET), which showed that a level of 0.24% of the total diet for 4 weeks can be used effectively for the prevention and treatment of metabolic disorders associated with obesity, dyslipidemia, imbalanced redox status and inflammation [74]. In vivo testing was also carried out by Sousa et al. (2020) on male Sprague-Dawley rats induced on a high-fat diet then given α-terpineol at a dose of 50 mg/kg, which improved insulin sensitivity and reduced (p < 0.05) serum levels of the proinflammatory cytokines TNF-α and IL-1β, when compared with the control group [75].
5. Effect of Antioxidants on Metabolic Disorders of Obesity, Dyslipidemia and Diabetes
5.1. Relationship between Obesity, Dyslipidemia and Diabetes
In the obese population, there is a decrease in skeletal muscle strength and function as well as impaired skeletal muscle mitochondrial respiratory function that contributes to increased mitochondrial ROS production compared to normal-weight individuals [76]. It has been reported that the ratio of type II and type I skeletal muscle fibers is higher than that of normal individuals, with two to three-fold ROS production [77]. Tumor necrosis factor alpha (TNF-α) functions as a catalyst in oxidative stress which is only expressed by type II muscle fibers. Based on studies, systemic administration of TNF-α has been shown to reduce the production of skeletal muscle strength in test animals and can increase muscle protein loss through oxidative activation of the TNF-α/nuclear factor kappa B (NF-κB) signaling pathway. Skeletal muscle oxidative stress induced by TNF-α is preventable by the administration of antioxidants, suggesting that TNF-α may provide an important target for confirming obesity-associated oxidative stress [78].
Obesity can trigger various complications, as shown in Figure 2. Obesity leads to increased and dysfunctional adipocytes [79]. Adipocytes produce adipokines and hormones whose rate and effect of secretion are influenced by the distribution and amount of available adipose tissue. High secretion of pro-inflammatory adipokines by adipocytes and macrophages may cause systemic inflammation in some obese patients [80]. The accumulation of excess lipid intermediates (such as ceramides) triggers lipotoxicity with cell dysfunction and apoptosis in some non-obese tissues. Inflammatory cytokines that are elevated in non-adipose tissue cause impaired signaling and insulin resistance, especially in obese patients, leading to type 2 DM [81].
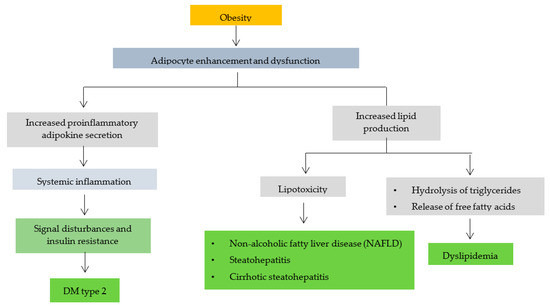
Figure 2.
The relationship of obesity with other disease complications (modification of B. Heymsfield et al., 2017) [81].
Increased adipocytes can also trigger increased lipid production, causing the triglycerides in adipocytes to hydrolyze and release free fatty acids (FFA). Dilation of adipose tissue causes high plasma FFA levels in some patients. Apart from adipose tissue, lipids can also be found in liposomes [82]. Too many fat cells can cause liposomes (steatosis) in liver cells to expand and form large vacuoles associated with diseases, including non-alcoholic fatty liver disease (NAFLD), steatohepatitis and cirrhotic steatohepatitis [83]. This finding is one of many pathophysiological mechanisms of obesity-induced dyslipidemia (increased triglyceride levels, LDL, decreased HDL), type 2 DM, obesity-associated liver disease and osteoarthritis [84,85,86].
In obese patients, there is an increase in reactive oxygen species (ROS) and a decrease in antioxidant defense. Increased oxidative stress in obesity can cause inflammation. In addition, the hormone leptin secreted by adipocytes also plays a role in inducing oxidative stress [87]. Oxidative stress plays a role in causing insulin resistance leading to type 2 diabetes and dyslipidemia [88].
Compounds with antioxidant activity in the treatment of type 2 diabetes can activate the 5′adenosine monophosphate-activated protein kinase (AMPK) pathways, down-regulate the expression of cyclooxygenase-2 (COX2) related genes to release pro-inflammatory mediators, increase glucose tolerance and insulin sensitivity, reduce inflammatory cells and reduce cytokines levels. Pro-inflammatory agents in the serum, such as IL-1B, IL-6 and TNF-α, can inhibit the activation of NF-κB and inhibit the expression of macrophage chemotactic protein (MCP1) [89]. It is reported that the use of antioxidants in patients with type 2 diabetes can effectively prevent complications, which is supported by various studies on antioxidants and the pathological process of diabetes caused by increased oxidative stress [13].
5.2. Antioxidant Mechanisms Associated with Obesity, Dyslipidemia and Diabetes
Several antioxidant compounds have been studied and have activity against obesity, oxidative stress, dyslipidemia and diabetes. Kukoamine B compounds are known to have activity in preventing inflammation and reducing lipid accumulation and oxidative stress [90]. Another antioxidant compound, salidroside, has also been investigated to have activity in inhibiting the formation of ROS that leads to oxidative stress [91,92]. In addition, salidroside can also protect cells from apoptosis caused by H2O2 induction [93]. The antioxidant compound polysaccharide okra (OP) is known to prevent an increase in levels of free fatty acids (FFA), triglycerides and LDL and can prevent a decrease in HDL levels [72].
Kahweol is an antioxidant diterpene compound derived from coffee. Based on research, kahweol can inhibit adipogenesis and lipid accumulation while lowering blood glucose levels in rats induced by hyperglycemia [14,94]. Besides playing a role in inhibiting ROS and suppressing lipid accumulation, antioxidant compounds also have activity against insulin resistance. An antioxidant compound known to affect insulin resistance is asphalathin. Asphalathin can improve insulin resistance in in vitro testing with palmitate induction [95,96], while other studies have also shown that asphalathin can treat hyperglycemia accompanied by inflammation and apoptosis [97].
Another antioxidant compound that also affects insulin resistance is isothiocyanate. This compound reduced lipid accumulation and inflammation in the palmitate-induced test [98]. In another study, it was also reported that isothiocyanate compounds can suppress inflammation caused by an increase in pro-inflammatory cytokines and can reduce oxidative stress levels [99]. The activities of some of these antioxidant compounds against obesity, oxidative stress, dyslipidemia and diabetes are summarized in Figure 3.
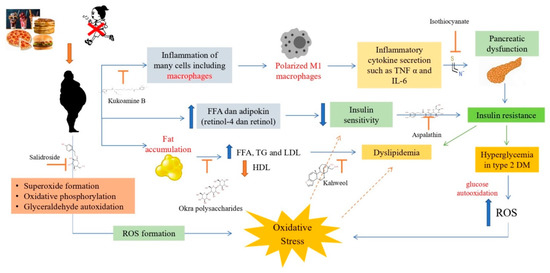
Figure 3.
Antioxidant mechanisms in obesity, dyslipidemia and diabetes [100]. Obesity can cause inflammation of macrophage cells so macrophages will be polarized into M1 macrophages due to inflammation. M1 polarized macrophages will secrete inflammatory cytokines such as TNFα and IL-6, which can cause pancreatic dysfunction that leads to insulin resistance. Insulin resistance will cause type 2 DM with hyperglycemia, which can increase ROS, causing oxidative stress. Obesity can form ROS through the formation of superoxide, oxidative phosphorylation and auto-oxidation of glyceraldehyde, causing oxidative stress. Obesity can also cause an increase in FFA and adipokines, which can reduce insulin sensitivity and lead to type 2 diabetes and dyslipidemia. In addition, obesity also affects fat accumulation, which can cause an increase in FFA, TG and LDL and a decrease in HDL, which can cause dyslipidemia.
6. Antioxidant Compound Signaling Pathways
Based on the previous discussion, it is known that several antioxidant compounds have antiobesity, antidyslipidemic and antidiabetic activities both in vitro and in vivo and clinically. Furthermore, this review discusses the signaling pathways of antioxidant compounds derived from natural products against obesity, dyslipidemia and diabetes as well as oxidative stress that can trigger the emergence of these metabolic disorders (Table 1).

Table 1.
Antioxidant signaling pathways of natural product.
6.1. The Phosphoinositide 3-kinase/Protein Kinase B (PI3K/AKT)
The phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling pathway is a regulator of physiological processes associated with type 2 diabetes mellitus. Most studies reported that the PI3K/AKT pathway not only promotes insulin signal transduction but can also stimulate glucose uptake in adipose and liver [72]. Phosphorylated protein kinases can activate glycogen synthase kinase 3 beta (GSK3β), which then triggers NF-E2-related factor (Nrf2) from the binding of Keap1 to the nucleus. Then, target genes are transactivated through antioxidant response elements (AREs) to inhibit oxidative stress. Some of the compounds in Table 1 that can induce vasodilation through the PI3K/AKT signaling pathway are polysaccharides [72], anthocyanin [101], resveratrol [120], gossypol [104], procyanidins [117] and polyphenol [114].
6.2. The Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Signaling
The NFE2 system associated with kelch-like ECH-associated protein 1 (Keap1) factor 2 (Nrf2) is a defense system for cellular homeostasis. The interaction between Nrf2 and Keap1 can trigger the expression of the B globin gene known as a key marker of oxidative stress in cells [125]. Levels of oxidative stress and inflammation in cells are common in most tissues. Nrf2 and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) are the two main transcription factors that play a role in regulating cellular responses to oxidative stress and inflammation. There is functional crosstalk between these two pathways based on pharmacological and genetic studies which stated that NF-κB activity will be disrupted with the absence of Nrf2, causing an increase in cytokine production. In addition, NF-B also plays a role in modulating the activity and transcription of Nrf2 [126]. Based on Table 1, several natural compounds were reported to act on the Nrf2 cell homeostasis system such as isothiocyanates [99], ocra polysaccharides [72], simmondsine [123] and puerarine [118].
Moringa isothiocyanate (MIC1) is the main isothiocyanate found in Moringa oleifera. MIC-1 can activate Nrf2-ARE at levels similar to sulforaphane (SFN), suppress pro-inflammatory cytokines, reduce ROS and inhibit high glucose (HG)-induced transforming growth factor beta 1 (TGFβ1) [99]. Another antioxidant compound, namely, okra polysaccharide (OP), significantly reduces the increase in blood sugar, cholesterol, triglycerides and LDL. OP also decreases ROS and mitochondrial dysfunction by inhibiting activation of NADPH oksidase 2 (Nox2). In summary, OP has activity against type 2 DM via Nrf2 transport of the PI3K/AKT pathway [72]. Simmondsin and puerarin are also reported to reduce oxidative stress via the same mechanism, namely, by activating the Nrf2 pathway [118,123].
6.3. The Peroxisome Proliferation Activated Receptor γ (PPARγ)
The peroxisome proliferation activated receptor γ (PPARγ) is a transmembrane transcription factor. When activated by the ligand, PPARγ inhibits the transcription of NF-κB and reduces the expression of the cytokine gene in inflammation, which may decrease the inflammatory response. In vivo studies have shown that the induction of a high-fat diet can lead to an increase in glucose levels and insulin resistance accompanied by a decrease in PPAR activation [127]. Compounds reported in Table 1 with activity to increase PPARγ expression are kahweol [14], Angelica sinensis polysaccharide (ASP) [115] and toosendanin [128].
6.4. The Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB) Signaling
NF-κB is a transcription factor consisting of seven transcription factors that are structurally related and play a role in regulating the expression of many genes. NF-κB usually represents the p50-p65 heterodimer, which is the major Rel/NF-κB complex in most cells. The NF-κB subunit is expressed in many places but its induction and expression depend on the stimulus and cell type. NF-κB can be activated by cytokines, ROS, viral infection, vasopressors and DNA damage. NF-κB and Nrf2 play a role in cellular homeostasis and responses to stress and inflammation, whose molecular mechanisms depend on cell type and tissue context [129]. Based on Table 1, several natural compounds are reported to act on the NFκB cell homeostasis system such as kahweol [14], kukoamine B [106], saponins [122], oligopeptides [103] and hyperoside [105]. These compounds can prevent cell apoptosis due to the induction of hyperglycemia and downregulation of NFκB to protect against inflammation caused by diabetes [14,103].
6.5. 5′AMP-Activated Protein Kinase (AMPK) Signaling
AMP-activated protein kinase (AMPK) plays a role in regulating energy metabolism through the inhibition of anabolic pathways and stimulation of catabolic pathways. In addition, AMPK also plays a role in enzyme regulation through phosphorylation and regulation of transcription factors and coactivators [130]. Based on Table 1, several natural compounds can increase AMPK levels, such as salidroside [121], aspalathin [102], nodakenine [110], anthocyanins [101], saponin [122], peptic bee pollen polysaccharide [112] and bouchardatine [102]. Salidroside (Figure 4) and other antioxidant compounds that act in the AMPK pathways can decrease ROS production, improve mitochondrial function by reducing NADPH oxidase-2 (NOX2) expression and inhibit the JNK-caspase 3 apoptotic cascade by activating AMPK [121].

Figure 4.
The salidroside mechanism in preventing oxidative diabetes (modification from Ju L et al., 2017) [121].
6.6. AGE/RAGE
In conditions of persistent hyperglycemia of uncontrolled diabetes mellitus, the end product is advanced glycation end (AGE). Glycation is one of the mechanisms that contribute to diabetes complications such as cardiomyopathy, nephropathy, retinopathy and neuropathy [131]. Advanced glycation ends (AGEs) play a role in the disruption of cellular functions including denaturation of target proteins and reduced function of AGEs. The accumulation of AGEs in tissues can damage organs and trigger chemical oxidative stress. Another mechanism of AGEs is the receptor associated with the receptor for the advanced glycation end product (RAGE). The increase in RAGE causes an increase in ROS synthesis and oxidative stress. One of the oxidative stresses is the phosphorylation of the primary signal transduction cascade, which is the molecularly activated protein kinase (MAPK) that activates NF-κB [132]. In Table 1, the compounds with a AGE/RAGE inhibitory mechanism are mangiferin [108], morroniside [109] and pyrogallol-phloroglucinol-6,6-bieckol (PPB) [119].
6.7. SIRT (Sirtuin)
Sirtuin 1 (SIRT1) and sirtuin 3 (SIRT3) proteins play an important role in counteracting oxidative stress. In degenerative diseases such as type 2 diabetes, especially in women, there is a high risk of death from myocardial infarction, even with drug therapy for diabetes. Compounds that can increase SIRT1 are resveratrol and bouchardatine [102] (Table 1). Resveratrol (RSV) is a natural polyphenol that has antioxidant activity and can improve mitochondrial dysfunction. RSV has been shown to increase NO production, increase NOS expression and activity, prevent eNOS release and increase NO bioavailability [120].
7. Conclusions
Various antioxidant compounds have been reported to have beneficial activities against obesity, dyslipidemia and diabetes in the literature. The molecular signaling mechanism of the reported compounds associated with obesity, dyslipidemia and diabetes has also been discussed. However, further research is needed to determine the optimal dose of these antioxidant compounds so as to provide optimal effects on these metabolic disorders. Furthermore, the review also provides insights into antioxidant compounds that act simultaneously against obesity, dyslipidemia and diabetes to minimize the use of drugs and the risk of side effects. Further research on the side effects of long-term use of these antioxidant compounds should also be explored, thereby increasing the safety of long-term use of these compounds.
Author Contributions
C.K. conceived and drafted the manuscript. S.A.S., N.K.K.I. and M.M. were involved in the editing process. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Universitas Padjadjaran.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data included in the article.
Acknowledgments
We thank Rector of Universitas Padjadjaran for supporting this study through Academic Leadership Grant No. 1959/UN6.3.1/PT.00/2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leibowitz, K.L.; Moore, R.H.; Ahima, R.S.; Stunkard, A.J.; Stallings, V.A.; Berkowitz, R.I.; Chittams, J.L.; Faith, M.S.; Stettler, N. Maternal obesity associated with inflammation in their children. World J. Pediatr. 2012, 8, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Oyenihi, A.B.; Ayeleso, A.O.; Mukwevho, E.; Masola, B. Antioxidant strategies in the management of diabetic neuropathy. Biomed Res. Int. 2015, 2015, 515042. [Google Scholar] [CrossRef]
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: A randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef]
- Atlas, D. International Diabetes Federation, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Mohieldein, A.H.; Hasan, M.; Al-Harbi, K.K.; Alodailah, S.S.; Azahrani, R.M.; Al-Mushawwah, S.A. Dyslipidemia and reduced total antioxidant status in young adult Saudis with prediabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 287–291. [Google Scholar] [CrossRef]
- Rambhade, S.; Chakraborty, A.K.; Patil, U.K.; Rambhade, A. Diabetes Mellitus-Its complications, factors influencing complications and prevention-An Overview. J. Chem. Pharm. Res. 2010, 2, 7–25. [Google Scholar]
- Kashiyama, K.; Sonoda, S.; Otsuji, Y. Reconsideration of Secondary Risk Management Strategies in Patients with Ischemic Heart Disease. J. UOEH 2017, 39, 11–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Pechánová, O.; Varga, Z.V.; Cebová, M.; Giricz, Z.; Pacher, P.; Ferdinandy, P. Cardiac NO signalling in the metabolic syndrome. Br. J. Pharmacol. 2015, 172, 1415–1433. [Google Scholar] [CrossRef]
- Kuk, J.L.; Rotondi, M.; Sui, X.; Blair, S.N.; Ardern, C.I. Individuals with obesity but no other metabolic risk factors are not at significantly elevated all-cause mortality risk in men and women. Clin. Obes. 2018, 8, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Prawitasari, D.S. Diabetes Melitus dan Antioksidan. Keluwih J. Kesehat. Kedokt. 2019, 1, 48–52. [Google Scholar] [CrossRef]
- El-Huneidi, W.; Anjum, S.; Bajbouj, K.; Abu-Gharbieh, E.; Taneera, J. The coffee diterpene, kahweol, ameliorates pancreatic β-cell function in streptozotocin (Stz)-treated rat ins-1 cells through nf-kb and p-akt/bcl-2 pathways. Molecules 2021, 26, 5167. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Ge, L.; Xun, Y.Q.; Chen, Y.J.; Gao, C.Y.; Han, X.; Zuo, L.Q.; Shan, H.Q.; Yang, K.H.; Ding, G.W.; et al. Exercise training modalities in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 1–14. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.S. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef]
- Dal, S.; Sigrist, S. The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications. Diseases 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.; Chelliah, R.; Javed, A.; Oh, D. Curcumin, Quercetin, Catechins and Metabolic Diseases : The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care 2008, 31 (Suppl. 2), S170–S180. [Google Scholar] [CrossRef]
- Corbi, S.C.T.; Bastos, A.S.; Orrico, S.R.P.; Secolin, R.; Dos Santos, R.A.; Takahashi, C.S.; Scarel-Caminaga, R.M. Elevated micronucleus frequency in patients with type 2 diabetes, dyslipidemia and periodontitis. Mutagenesis 2014, 29, 433–439. [Google Scholar] [CrossRef]
- Ren, W.; Xia, Y.; Chen, S.; Wu, G.; Bazer, F.W.; Zhou, B.; Tan, B.; Zhu, G.; Deng, J.; Yin, Y. Glutamine Metabolism in Macrophages: A Novel Target for Obesity/Type 2 Diabetes. Adv. Nutr. 2019, 10, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Flock, M.R.; Green, M.H.; Kris-Etherton, P.M. Effects of adiposity on plasma lipid response to reductions in dietary saturated fatty acids and cholesterol. Adv. Nutr. 2011, 2, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Na, I.J.; Park, J.S.; Park, S.B. Association between abdominal obesity and oxidative stress in Korean adults. Korean J. Fam. Med. 2019, 40, 395–398. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J.C. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 230–251. [Google Scholar] [CrossRef]
- Wang, H.; Peng, D.Q. New insights into the mechanism of low high-density lipoprotein cholesterol in obesity. Lipids Health Dis. 2011, 10, 176. [Google Scholar] [CrossRef]
- Li, K.; Deng, Y.; Deng, G.; Chen, P.; Wang, Y.; Wu, H.; Ji, Z.; Yao, Z.; Zhang, X.; Yu, B.; et al. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res. Ther. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef]
- Czaja, A.J. Autoimmune Hepatitis: Focusing on Treatments other than Steroids. Can. J. Gastroenterol. 2012, 26, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From pre-diabetes to diab. Medicine 2019, 55, 1–30. [Google Scholar]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. ISRN Inflamm. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, M.; Xiao, M.; Ruan, Q.; Chu, Z.; Ye, Z.; Zhong, L.; Zhang, H.; Huang, X.; Xie, W.; et al. ERβ Accelerates Diabetic Wound Healing by Ameliorating Hyperglycemia-Induced Persistent Oxidative Stress. Front. Endocrinol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Sridhar, C.; Reddy, Y.S.R.; De, B. Free radicals, antioxidants, diseases and phytomedicines: Current status and future prospect. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 91–100. [Google Scholar]
- Kan, N.W.; Lee, M.C.; Tung, Y.T.; Chiu, C.C.; Huang, C.C.; Huang, W.C. The synergistic effects of resveratrol combined with resistant training on exercise performance and physiological adaption. Nutrients 2018, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Raj, P.; Nabipour, I.; Mahmoodi, M.; Ostovar, A. Efficacy and Safety of Resveratrol in Type 1 Diabetes. Nutrients 2020, 12, 161. [Google Scholar] [CrossRef]
- Batista-Jorge, G.C.; Barcala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimarães, A.L.S.; Santos, S.H.S. Oral resveratrol supplementation improves Metabolic Syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Martin, O.W.; Thornalley, P.J. Subjects by trans -Resveratrol and Hesperetin. Nutrients 2021, 13, 2374. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, J.; Zhu, W.; Yin, X.; Yang, B.; Wei, Y.; Guo, X. Combination of berberine with resveratrol improves the lipid-lowering efficacy. Int. J. Mol. Sci. 2018, 19, 3903. [Google Scholar] [CrossRef]
- Campbell, L.; Yu, R.; Li, F.; Zhou, Q.; Chen, D.; Qi, C.; Yin, Y.; Sun, J. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy Dovepress Modulation of fat metabolism and gut microbiota by resveratrol on high-fat diet-induced obese mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 97–107. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, K.Y.; Peng, K.Y.; Day, Y.J.; Hung, L.M. Resveratrol exerts anti-obesity effects in high-fat diet obese mice and displays differential dosage effects on cytotoxicity, differentiation, and lipolysis in 3T3-L1 cells. Endocr. J. 2016, 63, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Choi, J.H. Korean Curcuma longa L. induces lipolysis and regulates leptin in adipocyte cells and rats. Nutr. Res. Pract. 2016, 10, 487–493. [Google Scholar] [CrossRef][Green Version]
- Ferguson, B.S.; Nam, H.; Morrison, R.F. Curcumin inhibits 3T3-L1 preadipocyte proliferation by mechanisms involving post-transcriptional p27 regulation. Biochem. Biophys. Rep. 2016, 5, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Pan, Y.; Yu, N.; Bai, Y.; Ma, R.; Mo, F.; Zuo, J.; Chen, B.; Jia, Q.; Zhang, D.; et al. Curcumin improves adipocytes browning and mitochondrial function in 3T3-L1 cells and obese rodent model. R. Soc. Open Sci. 2021, 8, 200974. [Google Scholar] [CrossRef] [PubMed]
- Labban, R.S.M.; Alfawaz, H.A.; Almnaizel, A.T.; Al-Muammar, M.N.; Bhat, R.S.; El-Ansary, A. Garcinia mangostana extract and curcumin ameliorate oxidative stress, dyslipidemia, and hyperglycemia in high fat diet-induced obese Wistar albino rats. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.N.; Garg, M.L. Dietary supplementation with curcumin reduce circulating levels of glycogen synthase kinase-3Β and islet amyloid polypeptide in adults with high risk of type 2 diabetes and Alzheimer’s disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Shamsi-Goushki, A.; Mortazavi, Z.; Mirshekar, M.A.; Mohammadi, M.; Moradi-Kor, N.; Jafari-Maskouni, S.; Shahraki, M. Comparative effects of curcumin versus nano-curcumin on insulin resistance, serum levels of apelin and lipid profile in type 2 diabetic rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Roxo, D.F.; Arcaro, C.A.; Gutierres, V.O.; Costa, M.C.; Oliveira, J.O.; Lima, T.F.O.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Curcumin combined with metformin decreases glycemia and dyslipidemia, and increases paraoxonase activity in diabetic rats. Diabetol. Metab. Syndr. 2019, 11, 1–8. [Google Scholar] [CrossRef]
- Xie, T.; Chen, X.; Chen, W.; Huang, S.; Peng, X.; Tian, L.; Wu, X.; Huang, Y. Curcumin is a Potential Adjuvant to Alleviates Diabetic Retinal Injury via Reducing Oxidative Stress and Maintaining Nrf2 Pathway Homeostasis. Front. Pharmacol. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L.; et al. Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxid. Med. Cell. Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef]
- Lima, T.F.O.; Costa, M.C.; Figueiredo, I.D.; Inácio, M.D.; Rodrigues, M.R.; Assis, R.P.; Baviera, A.M.; Brunetti, I.L. Curcumin, Alone or in Combination with Aminoguanidine, Increases Antioxidant Defenses and Glycation Product Detoxification in Streptozotocin-Diabetic Rats: A Therapeutic Strategy to Mitigate Glycoxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 1036360. [Google Scholar] [CrossRef]
- Li, J.; Wu, N.; Chen, X.; Chen, H.; Yang, X.; Liu, C. Curcumin protects islet cells from glucolipotoxicity by inhibiting oxidative stress and NADPH oxidase activity both in vitro and in vivo. Islets 2019, 11, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.; Leibel, L.; Tortoriello, D. Proteasome inhibitors, including curcumin, improve pancreatic β-cell function and insulin sensitivity in diabetic mice. Nutr. Diabetes 2016, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Sultana, M.; Raina, R.; Pankaj, N.K.; Verma, P.K.; Prawez, S. Hypoglycemic, Hypolipidemic, and Wound Healing Potential of Quercetin in Streptozotocin-Induced Diabetic Rats. Pharmacogn. Mag. 2017, 13, 633–639. [Google Scholar] [CrossRef]
- Han, Y.; Wu, J.Z.; Shen, J.Z.; Chen, L.; He, T.; Jin, M.W.; Liu, H. Pentamethylquercetin induces adipose browning and exerts beneficial effects in 3T3-L1 adipocytes and high-fat diet-fed mice. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Srinivasan, P.; Vijayakumar, S.; Kothandaraman, S.; Palani, M. Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L. fruit: In silico and in vivo approaches. J. Pharm. Anal. 2018, 8, 109–118. [Google Scholar] [CrossRef]
- Zhuang, M.; Qiu, H.; Li, P.; Hu, L.; Wang, Y.; Rao, L. Islet protection and amelioration of type 2 diabetes mellitus by treatment with quercetin from the flowers of Edgeworthia gardneri. Drug Des. Devel. Ther. 2018, 12, 955–966. [Google Scholar] [CrossRef]
- Lee, J.S.; Cha, Y.J.; Lee, K.H.; Yim, J.E. Onion peel extract reduces the percentage of body fat in overweight and obese subjects: A 12-week, randomized, double-blind, placebo-controlled study. Nutr. Res. Pract. 2016, 10, 175–181. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Guo, X.; Zhang, M.; Gong, L. Blackberry and blueberry anthocyanin supplementation counteract high-fat-diet-induced obesity by alleviating oxidative stress and inflammation and accelerating energy expenditure. Oxid. Med. Cell. Longev. 2018, 2018, 4051232. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Shin, T.S.; Kim, M.Y.; Cho, N.J.; Kim, J.D. Anthocyanins from Cornus kousa ethanolic extract attenuate obesity in association with anti-angiogenic activities in 3T3-L1 cells by down-regulating adipogeneses and lipogenesis. PLoS ONE 2018, 13, e0208556. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Kim, H.J.; Jeong, J.W.; Park, C.; Kim, B.W.; Choi, Y.H. Inhibition of adipocyte differentiation by anthocyanins isolated from the fruit of Vitis coignetiae Pulliat is associated with the activation of AMPK signaling pathway. Toxicol. Res. 2018, 34, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid. Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef] [PubMed]
- Suantawee, T.; Elazab, S.T.; Hsu, W.H.; Yao, S.; Cheng, H.; Adisakwattana, S. Cyanidin stimulates insulin secretion and pancreatic β-cell gene expression through activation of L-type voltage-dependent ca2+ channels. Nutrients 2017, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Kongthitilerd, P.; Thilavech, T.; Marnpae, M.; Rong, W.; Yao, S.; Adisakwattana, S.; Cheng, H.; Suantawee, T. Cyanidin-3-rutinoside stimulated insulin secretion through activation of L-type voltage-dependent Ca2+ channels and the PLC-IP3 pathway in pancreatic β-cells. Biomed. Pharmacother. 2022, 146, 112494. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose–response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef] [PubMed]
- Rowicka, G.; Dyląg, H.; Ambroszkiewicz, J.; Riahi, A.; Weker, H.; Chełchowska, M. Total Oxidant and Antioxidant Status in Prepubertal Children with Obesity. Oxid. Med. Cell. Longev. 2017, 2017, 5621989. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, Y.W.; Hou, C.Y.; Chen, Y.T.; Di Dong, C.; Chen, C.W.; Singhania, R.R.; Leang, J.Y.; Hsieh, S.L. The anti-obesity effects of lemon fermented products in 3t3-l1 preadipocytes and in a rat model with high-calorie diet-induced obesity. Nutrients 2021, 13, 2809. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, J.; Liu, B.; Yan, T.; Xu, F.; Xiao, F. Polysaccharide from Okra (Abelmoschus esculentus). Molecules 2019, 24, 1906. [Google Scholar] [CrossRef]
- Yoshitomi, R.; Yamamoto, M.; Kumazoe, M.; Fujimura, Y.; Yonekura, M.; Shimamoto, Y.; Nakasone, A.; Kondo, S.; Hattori, H.; Haseda, A.; et al. The combined effect of green tea and α-glucosyl hesperidin in preventing obesity: A randomized placebo-controlled clinical trial. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Jurgo, A.; Kosmala, M.; Majewski, M.; Ognik, K. Extract against Pro-Oxidative and Pro-Inflammatory Rat Model. Molecules 2020, 25, 5874. [Google Scholar] [CrossRef]
- de Sousa, G.M.; Cazarin, C.B.B.; Maróstica Junior, M.R.; Lamas, C.d.A.; Quitete, V.H.A.C.; Pastore, G.M.; Bicas, J.L. The effect of α-terpineol enantiomers on biomarkers of rats fed a high-fat diet. Heliyon 2020, 6, e03752. [Google Scholar] [CrossRef] [PubMed]
- Hey-Mogensen, M.; Højlund, K.; Vind, B.F.; Wang, L.; Dela, F.; Beck-Nielsen, H.; Fernström, M.; Sahlin, K. Effect of physical training on mitochondrial respiration and reactive oxygen species release in skeletal muscle in patients with obesity and type 2 diabetes. Diabetologia 2010, 53, 1976–1985. [Google Scholar] [CrossRef]
- Anderson, J.C.; Clarke, E.J.; Arkin, A.P.; Voigt, C.A. Environmentally controlled invasion of cancer cells by engineered bacteria. J. Mol. Biol. 2006, 355, 619–627. [Google Scholar] [CrossRef]
- Li, Y.P.; Reid, M.B. NF-κB mediates the protein loss induced by TNF-α in differentiated skeletal muscle myotubes. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 279, 1165–1170. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Tchkonia, T.; Thomou, T.; Zhu, Y.I.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013, 17, 644–656. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Hu, H.H.; Shen, W.; Carmichael, O. Emerging Technologies and Their Applications in Lipid Compartment Measurement Lipid Compartment Measurement Advances HHS Public Access. Trends Endocrinol Metab 2015, 26, 688–698. [Google Scholar] [CrossRef]
- McCullough, A.J. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin. Liver Dis. 2004, 8, 521–533. [Google Scholar] [CrossRef]
- Calle, E.E.; Thun, M.J. Obesity and cancer. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ma, P.; Chen, J.; Xie, W. Ficus carica leaves extract inhibited pancreatic β-cell apoptosis by inhibiting AMPK/JNK/caspase-3 signaling pathway and antioxidation. Biomed. Pharmacother. 2020, 122, 109689. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; McAllister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-Related Oxidative Stress: The Impact of Physical Activity and Diet Manipulation. Sport. Med.-Open 2015, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Drago, D.; Manea, M.M.; Timofte, D.; Ionescu, D. Mechanisms of Herbal Nephroprotection in diabetes mellitus. J. Diabetes Res. 2020, 2020. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Zhu, Y.; Hou, D.; Li, Y.; Guo, X.; Wang, Y.; Olatunji, O.J.; Wan, P.; Gong, K. Kukoamine B ameliorate insulin resistance, oxidative stress, inflammation and other metabolic abnormalities in high-fat/high-fructose-fed rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1843–1853. [Google Scholar] [CrossRef]
- Chen, L.; Liu, P.; Feng, X.; Ma, C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J. Cell. Mol. Med. 2017, 21, 3178–3189. [Google Scholar] [CrossRef]
- Zheng, T.; Yang, X.; Li, W.; Wang, Q.; Chen, L.; Wu, D.; Bian, F.; Xing, S.; Jin, S. Salidroside attenuates high-fat diet-induced nonalcoholic fatty liver disease via AMPK-dependent TXNIP/NLRP3 pathway. Oxid. Med. Cell. Longev. 2018, 2018, 8597897. [Google Scholar] [CrossRef]
- Qi, Z.L.; Liu, Y.H.; Qi, S.M.; Ling, L.F.; Feng, Z.Y.; Li, Q. Salidroside protects PC12 cells from H2O2-induced apoptosis via suppressing NOX2-ROS-MAPKs signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao 2016, 37, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, N.J.; Song, J.K.; Chun, K.H. Kahweol inhibits lipid accumulation and induces Glucose-uptake through activation of AMP-activated protein kinase (AMPK). BMB Rep. 2017, 50, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Johnson, R.; Joubert, E.; Louw, J.; Ziqubu, K.; Tiano, L.; Silvestri, S.; Orlando, P.; Opoku, A.R.; et al. Aspalathin, a natural product with the potential to reverse hepatic insulin resistance by improving energy metabolism and mitochondrial respiration. PLoS ONE 2019, 14, e0216172. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Roux, C.; Johnson, R.; Ghoor, S.; Joubert, E.; Louw, J.; Opoku, A.R.; Muller, C.J.F. Aspalathin-enriched green rooibos extract reduces hepatic insulin resistance by modulating PI3K/AKT and AMPK pathways. Int. J. Mol. Sci. 2019, 20, 633. [Google Scholar] [CrossRef]
- Johnson, R.; Dludla, P.V.; Muller, C.J.F.; Huisamen, B.; Essop, M.F.; Louw, J. The Transcription Profile Unveils the Cardioprotective Effect of Aspalathin against Lipid Toxicity in an in Vitro H9c2 Model. Molecules 2017, 22, 219. [Google Scholar] [CrossRef]
- Li, C.X.; Gao, J.G.; Wan, X.Y.; Chen, Y.; Xu, C.F.; Feng, Z.M.; Zeng, H.; Lin, Y.M.; Ma, H.; Xu, P.; et al. Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J. Gastroenterol. 2019, 25, 5120–5133. [Google Scholar] [CrossRef]
- Cheng, D.; Gao, L.; Su, S.; Sargsyan, D.; Wu, R.; Raskin, I.; Kong, A.N. Moringa Isothiocyanate Activates Nrf2: Potential Role in Diabetic Nephropathy. AAPS J. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Khutami, C.; Sumiwi, S.A.; Ikram, N.K.K.; Muchtaridi, M. The Effect of Antioxidants from Natural Products on Obesity, Dyslipidemia and Diabetes and Their Molecular Signaling Mechanism; Universitas Padjadjaran: Bandung, Indonesia, 2021. [Google Scholar]
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.; Li, D.; Huang, W. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol. 2021, 46, 102100. [Google Scholar] [CrossRef]
- Rao, Y.; Yu, H.; Gao, L.; Lu, Y.T.; Xu, Z.; Liu, H.; Gu, L.Q.; Ye, J.M.; Huang, Z.S. Natural alkaloid bouchardatine ameliorates metabolic disorders in high-fat diet-fed mice by stimulating the sirtuin 1/liver kinase B-1/AMPK axis. Br. J. Pharmacol. 2017, 174, 2457–2470. [Google Scholar] [CrossRef]
- Xu, M.; Sun, B.; Li, D.; Mao, R.; Li, H.; Li, Y.; Wang, J. Beneficial effects of small molecule oligopeptides isolated from Panax ginseng meyer on pancreatic beta-cell dysfunction and death in diabetic rats. Nutrients 2017, 9, 1061. [Google Scholar] [CrossRef]
- Alam, M.B.; An, H.; Ra, J.S.; Lim, J.Y.; Lee, S.H.; Yoo, C.Y.; Lee, S.H. Gossypol from cottonseeds ameliorates glucose uptake by mimicking insulin signaling and improves glucose homeostasis in mice with streptozotocin-induced diabetes. Oxid. Med. Cell. Longev. 2018, 2018, 5796102. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Dong, H.; Yu, X.; Zhang, J. Anti-hypoglycemic and hepatocyte-protective effects of hyperoside from Zanthoxylum bungeanum leaves in mice with high-carbohydrate/high-fat diet and alloxan-induced diabetes. Int. J. Mol. Med. 2018, 41, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Stewart, D.A.; Ye, X.M.; Yin, L.H.; Pathmasiri, W.W.; McRitchie, S.L.; Fennell, T.R.; Cheung, H.Y.; Sumner, S.J. A metabolomics approach to investigate kukoamine B—A potent natural product with anti-diabetic properties. Front. Pharmacol. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Hu, X.; Kou, L.; Zhang, B.; Zhang, C. Lycium barbarum Polysaccharide Mediated the Antidiabetic and Antinephritic Effects in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats via Regulation of NF- B. Biomed Res. Int. 2016, 2016, 3140290. [Google Scholar] [CrossRef] [PubMed]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Kumari, S.; Ojha, S.; Arya, D.S. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK pathways. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Gao, J.; Liu, P.; Shen, Z.; Xu, K.; Wu, C.; Tian, F.; Chen, M.; Wang, L.; Li, P. Morroniside Promotes PGC-1 α -Mediated Cholesterol Efflux in Sodium Palmitate or High Glucose-Induced Mouse Renal Tubular Epithelial Cells. Biomed Res. Int. 2021, 2021, 9942152. [Google Scholar] [CrossRef]
- Jin, B.R.; Lee, M.; An, H.J. Nodakenin represses obesity and its complications via the inhibition of the VLDLR signalling pathway in vivo and in vitro. Cell Prolif. 2021, 54, 1–15. [Google Scholar] [CrossRef]
- El Khatib, N.; Morel, S.; Hugon, G.; Rapior, S.; Carnac, G.; Saint, N. Identification of a sesquiterpene lactone from arctium lappa leaves with antioxidant activity in primary human muscle cells. Molecules 2021, 26, 1328. [Google Scholar] [CrossRef]
- Li, X.; Gong, H.; Yang, S.; Yang, L.; Fan, Y.; Zhou, Y. Pectic Bee Pollen Polysaccharide from Rosa rugosa Alleviates Diet-Induced Hepatic Steatosis and Insulin Resistance via Induction of AMPK/mTOR-Mediated Autophagy. Molecules 2017, 22, 699. [Google Scholar] [CrossRef]
- Liu, S.; Huang, S.; Wu, X.; Feng, Y.; Shen, Y.; Zhao, Q.S.; Leng, Y. Activation of SIK1 by phanginin A inhibits hepatic gluconeogenesis by increasing PDE4 activity and suppressing the cAMP signaling pathway. Mol. Metab. 2020, 41, 101045. [Google Scholar] [CrossRef]
- Torabi, S.; DiMarco, N.M. Original Research: Polyphenols extracted from grape powder induce lipogenesis and glucose uptake during differentiation of murine preadipocytes. Exp. Biol. Med. 2016, 241, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cao, P.; Wang, H.; Tang, Z.; Wang, N.; Wang, J.; Zhang, Y. Chronic administration of Angelica sinensis polysaccharide effectively improves fatty liver and glucose homeostasis in high-fat diet-fed mice. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Lu, K.; Wang, L.; Lv, M.; Fu, W. Astraglaus polysaccharide protects diabetic cardiomyopathy by activating NRG1/ErbB pathway. Biosci. Trends 2018, 12, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhu, T.; Tong, J.; Caidan, R.; Wang, K.; Kai, G.; Zhang, W.; Ru, L.; Pengcuo, J.; Tong, L. Screening active components from Rubus amabilis for pancreatic β-cells protection. Pharm. Biol. 2020, 58, 674–685. [Google Scholar] [CrossRef]
- Zhang, D.; Li, M. Puerarin prevents cataract development and progression in diabetic rats through Nrf2/HO-1 signaling. Mol. Med. Rep. 2019, 20, 1017–1024. [Google Scholar] [CrossRef]
- Choi, J.; Oh, S.; Son, M.; Byun, K. Pyrogallol-phloroglucinol-6,6-bieckol alleviates obesity and systemic inflammation in a mouse model by reducing expression of RAGE and RAGE ligands. Mar. Drugs 2019, 17, 612. [Google Scholar] [CrossRef]
- Fourny, N.; Lan, C.; Sérée, E.; Bernard, M.; Desrois, M. Protective effect of resveratrol against ischemia-reperfusion injury via enhanced high energy compounds and eNOS-SIRT1 expression in type 2 diabetic female rat heart. Nutrients 2019, 11, 105. [Google Scholar] [CrossRef]
- Ju, L.; Wen, X.; Wang, C.; Wei, Y.; Peng, Y.; Ding, Y.; Feng, L.; Shu, L. Salidroside, a natural antioxidant, improves β-cell survival and function via activating AMPK pathway. Front. Pharmacol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Shi, F.; Liu, Y. Comparison of antidiabetic effects of saponins and polysaccharides from Momordica charantia L. in STZ-induced type 2 diabetic mice. Biomed. Pharmacother. 2019, 109, 744–750. [Google Scholar] [CrossRef]
- Belhadj, S.; Hentati, O.; Hamdaoui, G.; Fakhreddine, K.; Maillard, E.; Dal, S.; Sigrist, S. Beneficial effect of Jojoba seed extracts on hyperglycemia-induced oxidative stress in RINm5f beta cells. Nutrients 2018, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.X.; Cheng, X.Y.; Wang, Y.; Yin, W. Toosendanin inhibits adipogenesis by activating Wnt/β-catenin signaling. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Albrahim, T.; Alonazi, M.A. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed. Pharmacother. 2021, 141, 111831. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, Y.; Yang, Z.; Hu, W.; Xiong, L.; Wang, N.; Liu, X.; Zheng, G.; Ouyang, K.; Wang, W. Effects of Chimonanthus nitens Oliv. Leaf Extract on Glycolipid Metabolism and Antioxidant Capacity in Diabetic Model Mice. Oxid. Med. Cell. Longev. 2017, 2017, 7648505. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R. The NF-κB regulatory network. Cardiovasc. Toxicol. 2006, 6, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.L.; Zhao, K.C.; Yuan, W.; Zhou, F.; Song, H.Y.; Liu, G.L.; Huang, J.; Zou, J.J.; Zhao, B.; Xie, S.P. MicroRNA-31-5p Exacerbates Lipopolysaccharide-Induced Acute Lung Injury via Inactivating Cab39/AMPK α Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8822361. [Google Scholar] [CrossRef] [PubMed]
- Tayanloo-Beik, A.; Roudsari, P.P.; Rezaei-Tavirani, M.; Biglar, M.; Tabatabaei-Malazy, O.; Arjmand, B.; Larijani, B. Diabetes and Heart Failure: Multi-Omics Approaches. Front. Physiol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Kay, A.M.; Simpson, C.L.; Stewart, J.A. The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).