MicroRNA Cross-Involvement in Acne Vulgaris and Hidradenitis Suppurativa: A Literature Review
Abstract
:1. Introduction
1.1. Pathogenesis of AV
1.2. Pathogenesis of HS
1.3. MicroRNAs Biogenesis and Skin Function
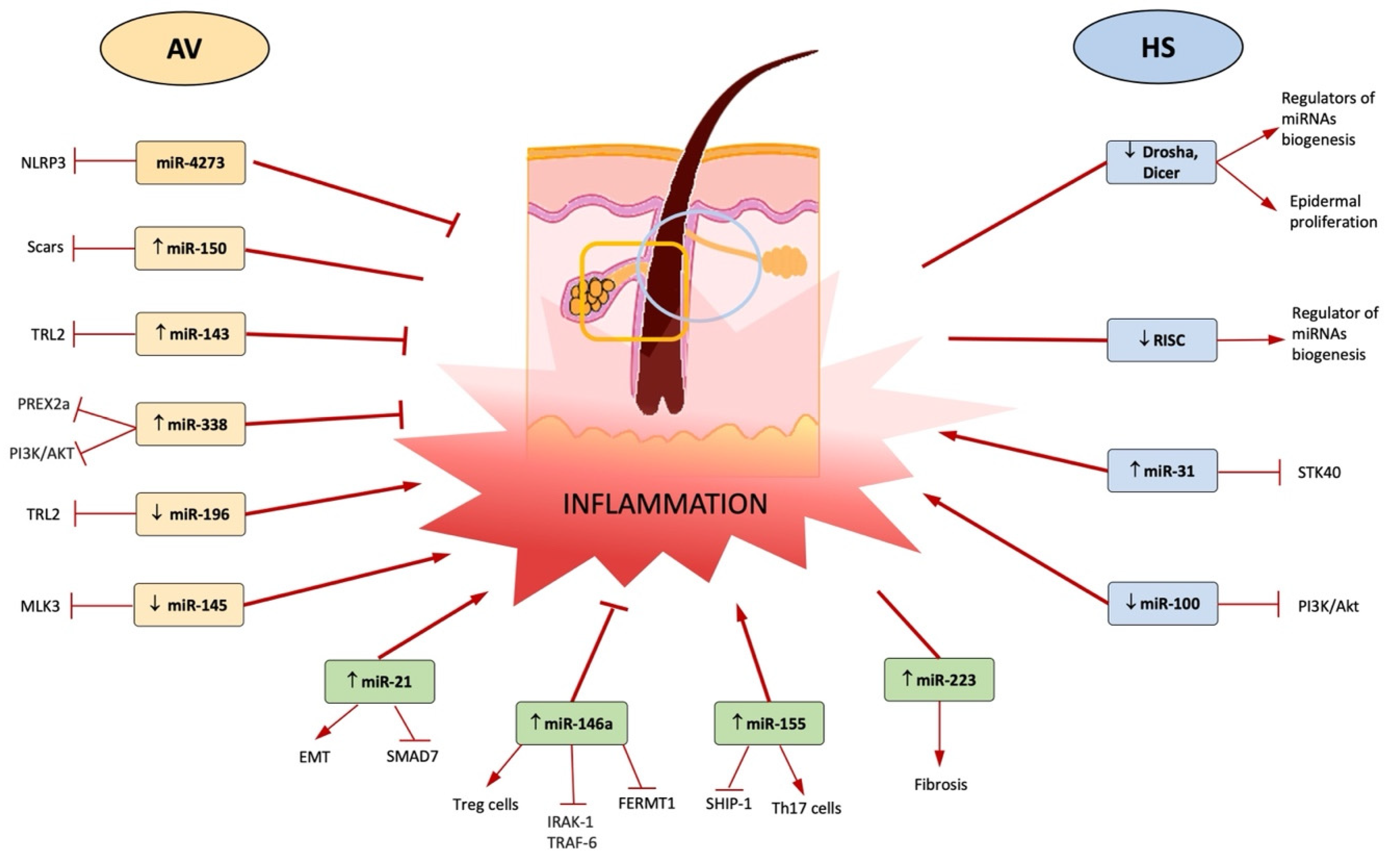
2. MiRNAs in AV and HS: Pathogenetic Role and Therapeutic Strategies
2.1. Anti-Inflammatory MiRNAs in AV and HS
2.2. Pro Inflammatory MiRNAs in AV and HS
2.3. MiRNAs and Inflammasome in AV and HS
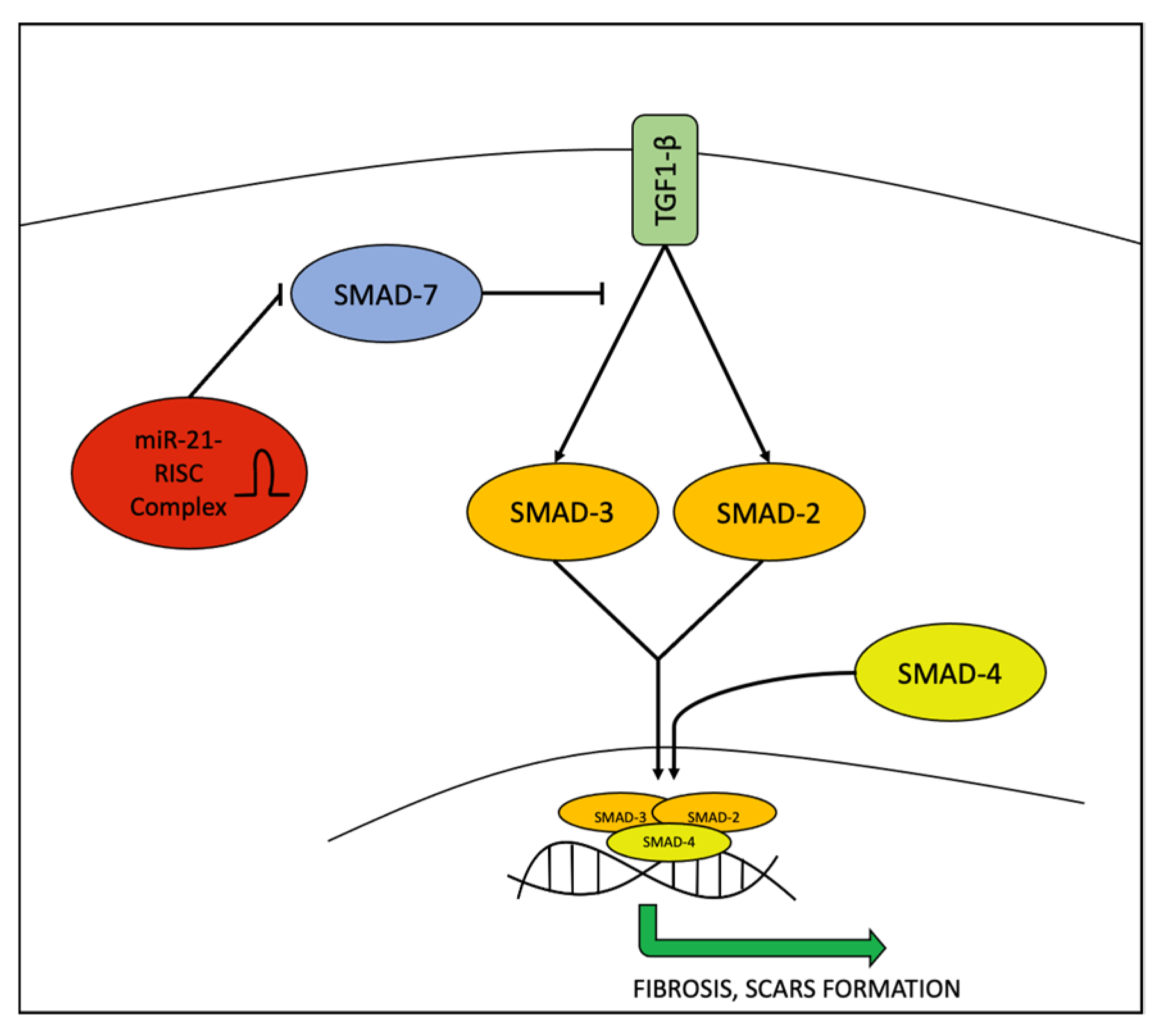
2.4. MiRNAs and Fibrosis Process in AV and HS
2.5. MiRNAs and Keratinization
2.6. AV Studies
2.7. HS Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moradi Tuchayi, S.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Primers 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.N.; Harper, J.C.; Cantrell, W.C.; Wang, W.; Foster, K.W.; Elewski, B.E. The prevalence of acne in adults 20 years and older. J. Am. Acad. Dermatol. 2008, 58, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Bhate, K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 2015, 172, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.; Göbel, K.; Niessen, C.; Paus, R.; Van Steensel, M.; Lim, X. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. Br. J. Dermatol. 2019, 181, 677–690. [Google Scholar] [CrossRef]
- Kelhälä, H.L.; Palatsi, R.; Fyhrquist, N.; Lehtimäki, S.; Väyrynen, J.P.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H.; et al. IL-17/Th17 pathway is activated in acne lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef] [PubMed]
- Scala, E.; Cacciapuoti, S.; Garzorz-Stark, N.; Megna, M.; Marasca, C.; Seiringer, P.; Volz, T.; Eyerich, K.; Fabbrocini, G. Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells 2021, 10, 2094. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Damiani, G.; Orenstein, L.A.; Hamzavi, I.; Jemec, G. Hidradenitis suppurativa: An update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J. Eur. Acad. Dermatol. Venereol. 2020, 35, 50–61. [Google Scholar] [CrossRef]
- Wolk, K.; Join-Lambert, O.; Sabat, R. Aetiology and pathogenesis of hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 999–1010. [Google Scholar] [CrossRef]
- Shah, A.; Alhusayen, R.; Amini-Nik, S. The critical role of macrophages in the pathogenesis of hidradenitis suppurativa. Inflamm. Res. 2017, 11, 931–945. [Google Scholar] [CrossRef]
- Fan, R.; Xiao, C.; Wan, X.; Cha, W.; Miao, Y.; Zhou, Y.; Qin, C.; Cui, T.; Su, F.; Shan, X. Small molecules with big roles in microRNA chemical biology and microRNA-targeted therapeutics. RNA Biol. 2019, 16, 707–718. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Yi, R.; O’Carroll, D.; Pasolli, H.A.; Zhang, Z.; Dietrich, F.S.; Tarakhovsky, A.; Fuchs, E. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat. Genet. 2006, 38, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Sand, M.; Gambichler, T.; Sand, D.; Skrygan, M.; Altmeyer, P.; Bechara, F.G. MicroRNAs and the skin: Tiny players in the body’s largest organ. J. Dermatol. Sci. 2009, 53, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, C.; Casciaro, M.; Minciullo, P.L.; Calapai, G.; Navarra, M.; Gangemi, S. Involvement of microRNAs in skin disorders: A literature review. Allergy Asthma Proc. 2017, 38, 9–15. [Google Scholar] [CrossRef]
- Kircik, L.H. Advances in the Understanding of the Pathogenesis of Inflammatory Acne. J. Drugs Dermatol. 2016, 15 (Suppl. 1), s7–s10. [Google Scholar]
- Hessam, S.; Sand, M.; Skrygan, M.; Gambichler, T.; Bechara, F.G. Expression of miRNA-155, miRNA-223, miRNA-31, miRNA-21, miRNA-125b, and miRNA-146a in the Inflammatory Pathway of Hidradenitis Suppurativa. Inflammation 2016, 40, 464–472. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.; Xu, H.; Liu, Y.; Du, L.; Duan, Z.; Tong, J.; He, Y.; Chen, Q.; Chen, X.; Li, M. miR-146a Inhibits Biofilm-Derived Cutibacterium acnes–Induced Inflammatory Reactions in Human Keratinocytes. J. Investig. Dermatol. 2019, 139, 2488–2496.e4. [Google Scholar] [CrossRef]
- Dull, K.; Fazekas, F.; Deák, D.; Kovács, D.; Póliska, S.; Szegedi, A.; Zouboulis, C.C.; Törőcsik, D. miR-146a modulates TLR1/2 and 4 induced inflammation and links it with proliferation and lipid production via the indirect regulation of GNG7 in human SZ95 sebocytes. Sci. Rep. 2021, 11, 21510. [Google Scholar] [CrossRef]
- Lu, L.-F.; Boldin, M.; Chaudhry, A.; Lin, L.-L.; Taganov, K.D.; Hanada, T.; Yoshimura, A.; Baltimore, D.; Rudensky, A.Y. Function of miR-146a in Controlling Treg Cell-Mediated Regulation of Th1 Responses. Cell 2010, 142, 914–929. [Google Scholar] [CrossRef] [Green Version]
- Løvendorf, M.B.; Mitsui, H.; Zibert, J.R.; Røpke, M.A.; Hafner, M.; Dyring-Andersen, B.; Bonefeld, C.M.; Krueger, J.G.; Skov, L. Laser capture microdissection followed by next-generation sequencing identifies disease-related microRNAs in psoriatic skin that reflect systemic microRNA changes in psoriasis. Exp. Dermatol. 2015, 24, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, Z.; Liu, K.; Wu, Y.; Jiang, D.; Lai, Y. Staphylococcal LTA-Induced miR-143 Inhibits Propionibacterium acnes-Mediated Inflammatory Response in Skin. J. Investig. Dermatol. 2016, 136, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Cao, L.; Feng, Y.; Li, Y.; Li, T. MiR-338-3p inhibits TNF-α-induced lipogenesis in human sebocytes. Biotechnol. Lett. 2017, 39, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, X.; Sun, S.; Chen, P.; Liang, X.; Wang, J.; Ruan, J.; Zhang, S.; Zhang, X. Circular RNA expression profile analysis of severe acne by RNA-Seq and bioinformatics. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1986–1992. [Google Scholar] [CrossRef]
- Yan, J.; Qiao, M.; Li, R.; Zhao, X.; Wang, X.; Sun, Q. Downregulation of miR-14-5p contributes to hyperproliferation of keratinocytes and skin inflammation in psoriasis. Br. J. Dermatol. 2018, 180, 365–372. [Google Scholar] [CrossRef]
- Yang, S.; Fang, F.; Yu, X.; Yang, C.; Zhang, X.; Wang, L.; Zhu, L.; Shao, K.; Zhu, T. Knockdown of H19 Inhibits the Pathogenesis of Acne Vulgaris by Targeting the miR-196a/TLR2/NF-kB Axis. Inflammation 2020, 43, 1936–1947. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef] [Green Version]
- Moran, B.; Sweeney, C.M.; Hughes, R.; Malara, A.; Kirthi, S.; Tobin, A.M.; Kirby, B.; Fletcher, J.M. Hidradenitis Suppurativa Is Characterized by Dysregulation of the Th17:Treg Cell Axis, Which Is Corrected by Anti-TNF Therapy. J. Investig. Dermatol. 2017, 137, 2389–2395. [Google Scholar] [CrossRef] [Green Version]
- Verma, G.; Sardana, K. Propionibacterium acnes and the Th1/Th17 Axis, implications in acne pathogenesis and treatment. Indian J. Dermatol. 2017, 62, 392–394. [Google Scholar] [CrossRef]
- Ghumra, W.; Lee, N.; Whitehouse, H.; Bhutani, R.; Lagos, D.; Layton, A.M. MicroRNAs as biomarkers of atrophic scarring in acne: A cross-sectional analysis of 41 patients. Clin. Exp. Dermatol. 2021, 46, 1495–1503. [Google Scholar] [CrossRef]
- Guinea-Viniegra, J.; Jiménez, M.; Schonthaler, H.B.; Navarro, R.; Delgado, Y.; José Concha-Garzón, M.; Tschachler, E.; Obad, S.; Daudén, E.; Wagner, E.F. Targeting miR-21 to Treat Psoriasis. Sci. Transl. Med. 2014, 6, 225re1. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, G.; Da Cunha, A.P.; Ajay, A.K.; Joller, N.; Garo, L.P.; Kumaradevan, S.; Yosef, N.; Vaidya, V.S.; Weiner, H.L. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J. Clin. Investig. 2015, 125, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Mehta, A.; Zhao, J.L.; Lee, K.; Marinov, G.K.; Garcia-Flores, Y.; Lu, L.F.; Rudensky, A.Y.; Baltimore, D. An NF-kB-microRNA regulatory network tunes macrophage inflammatory responses. Nat. Commun. 2017, 8, 851. [Google Scholar] [CrossRef] [Green Version]
- Genovese, G.; Moltrasio, C.; Garcovich, S.; Marzano, A.V. PAPA spectrum disorders. G. Ital. Dermatol. Venereol. 2020, 155, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, Q.Z.; Shen, Z.Y.; Yuan, H.Y.; Yu, W.J.; Chen, X.D.; Xu, H. Genetic association between the NLRP3 gene and acne vulgaris in a Chinese population. Clin. Exp. Dermatol. 2019, 44, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Di Vincenzo, S.; Di Salvo, E.; Genovese, S.; Dino, P.; Sangiorgi, C.; Ferraro, M.; Gangemi, S. MiR-21 upregulation increases IL-8 expression and tumorigenesis program in airway epithelial cells exposed to cigarette smoke. J. Cell. Physiol. 2019, 234, 22183–22194. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, J. Microrna-21: A Central Regulator of Fibrotic Diseases Via Various Targets. Curr. Pharm. Des. 2015, 21, 2236–2242. [Google Scholar]
- Bagnato, G.; Roberts, W.N.; Roman, J.; Gangemi, S. A systematic review of overlapping microRNA patterns in systemic sclerosis and idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2017, 26, 160125. [Google Scholar] [CrossRef] [Green Version]
- Timis, T.L.; Orasan, R.I. Understanding psoriasis: Role of miRNAs (Review). Biomed. Rep. 2018, 9, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Reyfman, P.A.; Gottardi, C.J. Idiopathic Pulmonary Fibrosis and Lung Cancer: Finding Similarities within Differences. Am. J. Respir. Cell Mol. Biol. 2019, 61, 667–668. [Google Scholar] [CrossRef]
- Muñoz-Félix, J.M.; González-Núñez, M.; López-Novoa, J.M. ALK1-Smad1/5 signaling pathway in fibrosis development: Friend or foe? Cytokine Growth Factor Rev. 2013, 24, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Dai, C. Ablation of FGFR2 in Fibroblasts Ameliorates Kidney Fibrosis after Ischemia/Reperfusion Injury in Mice. Kidney Dis. 2017, 3, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Tamari, H.M.; Dabral, S.; Schmall, A.; Sarvari, P.; Ruppert, C.; Paik, J.; DePinho, R.; Grimminger, F.; Eickelberg, O.; Guenther, A.; et al. FoxO3 an important player in fibrogenesis and therapeutic target for idiopathic pulmonary fibrosis. EMBO Mol. Med. 2017, 10, 276–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Ding, H.-S.; Guo, X.; Shen, J.-J.; Fan, D.; Huang, Y.; Huang, C.-X. MiR-33 promotes myocardial fibrosis by inhibiting MMP16 and stimulating p38 MAPK signaling. Oncotarget 2018, 9, 22047–22057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivarcsi, A.; Meisgen, F.; Xu, N.; Stähle, M.; Sonkoly, E. Changes in the level of serum microRNAs in patients with psoriasis after antitumour necrosis factor-α therapy. Br. J. Dermatol. 2013, 169, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Hessam, S.; Sand, M.; Skrygan, M.; Gambichler, T.; Bechara, F.G. Inflammation induced changes in the expression levels of components of the microRNA maturation machinery Drosha, Dicer, Drosha co-factor DGRC8 and Exportin-5 in inflammatory lesions of hidradenitis suppurativa patients. J. Dermatol. Sci. 2016, 82, 166–174. [Google Scholar] [CrossRef]
- Hessam, S.; Sand, M.; Skrygan, M.; Bechara, F.G. The microRNA effector RNA-induced silencing complex in hidradenitis suppurativa: A significant dysregulation within active inflammatory lesions. Arch. Dermatol. Res. 2017, 309, 557–565. [Google Scholar] [CrossRef]
- Dedes, K.J.; Natrajan, R.; Lambros, M.B.; Geyer, F.C.; Lopez-Garcia, M.A.; Savage, K.; Jones, R.L.; Reis-Filho, J.S. Down-regulation of the miRNA master regulators Drosha and Dicer is associated with specific subgroups of breast cancer. Eur. J. Cancer 2011, 47, 138–150. [Google Scholar] [CrossRef]
- He, Y.; Li, C.; Xu, H.; Duan, Z.; Liu, Y.; Zeng, R.; Li, M.; Wang, B. AKT-dependent hyperproliferation of keratinocytes in familial hidradenitis suppurativa with a NCSTN mutation: A potential role of defective miR-100-5p. Br. J. Dermatol. 2020, 182, 500–502. [Google Scholar] [CrossRef]
- Teng, Y.; Fan, Y.; Ma, J.; Lu, W.; Liu, N.; Chen, Y.; Pan, W.; Tao, X. The PI3K/Akt Pathway: Emerging Roles in Skin Homeostasis and a Group of Non-Malignant Skin Disorders. Cells 2021, 10, 1219. [Google Scholar] [CrossRef]

| miRNAs | Specimen | Method | Design | Subjects n | Function/Target | Year of Publication | Reference |
|---|---|---|---|---|---|---|---|
| miR-4273 | Humans | PCR, dual luciferase reporter assay | Case-control | AV = 428 Controls = 384 | Regulation of NLRP3 mRNA expression | 2019 | [35] |
| ↓ miR-196a | HaCaT cells, human keratinocytes | qPCR, western blotting; ELISA, FISH | Case-control | - | Regulation of TLR2/NF-kb pathway | 2020 | [26] |
| ↑ miR-146a and miR-146b | Sebocytes, AV and healthy skin biopsy, primary human keratinocytes | miR-146a-5p mimic, miR-146a-5p inhibitor; qPCR, western blotting; ELISA, FISH | Case-control | AV = 10 Controls = 10 | Regulation of TLR2/NF-kb pathway, sebocyte activation | 2021; 2019 | [18,19] |
| ↑ miR-155 | AV and healthy skin biopsy, primary human keratinocytes | qPCR | Case-control | AV = 10 Controls = 10 | Upregulation of TLR2/NF-kb pathway leading to expression of pro-inflammatory cytokines | 2019 | [18] |
| MiR-338-3p | Human sebocytes | Thin-layer chromatography, immunofluorescence, western blotting, flow cytometry | Case-control | - | Targets PREX2 and downregulates PI3K/AKT pathway leading to a reduction of lipogenesis | 2017 | [23] |
| ↑ miR-223 | AV samples, plasma | PCR | Case-control | AV = 8 Controls = 9 41 for circulating miRNAs | High levels in plasma of mild scarring cases, neutrophil activation and resolution of the acute inflammatory response in wound sites | 2021 | [30] |
| ↑ miR-21 | AV samples, plasma | PCR | Case-control | AV = 8 Controls = 9 41 for circulating miRNAs | Inhibits Smad7, a negative regulator of TGF-β1/Smad3 signalling | 2021 | [30] |
| ↑ miR-150 | AV samples, plasma | PCR | Case-control | AV = 8 Controls = 9 41 for circulating miRNAs | High levels in plasma of scarring cases | 2021 | [30] |
| miR-143 | Keratinocytes Tlr2-/- mice, Hela cells, human epidermal keratinocytes | qRT-PCR, ELISA, western blot | Case-control | - | targets 3′ UTR of TLR2 mRNA | 2016 | [22] |
| miR-145-5p | AV samples, human keratinocytes | qRT-PCR, Sanger sequencing, PCR | Case-control | AV = 3 Controls = 3 | Targets MLK3, a kinase involved in the control of the transcription activity of JNK and NF-kB | 2018 | [24] |
| Type of Cells | miRNAs | Method | Design | Subjects n | Function/Target | Year of Publication | Reference |
|---|---|---|---|---|---|---|---|
| Keratinocytes from HS samples | ↑ miR-31-5p | qPCR | Prospective study | HS = 15 Controls = 10 | Enhances expression of proinflammatory mediators in keratinocytes by blocking STK40, a negative regulator of NF-kB signalling | 2017 | [16] |
| Keratinocytes from HS samples | ↑ miR-21-5p | qPCR | Prospective study | HS = 15 Controls = 10 | Maintains T cell-derived skin inflammation, upregulation of TH17 cells and related cytokines, EMT, SMAD 7 | 2017 | [16] |
| Keratinocytes from HS samples | ↑ miR-146a-5p | qPCR | Prospective study | HS = 15 Controls = 10 | Regulation of TLR2/NF-kb pathway | 2017 | [16] |
| Keratinocytes from HS samples | ↑ miR-223-5p | qPCR | Prospective study | HS = 15 Controls = 10 | Negatively regulates the proliferation and differentiation of precursor granulocytes into neutrophils | 2017 | [16] |
| Keratinocytes from HS samples | ↓ RISC | RT-PCR | Prospective study | HS = 18 HS perilesional skin = 10 Controls: 10 healthy controls 10 = psoriatic patients | Regulation of miRNA formation and function | 2017 | [47] |
| Keratinocytes from HS samples | ↓ Drosha, Dicer, Drosha co-factor DGRC8, Exportin-5 | RT-PCR, immunohistochemistry | Prospective pilot study | HS = 18 HS perilesional skin = 7 Controls: 10 healthy controls 10 = psoriatic patients | miRNAs key regulators of biogenesis of miRNAs | 2016 | [46] |
| Keratinocytes from HS samples | ↓ miR-100-5p | qRT-PCR, in situ hybridization, immunofluorescence, western blotting, cell transfection and cell counting kit-8 assays | Case-control | HS = 5 Controls = 5 | Proliferation of keratinocytes targeting AKT pathway | 2020 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgia, F.; Peterle, L.; Custurone, P.; Vaccaro, M.; Pioggia, G.; Gangemi, S. MicroRNA Cross-Involvement in Acne Vulgaris and Hidradenitis Suppurativa: A Literature Review. Int. J. Mol. Sci. 2022, 23, 3241. https://doi.org/10.3390/ijms23063241
Borgia F, Peterle L, Custurone P, Vaccaro M, Pioggia G, Gangemi S. MicroRNA Cross-Involvement in Acne Vulgaris and Hidradenitis Suppurativa: A Literature Review. International Journal of Molecular Sciences. 2022; 23(6):3241. https://doi.org/10.3390/ijms23063241
Chicago/Turabian StyleBorgia, Francesco, Lucia Peterle, Paolo Custurone, Mario Vaccaro, Giovanni Pioggia, and Sebastiano Gangemi. 2022. "MicroRNA Cross-Involvement in Acne Vulgaris and Hidradenitis Suppurativa: A Literature Review" International Journal of Molecular Sciences 23, no. 6: 3241. https://doi.org/10.3390/ijms23063241
APA StyleBorgia, F., Peterle, L., Custurone, P., Vaccaro, M., Pioggia, G., & Gangemi, S. (2022). MicroRNA Cross-Involvement in Acne Vulgaris and Hidradenitis Suppurativa: A Literature Review. International Journal of Molecular Sciences, 23(6), 3241. https://doi.org/10.3390/ijms23063241










