Potential of a Novel Chemical Compound Targeting Matrix Metalloprotease-13 for Early Osteoarthritis: An In Vitro Study
Abstract
:1. Introduction
2. Results
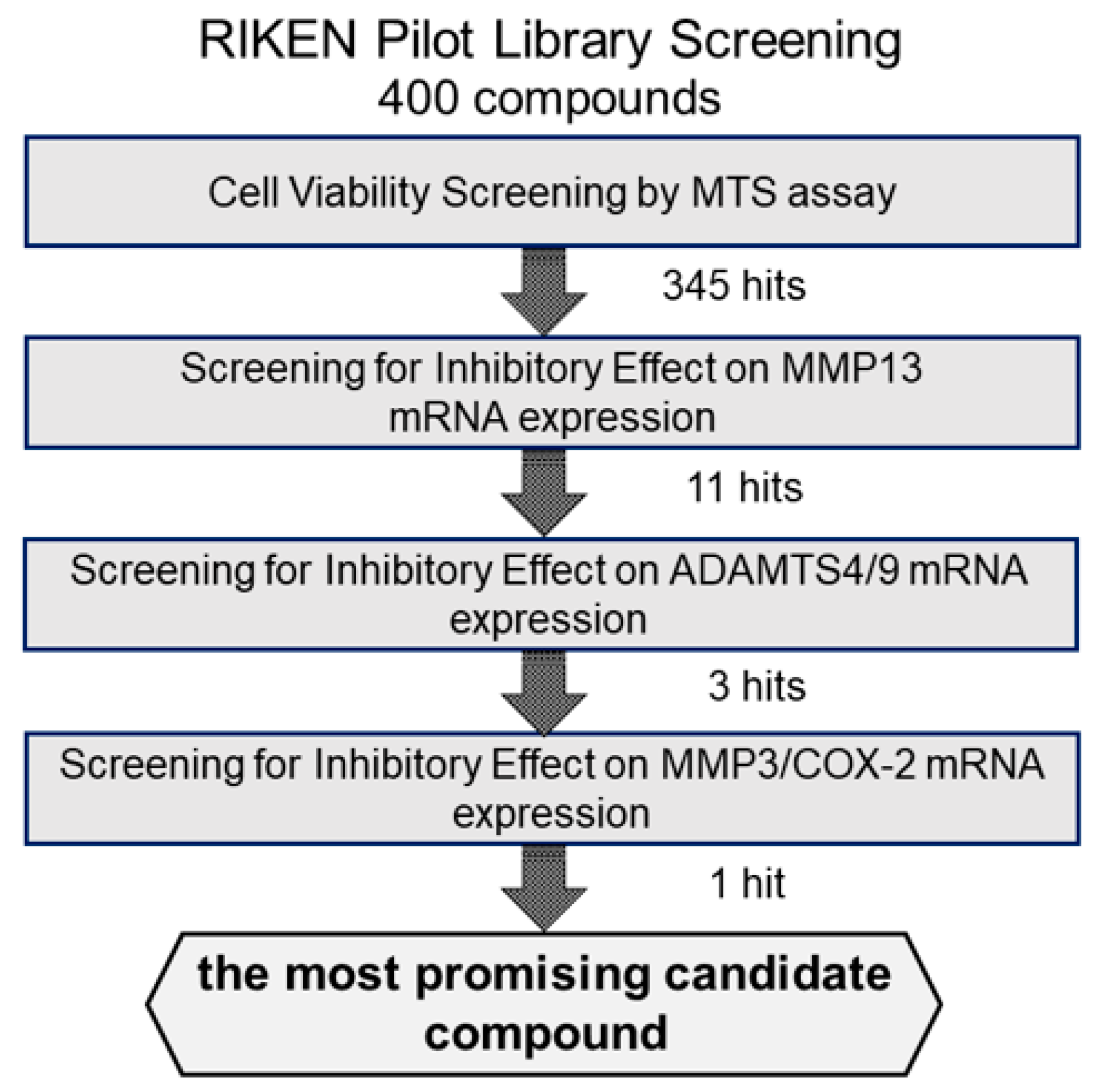
2.1. Screening of Candidate Compounds Based on Cytotoxicity
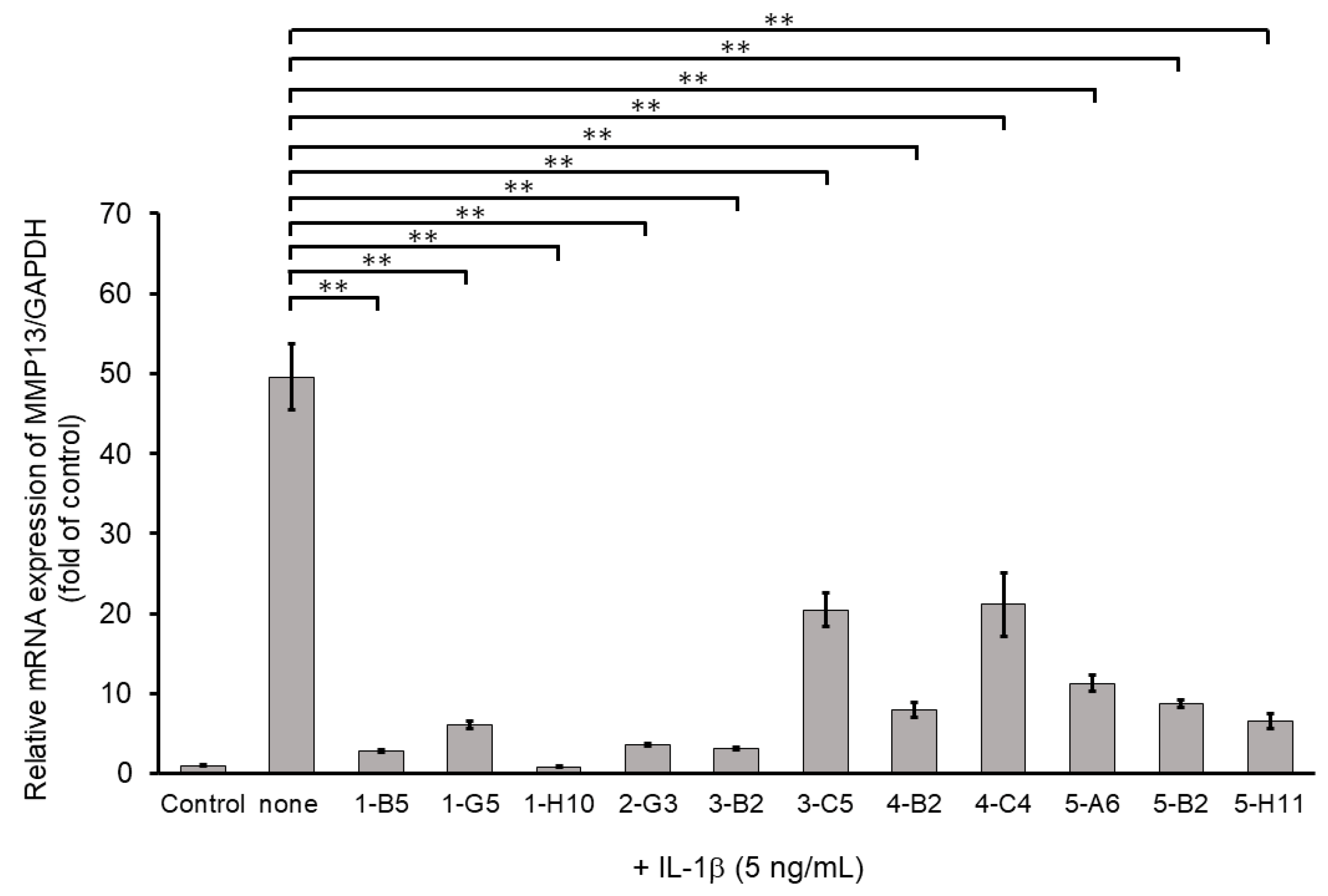
2.2. Screening via Inhibitory Effects on IL-1β-Induced MMP13 mRNA Expression
2.3. Screening Based on Inhibitory Effects on IL-1β-Induced ADAMTS4 and ADAMTS9 mRNA Expression
2.4. Screening via Inhibitory Effects on IL-1β-Induced MMP3 and COX-2 mRNA Expressions
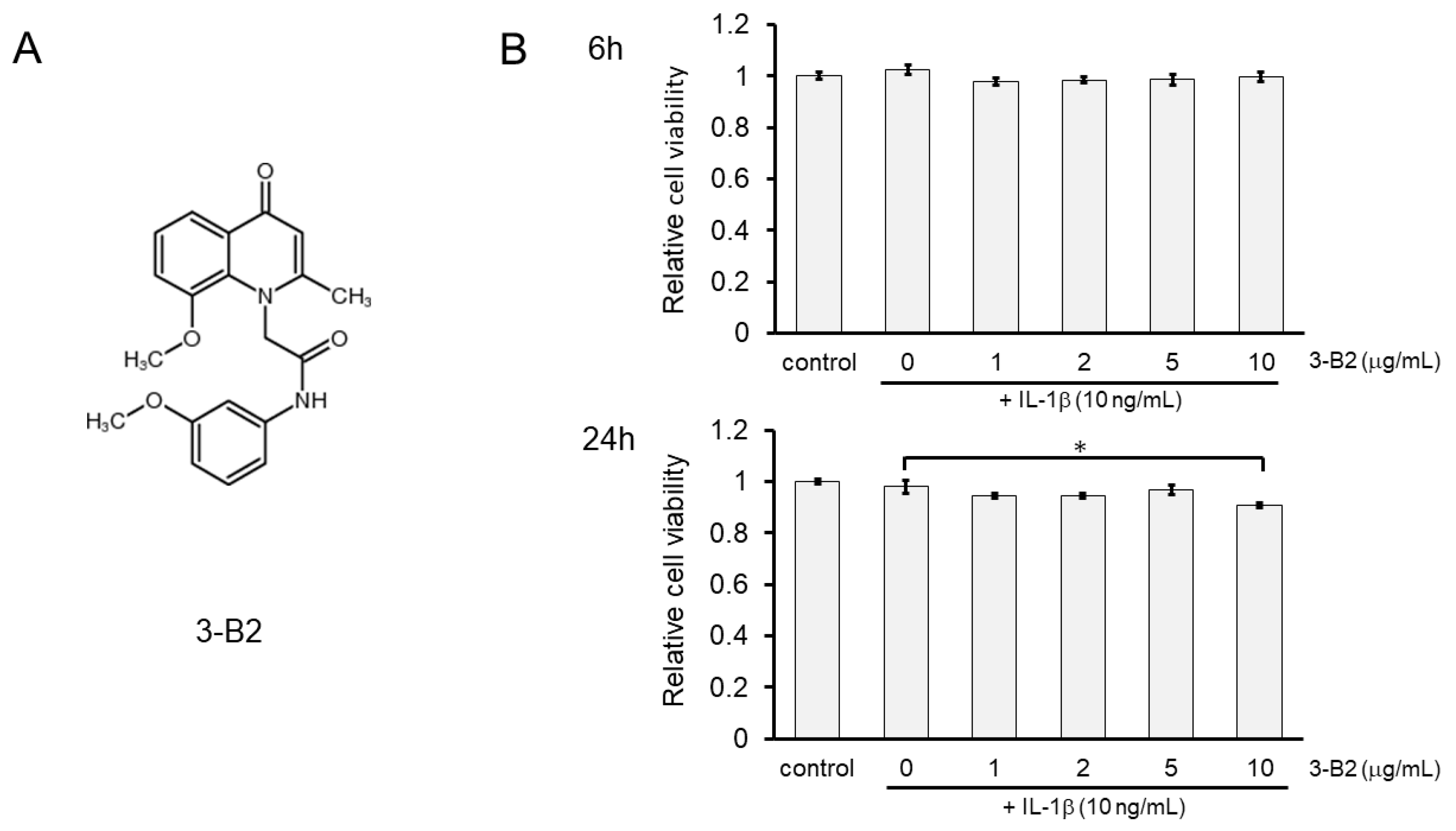
2.5. Effects of Compound 3-B2 on the Viability of IL-1β-Treated OUMS27 Cells
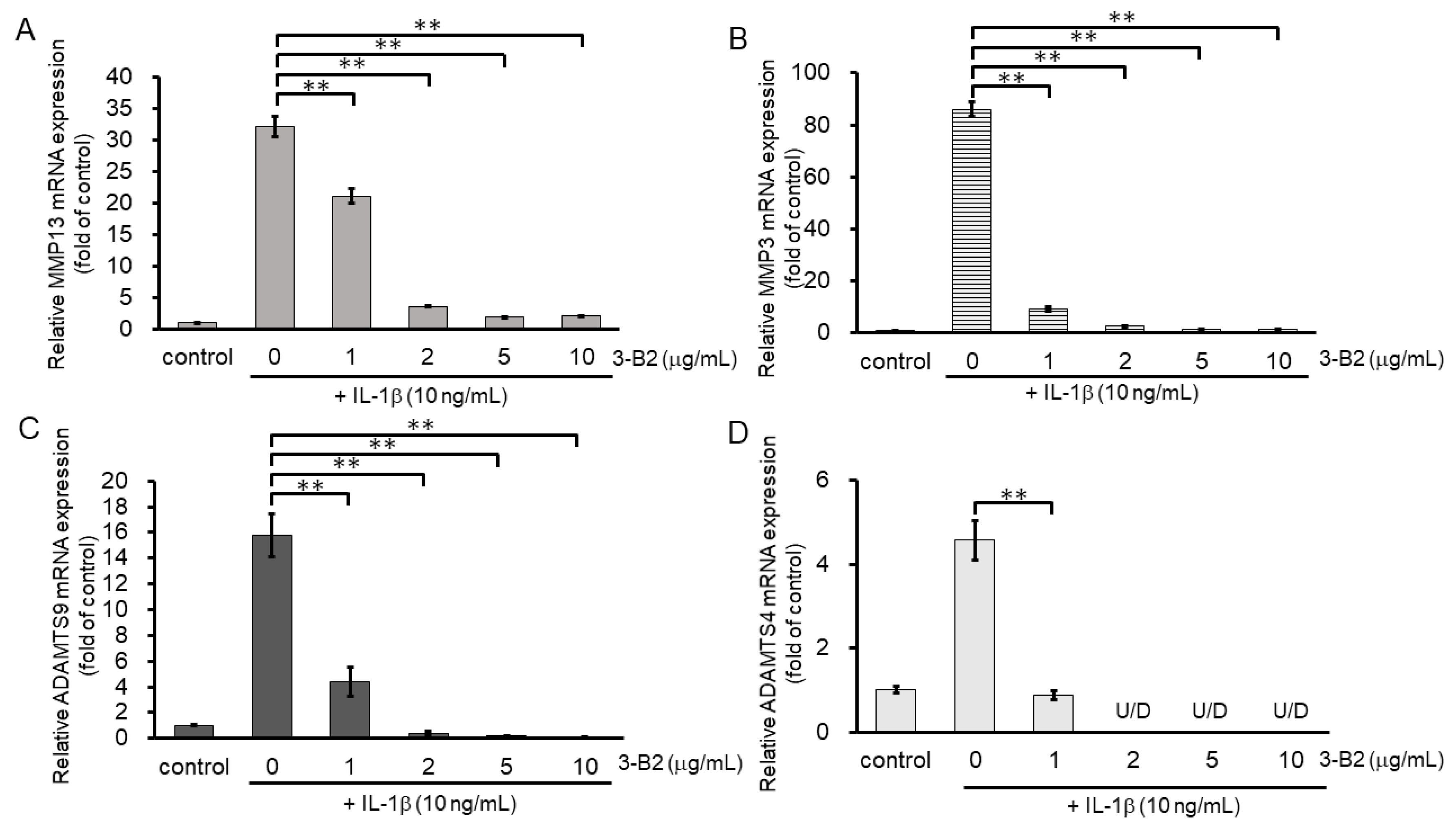
2.6. 3-B2 Inhibited IL-1β-Induced Gene Expression of Articular Cartilage ECM-Degrading Proteases in OUMS27 Cells
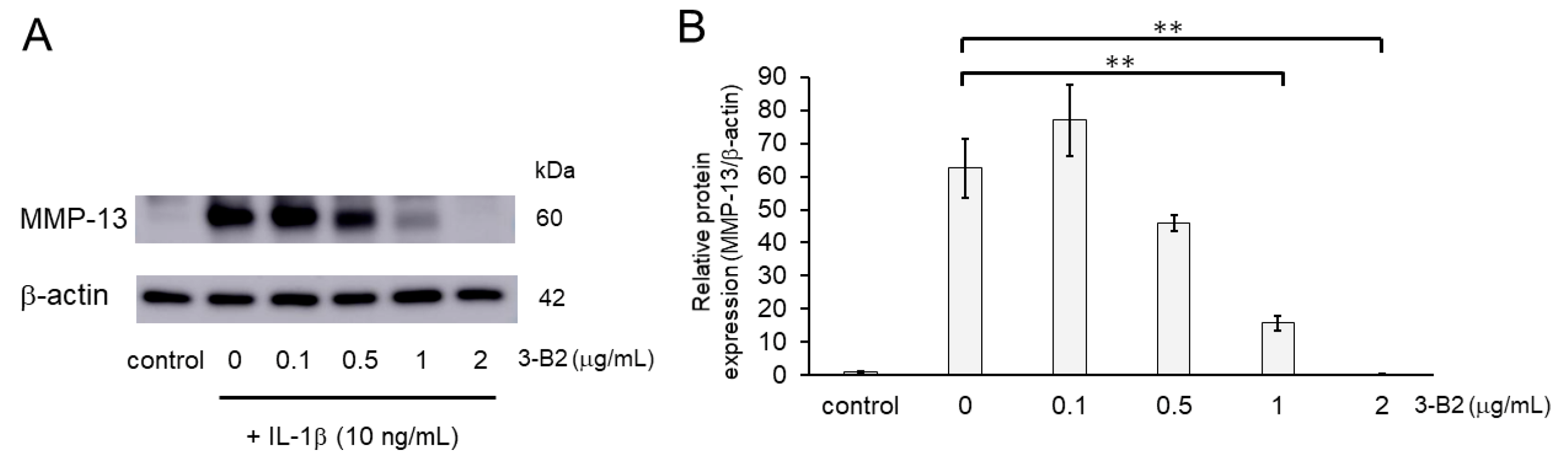
2.7. Inhibitory Effects of 3-B2 on the Protein Expression of Articular Cartilage ECM-Degrading Proteases in OUMS27 Cells
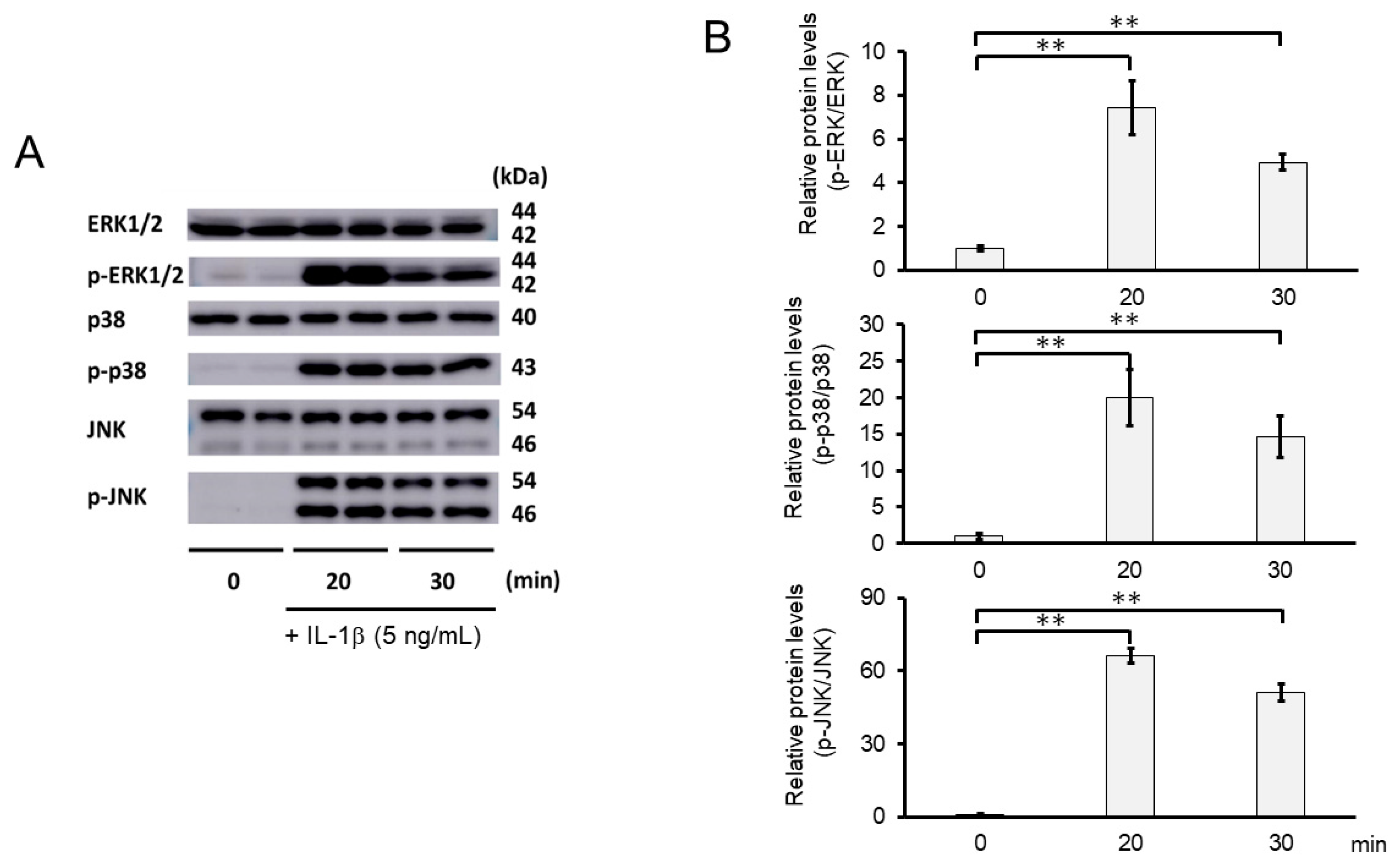
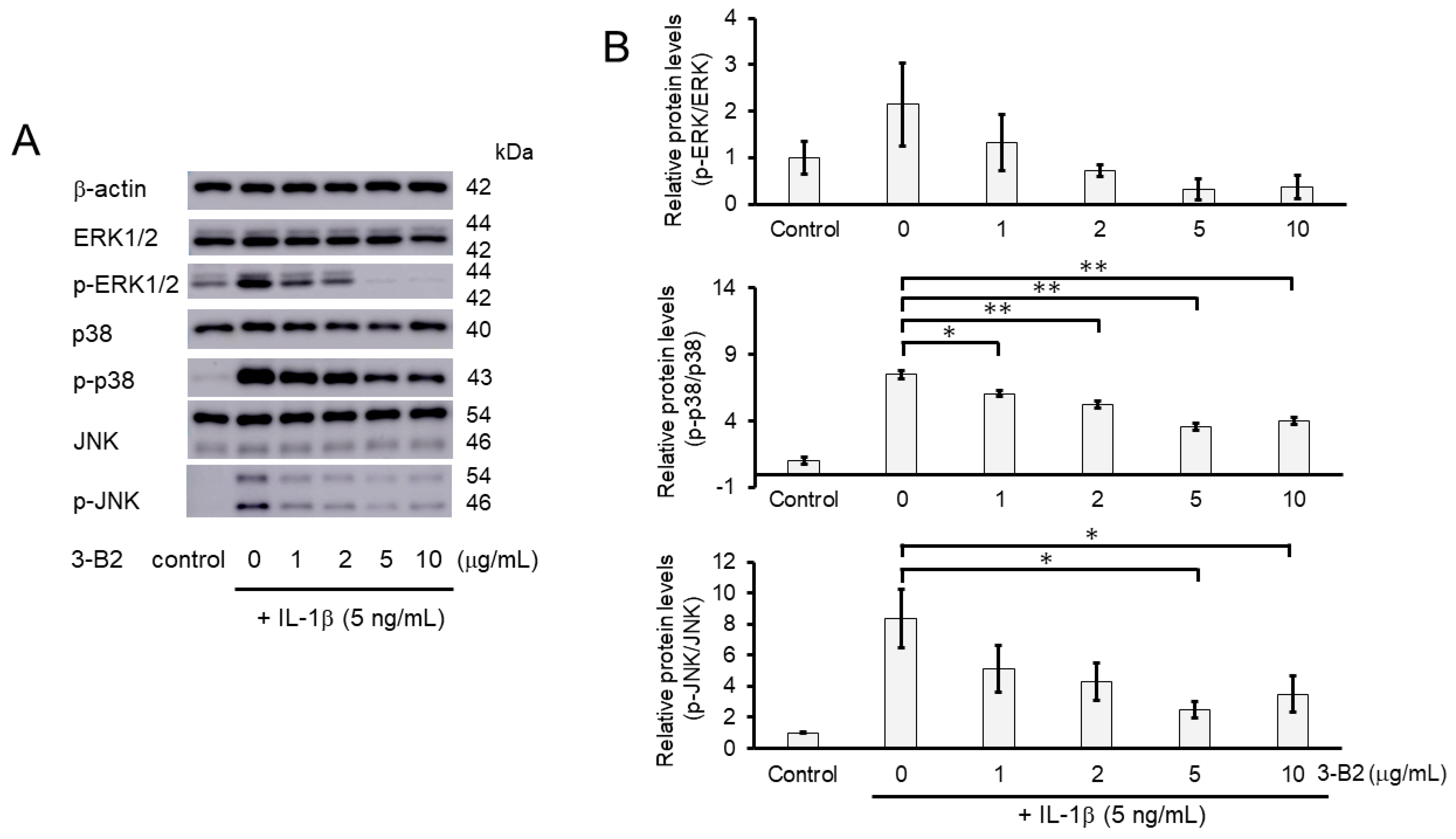
2.8. 3-B2 Inhibited IL-1β-Induced MAPK Activation in OUMS27 Cells
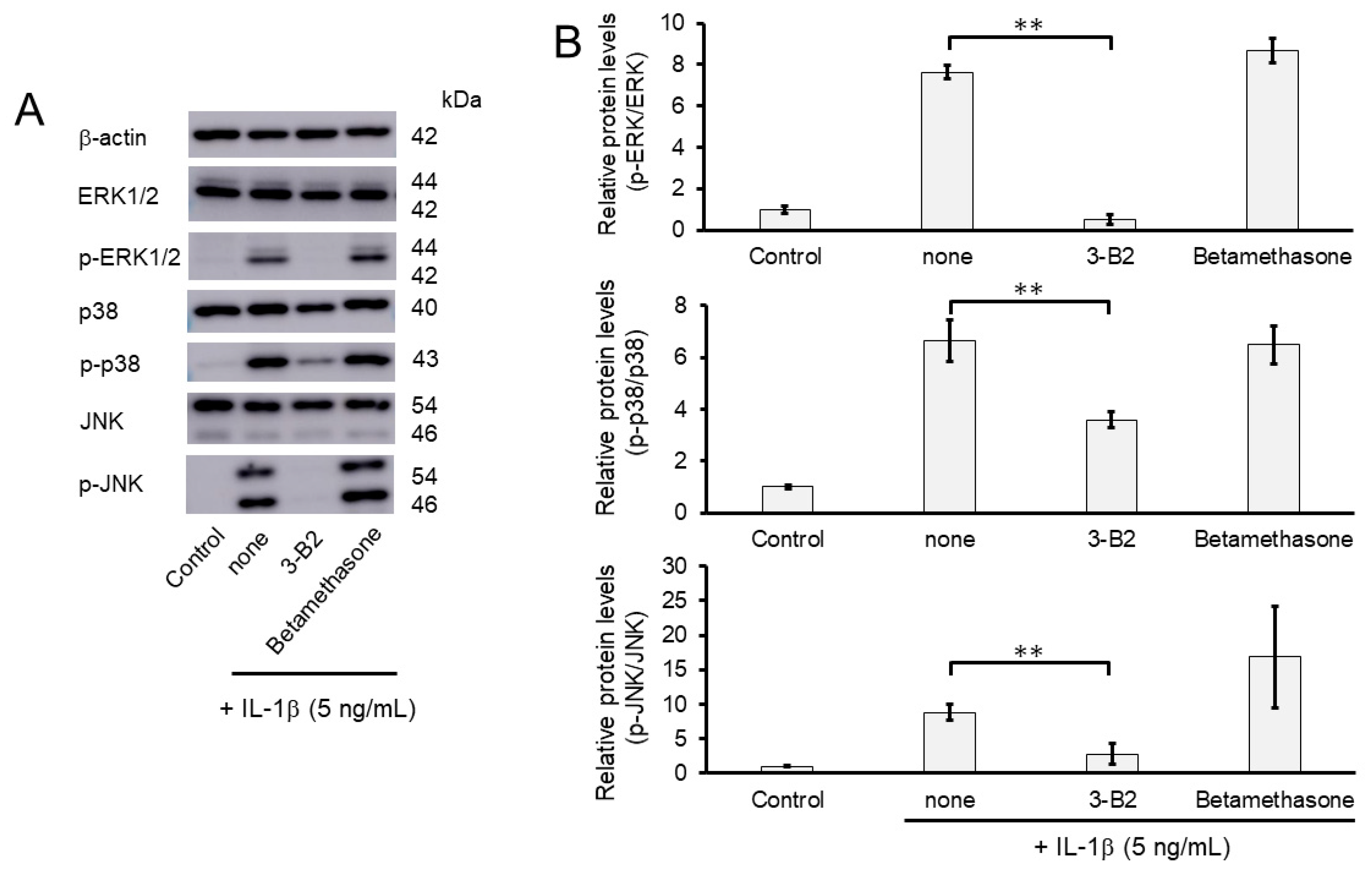
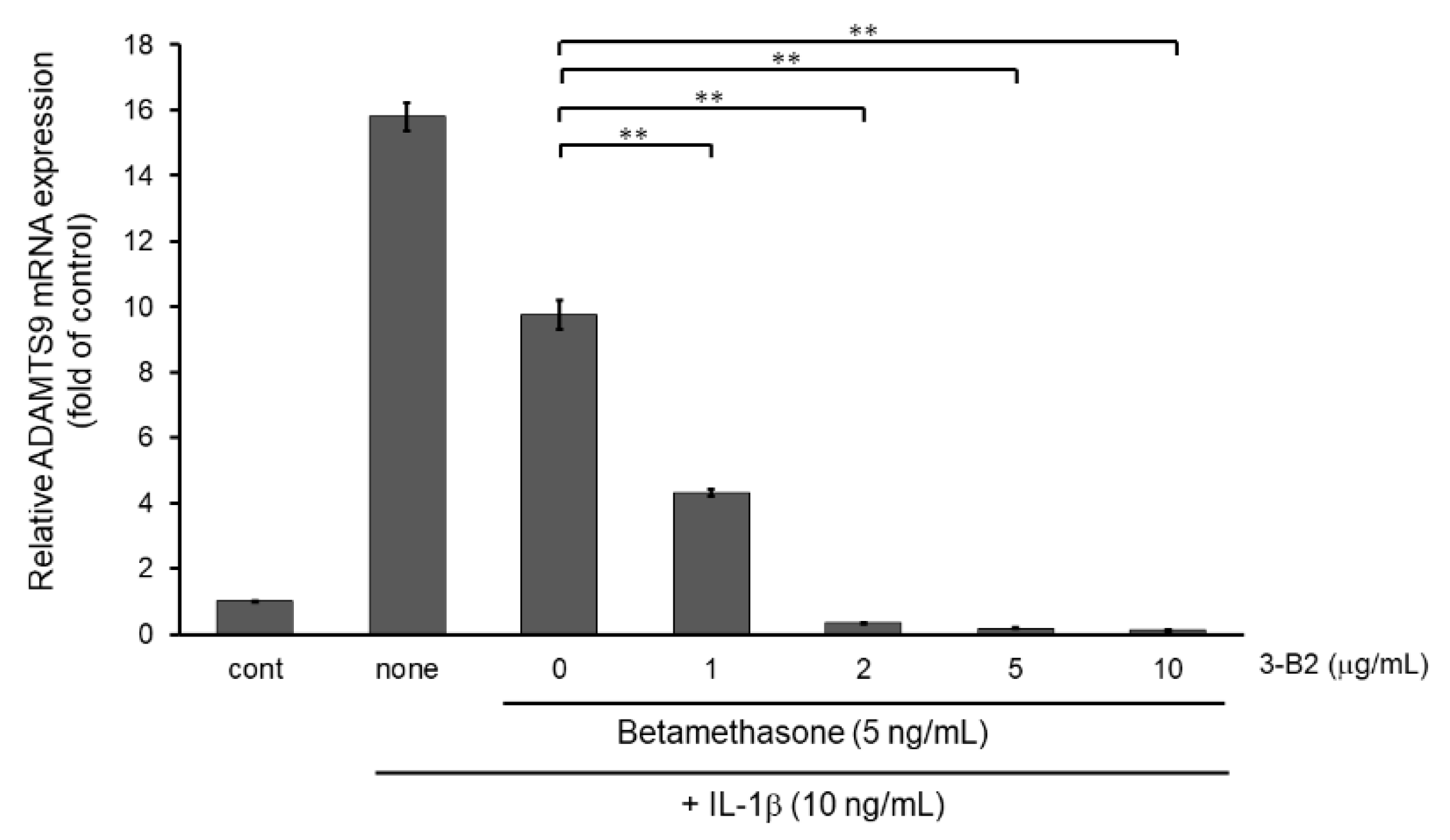
2.9. 3-B2 Synergistically Attenuated MMP13 and ADAMTS9 Expression with Betamethasone in IL-1β-Stimulated OUMS-27 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay
4.4. RNA Extraction and Quantitative Real-Time (qRT) -PCR
4.5. Western Blot Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Bortoluzzi, A.; Furini, F.; Scirè, C.A. Osteoarthritis and its management—Epidemiology, nutritional aspects and environmental factors. Autoimmun. Rev. 2018, 17, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Sacitharan, P.K.; Vincent, T.L. Cellular ageing mechanisms in osteoarthritis. Mamm. Genome 2016, 27, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Schroeppel, J.P.; Crist, J.D.; Anderson, H.C.; Wang, J. Molecular regulation of articular chondrocyte function and its significance in osteoarthritis. Histol. Histopathol. 2011, 26, 377–394. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, M.H.J. Osteoarthritis year in review 2020: Biology. Osteoarthr. Cartil. 2021, 29, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM metalloproteinases in osteoarthritis—Looking beyond the “usual suspects”. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, R.; Yang, X.; Zhang, D.; Li, B.; Zhang, D.; Li, Q.; Xiong, Y. Analysis of gene expression and methylation datasets identified ADAMTS9, FKBP5, and PFKBF3 as biomarkers for osteoarthritis. J. Cell. Physiol. 2019, 234, 8908–8917. [Google Scholar] [CrossRef]
- Demircan, K.; Hirohata, S.; Nishida, K.; Hatipoglu, O.F.O.F.; Oohashi, T.; Yonezawa, T.; Apte, S.S.S.; Ninomiya, Y. ADAMTS-9 is synergistically induced by interleukin-1β and tumor necrosis factor α in OUMS-27 chondrosarcoma cells and in human chondrocytes. Arthritis Rheum. 2005, 52, 1451–1460. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Okano, H.; Miyagawa, W.; Visse, R.; Shitomi, Y.; Santamaria, S.; Dudhia, J.; Troeberg, L.; Strickland, D.K.; Hirohata, S.; et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016, 56, 57–73. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Chen, D.; Liu-Bryan, R. Oral administration of berberine limits post-traumatic osteoarthritis development and associated pain via AMP-activated protein kinase (AMPK) in mice. Osteoarthr. Cartil. 2021, 30, 160–171. [Google Scholar] [CrossRef]
- Liu, S.-C.; Lee, H.-P.; Hung, C.-Y.; Tsai, C.-H.; Li, T.-M.; Tang, C.-H. Berberine attenuates CCN2-induced IL-1β expression and prevents cartilage degradation in a rat model of osteoarthritis. Toxicol. Appl. Pharmacol. 2015, 289, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, T.; Xia, C.; Shi, L.; Wang, S.; Zheng, X.; Hu, T.; Zhang, B. Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signalling. J. Cell. Mol. Med. 2014, 18, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Berberine and musculoskeletal disorders: The therapeutic potential and underlying molecular mechanisms. Phytomedicine 2020, 73, 152892. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Hatipoglu, O.F.; Asano, K.; Inagaki, J.; Nishida, K.; Hirohata, S. Induction of CEMIP in Chondrocytes by Inflammatory Cytokines: Underlying Mechanisms and Potential Involvement in Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3140. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Ünal, Z.N.; Erdoğan, S.; Akyol, S.; Ramazan Yiğitoğlu, M.; Hirohata, S.; Işık, B.; Demircan, K. Augmentation of ADAMTS9 gene expression by IL-1β is reversed by NFκB and MAPK inhibitors, but not PI3 kinase inhibitors. Cell Biochem. Funct. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1426530. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1426530 (accessed on 13 December 2021).
- Fu, L.; Hu, Y.; Song, M.; Liu, Z.; Zhang, W.; Yu, F.X.; Wu, J.; Wang, S.; Belmonte, J.C.I.; Chan, P.; et al. Up-regulation of FOXD1 by yap alleviates senescence and osteoarthritis. PLoS Biol. 2019, 17, e3000201. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, D.; Zhou, F.; Yu, J.; Wu, X.; Yu, D.; Zou, Y.; Hong, Y.; Yuan, C.; Chen, Y.; et al. Targeting downstream subcellular YAP activity as a function of matrix stiffness with Verteporfin-encapsulated chitosan microsphere attenuates osteoarthritis. Biomaterials 2020, 232, 119724. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, J.; Li, W.; Wu, A.; Zhang, X.; Tong, W.; Ho, K.K.; Qin, L.; Song, H.; Mak, K.K. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat. Commun. 2018, 9, 4564. [Google Scholar] [CrossRef] [Green Version]
- Kunisada, T.; Miyazaki, M.; Mihara, K.; Gao, C.; Kawai, A.; Inoue, H.; Namba, M. A new human chondrosarcoma cell line (OUMS-27) that maintains chondrocytic differentiation. Int. J. Cancer 1998, 77, 854–859. [Google Scholar] [CrossRef]
- Nishida, K.; Kunisada, T.; Shen, Z.N.; Kadota, Y.; Hashizume, K.; Ozaki, T. Chondrosarcoma and peroxisome proliferator-activated receptor. PPAR Res. 2008, 2008, 250568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, P.; Chen, W.; Tang, J.; Bao, J.; Wu, L. Protective effects of berberine in an experimental rat osteoarthritis model. Phytother. Res. 2011, 25, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, P.; Hou, Y.; Chen, S.; Xiao, Z.; Zhan, J.; Luo, D.; Gu, M.; Lin, D. Berberine inhibits the interleukin-1 beta-induced inflammatory response via MAPK downregulation in rat articular chondrocytes. Drug Dev. Res. 2019, 80, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Pang, Z.Y.; Chen, X.M.; Li, Z.; Liu, X.X.; Zhai, Q.L.; Huang, J.M.; Ruan, Z.Y. Urolithin a attenuates IL-1β-induced inflammatory responses and cartilage degradation via inhibiting the MAPK/NF-κB signaling pathways in rat articular chondrocytes. J. Inflamm. 2020, 17, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondoh, Y.; Honda, K.; Osada, H. Construction and application of a photo-cross-linked chemical array. Methods Mol. Biol. 2015, 1263, 29–41. [Google Scholar] [CrossRef]
- Hatipoglu, O.F.; Uctepe, E.; Opoku, G.; Wake, H.; Ikemura, K.; Ohtsuki, T.; Inagaki, J.; Gunduz, M.; Gunduz, E.; Watanabe, S.; et al. Osteopontin silencing attenuates bleomycin-induced murine pulmonary fibrosis by regulating epithelial-mesenchymal transition. Biomed. Pharmacother. 2021, 139, 111633. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Shinaoka, A.; Kumagishi-Shinaoka, K.; Asano, K.; Hatipoglu, O.F.; Inagaki, J.; Takahashi, K.; Oohashi, T.; Nishida, K.; Naruse, K.; et al. Mechanical strain attenuates cytokine-induced ADAMTS9 expression via transient receptor potential vanilloid type 1. Exp. Cell Res. 2019, 383, 111556. [Google Scholar] [CrossRef]
- Obika, M.; Ogawa, H.; Takahashi, K.; Li, J.; Hatipoglu, O.F.; Cilek, M.Z.; Miyoshi, T.; Inagaki, J.; Ohtsuki, T.; Kusachi, S.; et al. Tumor growth inhibitory effect of ADAMTS1 is accompanied by the inhibition of tumor angiogenesis. Cancer Sci. 2012, 103, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, J.; Takahashi, K.; Ogawa, H.; Asano, K.; Faruk Hatipoglu, O.; Cilek, M.Z.; Obika, M.; Ohtsuki, T.; Hofmann, M.; Kusachi, S.; et al. ADAMTS1 inhibits lymphangiogenesis by attenuating phosphorylation of the lymphatic endothelial cell-specific VEGF receptor. Exp. Cell Res. 2014, 323, 263–275. [Google Scholar] [CrossRef]
- Demircan, K.; Yonezawa, T.; Takigawa, T.; Topcu, V.; Erdogan, S.; Ucar, F.; Armutcu, F.; Yigitoglu, M.R.; Ninomiya, Y.; Hirohata, S. ADAMTS1, ADAMTS5, ADAMTS9 and aggrecanase-generated proteoglycan fragments are induced following spinal cord injury in mouse. Neurosci. Lett. 2013, 544, 25–30. [Google Scholar] [CrossRef]
- Komata, T.; Koga, S.; Hirohata, S.; Takakura, M.; Germano, I.; Inoue, M.; Kyo, S.; Kondo, S.; Kondo, Y. A novel treatment of human malignant gliomas in vitro and in vivo: FADD gene transfer under the control of the human telomerase reverse transcriptase gene promoter. Int. J. Oncol. 2001, 19, 1015–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asano, K.; Nelson, C.M.; Nandadasa, S.; Aramaki-Hattori, N.; Lindner, D.J.; Alban, T.; Inagaki, J.; Ohtsuki, T.; Oohashi, T.; Apte, S.S.; et al. Stromal Versican Regulates Tumor Growth by Promoting Angiogenesis. Sci. Rep. 2017, 7, 17225. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, O.F.; Miyoshi, T.; Yonezawa, T.; Kondo, M.; Amioka, N.; Yoshida, M.; Akagi, S.; Nakamura, K.; Hirohata, S.; Ito, H. Deficiency of CD44 prevents thoracic aortic dissection in a murine model. Sci. Rep. 2020, 10, 6869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtsuki, T.; Asano, K.; Inagaki, J.; Shinaoka, A.; Kumagishi-Shinaoka, K.; Cilek, M.Z.; Hatipoglu, O.F.; Oohashi, T.; Nishida, K.; Komatsubara, I.; et al. High molecular weight hyaluronan protects cartilage from degradation by inhibiting aggrecanase expression. J. Orthop. Res. 2018, 36, 3247–3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Hirohata, S.; Murakami, T.; Miyoshi, T.; Demircan, K.; Oohashi, T.; Ogawa, H.; Koten, K.; Toeda, K.; Kusachi, S.; et al. Dynamic induction of ADAMTS1 gene in the early phase of acute myocardial infarction. J. Biochem. 2004, 136, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, T.; Hirohata, S.; Ogawa, H.; Doi, M.; Obika, M.; Yonezawa, T.; Sado, Y.; Kusachi, S.; Kyo, S.; Kondo, S.; et al. Tumor-specific expression of the RGD-alpha3(IV)NC1 domain suppresses endothelial tube formation and tumor growth in mice. FASEB J. 2006, 20, 1904–1906. [Google Scholar] [CrossRef]
- Demircan, K.; Gunduz, E.; Gunduz, M.; Beder, L.B.; Hirohata, S.; Nagatsuka, H.; Cengiz, B.; Cilek, M.Z.; Yamanaka, N.; Shimizu, K.; et al. Increased mRNA expression of ADAMTS metalloproteinases in metastatic foci of head and neck cancer. Head Neck 2009, 31, 793–801. [Google Scholar] [CrossRef]
- Yaykasli, K.O.; Oohashi, T.; Hirohata, S.; Hatipoglu, O.F.; Inagawa, K.; Demircan, K.; Ninomiya, Y. ADAMTS9 activation by interleukin 1 beta via NFATc1 in OUMS-27 chondrosarcoma cells and in human chondrocytes. Mol. Cell. Biochem. 2009, 323, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inagaki, J.; Nakano, A.; Hatipoglu, O.F.; Ooka, Y.; Tani, Y.; Miki, A.; Ikemura, K.; Opoku, G.; Ando, R.; Kodama, S.; et al. Potential of a Novel Chemical Compound Targeting Matrix Metalloprotease-13 for Early Osteoarthritis: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 2681. https://doi.org/10.3390/ijms23052681
Inagaki J, Nakano A, Hatipoglu OF, Ooka Y, Tani Y, Miki A, Ikemura K, Opoku G, Ando R, Kodama S, et al. Potential of a Novel Chemical Compound Targeting Matrix Metalloprotease-13 for Early Osteoarthritis: An In Vitro Study. International Journal of Molecular Sciences. 2022; 23(5):2681. https://doi.org/10.3390/ijms23052681
Chicago/Turabian StyleInagaki, Junko, Airi Nakano, Omer Faruk Hatipoglu, Yuka Ooka, Yurina Tani, Akane Miki, Kentaro Ikemura, Gabriel Opoku, Ryosuke Ando, Shintaro Kodama, and et al. 2022. "Potential of a Novel Chemical Compound Targeting Matrix Metalloprotease-13 for Early Osteoarthritis: An In Vitro Study" International Journal of Molecular Sciences 23, no. 5: 2681. https://doi.org/10.3390/ijms23052681






