Coagulopathy and Fibrinolytic Pathophysiology in COVID-19 and SARS-CoV-2 Vaccination
Abstract
:1. Introduction
2. COVID-19-Associated Coagulopathy
2.1. COVID-19 and Physical Findings
2.2. D-Dimer and Prognostic Factors for Poor Clinical Outcome in COVID-19
2.3. COVID-19 and Thrombotic/Hemorrhagic Disease
2.3.1. Macro-Thrombosis in COVID-19
2.3.2. Micro-Thrombosis in COVID-19
2.4. Dynamic Changes in Coagulation/Fibrinolysis in COVID-19
2.5. Treatment of COVID-19-Associated Coagulopathy
2.5.1. Antiplatelet Agents
2.5.2. Heparin Therapy
2.5.3. Combination Therapy with Heparin and Nafamostat (Attention to Fibrinolytic Pathophysiology)
- (1)
- Anti-thrombin effects
- (2)
- Anti-plasmin activity
- (3)
- Anti-transmembrane serine protease 2 (TMPRSS2) action
2.5.4. Direct Oral Anticoagulant
2.5.5. Fibrinolytic Treatment
2.5.6. Treatments to Avoid
- (a)
- Warfarin
- (b)
- Tranexamic acid
3. SARS-CoV-2 Vaccination-Associated Coagulopathy
3.1. Safety of Vaccination in Persons with Coagulation Abnormalities
3.1.1. Preventing Bleeding Due to Vaccination in Patients with Coagulation Abnormalities
3.1.2. Vaccination and Thrombotic/Hemorrhagic Disease Exacerbations and Relapses
3.2. Novel Clotting Abnormalities after Vaccination
3.2.1. Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT)
3.2.2. Thrombotic/Bleeding Disorders Other Than VITT
- (1)
- Hemorrhagic disease
- (2)
- Thrombotic disease
- (3)
- Thrombophilia with low platelet count
3.3. Pathophysiology of VITT
3.4. Diagnosis of VITT
3.5. Treatment of VITT (Attention to Fibrinolytic Pathophysiology)
4. Summary
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Geerts, W.; Selby, R. Prevention of Venous Thromboembolism in the ICU. Chest 2003, 124, 357S–363S. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, H.; Morishita, E.; Urano, T.; Yokoyama, K. The Questionnaire-survey Joint Team on The COVID-19-related thrombosis COVID-19-Related Thrombosis in Japan: Final Report of a Questionnaire-Based Survey in 2020. J. Atheroscler. Thromb. 2021, 28, 406–416. [Google Scholar] [CrossRef]
- Kerbikov, O.; Orekhov, P.; Borskaya, E.; Nosenko, N. High incidence of venous thrombosis in patients with moderate-to-severe COVID-19. Int. J. Hematol. 2021, 113, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R. Thrombosis after COVID-19 vaccination. BMJ 2021, 373, n958. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, R.F.; Ligresti, G.; Caporarello, N.; Akilesh, S.; Ribatti, D. COVID-19 Vasculopathy: Mounting Evidence for an Indirect Mechanism of Endothelial Injury. Am. J. Pathol. 2021, 191, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Coppens, M. Vascular mechanisms and manifestations of COVID-19. Lancet Respir. Med. 2021, 9, 551–553. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Martines, R.B.; Ritter, J.M.; Matkovic, E.; Gary, J.; Bollweg, B.C.; Bullock, H.; Goldsmith, C.S.; Silva-Flannery, L.; Seixas, J.N.; Reagan-Steiner, S.; et al. Pathology and Pathogenesis of SARS-CoV-2 Associated with Fatal Coronavirus Disease, United States. Emerg. Infect. Dis. 2020, 26, 2005–2015. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Magro, C.M.; Mulvey, J.; Kubiak, J.; Mikhail, S.; Suster, D.; Crowson, A.N.; Laurence, J.; Nuovo, G. Severe COVID-19: A multifaceted viral vasculopathy syndrome. Ann. Diagn. Pathol. 2020, 50, 151645. [Google Scholar] [CrossRef]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, J.; Gary, J.; Reagan-Steiner, S.; Estetter, L.B.; Tong, S.; Tao, Y.; Denison, A.M.; Lee, E.; DeLeon-Carnes, M.; Li, Y.; et al. Evidence of Severe Acute Respiratory Syndrome Coronavirus 2 Replication and Tropism in the Lungs, Airways, and Vascular Endothelium of Patients with Fatal Coronavirus Disease 2019: An Autopsy Case Series. J. Infect. Dis. 2021, 223, 752–764. [Google Scholar] [CrossRef]
- Scudellari, M. How the coronavirus infects cells and why Delta is so dangerous. Nature 2021, 595, 640–644. [Google Scholar] [CrossRef]
- Akilesh, S.; Nicosia, R.F.; Alpers, C.E.; Tretiakova, M.; Hsiang, T.-Y.; Gale, M.; Smith, K.D. Characterizing Viral Infection by Electron Microscopy: Lessons from the coronavirus disease 2019 pandemic. Am. J. Pathol. 2021, 191, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Dittmayer, C.; Meinhardt, J.; Radbruch, H.; Radke, J.; Happner, B.I.; Happner, F.L.; Stenzel, W.; Holland, G.; Laue, M. Why misin-terpretation of electron micrographs in SARS-CoV-2-infected tissue goes viral. Lancet 2020, 396, e64–e65. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- McCracken, I.R.; Saginc, G.; He, L.; Huseynov, A.; Daniels, A.; Fletcher, S.; Peghaire, C.; Kalna, V.; Andaloussi-Mäe, M.; Muhl, L.; et al. Lack of Evidence of Angiotensin-Converting Enzyme 2 Expression and Replicative Infection by SARS-CoV-2 in Human Endothelial Cells. Circulation 2021, 143, 865–868. [Google Scholar] [CrossRef]
- Singh, M.; Bansal, V.; Feschotte, C. A Single-Cell RNA Expression Map of Human Coronavirus Entry Factors. Cell Rep. 2020, 32, 108175. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 202, 756–759. [Google Scholar] [CrossRef]
- Conde, J.N.; Schutt, W.R.; Gorbunova, E.E.; Mackow, E.R. Recombinant ACE2 Expression Is Required for SARS-CoV-2 To Infect Primary Human Endothelial Cells and Induce Inflammatory and Procoagulative Responses. mBio 2020, 11. [Google Scholar] [CrossRef]
- Huang, J.; Hume, A.J.; Abo, K.M.; Werder, R.B.; Villacorta-Martin, C.; Alysandratos, K.-D.; Beermann, M.L.; Simone-Roach, C.; Lindstrom-Vautrin, J.; Olejnik, J.; et al. SARS-CoV-2 Infection of Pluripotent Stem Cell-Derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response. Cell Stem Cell 2020, 27, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Angriman, F.; Ferreyro, B.L.; Burry, L.; Fan, E.; Ferguson, N.D.; Husain, S.; Keshavjee, S.H.; Lupia, E.; Munshi, L.; Renzi, S.; et al. Interleukin-6 recep-tor blockade in patients with COVID-19: Placing clinical trials into context. Lancet Respir. Med. 2021, 9, 655–664. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Lei, T.; Patel, P.S.; Lee, C.H.; Monaghan-Nichols, P.; Xin, H.-B.; Qiu, J.; Fu, M. Direct Activation of Endothelial Cells by SARS-CoV-2 Nucleocapsid Protein Is Blocked by Simvastatin. J. Virol. 2021, 95, e0139621. [Google Scholar] [CrossRef] [PubMed]
- Flaumenhaft, R.; Enjyoji, K.; Schmaier, A.A. Vasculopathy in COVID-19. Blood 2022. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S. Cutaneous manifestations in COVID-19: A first perspective. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e212–e213. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.E.; McMahon, D.E.; Lipoff, J.B.; Rosenbach, M.; Kovarik, C.; Desai, S.R.; Harp, J.; Takeshita, J.; French, L.E.; Lim, H.W.; et al. The spectrum of COVID-19–associated dermatologic manifestations: An international registry of 716 patients from 31 countries. J. Am. Acad. Dermatol. 2020, 83, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, I.; Santonja, C.; Alonso-Riaño, M.; Noguera-Morel, L.; Hernández-Martín, A.; Andina, D.; Wiesner, T.; Rodríguez-Peralto, J.; Requena, L.; Torrelo, A. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: Histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br. J. Dermatol. 2020, 183, 729–737. [Google Scholar] [CrossRef]
- Mazzotta, F.; Troccoli, T. Acute acro-ischemia in the child at the time of COVID-19. Eur. J. Pediat. Dermatol. 2020, 30, 71–74. [Google Scholar] [CrossRef]
- Colonna, C.; Genovese, G.; Monzani, N.A.; Picca, M.; Boggio, F.; Gianotti, R.; Marzano, A.V. Outbreak of chilblain-like acral lesions in children in the metropolitan area of Milan, Italy, during the COVID-19 pandemic. J. Am. Acad. Dermatol. 2020, 83, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Nieto, D.; Jimenez-Cauhe, J.; Suarez-Valle, A.; Moreno-Arrones, O.M.; Saceda-Corralo, D.; Arana-Raja, A.; Ortega-Quijano, D. Characterization of acute acral skin lesions in nonhospitalized patients: A case series of 132 patients during the COVID-19 outbreak. J. Am. Acad. Dermatol. 2020, 83, e61–e63. [Google Scholar] [CrossRef] [PubMed]
- Kanitakis, J.; Lesort, C.; Danset, M.; Jullien, D. Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): Histologic, immunofluorescence, and immunohistochemical study of 17 cases. J. Am. Acad. Dermatol. 2020, 83, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Manalo, I.F.; Smith, M.K.; Cheeley, J.; Jacobs, R. A dermatologic manifestation of COVID-19: Transient livedo reticularis. J. Am. Acad. Dermatol. 2020, 83, 700. [Google Scholar] [CrossRef]
- Sahara, T.; Yokota, K. Livedo Reticularis Associated with COVID-19. Intern. Med. 2022, 61, 441. [Google Scholar] [CrossRef]
- Tusheva, I.; Damevska, K.; Dimitrovska, I.; Markovska, Z.; Malinovska-Nikolovska, L. Unilateral livedo reticularis in a COVID-19 patient: Case with fatal outcome. JAAD Case Rep. 2021, 7, 120–121. [Google Scholar] [CrossRef]
- Khalil, S.; Hinds, B.R.; Manalo, I.F.; Vargas, I.M.; Mallela, S.; Jacobs, R. Livedo reticularis as a presenting sign of severe acute respiratory syndrome coronavirus 2 infection. JAAD Case Rep. 2020, 6, 871–874. [Google Scholar] [CrossRef]
- Chibane, S.; Gibeau, G.; Poulin, F.; Tessier, P.; Goulet, M.; Carrier, M.; Lanthier, S. Hyperacute multi-organ thromboembolic storm in COVID-19: A case report. J. Thromb. Thrombolysis 2020, 51, 25–28. [Google Scholar] [CrossRef]
- Favia, G.; Tempesta, A.; Barile, G.; Brienza, N.; Capodiferro, S.; Vestito, M.C.; Crudele, L.; Procacci, V.; Ingravallo, G.; Maiorano, E.; et al. COVID-19 Symptomatic Patients with Oral Lesions: Clinical and Histopathological Study on 123 Cases of the University Hospital Policlinic of Bari with a Purpose of a New Classification. J. Clin. Med. 2021, 10, 757. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Hübner, D.; Marx, G.; Bickenbach, J.; Bootsveld, A. Primary presentation of COVID-19 solely with gastrointestinal symptoms: A problem for the containment of the disease. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1475–1478. [Google Scholar] [CrossRef]
- Li, T.; Lu, H.; Zhang, W. Clinical observation and management of COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 687–690. [Google Scholar] [CrossRef]
- Zhou, B.; She, J.; Wang, Y.; Ma, X. Venous thrombosis and arteriosclerosis obliterans of lower extremities in a very severe patient with 2019 novel coronavirus disease: A case report. J. Thromb. Thrombolysis 2020, 50, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, R.; Luzzani, L.; Natalini, G.; Pegorer, M.A.; Attisani, L.; Cossu, L.G.; Ferrandina, C.; Fossati, A.; Conti, E.; Bush, R.L.; et al. Acute limb ischemia in patients with COVID-19 pneumonia. J. Vasc. Surg. 2020, 72, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Galyfos, G.; Sianou, A.; Frountzas, M.; Vasilios, K.; Vouros, D.; Theodoropoulos, C.; Michalopoulou, V.; Sigala, F.; Filis, K. Acute limb ischemia among patients with COVID-19 infection. J. Vasc. Surg. 2021, 75, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippi, G.; Favaloro, E.J. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb. Haemost. 2020, 120, 876–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhao, K.; Wei, H.; Chen, W.; Wang, W.; Jia, L.; Liu, Q.; Zhang, J.; Shan, T.; Peng, Z.; et al. Dynamic relationship between D-dimer and COVID-19 severity. Br. J. Haematol. 2020, 190, e24–e27. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Ozen, M.; Yilmaz, A.; Cakmak, V.; Beyoglu, R.; Oskay, A.; Seyit, M.; Senol, H. D-Dimer as a potential biomarker for disease severity in COVID-19. Am. J. Emerg. Med. 2021, 40, 55–59. [Google Scholar] [CrossRef]
- Sakka, M.; Connors, J.; Hékimian, G.; Martin-Toutain, I.; Crichi, B.; Colmegna, I.; Bonnefont-Rousselot, D.; Farge, D.; Frere, C. Association between D-Dimer levels and mortality in patients with coronavirus disease 2019 (COVID-19): A systematic review and pooled analysis. J. Med. Vasc. 2020, 45, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; Wehmeyer, G.T.; Li, H.A.; Alshak, M.N.; Nahid, M.; Rajan, M.; Liu, B.; Schatoff, E.M.; Elahjji, R.; Abdelghany, Y.; et al. D-dimer cut-off points and risk of venous thromboembolism in adult hospitalized patients with COVID-19. Thromb. Res. 2020, 196, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.O.; Jensen, A.; Khan, M.; Chin, J.; Chin, K.; Saad, J.; Parnell, R.; Awwad, C.; Patel, D. Pulmonary Embolism and Increased Levels of d-Dimer in Patients with Coronavirus Disease. Emerg. Infect. Dis. 2020, 26, 1941–1943. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. The unique characteristics of COVID-19 coagulopathy. Crit. Care 2020, 24, 360. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of Coronavirus Disease 2019. Crit. Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef]
- Levi, M. Pathophysiology of Coagulopathy in Hematological Malignancies and in COVID-19. HemaSphere 2021, 5, e571. [Google Scholar] [CrossRef]
- Kermali, M.; Khalsa, R.K.; Pillai, K.; Ismail, Z.; Harky, A. The role of biomarkers in diagnosis of COVID-19—A systematic review. Life Sci. 2020, 254, 117788. [Google Scholar] [CrossRef]
- Liao, D.; Zhou, F.; Luo, L.; Xu, M.; Wang, H.; Xia, J.; Gao, Y.; Cai, L.; Wang, Z.; Yin, P.; et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol. 2020, 7, e671–e678. [Google Scholar] [CrossRef]
- Zeng, F.; Huang, Y.; Guo, Y.; Yin, M.; Chen, X.; Xiao, L.; Deng, G. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int. J. Infect. Dis. 2020, 96, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Torretta, E.; Garziano, M.; Poliseno, M.; Capitanio, D.; Biasin, M.; Santantonio, T.A.; Clerici, M.; Caputo, S.L.; Trabattoni, D.; Gelfi, C. Severity of COVID-19 Patients Predicted by Serum Sphingolipids Signature. Int. J. Mol. Sci. 2021, 22, 10198. [Google Scholar] [CrossRef] [PubMed]
- Palacios, Y.; Ruiz, A.; Ramón-Luing, L.; Ocaña-Guzman, R.; Barreto-Rodriguez, O.; Sánchez-Monciváis, A.; Tecuatzi-Cadena, B.; Regalado-García, A.; Pineda-Gudiño, R.; García-Martínez, A.; et al. Severe COVID-19 Patients Show an Increase in Soluble TNFR1 and ADAM17, with a Relationship to Mortality. Int. J. Mol. Sci. 2021, 22, 8423. [Google Scholar] [CrossRef] [PubMed]
- Tamara, A.; Tahapary, D.L. Obesity as a predictor for a poor prognosis of COVID-19: A systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Di Lecce, V.; Quaranta, V.N.; Zito, A.; Buonamico, E.; Capozza, E.; Palumbo, A.; Di Gioia, G.; Valerio, V.N.; Resta, O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Investig. 2021, 44, 765–771. [Google Scholar] [CrossRef]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Natarelli, L.; Virgili, F.; Weber, C. SARS-CoV-2, Cardiovascular Diseases, and Noncoding RNAs: A Connected Triad. Int. J. Mol. Sci. 2021, 22, 12243. [Google Scholar] [CrossRef]
- Song, X.; Ji, J.; Reva, B.; Joshi, H.; Calinawan, A.P.; Mazumdar, M.; Wisnivesky, J.P.; Taioli, E.; Wang, P.; Veluswamy, R.R. Post-anticoagulant D-dimer is a highly prognostic biomarker of COVID-19 mortality. ERJ Open Res. 2021, 7, 00018–02021. [Google Scholar] [CrossRef]
- Long, H.; Nie, L.; Xiang, X.; Li, H.; Zhang, X.; Fu, X.; Ren, H.; Liu, W.; Wang, Q.; Wu, Q. D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis. BioMed Res. Int. 2020, 2020, 6159720. [Google Scholar] [CrossRef] [PubMed]
- Gazzaruso, C.; Paolozzi, E.; Valenti, C.; Brocchetta, M.; Naldani, D.; Grignani, C.; Salvucci, F.; Marino, F.; Coppola, A.; Gallotti, P. Association between antithrombin and mortality in patients with COVID-19. A possible link with obesity. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Bazzan, M.; Montaruli, B.; Sciascia, S.; Cosseddu, D.; Norbiato, C.; Roccatello, D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern. Emerg. Med. 2021, 15, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Ladikou, E.E.; Sivaloganathan, H.; Milne, K.M.; Arter, W.E.; Ramasamy, R.; Saad, R.; Stoneham, S.M.; Philips, B.; Eziefula, A.C.; Chevassut, T. Von Willebrand factor (vWF): Marker of endothelial damage and thrombotic risk in COVID-19? Clin. Med. 2020, 20, e178–e182. [Google Scholar] [CrossRef]
- Joly, B.S.; Darmon, M.; Dekimpe, C.; Dupont, T.; Dumas, G.; Yvin, E.; Beranger, N.; Vanhoorelbeke, K.; Azoulay, E.; Veyradier, A. Imbalance of von Willebrand factor and ADAMTS13 axis is rather a biomarker of strong inflammation and endothelial damage than a cause of thrombotic process in critically ill COVID-19 patients. J. Thromb. Haemost. 2021, 19, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Kanno, H.; Zhou, Y.; Xiao, T.-H.; Suzuki, T.; Ibayashi, Y.; Harmon, J.; Takizawa, S.; Hiramatsu, K.; Nitta, N.; et al. Massive image-based single-cell profiling reveals high levels of circulating platelet aggregates in patients with COVID-19. Nat. Commun. 2021, 12, 7135. [Google Scholar] [CrossRef]
- Barrett, T.J.; Bilaloglu, S.; Cornwell, M.; Burgess, H.M.; Virginio, V.W.; Drenkova, K.; Ibrahim, H.; Yuriditsky, E.; Aphinyanaphongs, Y.; Lifshitz, M.; et al. Platelets contribute to disease severity in COVID-19. J. Thromb. Haemost. 2021, 19, 3139–3153. [Google Scholar] [CrossRef]
- Cohen, A.; Harari, E.; Yahud, E.; Cipok, M.; Bryk, G.; Lador, N.K.; Mann, T.; Mayo, A.; Lev, E.I. Immature platelets in patients with Covid-19: Association with disease severity. J. Thromb. Thrombolysis 2021, 52, 708–714. [Google Scholar] [CrossRef]
- Devreese, K.M.J.; Linskens, E.A.; Benoit, D.; Peperstraete, H. Antiphospholipid antibodies in patients with COVID-19: A relevant observation? J. Thromb. Haemost. 2020, 18, 2191–2201. [Google Scholar] [CrossRef]
- Dandu, H.; Yadav, G.; Malhotra, H.S.; Pandey, S.; Sachu, R.; Dubey, K. Hemophagocytic histiocytosis in severe SARS-CoV-2 infection: A bone marrow study. Int. J. Lab. Hematol. 2021, 43, 1291–1301. [Google Scholar] [CrossRef]
- Tang, L.V.; Hu, Y. Hemophagocytic lymphohistiocytosis after COVID-19 vaccination. J. Hematol. Oncol. 2021, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Maruyama, Y.; Satokawa, H.; Nishimoto, Y.; Tsujino, I.; Sakashita, H.; Nakata, H.; Okuno, Y.; Ogihara, Y.; Yachi, S.; et al. Incidence and Clinical Features of Venous Thromboembolism in Hospitalized Patients with Coronavirus Disease 2019 (COVID-19) in Japan. Circ. J. 2021, 85, 2208–2214. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Brown, J.Q.; Heide, R.S.V. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Lax, S.F.; Skok, K.; Zechner, P.; Kessler, H.H.; Kaufmann, N.; Koelblinger, C.; Vander, K.; Bargfrieder, U.; Trauner, M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome: Results from a prospective, single-center, clinico-pathologic case series. Ann. Intern. Med. 2020, 173, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Francischetti, I.M.; Toomer, K.; Zhang, Y.; Jani, J.; Siddiqui, Z.; Brotman, D.J.; Hooper, J.E.; Kickler, T.S. Upregulation of pulmonary tissue factor, loss of thrombomodulin and immunothrombosis in SARS-CoV-2 infection. EClinicalMedicine 2021, 39, 101069. [Google Scholar] [CrossRef]
- McGonagle, D.; Plein, S.; O’Donnell, J.S.; Sharif, K.; Bridgewood, C. Increased cardiovascular mortality in African Americans with COVID-19. Lancet Respir. Med. 2020, 8, 649–651. [Google Scholar] [CrossRef]
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445. [Google Scholar] [CrossRef]
- Belen-Apak, F.B.; Sarıalioğlu, F. Pulmonary intravascular coagulation in COVID-19: Possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J. Thromb. Thrombolysis 2020, 50, 278–280. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H. Classifying types of disseminated intravascular coagulation: Clinical and animal models. J. Intensiv. Care 2014, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikura, H.; Maruyama, J.; Irie, Y.; Izutani, Y.; Naito, M.; Koie, M.; Hoshino, K.; Nakamura, Y. Characteristics of coagula-tion/fibrinolysis abnormalities in severe novel coronavirus disease 2019 (COVID-19) patients-Case series. Jpn. J. Thromb. Haemost. 2020, 31, 398–408. [Google Scholar] [CrossRef]
- Asakura, H.; Ogawa, H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 2021, 113, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Kadohira, Y.; Yamada, S.; Matsuura, E.; Hayashi, T.; Morishita, E.; Nakao, S.; Asakura, H. Aortic Aneurysm-associated Disseminated Intravascular Coagulation that Responded Well to a Switch from Warfarin to Rivaroxaban. Intern. Med. 2017, 56, 2913–2917. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Okumura, H.; Morishita, E.; Asakura, H. Complete hemostasis achieved by factor XIII concentrate administration in a patient with bleeding after teeth extraction as a complication of aplastic anemia and chronic disseminated intravascular coagulation. Blood Coagul. Fibrinolysis 2020, 31, 274–278. [Google Scholar] [CrossRef]
- Yamada, S.; Arahata, M.; Morishita, E.; Asakura, H. Blue Rubber Bleb Nevus Syndrome Complicated by Enhanced-Fibrinolytic-Type DIC: A Case Report. Ann. Vasc. Dis. 2021, 14, 252–255. [Google Scholar] [CrossRef]
- Hayakawa, M.; Takano, K.; Kayashima, M.; Kasahara, K.; Fukushima, H.; Matsumoto, M. Management of a COVID-19 Patient during ECMO: Paying Attention to Acquired von Willebrand Syndrome. J. Atheroscler. Thromb. 2021, 28, 396–401. [Google Scholar] [CrossRef]
- Yamada, S.; Ogawa, H.; Asakura, H. Etiology and Management of Bleeding during ECMO in a COVID-19 Patient. J. Atheroscler. Thromb. 2021, 28, 402–403. [Google Scholar] [CrossRef]
- Asakura, H. Diversity of disseminated intravascular coagulation and selection of appropriate treatments. Int. J. Hematol. 2021, 113, 10–14. [Google Scholar] [CrossRef] [PubMed]
- ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators; Lawler, P.R.; Goligher, E.C.; Berger, J.S. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar] [CrossRef] [PubMed]
- REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators; Goligher, E.C.; Bradbury, C.A.; McVerry, B.J. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 385, 777–789. [Google Scholar] [CrossRef]
- Yamada, S.; Asakura, H. Management of disseminated intravascular coagulation associated with aortic aneurysm and vascular malformations. Int. J. Hematol. 2020, 113, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kalbhenn, J.; Schmidt, R.; Nakamura, L.; Schelling, J.; Rosenfelder, S.; Zieger, B. Early Diagnosis of Acquired von Willebrand Syndrome (AVWS) is Elementary for Clinical Practice in Patients Treated with ECMO Therapy. J. Atheroscler. Thromb. 2015, 22, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Kalbhenn, J.; Schlagenhauf, A.; Rosenfelder, S.; Schmutz, A.; Zieger, B. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J. Heart Lung Transplant. 2018, 37, 985–991. [Google Scholar] [CrossRef]
- Asakura, H.; Ogawa, H. Overcoming bleeding events related to extracorporeal membrane oxygenation in COVID-19. Lancet Respir. Med. 2020, 8, e87–e88. [Google Scholar] [CrossRef]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Mezgebe, M.; Jacobson, B.F.; Mayne, E.S.; Low, S. Change in platelet indices in patients with coro-navirus disease-2019 (COVID-19): A reflection of platelet activation and contribution to immuno-thrombosis? Int. J. Lab. Hematol. 2021, 44, e46–e48. [Google Scholar] [CrossRef]
- Sisinni, A.; Rossi, L.; Battista, A.; Poletti, E.; Battista, F.; Battista, R.A.; Malagoli, A.; Biagi, A.; Zanni, A.; Sticozzi, C.; et al. Pre-admission acetylsalicylic acid therapy and impact on in-hospital outcome in COVID-19 patients: The ASA-CARE study. Int. J. Cardiol. 2021, 344, 240–245. [Google Scholar] [CrossRef]
- Santoro, F.; Nuñez-Gil, I.J.; Vitale, E.; Viana-Llamas, M.C.; Reche-Martinez, B.; Romero-Pareja, R.; Guzman, G.F.; Rozas, I.F.; Uribarri, A.; Becerra-Muñoz, V.M.; et al. Antiplatelet therapy and outcome in COVID-19: The Health Outcome Predictive Evaluation Registry. Heart 2022, 108, 130–136. [Google Scholar] [CrossRef]
- Chow, J.H.; Yin, Y.; Yamane, D.P.; Davison, D.; Keneally, R.J.; Hawkins, K.; Parr, K.G.; Al-Mashat, M.; Berger, J.S.; Bushardt, R.L.; et al. Association of prehospital antiplatelet therapy with survival in patients hospitalized with COVID-19: A propensity score-matched analysis. J. Thromb. Haemost. 2021, 19, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2022, 399, 143–151. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. Meta-Analysis of the Effect of Aspirin on Mortality in COVID-19. Am. J. Cardiol. 2021, 142, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ao, G.; Nasr, B.; Qi, X. Effect of antiplatelet treatments on patients with COVID-19 infection: A systematic review and meta-analysis. Am. J. Emerg. Med. 2021, 43, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Yoon, S.; Kim, M.; Lee, H.; Park, S.; Kim, W.; Lee, S. Aspirin Is Related to Worse Clinical Outcomes of COVID-19. Medicina 2021, 57, 931. [Google Scholar] [CrossRef]
- Weiler, J.M.; Edens, R.E.; Linhardt, R.J.; Kapelanski, D.P. Heparin and modified heparin inhibit complement activation in vivo. J. Immunol. 1992, 148, 3210–3215. [Google Scholar]
- Linhardt, R.J.; Rice, K.G.; Kim, Y.S.; Engelken, J.D.; Weiler, J.M. Homogeneous, structurally defined heparin-oligosaccharides with low anticoagulant activity inhibit the generation of the amplification pathway C3 convertase in vitro. J. Biol. Chem. 1998, 263, 13090–13096. [Google Scholar] [CrossRef]
- Proudfoot, A.E.I.; Fritchley, S.; Borlat, F.; Shaw, J.P.; Vilbois, F.; Zwahlen, C.; Trkola, A.; Marchant, D.; Clapham, P.R.; Wells, T. The BBXB Motif of RANTES Is the Principal Site for Heparin Binding and Controls Receptor Selectivity. J. Biol. Chem. 2001, 276, 10620–10626. [Google Scholar] [CrossRef] [Green Version]
- Kuschert, G.S.V.; Coulin, F.; Power, C.A.; Proudfoot, A.E.I.; Hubbard, R.E.; Hoogewerf, A.J.; Wells, T.N.C. Glycosaminoglycans Interact Selectively with Chemokines and Modulate Receptor Binding and Cellular Responses. Biochemistry 1999, 38, 12959–12968. [Google Scholar] [CrossRef]
- Baba, M.; Pauwels, R.; Balzarini, J.; Arnout, J.; Desmyter, J.; De Clercq, E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 1988, 85, 6132–6136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lederman, S.; Gulick, R.; Chess, L. Dextran sulfate and heparin interact with CD4 molecules to in-hibit the binding of coat protein (gp120) of HIV. J. Immunol. Immunol. 1989, 143, 1149–1154. [Google Scholar]
- Hu, Q.-Y.; Fink, E.; Grant, C.K.; Elder, J.H. Selective Interaction of Heparin with the Variable Region 3 within Surface Glycoprotein of Laboratory-Adapted Feline Immunodeficiency Virus. PLoS ONE 2014, 9, e115252. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Ogawa, H. Potential of heparin and nafamostat combination therapy for COVID-19. J. Thromb. Haemost. 2020, 18, 1521–1522. [Google Scholar] [CrossRef]
- Turshudzhyan, A. Anticoagulation Options for Coronavirus Disease 2019 (COVID-19)-Induced Coagulopathy. Cureus 2020, 12, e8150. [Google Scholar] [CrossRef]
- Takahashi, W.; Yoneda, T.; Koba, H.; Ueda, T.; Tsuji, N.; Ogawa, H.; Asakura, H. Potential mechanisms of nafamostat therapy for severe COVID-19 pneumonia with disseminated intravascular coagulation. Int. J. Infect. Dis. 2021, 102, 529–531. [Google Scholar] [CrossRef]
- Doi, K.; the COVID-UTH Study Group; Ikeda, M.; Hayase, N.; Moriya, K.; Morimura, N. Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: A case series. Crit. Care 2020, 24, 392. [Google Scholar] [CrossRef]
- Yamada, S.; Asakura, H. Therapeutic Strategies for Disseminated Intravascular Coagulation Associated with Aortic Aneurysm. Int. J. Mol. Sci. 2022, 23, 2396. [Google Scholar] [CrossRef]
- Coutard, B.; Valle, C.; de Lamballerie, X.; Canard, B.; Seidah, N.G.; Decroly, E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020, 176, 104742. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kiso, M.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Takeda, M.; Kinoshita, N.; Ohmagari, N.; Gohda, J.; Semba, K.; et al. The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner. Viruses 2020, 12, 629. [Google Scholar] [CrossRef]
- Ragia, G.; Manolopoulos, V.G. Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: A promising approach for uncovering early COVID-19 drug therapies. Eur. J. Clin. Pharmacol. 2020, 76, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Chakrabarti, S.S.; Ojha, B.; Pathak, B.K.; Singh, A.; Saso, L.; Chakrabarti, S. Targeting Host Cell Proteases to Prevent SARS-CoV-2 Invasion. Curr. Drug Targets 2021, 22, 192–201. [Google Scholar] [CrossRef]
- Hoffmann, M.; Schroeder, S.; Kleine-Weber, H.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuravel, S.V.; Khmelnitskiy, O.K.; Burlaka, O.O.; Gritsan, A.I.; Goloshchekin, B.M.; Kim, S.; Hong, K.Y. Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: A randomised Phase II clinical trial. eClinicalMedicine 2021, 41, 101169. [Google Scholar] [CrossRef] [PubMed]
- Muto, S.; Imai, M.; Asano, Y. Mechanisms of hyperkalemia caused by nafamostat mesilate. Gen. Pharmacol. Vasc. Syst. 1995, 26, 1627–1632. [Google Scholar] [CrossRef]
- Okajima, M.; Takahashi, Y.; Kaji, T.; Ogawa, N.; Mouri, H. Nafamostat mesylate-induced hyperkalemia in critically ill patients with COVID-19: Four case reports. World J. Clin. Cases 2020, 8, 5320–5325. [Google Scholar] [CrossRef]
- Talasaz, A.H.; Sadeghipour, P.; Aghakouchakzadeh, M.; Kakavand, H.; Ariannejad, H.; Connors, J.M.; Hunt, B.J.; Berger, J.S.; Van Tassell, B.W.; Middeldorp, S.; et al. Use of novel antithrombotic agents for COVID-19: Systematic summary of ongoing randomized controlled trials. J. Thromb. Haemost. 2021, 19, 3080–3089. [Google Scholar] [CrossRef]
- Patell, R.; Bogue, T.; Koshy, A.; Bindal, P.; Merrill, M.; Aird, W.C.; Bauer, K.A.; Zwicker, J.I. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood 2020, 136, 1342–1346. [Google Scholar] [CrossRef]
- Roberts, L.N.; Whyte, M.B.; Georgiou, L.; Giron, G.; Czuprynska, J.; Rea, C.; Vadher, B.; Patel, R.K.; Gee, E.; Arya, R. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood 2020, 136, 1347–1350. [Google Scholar] [CrossRef]
- Giannis, D.; Allen, S.L.; Tsang, J.; Flint, S.; Pinhasov, T.; Williams, S.; Tan, G.; Thakur, R.; Leung, C.; Snyder, M.; et al. Postdischarge throm-boembolic outcomes and mortality of hospitalized patients with COVID-19: The CORE-19 registry. Blood 2021, 137, 2838–2847. [Google Scholar] [CrossRef]
- Ramacciotti, E.; Agati, L.B.; Calderaro, D.; Aguiar, V.C.R.; Spyropoulos, A.C.; de Oliveira, C.C.C.; dos Santos, J.L.; Volpiani, G.G.; Sobreira, M.L.; Joviliano, E.E.; et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): An open-label, multicentre, randomised, controlled trial. Lancet 2021, 399, 50–59. [Google Scholar] [CrossRef]
- Testa, S.; Prandoni, P.; Paoletti, O.; Morandini, R.; Tala, M.; Dellanoce, C.; Giorgi-Pierfranceschi, M.; Betti, M.; Danzi, G.B.; Pan, A.; et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: The Cremona experience. J. Thromb. Haemost. 2020, 18, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; de Barros, E.; Silva, P.G.M.; Furtado, R.H.M.; Macedo, A.V.S.; Bronhara, B.; Damiani, L.P.; Barbosa, L.M.; de Aveiro Morata, J.; Ramacciotti, E.; et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): An open-label, multicentre, randomised, controlled trial. Lancet 2021, 397, 2253–2263. [Google Scholar] [CrossRef]
- Dunois, C. Laboratory Monitoring of Direct Oral Anticoagulants (DOACs). Biomedicines 2021, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hajizadeh, N.; Moore, E.E.; McIntyre, R.C.; Moore, P.; Veress, L.A.; Yaffe, M.B.; Moore, H.B.; Barrett, C.D. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J. Thromb. Haemost. 2020, 18, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.; Barrett, C.D.; Moore, H.B.; Moore, E.E.; McIntyre, R.C.; Moore, P.K.; Talmor, D.S.; Nydam, T.L.; Yaffe, M.B. Salvage use of tissue plasminogen activator (tPA) in the setting of acute respiratory distress syndrome (ARDS) due to COVID-19 in the USA: A Markov decision analysis. World J. Emerg. Surg. 2020, 15, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, D.B.; Nemec, H.M.; Scott, A.M.; Buchanan, J.T.; Franklin, C.M.; Ahmed, A.; Khan, M.S.; Callender, C.W.; James, E.A.; Christie, A.B.; et al. Early outcomes with utilization of tissue plasminogen activator in COVID-19–associated respiratory distress: A series of five cases. J. Trauma Acute Care Surg. 2020, 89, 448–452. [Google Scholar] [CrossRef]
- Maier, C.L.; Sarker, T.; Szlam, F.; Sniecinski, R.M. COVID-19 patient plasma demonstrates resistance to tPA-induced fibrinolysis as measured by thromboelastography. J. Thromb. Thrombolysis 2021, 52, 766–771. [Google Scholar] [CrossRef]
- Jin, X.; Duan, Y.; Bao, T.; Gu, J.; Chen, Y.; Li, Y.; Mao, S.; Chen, Y.; Xie, W. The values of coagulation function in COVID-19 patients. PLoS ONE 2020, 15, e0241329. [Google Scholar] [CrossRef]
- Whyte, C.S.; Morrow, G.B.; Mitchell, J.L.; Chowdary, P.; Mutch, N.J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J. Thromb. Haemost. 2020, 18, 1548–1555. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T.; Guo, C.; Zhang, D.; Ge, X.; Huang, Z.; Zhou, X.; Li, Y.; Peng, Q.; Li, J. Plasminogen improves lung lesions and hypoxemia in patients with COVID-19. QJM Int. J. Med. 2020, 113, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Ogawa, H. Perspective on fibrinolytic therapy in COVID-19: The potential of inhalation therapy against suppressed-fibrinolytic-type DIC. J. Intensiv. Care 2020, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- van Haren, F.M.P.; van Loon, L.M.; Steins, A.; Smoot, T.L.; Sas, C.; Staas, S.; Vilaseca, A.B.; Barbera, R.A.; Vidmar, G.; Beccari, H.; et al. Inhaled nebulised unfractionated heparin for the treatment of hospitalised patients with COVID-19: A multicentre case series of 98 patients. Br. J. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Erelel, M.; Kaskal, M.; Akbal-Dagistan, O.; Issever, H.; Dagistanli, A.S.; Balkanci, H.; Oguz, M.S.; Qarayeva, A.; Culha, M.; Erturk, A.; et al. Early Effects of Low Molecular Weight Heparin Therapy with Soft-Mist Inhaler for COVID-19-Induced Hypoxemia: A Phase IIb Trial. Pharmaceutics 2021, 13, 1768. [Google Scholar] [CrossRef]
- Agarwal, R.N.; Aggarwal, H.; Verma, A.; Tripathi, M.K. A Case Report of a Patient on Therapeutic Warfarin Who Died of COVID-19 Infection with a Sudden Rise in D-Dimer. Biomedicines 2021, 9, 1382. [Google Scholar] [CrossRef]
- Garg, A.; Goyal, S.; Patel, P. A Case of COVID-19 Infection with Delayed Thromboembolic Complication on Warfarin. Cureus 2020, 12, e8847. [Google Scholar] [CrossRef]
- Irwin, M.N.; Adie, S.; Sandison, K.; Alsomairy, S.A.; Brancaccio, A. Warfarin Dose Requirements in Adults Hospitalized With COVID-19 Infection: A Retrospective Case Series. J. Pharm. Pract. 2021. [Google Scholar] [CrossRef]
- Landayan, R.P.; Saint-Felix, S.; Williams, A. Probable Interaction Between Warfarin and the Combination of Remdesivir with Dexamethasone for Coronavirus Disease 2019 (COVID-19) Treatment: A2 Case Report. J. Pharm. Pract. 2021. [Google Scholar] [CrossRef]
- Barker, A.B.; Wagener, B.M. An Ounce of Prevention May Prevent Hospitalization. Physiol. Rev. 2020, 100, 1347–1348. [Google Scholar] [CrossRef]
- Thierry, A.R. Anti-protease Treatments Targeting Plasmin(ogen) and Neutrophil Elastase May Be Beneficial in Fighting COVID-19. Physiol. Rev. 2020, 100, 1597–1598. [Google Scholar] [CrossRef]
- Ogawa, H.; Asakura, H. Consideration of Tranexamic Acid Administration to COVID-19 Patients. Physiol. Rev. 2020, 100, 1595–1596. [Google Scholar] [CrossRef] [PubMed]
- Perrin, G.; Le Beller, C.; Darnige, L.; Khider, L.; Smadja, D.M.; Louet, A.L.-L.; Planquette, B.; Lebeaux, D.; Sanchez, O.; Sabatier, B.; et al. Intramuscular Vaccination in Adults with Therapeutic Anticoagulation in the Era of COVID-19 Vaccines Outbreak: A Practical Review. TH Open 2021, 5, e166–e170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Portuguese, A.J.; Weatherford, A.; Garcia, D.; Gernsheimer, T. Platelet trends after Covid-19 vaccination in patients with chronic or persistent immune thrombocytopenia. Am. J. Hematol. 2021, 96, E472–E474. [Google Scholar] [CrossRef] [PubMed]
- Visser, C.; Swinkels, M.; van Werkhoven, E.D.; Croles, F.N.N.; Noordzij-Nooteboom, H.S.; Eefting, M.; Last-Koopmans, S.M.; Idink, C.; Westerweel, P.E.; Santbergen, B.; et al. COVID-19 vaccination in patients with immune thrombocytopenia. Blood Adv. 2022, 6, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Beltrami-Moreira, M.; Al-Samkari, H.; Cuker, A.; DiRaimo, J.; Gernsheimer, T.; Kruse, A.; Kessler, C.M.; Kruse, C.; Leavitt, A.D.; et al. SARS-CoV-2 vaccination and ITP in patients with de novo or preexisting ITP. Blood 2022, 139, 1564–1574. [Google Scholar] [CrossRef]
- Crickx, E.; Moulis, G.; Ebbo, M.; Terriou, L.; Briantais, A.; Languille, L.; Limal, N.; Guillet, S.; Michel, M.; Mahevas, M.; et al. Safety of anti-SARS-CoV-2 vaccination for patients with immune thrombocytopenia. Br. J. Haematol. 2021, 195, 703–705. [Google Scholar] [CrossRef]
- Kuter, D.J. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br. J. Haematol. 2021, 195, 365–370. [Google Scholar] [CrossRef]
- Immune thrombocytopenia associated with COVID-19 mRNA vaccine tozinameran—a clinical case and global pharmacovigilance data. Swiss Med Wkly. 2021, 151, w30084. [CrossRef]
- Gerber, G.F.; Yuan, X.; Yu, J.; Cher, B.A.Y.; Braunstein, E.M.; Chaturvedi, S.; Brodsky, R.A. COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria. Blood 2021, 137, 3670–3673. [Google Scholar] [CrossRef]
- Our World in Data. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 28 February 2022).
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Tiede, A.; Sachs, U.J.; Czwalinna, A.; Werwitzke, S.; Bikker, R.; Krauss, J.K.; Donnerstag, F.G.; Weißenborn, K.; Höglinger, G.U.; Maasoumy, B.; et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood 2021, 138, 350–353. [Google Scholar] [CrossRef] [PubMed]
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA 2021, 325, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef]
- Favaloro, E.J. Laboratory testing for suspected COVID-19 vaccine–induced (immune) thrombotic thrombocytopenia. Int. J. Lab. Hematol. 2021, 43, 559–570. [Google Scholar] [CrossRef]
- Waraich, A.; Williams, G. Haematuria, a widespread petechial rash, and headaches following the Oxford AstraZeneca ChAdOx1 nCoV-19 Vaccination. BMJ Case Rep. 2021, 14, e245440. [Google Scholar] [CrossRef]
- Paulsen, F.-O.; Schaefers, C.; Langer, F.; Frenzel, C.; Wenzel, U.; Hengel, F.E.; Bokemeyer, C.; Seidel, C. Immune Thrombocytopenic Purpura after vaccination with COVID-19 Vaccine (ChAdOx1 nCov-19). Blood 2021, 138, 996–999. [Google Scholar] [CrossRef]
- Choi, P.Y.-I.; Hsu, D.; Tran, H.A.; Tan, C.W.; Enjeti, A.; Chen, V.M.Y.; Chong, B.H.; Curnow, J.; Pepperell, D.; Bird, R. Immune thrombocytopenia following vaccination during the COVID-19 pandemic. Haematologica 2021. [Google Scholar] [CrossRef]
- Tarawneh, O.; Tarawneh, H. Immune thrombocytopenia in a 22-year-old post COVID-19 vaccine. Am. J. Hematol. 2021, 96, E133–E134. [Google Scholar] [CrossRef]
- Nakamura, T.; Morodomi, Y.; Kanaji, S.; Okamura, T.; Nagafuji, K.; Kanaji, T. Detection of anti-GPIbα autoantibodies in a case of immune thrombocytopenia following COVID-19 vaccination. Thromb. Res. 2021, 209, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Sakai, R.; Sato-Fitoussi, M.; Nodera, M.; Yoshinaga, S.; Shibata, A.; Kurasawa, T.; Kondo, T.; Amano, K. Potential Triggers for Thrombocytopenia and/or Hemorrhage by the BNT162b2 Vaccine, Pfizer-BioNTech. Front. Med. 2021, 8, 751598. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, D.; Ogasawara, R.; Sugimura, S.; Fujii, F.; Kojima, K.; Nagai, J.; Ebata, K.; Okada, K.; Kobayashi, N.; Ogasawara, M.; et al. New-onset Evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID-19 vaccination. Int. J. Hematol. 2021, 115, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Radwi, M.; Farsi, S. A case report of acquired hemophilia following COVID-19 vaccine. J. Thromb. Haemost. 2021, 19, 1515–1518. [Google Scholar] [CrossRef]
- Shimoyama, S.; Kanisawa, Y.; Ono, K.; Souri, M.; Ichinose, A. First and fatal case of autoimmune acquired factor XIII /13 deficiency after COVID -19/ SARS-CoV -2 vaccination. Am. J. Hematol. 2021, 97, 243–245. [Google Scholar] [CrossRef]
- Goereci, Y.; Kleineberg, N.N.; Madlener, M.; Neuschmelting, H.; Fink, G.R.; Warnke, C.; Stetefeld, H. Successful treatment of thromboses of major arteries after ChAdOx1 nCov-19 vaccination. Neurol. Res. Pract. 2021, 3, 1–4. [Google Scholar] [CrossRef]
- Andraska, E.A.; Kulkarni, R.; Chaudhary, M.; Sachdev, U. Three cases of acute venous thromboembolism in females after vaccination for coronavirus disease 2019. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 10, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Carli, G.; Nichele, I.; Ruggeri, M.; Barra, S.; Tosetto, A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern. Emerg. Med. 2021, 16, 803–804. [Google Scholar] [CrossRef]
- D’Agostino, V.; Caranci, F.; Negro, A.; Piscitelli, V.; Tuccillo, B.; Fasano, F.; Sirabella, G.; Marano, I.; Granata, V.; Grassi, R.; et al. A Rare Case of Cerebral Venous Thrombosis and Disseminated Intravascular Coagulation Temporally Associated to the COVID-19 Vaccine Administration. J. Pers. Med. 2021, 11, 285. [Google Scholar] [CrossRef]
- Varona, J.F.; García-Isidro, M.; Moeinvaziri, M.; Ramos-López, M.; Fernández-Domínguez, M. Pri-mary adrenal insufficiency associated with Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia (VITT). Eur J Intern. Med. 2021, 91, 90–92. [Google Scholar] [CrossRef]
- Blauenfeldt, R.A.; Kristensen, S.R.; Ernstsen, S.L.; Kristensen, C.C.H.; Simonsen, C.Z.; Hvas, A. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J. Thromb. Haemost. 2021, 19, 1771–1775. [Google Scholar] [CrossRef]
- Shimazawa, R.; Ikeda, M. Potential adverse events in Japanese women who received tozinameran (BNT162b2, Pfizer-BioNTech). J. Pharm. Policy Pract. 2021, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Kirpalani, A.; Garabon, J.; Amos, K.; Patel, S.; Sharma, A.P.; Ganesan, S.L.; Barton, M.; Cacciotti, C.; Leppington, S.; Bakovic, L.; et al. Thrombotic thrombocytopenic purpura temporally associated with BNT162b2 vaccination in an adolescent successfully treated with caplacizumab. Br. J. Haematol. 2021, 196, e11–e14. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, S.; Maes, M.; De Waele, L.; Vanhoorelbeke, K.; Gadisseur, A. First report of a de novo iTTP episode associated with an mRNA-based anti-COVID-19 vaccination. J. Thromb. Haemost. 2021, 19, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Maayan, H.; Kirgner, I.; Gutwein, O.; Herzog-Tzarfati, K.; Rahimi-Levene, N.; Koren-Michowitz, M.; Blickstein, D. Acquired thrombotic thrombocytopenic purpura: A rare disease associated with BNT162b2 vaccine. J. Thromb. Haemost. 2021, 19, 2314–2317. [Google Scholar] [CrossRef] [PubMed]
- Aladdin, Y.; Algahtani, H.; Shirah, B. Vaccine-Induced Immune Thrombotic Thrombocytopenia with Disseminated Intravascular Coagulation and Death following the ChAdOx1 nCoV-19 Vaccine. J. Stroke Cerebrovasc. Dis. 2021, 30, 105938. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, D.; Elshafeey, A.; Mushannen, M.; Kawas, H.; Shafiq, A.; Mhaimeed, N.; Mhaimeed, O.; Mhaimeed, N.; Zeghlache, R.; Salameh, M.; et al. Cardiovascular and haematological events post COVID-19 vaccination: A systematic review. J. Cell. Mol. Med. 2021, 26, 636–653. [Google Scholar] [CrossRef]
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J. Neurol. Sci. 2021, 428, 117607. [Google Scholar] [CrossRef]
- Vayne, C.; Rollin, J.; Gruel, Y.; Pouplard, C.; Galinat, H.; Huet, O.; Mémier, V.; Geeraerts, T.; Marlu, R.; Pernod, G.; et al. PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 385, 376–378. [Google Scholar] [CrossRef]
- Tiede, A.; Althaus, K.; Sachs, U.J.; Cooper, N.; Czwalinna, A.; Müller, J.; Pötzsch, B. PF4-Dependent Immunoassays in Patients with Vaccine-Induced Immune Thrombotic Thrombocytopenia: Results of an Interlaboratory Comparison. Thromb. Haemost. 2021, 121, 1622–1627. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Politou, M.; Ntanasis-Stathopoulos, I.; Karalis, V.; Merkouri, E.; Fotiou, D.; Gavriatopoulou, M.; Malandrakis, P.; Kastritis, E.; Trougakos, I.; et al. High Prevalence of Anti-PF4 Antibodies Following ChAdOx1 nCov-19 (AZD1222) Vaccination Even in the Absence of Thrombotic Events. Vaccines 2021, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Hursting, M.J.; Pai, P.J.; McCracken, J.E.; Hwang, F.; Suvarna, S.; Lokhnygina, Y.; Bandarenko, N.; Arepally, G.M. Platelet Factor 4/Heparin Antibodies in Blood Bank Donors. Am. J. Clin. Pathol. 2010, 134, 774–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arepally, G.M.; Hursting, M.J. Platelet factor 4/heparin antibody (IgG/M/A) in healthy subjects: A literature analysis of commercial immunoassay results. J. Thromb. Thrombolysis 2008, 26, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Barefah, A.S.; Radhwi, O.O.; Alamri, S.S.; Alahwal, H.M.; Denetiu, I.; Almohammadi, A.T.; Bahashwan, S.M.; Qari, M.H.; Algaissi, A.; Alamer, E.; et al. Low clinical utility of testing for anti-platelet factor 4 in asymptomatic individuals after ChAdOx1 nCoV-19 vaccine. Int. J. Lab. Hematol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Kimihira, L.; Nagasawa, H.; Seo, K.; Wada, M. Cerebral Venous Sinus Thrombosis After BNT162b2 mRNA COVID-19 Vaccination. Cureus 2021, 13, e18775. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.E.; Shen, J.Y.; Lim, X.R.; Tu, T.M.; Chang, C.C.R.; Khin, H.S.W.; Koh, J.S.; Rao, J.P.; Lau, S.L.; Tan, G.B.; et al. Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV-2 vaccination: A black swan event. Am. J. Hematol. 2021, 96, E357–E361. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.; Soares-Dos-Reis, R.; Meira, J.; Ferrão, D.; Soares, P.R.; Pastor, A.; Gama, G.; Fonseca, L.; Fagundes, V.; Carvalho, M. Cerebral Venous Thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J. Stroke Cerebrovasc. Dis. 2021, 30, 105906. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greinacher, A.; Langer, F.; Makris, M.; Pai, M.; Pavord, S.; Tran, H.; Warkentin, T.E. Vaccine-induced immune thrombotic thrombocytopenia (VITT)—Update on diagnosis and management considering different resources: Response to Comment from Yamada et al. J. Thromb. Haemost. 2022, 20, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Langer, F.; Makris, M.; Pai, M.; Pavord, S.; Tran, H.; Warkentin, T.E. Vaccine-induced immune thrombotic thrombocytopenia (VITT): Update on diagnosis and management considering different resources. J. Thromb. Haemost. 2022, 20, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Nazy, I.; Sachs, U.J.; Arnold, D.M.; McKenzie, S.E.; Choi, P.; Althaus, K.; Ahlen, M.T.; Sharma, R.; Grace, R.F.; Bakchoul, T. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: Communication from the ISTH SSC Subcommittee on Platelet Immunology. J. Thromb. Haemost. 2021, 19, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Connors, J.M.; Cines, D.B.; Cines, D.B.; Dunbar, C.E.; Michaelis, L.C.; Kreuziger, L.B.; Lee, A.Y.Y.; Pabinger-Fasching, I. Throm-Bosis with Thrombocytopenia Syndrome (Also Termed Vaccine Induced Thrombotic Thrombocytopenia). Version 1.7. Available online: https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenialast (accessed on 17 December 2021).
- Salih, F.; Schönborn, L.; Kohler, S.; Franke, C.; Möckel, M.; Dörner, T.; Bauknecht, H.C.; Pille, C.; Graw, J.A.; Alonso, A.; et al. Vaccine-Induced Thrombocytopenia with Severe Headache. N. Engl. J. Med. 2021, 385, 2103–2105. [Google Scholar] [CrossRef] [PubMed]
- Khuhapinant, A.; Rungjirajittranon, T.; Suwanawiboon, B.; Chinthammitr, Y.; Ruchutrakool, T. Successful venous thromboprophylaxis in a patient with vaccine-induced immune thrombotic thrombocytopenia (VITT): A case report of the first reported case in Thailand. Thromb. J. 2021, 19, 1–5. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Wong, C.C.; Hong, J.M.; Peng, T.A.; Brondfield, S.; Reilly, L.M.; Cornett, P.; Leavitt, A.D. VITT following Ad26.COV2.S vaccination presenting without radiographically demonstrable thrombosis. Blood Adv. 2021, 5, 4662–4665. [Google Scholar] [CrossRef]
- Johansen, S.; Lægreid, I.J.; Ernstsen, S.L.; Azrakhsh, N.A.; Kittang, A.O.; Lindås, R.; Gjertsen, B.T.; Vetti, N.; Mørtberg, T.V.; Sørvoll, I.H.; et al. Thrombosis and thrombocytopenia after HPV vaccination. J. Thromb. Haemost. 2021, 20, 700–704. [Google Scholar] [CrossRef]
- Hwang, J.; Park, S.H.; Lee, S.W.; Lee, S.B.; Lee, M.H.; Jeong, G.H.; Kim, M.S.; Kim, J.Y.; Koyanagi, A.; Jacob, L.; et al. Predictors of mortality in thrombotic thrombocytopenia after adenoviral COVID-19 vaccination: The FAPIC score. Eur. Heart J. 2021, 42, 4053–4063. [Google Scholar] [CrossRef]
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2021, 9, e73–e80. [Google Scholar] [CrossRef]
- Arepally, G.M.; Ortel, T.L. Vaccine-induced immune thrombotic thrombocytopenia: What we know and do not know. Blood 2021, 138, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.; Ay, C.; Gleixner, K.V.; Hauswirth, A.W.; Cacioppo, F.; Grafeneder, J.; Quehenberger, P.; Pabinger, I.; Knöbl, P. Successful treatment of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT). J. Thromb. Haemost. 2021, 19, 1819–1822. [Google Scholar] [CrossRef]
- Wilting, F.N.; Kotsopoulos, A.M.; Platteel, A.C.; van Oers, J.A. Intracerebral Hemorrhage and Thrombocytopenia After AstraZeneca COVID-19 Vaccine: Clinical and Diagnostic Challenges of Vaccine-Induced Thrombotic Thrombocytopenia. Cureus 2021, 13, e17637. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, P.L.; Montague, S.J.; Smith, C.W.; Lodwick, C.S.; Stoneley, C.; Roberts, M.; Watson, S.P.; Lowe, G.C.; Lester, W.A. Anti-PF4 levels of patients with VITT do not reduce 4 months following AZD1222 vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Thaler, J.; Jilma, P.; Samadi, N.; Roitner, F.; Mikušková, E.; Kudrnovsky-Moser, S.; Rettl, J.; Preiss, R.; Quehenberger, P.; Pabinger, I.; et al. Long-term follow-up after successful treatment of vaccine-induced prothrombotic immune thrombocytopenia. Thromb. Res. 2021, 207, 126–130. [Google Scholar] [CrossRef]
- Lacy, J.; Pavord, S.; Brown, K.E. VITT and Second Doses of Covid-19 Vaccine. N. Engl. J. Med. 2022, 386, 95. [Google Scholar] [CrossRef]

| 1. COVID-19 per se [59,60] |
| 2. Disseminated intravascular coagulation (DIC) [45] |
| 3. Idiopathic thrombocytopenic purpura or immune thrombocytopenia (ITP) |
| 4. Thrombotic thrombocytopenic purpura (TTP) |
| 5. Antiphospholipid antibody syndrome (APS) [79] |
| 6. Hemophagocytic syndrome (HPS) [80,81] |
| 7. Heparin-induced thrombocytopenia (HIT) |
| 8. Drug-induced thrombocytopenia |
| 9. Pseudo-thrombocytopenia |
| 1. Side effects of anticoagulation therapy |
| 2. Complications of enhanced-fibrinolytic-type DIC |
| 3. Vascular endotheliitis, fragility of vessel walls |
| 4. Acquired von Willebrand syndrome (during ECMO) |
| 5. Thrombocytopenia |
| 6. Decreased coagulation factors (liver failure, vitamin K deficiency, etc.) |
| 7. Others |
| Disease Name | Abbreviation | Important Clinical and Laboratory Findings |
|---|---|---|
| Heparin-induced thrombocytopenia | HIT | History of exposure to heparin, 4T’s score |
| Thrombotic microangiopathy | TMA | Appearance of schizocytes (peripheral blood smear), marked decrease in haptoglobin |
| Thrombotic thrombocytopenic purpura | TTP | A type of TMA with markedly reduced ADAMTS13 activity with ADAMTS13 inhibitor |
| Immune thrombocytopenia | ITP | Diagnosis of exclusion. Increased megakaryocytes in bone marrow and positive antiplatelet antibodies assist in diagnosis |
| Antiphospholipid antibody syndrome | APS | Positive for at least one of the following antibodies: lupus anticoagulant; anticardiolipin antibody; and anti-β2 GPI antibody |
| Paroxysmal nocturnal hemoglobinuria | PNH | Hemolysis (normocytic anemia, elevated reticulocyte, elevated indirect bilirubin, elevated LDH, decreased haptoglobin), presence of PNH type-cells (CD55/59-negative) |
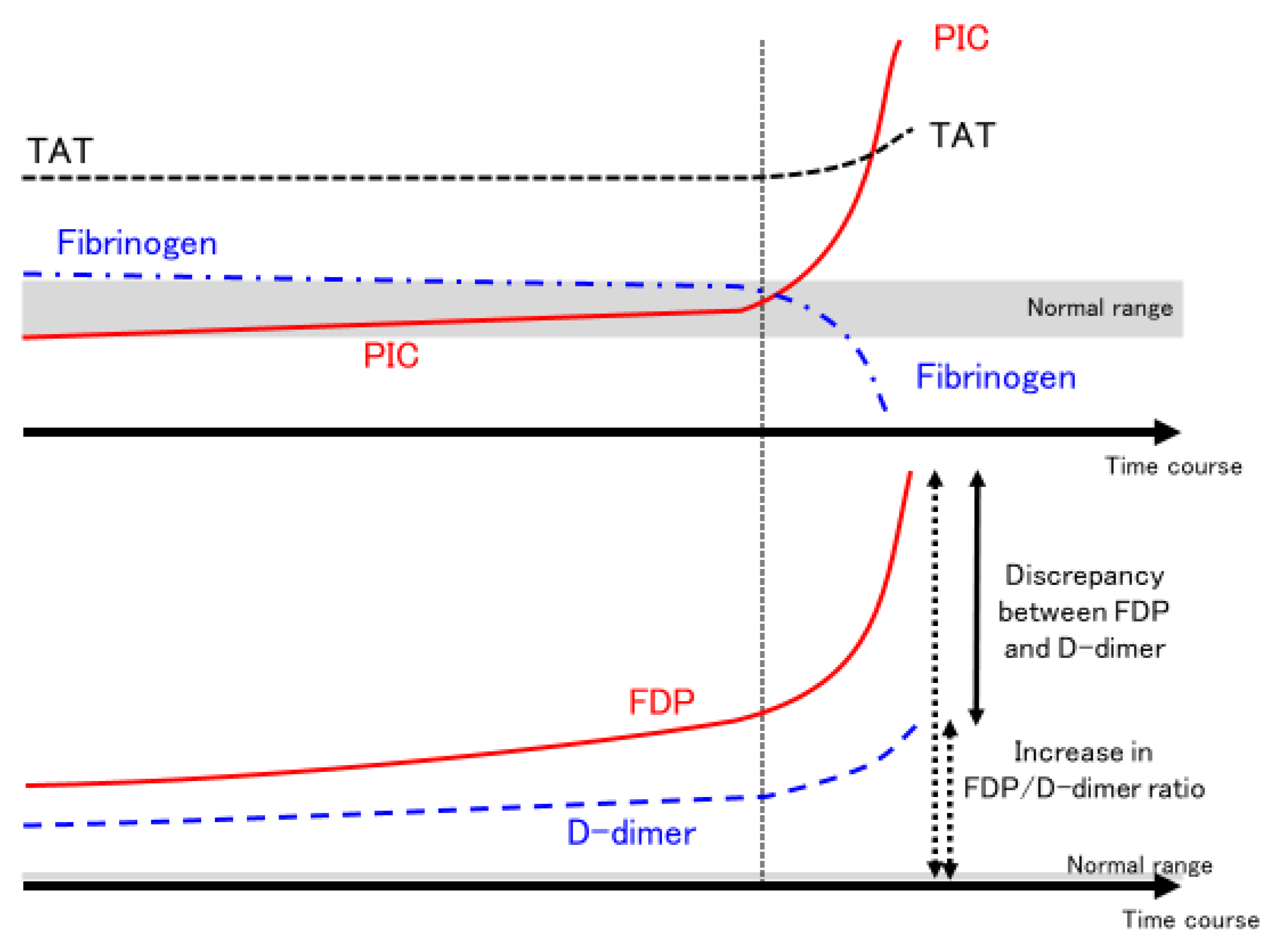
| Disseminated intravascular coagulation | DIC | PT, APTT, fibrinogen, FDP, D-dimer, AT, TAT, PIC, plasminogen, α2PI |
| Clinical Findings |
|---|
| (1) Onset 4–28 days after vaccination (counting the day of vaccination as day 0) |
| (2) Symptoms suggestive of stroke (unilateral facial palsy, unilateral motor palsy, language disorder, joint parallax, hemispheric neglect, etc.) |
| (3) Symptoms suggestive of cerebral venous sinus thrombosis (persistent headache, visual disturbance, seizure, nausea and vomiting, psychiatric symptoms, etc.) |
| (4) Symptoms suggestive of visceral vein thrombosis (persistent abdominal pain, nausea and vomiting, etc.) |
| (5) Symptoms suggestive of deep vein thrombosis or pulmonary thromboembolism (pain and swelling in lower limbs, chest and back pain, shortness of breath, etc.) |
| (6) Hemorrhagic tendencies such as hemorrhagic infarction, petechial hemorrhage, and mottled hemorrhage can also occur. |
| Disease | Suppressed-Fibrinolytic-Type DIC | Enhanced-Fibrinolytic-Type DIC | VITT | |
|---|---|---|---|---|
| Underlying disease/cause | Severe sepsis | APL, aortic aneurysm, prostate cancer, etc. | Adenovirus vector type vaccination | |
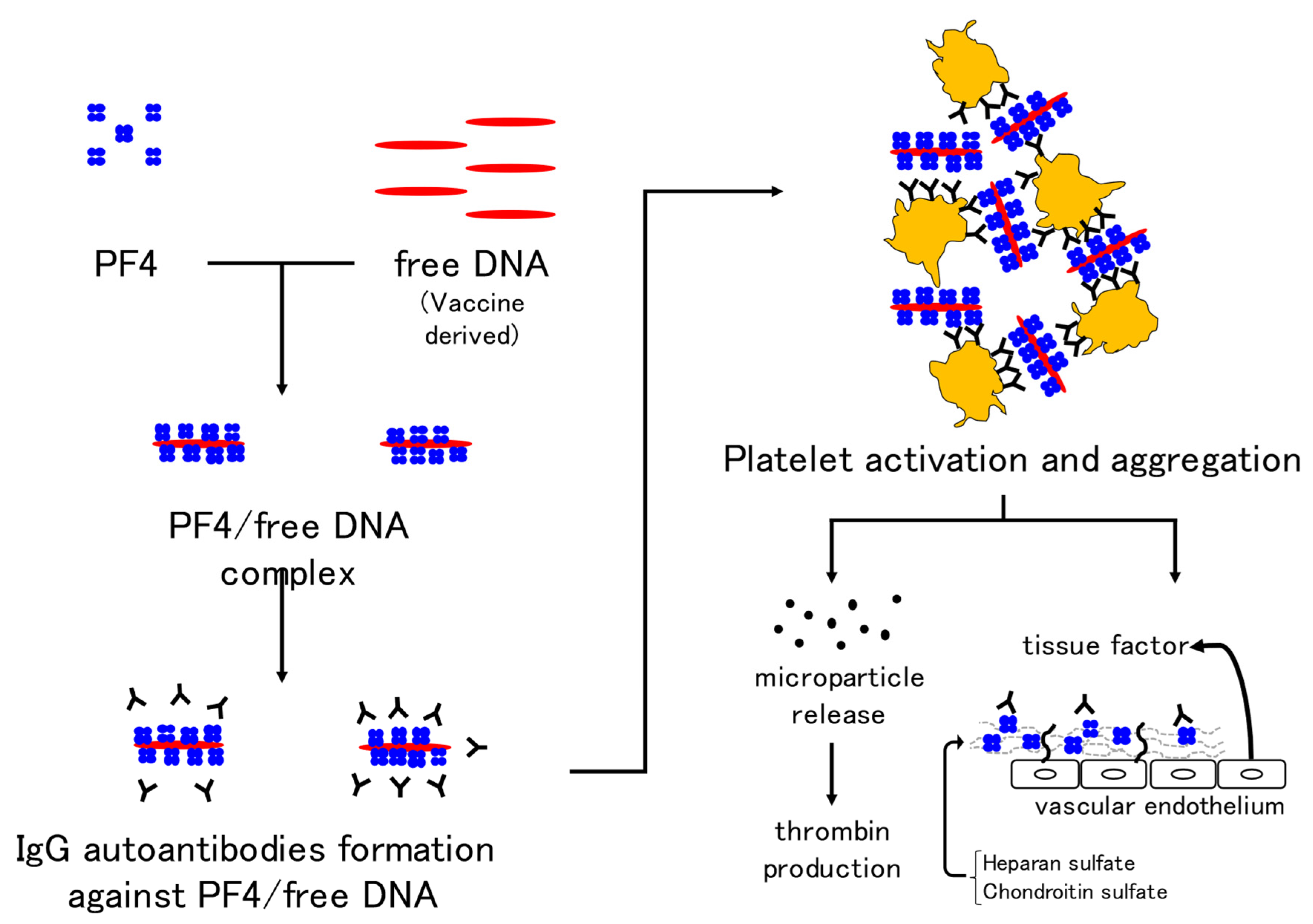
| Pathophysiology | Activation of coagulation and mild fibrinolysis activation | Activation of coagulation and enhanced fibrinolysis | Antibodies against PF4 are mediated platelet and coagulation activation | |
| Main symptom | Organ damage | Bleeding | Headache, abdominal pain, etc. | |
| Examination findings | Platelet count | Decreased | Decreased | Decreased |
| PT | Prolonged | Normal to prolonged | Normal to prolonged * | |
| APTT | Prolonged | Slightly shortened to prolonged | Normal to prolonged * | |
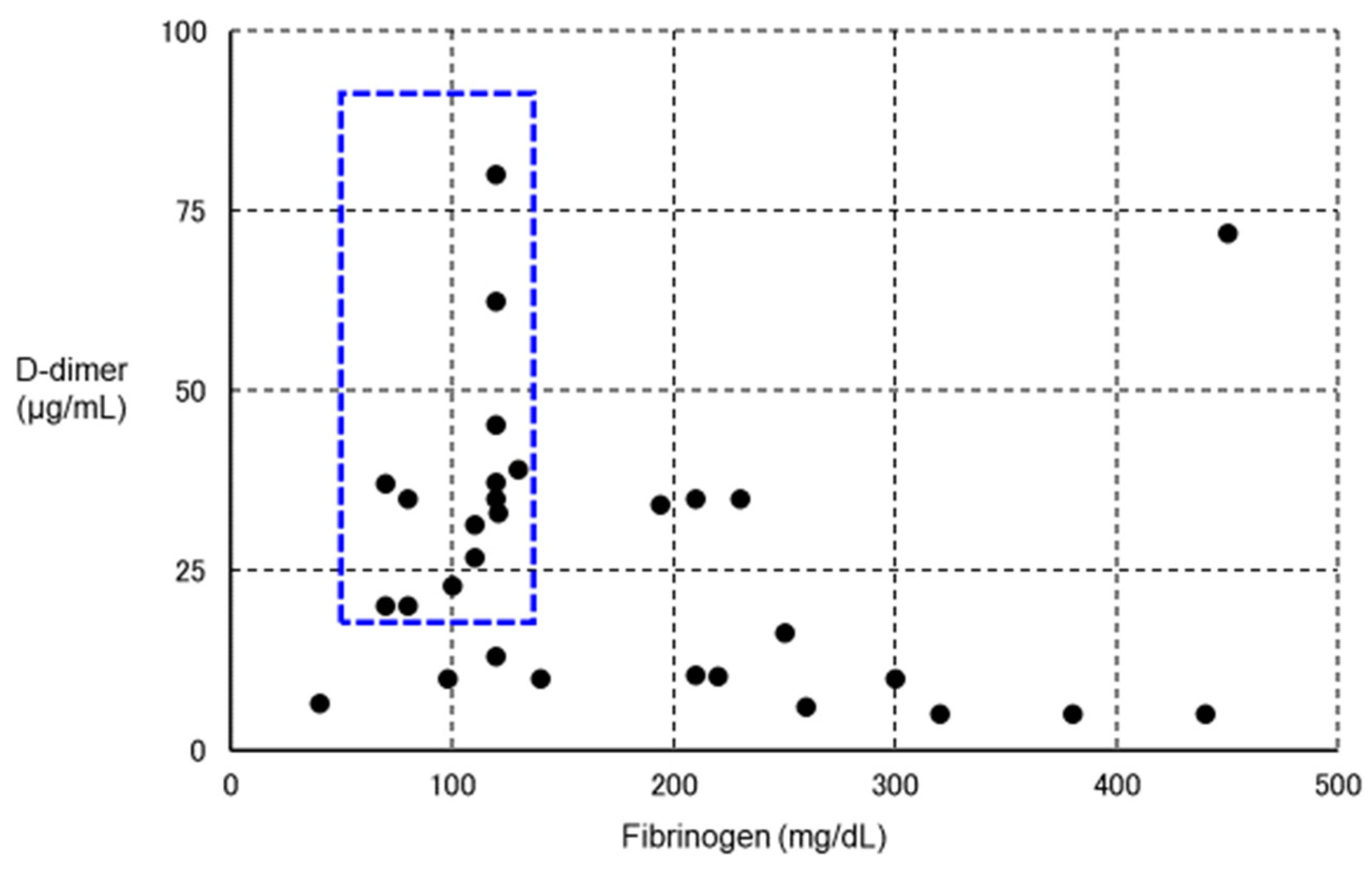
| Fibrinogen | Normal to elevated | Decreased | Significantly reduced to normal | |
| D-dimer | Increased | Increased | Increased | |
| FDP | Increased | Markedly increased | Increased—markedly increased * | |
| TAT | Increased | Increased | Increased * | |
| PIC | Slightly increased | Markedly increased | Increased—markedly increased * | |
| Medical treatment | Anticoagulant therapy | Anticoagulant therapy ± antifibrinolytic therapy | Anticoagulant therapy other than heparin, high-dose immunoglobulin therapy, etc. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Asakura, H. Coagulopathy and Fibrinolytic Pathophysiology in COVID-19 and SARS-CoV-2 Vaccination. Int. J. Mol. Sci. 2022, 23, 3338. https://doi.org/10.3390/ijms23063338
Yamada S, Asakura H. Coagulopathy and Fibrinolytic Pathophysiology in COVID-19 and SARS-CoV-2 Vaccination. International Journal of Molecular Sciences. 2022; 23(6):3338. https://doi.org/10.3390/ijms23063338
Chicago/Turabian StyleYamada, Shinya, and Hidesaku Asakura. 2022. "Coagulopathy and Fibrinolytic Pathophysiology in COVID-19 and SARS-CoV-2 Vaccination" International Journal of Molecular Sciences 23, no. 6: 3338. https://doi.org/10.3390/ijms23063338
APA StyleYamada, S., & Asakura, H. (2022). Coagulopathy and Fibrinolytic Pathophysiology in COVID-19 and SARS-CoV-2 Vaccination. International Journal of Molecular Sciences, 23(6), 3338. https://doi.org/10.3390/ijms23063338






