ISSVA Classification of Vascular Anomalies and Molecular Biology
Abstract
1. Introduction: ISSVA Classification
2. Structure of the ISSVA Classification
3. Gene Mutations and Molecular Biological Mechanisms in Vascular Anomalies
3.1. Venous Malformation
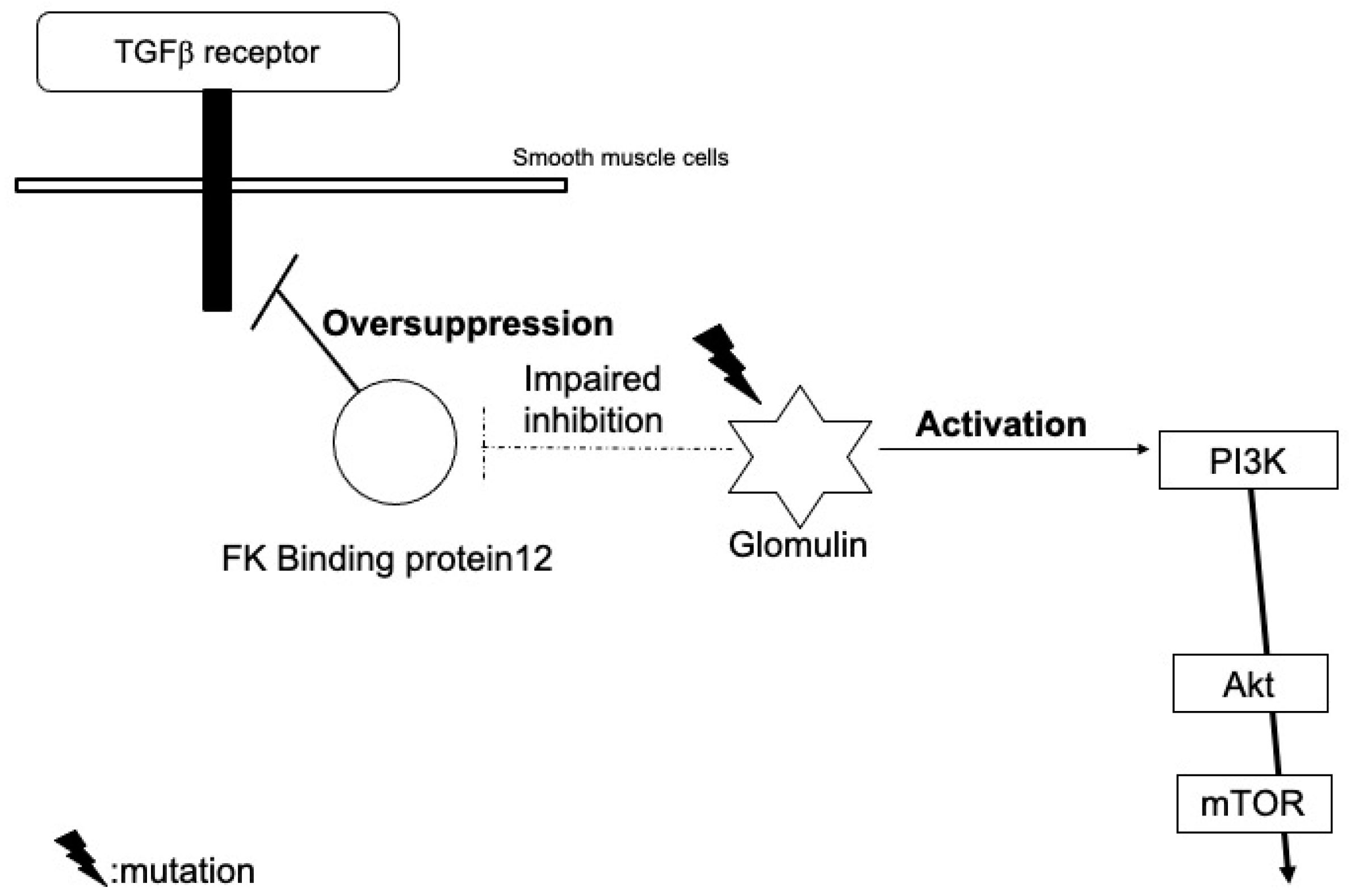
3.2. Glomuvenous Malformation
3.3. Lymphatic Malformation
3.4. Arteriovenous Malformation
3.5. Klippel–Trenaunay Syndrome
3.6. Sturge–Weber Syndrome
3.7. Infantile Hemangioma
3.8. Tufted Angioma and Kaposiform Hemangioendothelioma
4. Future Issues in Molecular Biologics of Vascular Anomalies
5. Future Perspective of Novel Therapies
6. Conclusions: Molecular Aspect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ECs | endothelial cells |
| HDMEC | human dermal microvascular ECs |
| hemECs | infantile hemangioma-derived ECs |
| hemEPCs | infantile hemangioma-derived endothelial progenitor cells |
| hemMSCs | infantile hemangioma-derived mesenchymal stem cells |
| hemSCs | infantile hemangioma-derived stem cells |
| ISSVA | International Society for the Study of Vascular Anomalies |
| KHE | kaposiform hemangioendothelioma |
| KMS | Kasabach-Merritt syndrome |
| MCAP | megalencephaly-capillary malformation-polymicrogyria |
| NFAT | nuclear factor in activated T cells |
| PROS | PIK3CA-related overgrowth spectrum |
| TA | tufted angioma |
| TEM8 | tumor endothelial marker-8 |
| VEGF | vascular endothelial growth factor |
| VEGFR1 | VEGF receptor type 1 |
| VEGFR2 | VEGF receptor type2 |
| VEGFR3 | VEGF receptor type3 |
References
- ISSVA Classification. Available online: https://www.issva.org/classification (accessed on 7 January 2022).
- Vikkula, M.; Boon, L.M.; Carraway, K.L., 3rd; Calvert, J.T.; Diamonti, A.J.; Goumnerov, B.; Pasyk, K.A.; Marchuk, D.A.; Warman, M.L.; Cantley, L.C.; et al. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell 1996, 87, 1181–1190. [Google Scholar] [CrossRef]
- Limaye, N.; Wouters, V.; Uebelhoer, M.; Tuominen, M.; Wirkkala, R.; Mulliken, J.B.; Eklund, L.; Boon, L.M.; Vikkula, M. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat. Genet. 2009, 41, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.D.; Tzouanacou, E.; Zaw-Thin, M.; Berenjeno, I.M.; Parker, V.E.; Chivite, I.; Mila-Guasch, M.; Pearce, W.; Solomon, I.; Angulo-Urarte, A.; et al. Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans. Sci. Transl. Med. 2016, 8, 332ra43. [Google Scholar] [CrossRef] [PubMed]
- Castel, P.; Carmona, F.J.; Grego-Bessa, J.; Berger, M.F.; Viale, A.; Anderson, K.V.; Bague, S.; Scaltriti, M.; Antonescu, C.R.; Baselga, E.; et al. Somatic PIK3CA mutations as a driver of sporadic venous malformations. Sci. Transl. Med. 2016, 8, 332ra42. [Google Scholar] [CrossRef] [PubMed]
- di Blasio, L.; Puliafito, A.; Gagliardi, P.A.; Comunanza, V.; Somale, D.; Chiaverina, G.; Bussolino, F.; Primo, L. PI3K/mTOR inhibition promotes the regression of experimental vascular malformations driven by PIK3CA-activating mutations. Cell Death Dis. 2018, 9, 45. [Google Scholar] [CrossRef]
- Brouillard, P.; Ghassibé, M.; Penington, A.; Boon, L.M.; Dompmartin, A.; Temple, I.K.; Cordisco, M.; Adams, D.; Piette, F.; Harper, J.I.; et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): Evidence for a founder effect. J. Med. Genet. 2005, 42, e13. [Google Scholar] [CrossRef]
- Fereydooni, A.; Dardik, A.; Nassiri, N. Molecular changes associated with vascular malformations. J. Vasc. Surg. 2019, 70, 314–326.e1. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.D.; Baselga, E.; Graupera, M. PIK3CA mutations in vascular malformations. Curr. Opin. Hematol. 2019, 26, 170–178. [Google Scholar] [CrossRef]
- Blesinger, H.; Kaulfuss, S.; Aung, T.; Schwoch, S.; Prantl, L.; Rossler, J.; Wilting, J.; Becker, J. PIK3CA mutations are specifically localized to lymphatic endothelial cells of lymphatic malformations. PLoS ONE 2018, 13, e0200343. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.A.; Huang, A.Y.; Konczyk, D.J.; Goss, J.A.; Fishman, S.J.; Mulliken, J.B.; Warman, M.L.; Greene, A.K. Somatic MAP2K1 mutations are associated with extracranial arteriovenous malformation. Am. J. Hum. Genet. 2017, 100, 546–554. [Google Scholar] [CrossRef]
- Palmieri, M.; Curro, A.; Tommasi, A.; Di Sarno, L.; Doddato, G.; Baldassarri, M.; Frullanti, E.; Giliberti, A.R.; Fallerini, C.; Spinazzola, A.; et al. Cell-free DNA next-generation sequencing liquid biopsy as a new revolutionary approach for arteriovenous malformation. JVS Vasc. Sci. 2020, 1, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Dekeuleneer, V.; Seront, E.; Van Damme, A.; Boon, L.M.; Vikkula, M. Theranostic advances in vascular malformations. J. Investig. Dermatol. 2020, 140, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Smits, P.J.; Konczyk, D.J.; Sudduth, C.L.; Goss, J.A.; Greene, A.K. Endothelial MAP2K1 mutations in arteriovenous malformation activate the RAS/MAPK pathway. Biochem. Biophys. Res. Commun. 2020, 529, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Flores Suarez, C.P.; Boudreau, E.; Herman, A.M.; Gutierrez, M.C.; Gustafson, D.; DiStefano, P.V.; Cui, M.; Chen, Z.; De Ruiz, K.B.; et al. Somatic gain of KRAS function in the endothelium is sufficient to cause vascular malformations that require MEK but not PI3K signaling. Circ. Res. 2020, 127, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Vahidnezhad, H.; Youssefian, L.; Uitto, J. Klippel-Trenaunay syndrome belongs to the PIK3CA-related overgrowth spectrum (PROS). Exp. Dermatol. 2016, 25, 17–19. [Google Scholar] [CrossRef]
- Shirley, M.D.; Tang, H.; Gallione, C.J.; Baugher, J.D.; Frelin, L.P.; Cohen, B.; North, P.E.; Marchuk, D.A.; Comi, A.M.; Pevsner, J. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N. Engl. J. Med. 2013, 368, 1971–1979. [Google Scholar] [CrossRef]
- Nguyen, V.; Hochman, M.; Mihm, M.C., Jr.; Nelson, J.S.; Tan, W. The pathogenesis of port wine stain and sturge weber syndrome: Complex interactions between genetic alterations and aberrant MAPK and PI3K activation. Int. J. Mol. Sci. 2019, 20, 2243. [Google Scholar] [CrossRef]
- Asadi, S. The role of genetic mutations in gene GNAQ in Sturge-Weber syndrome. J. Cell Signal Damage Assoc. Mol. Patterns 2020, 1, 15–19. [Google Scholar]
- Bichsel, C.; Bischoff, J. A somatic missense mutation in GNAQ causes capillary malformation. Curr. Opin. Hematol. 2019, 26, 179–184. [Google Scholar] [CrossRef]
- Ferrara, N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS 2005, 94, 209–231. [Google Scholar]
- Boye, E.; Yu, Y.; Paranya, G.; Mulliken, J.B.; Olsen, B.R.; Bischoff, J. Clonality and altered behavior of endothelial cells from hemangiomas. J. Clin. Investig. 2001, 107, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Jinnin, M.; Ishihara, T.; Boye, E.; Olsen, B.R. Recent progress in studies of infantile hemangioma. J. Dermatol. 2010, 37, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Pittman, K.M.; Losken, H.W.; Kleinman, M.E.; Marcus, J.R.; Blei, F.; Gurtner, G.C.; Marchuk, D.A. No evidence for maternal-fetal microchimerism in infantile hemangioma: A molecular genetic investigation. J. Investig. Dermatol. 2006, 126, 2533–2538. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Melero-Martin, J.M.; Wu, X.; Paruchuri, S.; Boscolo, E.; Mulliken, J.B.; Bischoff, J. Endothelial progenitor cells from infantile hemangioma and umbilical cord blood display unique cellular responses to endostatin. Blood 2006, 108, 915–921. [Google Scholar] [CrossRef]
- Kleinman, M.E.; Tepper, O.M.; Capla, J.M.; Bhatt, K.A.; Ceradini, D.J.; Galiano, R.D.; Blei, F.; Levine, J.P.; Gurtner, G.C. Increased circulating AC133+ CD34+ endothelial progenitor cells in children with hemangioma. Lymphat. Res. Biol. 2003, 1, 301–307. [Google Scholar] [CrossRef]
- Yu, Y.; Fuhr, J.; Boye, E.; Gyorffy, S.; Soker, S.; Atala, A.; Mulliken, J.B.; Bischoff, J. Mesenchymal stem cells and adipogenesis in hemangioma involution. Stem Cells 2006, 24, 1605–1612. [Google Scholar] [CrossRef]
- Khan, Z.A.; Boscolo, E.; Picard, A.; Psutka, S.; Melero-Martin, J.M.; Bartch, T.C.; Mulliken, J.B.; Bischoff, J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J. Clin. Investig. 2008, 118, 2592–2599. [Google Scholar] [CrossRef]
- Ritter, M.R.; Dorrell, M.I.; Edmonds, J.; Friedlander, S.F.; Friedlander, M. Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proc. Natl. Acad. Sci. USA 2002, 99, 7455–7460. [Google Scholar] [CrossRef]
- Melero-Martin, J.M.; De Obaldia, M.E.; Kang, S.Y.; Khan, Z.A.; Yuan, L.; Oettgen, P.; Bischoff, J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ. Res. 2008, 103, 194–202. [Google Scholar] [CrossRef]
- Jinnin, M.; Medici, D.; Park, L.; Limaye, N.; Liu, Y.; Boscolo, E.; Bischoff, J.; Vikkula, M.; Boye, E.; Olsen, B.R. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat. Med. 2008, 14, 1236–1246. [Google Scholar] [CrossRef]
- Fong, G.H.; Rossant, J.; Gertsenstein, M.; Breitman, M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995, 376, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006, 312, 549–560. [Google Scholar] [CrossRef] [PubMed]
- St Croix, B.; Rago, C.; Velculescu, V.; Traverso, G.; Romans, K.E.; Montgomery, E.; Lal, A.; Riggins, G.J.; Lengauer, C.; Vogelstein, B.; et al. Genes expressed in human tumor endothelium. Science 2000, 289, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, A.; Kunimoto, K.; Inaba, Y.; Mikita, N.; Kaminaka, C.; Kanazawa, N.; Yamamoto, Y.; Kakimoto, N.; Suenaga, T.; Takeuchi, T.; et al. Distribution analysis of infantile hemangioma or capillary malformation on the head and face in Japanese patients. J. Dermatol. 2019, 46, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Kunimoto, K.; Kawaguchi, A.; Inaba, Y.; Kaminaka, C.; Yamamoto, Y.; Kakimoto, N.; Suenaga, T.; Takeuchi, T.; Suzuki, H.; et al. Analysis of onset and clinical characteristics in Japanese patients with infantile hemangioma. Drug Discov. Ther. 2021, 15, 210–213. [Google Scholar] [CrossRef]
- Lim, Y.H.; Fraile, C.; Antaya, R.J.; Choate, K.A. Tufted angioma with associated Kasabach-Merritt phenomenon caused by somatic mutation in GNA14. Pediatr. Dermatol. 2019, 36, 963–964. [Google Scholar] [CrossRef]
- Croteau, S.E.; Liang, M.G.; Kozakewich, H.P.; Alomari, A.I.; Fishman, S.J.; Mulliken, J.B.; Trenor, C.C., 3rd. Kaposiform hemangioendothelioma: Atypical features and risks of Kasabach-Merritt phenomenon in 107 referrals. J. Pediatr. 2013, 162, 142–147. [Google Scholar] [CrossRef]
- Lewis, D.; Vaidya, R. Kasabach merritt syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Lim, Y.H.; Bacchiocchi, A.; Qiu, J.; Straub, R.; Bruckner, A.; Bercovitch, L.; Narayan, D.; Yale Center for Mendelian Genomics; McNiff, J.; Ko, C.; et al. GNA14 somatic mutation causes congenital and sporadic vascular tumors by MAPK activation. Am. J. Hum. Genet. 2016, 99, 443–450. [Google Scholar] [CrossRef]
- Ten Broek, R.W.; Koelsche, C.; Eijkelenboom, A.; Mentzel, T.; Creytens, D.; Vokuhl, C.; van Gorp, J.M.; Versleijen-Jonkers, Y.M.; van der Vleuten, C.J.; Kemmeren, P.; et al. Kaposiform hemangioendothelioma and tufted angioma-(epi)genetic analysis including genome-wide methylation profiling. Ann. Diagn. Pathol. 2020, 44, 151434. [Google Scholar] [CrossRef]
- Le Cras, T.D.; Boscolo, E. Cellular and molecular mechanisms of PIK3CA-related vascular anomalies. Vasc. Biol. 2019, 1, H33–H40. [Google Scholar] [CrossRef][Green Version]
- Venot, Q.; Blanc, T.; Rabia, S.H.; Berteloot, L.; Ladraa, S.; Duong, J.P.; Blanc, E.; Johnson, S.C.; Hoguin, C.; Boccara, O.; et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 2018, 558, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Freixo, C.; Ferreira, V.; Martins, J.; Almeida, R.; Caldeira, D.; Rosa, M.; Costa, J.; Ferreira, J. Efficacy and safety of sirolimus in the treatment of vascular anomalies: A systematic review. J. Vasc. Surg. 2020, 71, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Wang, Z.; Yao, W.; Dong, K.; Xiao, X. Sirolimus, a promising treatment for refractory Kaposiform hemangioendothelioma. J. Cancer Res. Clin. Oncol. 2014, 140, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, X.; Duan, Y.; Zheng, B.; Gao, Y. Sirolimus as initial therapy for kaposiform hemangioendothelioma and tufted angioma. Pediatr. Dermatol. 2018, 35, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.S.; Jia, W.; Phung, T.L.; Mihm, M.C. Observations on enhanced port wine stain blanching induced by combined pulsed dye laser and rapamycin administration. Lasers Surg. Med. 2011, 43, 939–942. [Google Scholar] [CrossRef]


| Vascular Anomalies | ||||
|---|---|---|---|---|
| Vascular Tumors | Vascular Malformations | |||
| Benign | Simple | Combined | Of Major Named Vessels | Associated with Other Anomalies |
| Capillary malformations | defined as two or more vascular malformations found in one lesion | abnormalities in the origin/course/number of major blood vessels that have anatomical names | syndromes in which vascular malformations are complicated by symptoms other than vascular anomalies | |
| Locally aggressive or Borderline | Lymphatic malformations | |||
| Venous malformations | ||||
| Malignant | Arteriovenous malformations * | |||
| Arteriovenous fistula * | ||||
| Diseases | Target | Drug |
|---|---|---|
| VEGF receptor | Pazopanib | |
| PROS | PI3K | Alpelisib |
| PROS | Akt | Miransertib |
| Venous malformation Lymphatic malformation | mTOR | Rapamycin |
| Arteriovenous malformation | BRAF | Vemurafenib |
| Arteriovenous malformation | MEK | Trametinib |
| Diseases | Causative Genes | Possible Function |
|---|---|---|
| Venous malformation | TIE2, PIK3CA, Akt | Affect cytokine expression, resulting in misguiding of smooth muscle cells to the surroundings of blood vessels and leading to abnormal venous dilation Induce both proliferation and senescence of endothelial cells, which leads to morphological abnormalities |
| Glomuvenous malformation | Glomulin | Inhibit TGF-β-mediated smooth muscle cell differentiation and induce the proliferation of immature glomus cells Activate PI3K signals through interactions with c-met |
| Lymphatic malformation | PIK3CA | Stimulate cytokine expression Induce the binding of PIK3CA to the cellular membrane, or increase endothelial cell proliferation, chemotaxis, and angiogenesis |
| Arteriovenous malformation | RAS | Induce morphological changes in endothelial cells Induce sprouting behavior, enlargement of the vessel lumen, and abnormal connections between arteries and veins |
| Klippel-Trenaunay syndrome | PIK3CA | Mosaic mutations in the early embryonic phase cause segmental hypergrowth |
| Capillary malformation Sturge-Weber syndrome | GNAQ, GNA11 | Impair the ability of endothelial cells to distinguish between laminar and disturbed flow Activate the PIK3/Akt pathway |
| Infantile hemangioma | VEGFR2 TEM8, etc. | Increase the interaction among VEGFR2, TEM8 and integrin Subsequent inactivation of the integrin-NFATc2-VEGFR1 pathway cause VEGFR2 phosphorylation and endothelial activation |
| Tufted angioma Kaposiform hemangioendothelioma | GNA14 | ? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunimoto, K.; Yamamoto, Y.; Jinnin, M. ISSVA Classification of Vascular Anomalies and Molecular Biology. Int. J. Mol. Sci. 2022, 23, 2358. https://doi.org/10.3390/ijms23042358
Kunimoto K, Yamamoto Y, Jinnin M. ISSVA Classification of Vascular Anomalies and Molecular Biology. International Journal of Molecular Sciences. 2022; 23(4):2358. https://doi.org/10.3390/ijms23042358
Chicago/Turabian StyleKunimoto, Kayo, Yuki Yamamoto, and Masatoshi Jinnin. 2022. "ISSVA Classification of Vascular Anomalies and Molecular Biology" International Journal of Molecular Sciences 23, no. 4: 2358. https://doi.org/10.3390/ijms23042358
APA StyleKunimoto, K., Yamamoto, Y., & Jinnin, M. (2022). ISSVA Classification of Vascular Anomalies and Molecular Biology. International Journal of Molecular Sciences, 23(4), 2358. https://doi.org/10.3390/ijms23042358





