Integration of Light and Auxin Signaling in Shade Plants: From Mechanisms to Opportunities in Urban Agriculture
Abstract
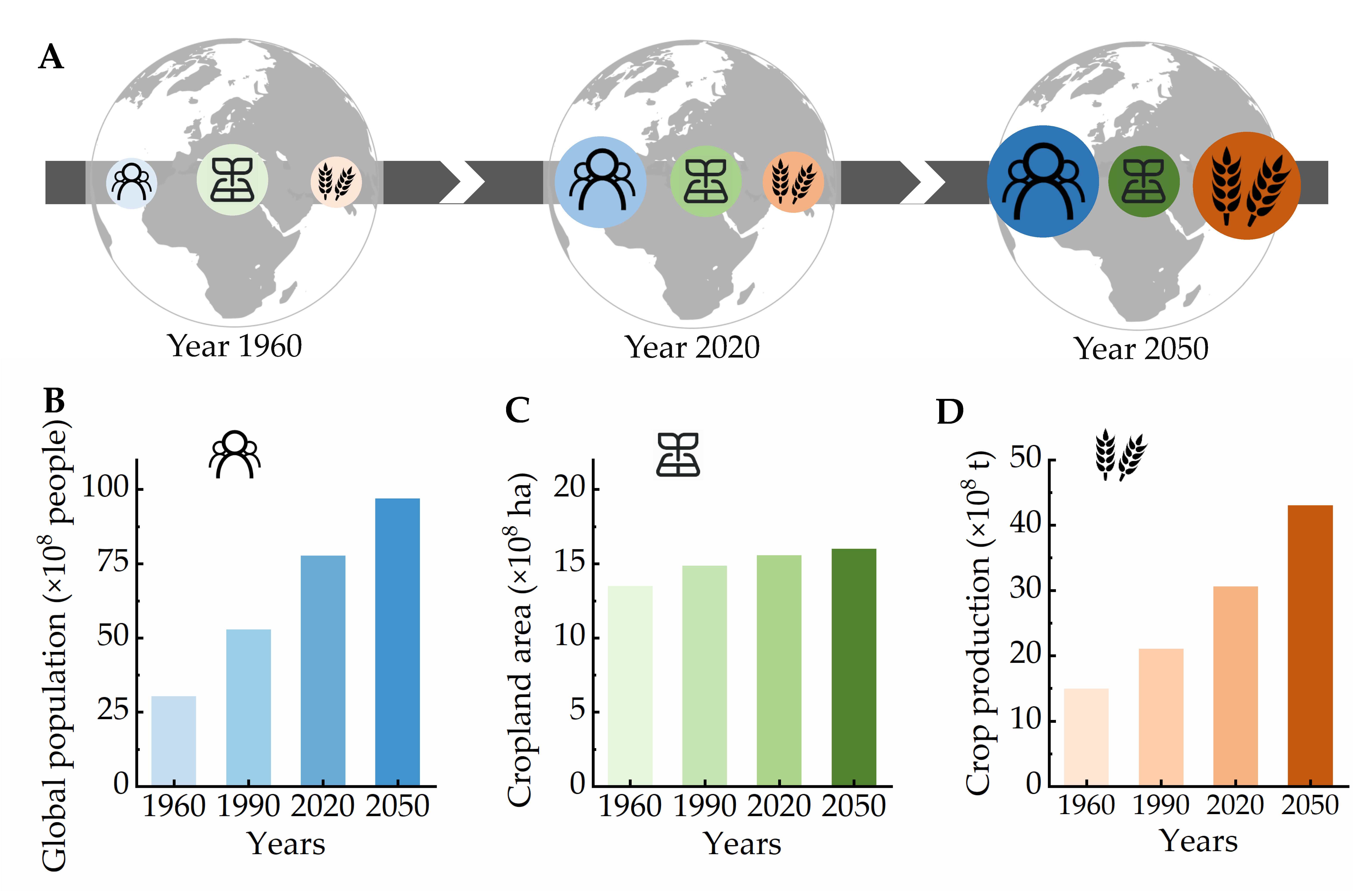
:1. Introduction
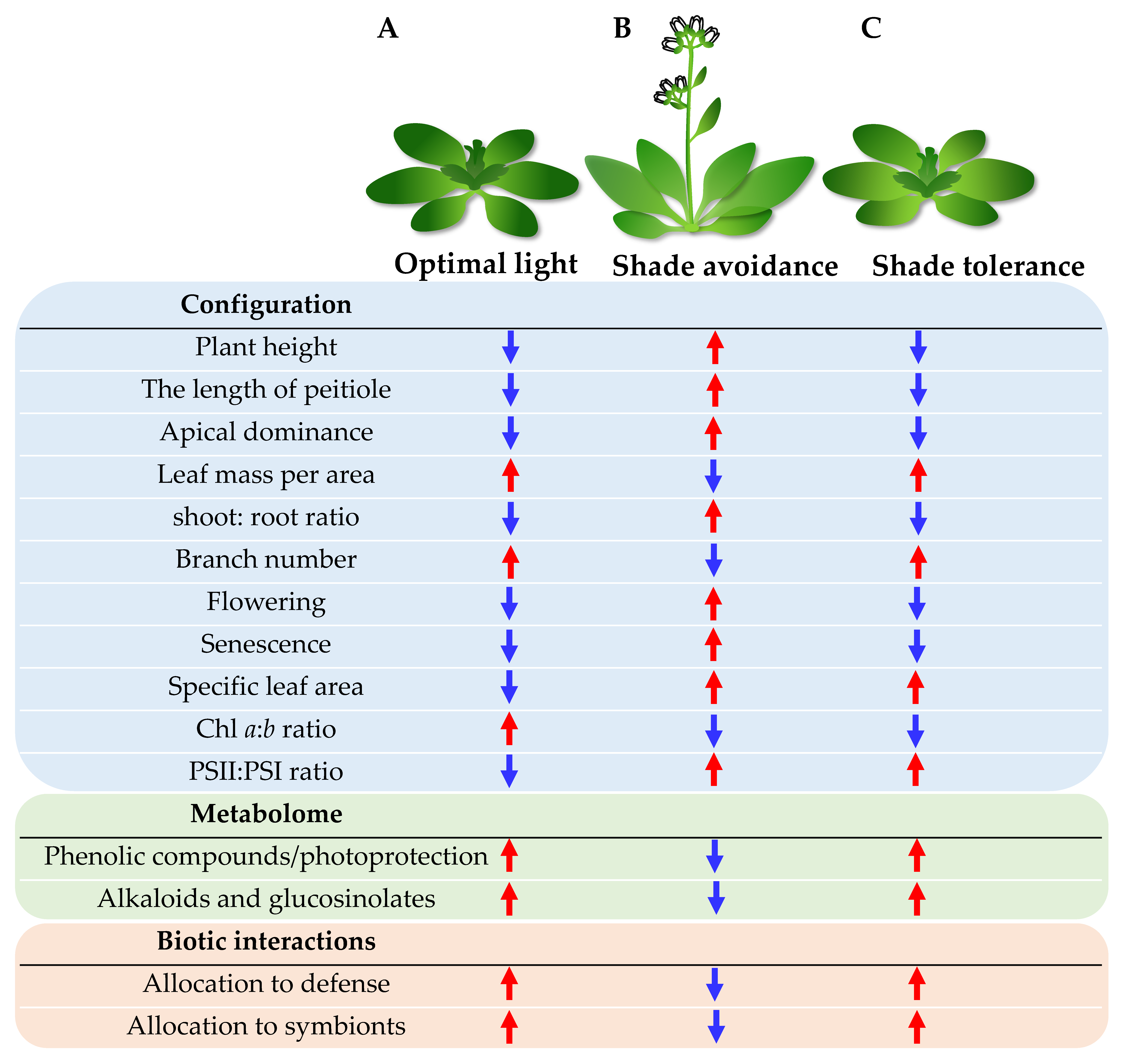
2. Shade Avoidance and Tolerance in Plants
3. Mechanism of Light and Auxin Integration in SAR and STR
3.1. Photomorphogenesis of Plants under Optimal Light Distribution
3.2. SAR and the Feedback Mechanism of Shade Avoiders under Shading or Suboptimal Light
3.3. STR Mechanism of Shade-Tolerant Species in Shading or Suboptimal Light
4. Prospects: From Shade Avoidance and Shade Tolerance Mechanisms to Development of Shade-Tolerant Plants for Urban Agriculture
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhou, D.; Li, Y.; Zhang, L. An integrated assessment on the warming effects of urbanization and agriculture in highly developed urban agglomerations of China. Sci. Total Environ. 2022, 804, 150119. [Google Scholar] [CrossRef] [PubMed]
- de Wit, M.; Galvao, V.C.; Fankhauser, C. Light-mediated hormonal regulation of plant growth and development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmulling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Milmanda, G.L.; Ballare, C.L. Shade avoidance: Expanding the color and hormone palette. Trends Plant Sci. 2021, 26, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Allorent, G.; Petroutsos, D. Photoreceptor-dependent regulation of photoprotection. Curr. Opin. Plant Biol. 2017, 37, 102–108. [Google Scholar] [CrossRef]
- Fiorucci, A.S.; Fankhauser, C. Plant strategies for enhancing access to sunlight. Curr. Biol. 2017, 27, R931–R940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, M.; Nieuwendijk, N.M.; Pantazopoulou, C.K.; Pierik, R. Light signalling shapes plant-plant interactions in dense canopies. Plant Cell Environ. 2021, 44, 1014–1029. [Google Scholar] [CrossRef]
- Liu, Y.; Jafari, F.; Wang, H. Integration of light and hormone signaling pathways in the regulation of plant shade avoidance syndrome. aBIOTECH 2021, 2, 131–145. [Google Scholar] [CrossRef]
- Ortiz-Alcaide, M.; Llamas, E.; Gomez-Cadenas, A.; Nagatani, A.; Martinez-Garcia, J.F.; Rodriguez-Concepcion, M. Chloroplasts modulate elongation responses to canopy shade by retrograde pathways involving HY5 and abscisic acid. Plant Cell 2019, 31, 384–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roig-Villanova, I.; Paulisic, S.; Martinez-Garcia, J.F. Shade avoidance and neighbor detection. Methods Mol. Biol. 2019, 2026, 157–168. [Google Scholar] [PubMed]
- Sng, B.J.R.; Singh, G.P.; Van Vu, K.; Chua, N.H.; Ram, R.J.; Jang, I.C. Rapid metabolite response in leaf blade and petiole as a marker for shade avoidance syndrome. Plant Methods 2020, 16, 144. [Google Scholar] [CrossRef]
- Ballare, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Gommers, C.M.; Visser, E.J.; St Onge, K.R.; Voesenek, L.A.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- Han, X.; Huang, X.; Deng, X.W. The photomorphogenic central repressor COP1: Conservation and functional diversification during evolution. Plant Commun. 2020, 1, 100044. [Google Scholar] [CrossRef]
- Podolec, R.; Ulm, R. Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr. Opin. Plant Biol. 2018, 45, 18–25. [Google Scholar] [CrossRef]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and phytochrome interacting factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef] [Green Version]
- Tavridou, E.; Pireyre, M.; Ulm, R. Degradation of the transcription factors PIF4 and PIF5 under UV-B promotes UVR8-mediated inhibition of hypocotyl growth in Arabidopsis. Plant J. 2020, 101, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, B.; Hayes, S.; Kerner, K.; Hoecker, U.; Jenkins, G.I.; Franklin, K.A. UVR8 disrupts stabilisation of PIF5 by COP1 to inhibit plant stem elongation in sunlight. Nat. Commun. 2019, 10, 4417. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liang, T.; Zhang, L.; Shao, K.; Gu, X.; Shang, R.; Shi, N.; Li, X.; Zhang, P.; Liu, H. UVR8 interacts with WRKY36 to regulate HY5 transcription and hypocotyl elongation in Arabidopsis. Nat. Plants 2018, 4, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, L. Hormonal regulation in shade avoidance. Front. Plant Sci. 2017, 8, 1527. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, Y.; Datla, R.; Ren, M. Auxin and target of rapamycin spatiotemporally regulate root organogenesis. Int. J. Mol. Sci. 2021, 22, 11357. [Google Scholar] [CrossRef]
- Leyser, O. Auxin signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanova-Saez, R.; Mateo-Bonmati, E.; Ljung, K. Auxin metabolism in plants. CSH Perspect. Biol. 2021, 13, a039867. [Google Scholar] [CrossRef]
- Huq, E. Direct convergence of light and auxin signaling pathways in Arabidopsis. Mol. Plant 2018, 11, 515–517. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; He, S.; Zhang, J.; Mao, Z.; Wang, W.; Li, T.; Hua, J.; Du, S.; Xu, P.; Li, L.; et al. Photoactivated CRY1 and phyB interact directly with AUX/IAA proteins to inhibit auxin signaling in Arabidopsi. Mol. Plant 2018, 11, 523–541. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.; He, S.; Xu, F.; Wei, X.; Jiang, L.; Liu, Y.; Wang, W.; Li, T.; Xu, P.; Du, S.; et al. Photoexcited CRY1 and phyB interact directly with ARF6 and ARF8 to regulate their DNA-binding activity and auxin-induced hypocotyl elongation in Arabidopsis. New Phytol. 2020, 225, 848–865. [Google Scholar] [CrossRef]
- Lv, B.; Zhu, J.; Kong, X.; Ding, Z. Light participates in the auxin-dependent regulation of plant growth. J. Integr. Plant Biol. 2021, 63, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, X.; Liu, Y.; Fan, S.; Ma, Q. Progress of research on the regulatory pathway of the plant shade-avoidance syndrome. Front. Plant Sci. 2020, 11, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holalu, S.V.; Reddy, S.K.; Blackman, B.K.; Finlayson, S.A. Phytochrome interacting factors 4 and 5 regulate axillary branching via bud abscisic acid and stem auxin signalling. Plant Cell Environ. 2020, 43, 2224–2238. [Google Scholar] [CrossRef] [PubMed]
- Pedmale, U.V.; Huang, S.-S.C.; Zander, M.; Cole, B.J.; Hetzel, J.; Ljung, K.; Reis, P.A.; Sridevi, P.; Nito, K.; Nery, J.R.; et al. Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 2016, 164, 233–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, B.; Kay, S.A.; Chory, J. Automated analysis of hypocotyl growth dynamics during shade avoidance in Arabidopsis. Plant J. 2011, 65, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Leivar, P.; Tepperman, J.M.; Cohn, M.M.; Monte, E.; Al-Sady, B.; Erickson, E.; Quail, P.H. Dynamic antagonism between phytochromes and PIF family Basic Helix-Loop-Helix factors induces selective reciprocal responses to light and shade in a rapidly responsive transcriptional network in Arabidopsis. Plant Cell 2012, 24, 1398–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, V.N.; Xu, X.; Huq, E. Molecular bases for the constitutive photomorphogenic phenotypes in Arabidopsi. Development 2018, 145, dev169870. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.; Li, Z.; Zhou, N.; Ma, M.; Jiang, Y.; Dong, A.; Shen, W.H.; Li, L. Linking Phytochrome-Interacting Factor to histone modification in plant shade avoidance. Plant Physiol. 2018, 176, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Leivar, P.; Martin, G.; Soy, J.; Dalton-Roesler, J.; Quail, P.H.; Monte, E. Phytochrome-imposed inhibition of PIF7 activity shapes photoperiodic growth in Arabidopsis together with PIF1, 3, 4 and 5. Physiol. Plant 2020, 169, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, O.; Legris, M.; Costigliolo Rojas, C.; Iglesias, M.J.; Hernando, C.E.; Dezar, C.; Vazquez, M.; Yanovsky, M.J.; Finlayson, S.A.; Prat, S.; et al. Rewiring of auxin signaling under persistent shade. Proc. Natl. Acad. Sci. USA 2018, 115, 5612–5617. [Google Scholar] [CrossRef] [Green Version]
- Keuskamp, D.H.; Pollmann, S.; Voesenek, L.A.; Peeters, A.J.; Pierik, R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc. Natl. Acad. Sci. USA 2010, 107, 22740–22744. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, M.J.; Sellaro, R.; Zurbriggen, M.D.; Casal, J.J. Multiple links between shade avoidance and auxin networks. J. Exp. Bot. 2018, 69, 213–228. [Google Scholar] [CrossRef]
- Reed, J.W.; Wu, M.F.; Reeves, P.H.; Hodgens, C.; Yadav, V.; Hayes, S.; Pierik, R. Three auxin response factors promote hypocotyl elongation. Plant Physiol. 2018, 178, 864–875. [Google Scholar] [CrossRef] [Green Version]
- Leivar, P.; Quail, P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011, 16, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Ye, M.; Sang, M.; Wu, R. A regulatory network for miR156-SPL module in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 6166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Liu, Y.; Ma, M.; Zhou, Q.; Zhao, Y.; Zhao, B.; Wang, B.; Wei, H.; Wang, H. Arabidopsis FHY3 and FAR1 integrate light and strigolactone signaling to regulate branching. Nat. Commun. 2020, 11, 1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Yang, C.; Jiang, Y.; Li, L. A PIF7-CONSTANS-Centered molecular regulatory network underlying shade-accelerated flowering. Mol. Plant 2019, 12, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, Q.; Zhao, Y.; Li, Q.; Liu, Y.; Ma, M.; Wang, B.; Shen, R.; Zheng, Z.; Wang, H. FHY3 and FAR1 integrate light signals with the miR156-SPL module-mediated aging pathway to regulate Arabidopsis flowering. Mol. Plant 2020, 13, 483–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Park, S.H.; Soh, M.Y.; Chua, N.H. Ubiquitin-specific proteases UBP12 and UBP13 promote shade avoidance response by enhancing PIF7 stability. Proc. Natl. Acad. Sci. USA 2021, 118, e2103633118. [Google Scholar] [CrossRef]
- Ma, L.; Li, G. Auxin-dependent cell elongation during the shade avoidance response. Front. Plant Sci. 2019, 10, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierik, R.; Ballare, C.L. Control of plant growth and defense by photoreceptors: From mechanisms to opportunities in agriculture. Mol. Plant 2021, 14, 61–76. [Google Scholar] [CrossRef]
- Hayes, S.; Pantazopoulou, C.K.; van Gelderen, K.; Reinen, E.; Tween, A.L.; Sharma, A.; de Vries, M.; Prat, S.; Schuurink, R.C.; Testerink, C.; et al. Soil salinity limits plant shade avoidance. Curr. Biol. 2019, 29, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kong, X.; Hu, K.; Cao, M.; Liu, J.; Ma, C.; Guo, S.; Yuan, X.; Zhao, S.; Robert, H.S.; et al. PIFs coordinate shade avoidance by inhibiting auxin repressor ARF18 and metabolic regulator QQS. New Phytol. 2020, 228, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Pantazopoulou, C.K.; Bongers, F.J.; Kupers, J.J.; Reinen, E.; Das, D.; Evers, J.B.; Anten, N.P.R.; Pierik, R. Neighbor detection at the leaf tip adaptively regulates upward leaf movement through spatial auxin dynamics. Proc. Natl. Acad. Sci. USA 2017, 114, 7450–7455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Gelderen, K.; Kang, C.; Paalman, R.; Keuskamp, D.; Hayes, S.; Pierik, R. Far-Red light detection in the shoot regulates lateral root development through the HY5 transcription factor. Plant Cell 2018, 30, 101–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, O.; Fiorucci, A.S.; Xenarios, I.; Fankhauser, C. Local auxin production underlies a spatially restricted neighbor-detection response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 7444–7449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.; Zhang, L.; Ding, Z. 26S proteasome: Hunter and prey in auxin signaling. Trends Plant Sci. 2016, 21, 546–548. [Google Scholar] [CrossRef]
- Zou, Y.; Li, R.; Baldwin, I.T. ZEITLUPE is required for shade avoidance in the wild tobacco Nicotiana attenuata. J. Integr. Plant Biol. 2020, 62, 1341–1351. [Google Scholar] [CrossRef]
- Yang, C.; Xie, F.; Jiang, Y.; Li, Z.; Huang, X.; Li, L. Phytochrome A negatively regulates the shade avoidance response by increasing auxin/indole acidic acid protein stability. Dev. Cell 2018, 44, 29–41.e4. [Google Scholar] [CrossRef] [Green Version]
- Buti, S.; Hayes, S.; Pierik, R. The bHLH network underlying plant shade-avoidance. Physiol. Plant 2020, 169, 312–324. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Li, X.; Xu, D.; Lin, X.; Liu, M.; Li, G.; Qin, X. FAR-RED ELONGATED HYPOCOTYLS3 negatively regulates shade avoidance responses in Arabidopsis. Plant Cell Environ. 2019, 42, 3280–3292. [Google Scholar] [CrossRef]
- Yang, C.; Huang, S.; Zeng, Y.; Liu, C.; Ma, Q.; Pruneda-Paz, J.; Kay, S.A.; Li, L. Two bHLH transcription factors, bHLH48 and bHLH60, associate with phytochrome interacting factor 7 to regulate hypocotyl elongation in Arabidopsis. Cell Rep. 2021, 35, 109054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pfeiffer, A.; Tepperman, J.M.; Dalton-Roesler, J.; Leivar, P.; Gonzalez Grandio, E.; Quail, P.H. Central clock components modulate plant shade avoidance by directly repressing transcriptional activation activity of PIF proteins. Proc. Natl. Acad. Sci. USA 2020, 117, 3261–3269. [Google Scholar] [CrossRef]
- Fraser, D.P.; Panter, P.E.; Sharma, A.; Sharma, B.; Dodd, A.N.; Franklin, K.A. Phytochrome A elevates plant circadian-clock components to suppress shade avoidance in deep-canopy shade. Proc. Natl. Acad. Sci. USA 2021, 118, e2108176118. [Google Scholar] [CrossRef]
- Buti, S.; Pantazopoulou, C.K.; van Gelderen, K.; Hoogers, V.; Reinen, E.; Pierik, R. A gas-and-brake mechanism of bHLH proteins modulates shade avoidance. Plant Physiol. 2020, 184, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Gruntman, M.; Gross, D.; Majekova, M.; Tielborger, K. Decision-making in plants under competition. Nat. Commun. 2017, 8, 2235. [Google Scholar] [CrossRef]
- Benkov, M.A.; Yatsenko, A.M.; Tikhonov, A.N. Light acclimation of shade-tolerant and sun-resistant tradescantia species: Photochemical activity of PSII and its sensitivity to heat treatment. Photosynth. Res. 2019, 139, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Gommers, C.M.; Keuskamp, D.H.; Buti, S.; van Veen, H.; Koevoets, I.T.; Reinen, E.; Voesenek, L.A.; Pierik, R. Molecular profiles of contrasting shade response strategies in wild plants: Differential control of immunity and shoot elongation. Plant Cell 2017, 29, 331–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranade, S.S.; Delhomme, N.; Garcia-Gil, M.R. Transcriptome analysis of shade avoidance and shade tolerance in conifers. Planta 2019, 250, 299–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelli, L.; Paulisic, S.; Qin, W.; Iglesias-Sanchez, A.; Roig-Villanova, I.; Florez-Sarasa, I.; Rodriguez-Concepcion, M.; Martinez-Garcia, J.F. Light signals generated by vegetation shade facilitate acclimation to low light in shade-avoider plants. Plant Physiol. 2021, 186, 2137–2151. [Google Scholar] [CrossRef] [PubMed]
- Molina-Contreras, M.J.; Paulisic, S.; Then, C.; Moreno-Romero, J.; Pastor-Andreu, P.; Morelli, L.; Roig-Villanova, I.; Jenkins, H.; Hallab, A.; Gan, X.; et al. Photoreceptor activity contributes to contrasting responses to shade in cardamine and Arabidopsis seedlings. Plant Cell 2019, 31, 2649–2663. [Google Scholar] [CrossRef]
- La Rocca, N.; Sciuto, K.; Meneghesso, A.; Moro, I.; Rascio, N.; Morosinotto, T. Photosynthesis in extreme environments: Responses to different light regimes in the Antarctic alga Koliella antarctica. Physiol. Plant 2015, 153, 654–667. [Google Scholar] [CrossRef]
- Paulišić, S.; Qin, W.; Verasztó, H.A.; Then, C.; Alary, B.; Nogue, F.; Tsiantis, M.; Hothorn, M.; Martínez-García, J.F. Adjustment of the PIF7-HFR1 transcriptional module activity controls plant shade adaptation. EMBO J. 2021, 40, e104273. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhao, Y.; Shen, R.; Wang, B.; Xie, Y.; Ma, X.; Zheng, Z.; Wang, H. Characterization of maize phytochrome-interacting factors in light signaling and photomorphogenesis. Plant Physiol. 2019, 181, 789–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gururani, M.A.; Ganesan, M.; Song, P.S. Photo-biotechnology as a tool to improve agronomic traits in crops. Biotechnol. Adv. 2015, 33, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, M.; Han, Y.J.; Bae, T.W.; Hwang, O.J.; Chandrasekhar, T.; Shin, A.Y.; Goh, C.H.; Nishiguchi, S.; Song, I.J.; Lee, H.Y.; et al. Overexpression of phytochrome A and its hyperactive mutant improves shade tolerance and turf quality in creeping bentgrass and zoysiagrass. Planta 2012, 236, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Sawers, R.J.; Wang, H.; Kim, J.K.; Walker, J.M.; Brutnell, T.P.; Parthasarathy, M.V.; Vierstra, R.D.; Wu, R.J. Light-regulated overexpression of an Arabidopsis phytochrome A gene in rice alters plant architecture and increases grain yield. Planta 2006, 223, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Heyer, A. Function of phytochrome A in potato plants as revealed through the study of transgenic plants. Plant Physiol. 1995, 109, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Boylan, M.T.; Quail, P.H. Oat phytochrome is biologically active in transgenic tomatoes. Plant Cell 1989, 1, 765–773. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, M.; Lee, H.Y.; Kim, J.I.; Song, P.S. Development of transgenic crops based on photo-biotechnology. Plant Cell Environ. 2017, 40, 2469–2486. [Google Scholar] [CrossRef]
- Bren d’Amour, C.; Reitsma, F.; Baiocchi, G.; Barthel, S.; Guneralp, B.; Erb, K.H.; Haberl, H.; Creutzig, F.; Seto, K.C. Future urban land expansion and implications for global croplands. Proc. Natl. Acad. Sci. USA 2017, 114, 8939–8944. [Google Scholar] [CrossRef] [Green Version]
- Asseng, S.; Guarin, J.R.; Raman, M.; Monje, O.; Kiss, G.; Despommier, D.D.; Meggers, F.M.; Gauthier, P.P.G. Wheat yield potential in controlled-environment vertical farms. Proc. Natl. Acad. Sci. USA 2020, 117, 19131–19135. [Google Scholar] [CrossRef] [PubMed]
- Slattery, R.A.; Ort, D.R. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol. 2021, 185, 34–48. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Liu, D.; Qin, S.; Sun, S.; Zhao, J.; Sui, S.F. Structure of phycobilisome from the red alga Griffithsia pacifica. Nature 2017, 551, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; You, X.; Sun, S.; Wang, X.; Qin, S.; Sui, S.F. Structural basis of energy transfer in Porphyridium purpureum phycobilisome. Nature 2020, 579, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Zhao, S.; Wang, W.; Liu, D.; Xu, C.; Han, G.; Kuang, T.; Sui, S.F.; Shen, J.R. The pigment-protein network of a diatom photosystem II-light-harvesting antenna supercomplex. Science 2019, 365, eaax4406. [Google Scholar] [CrossRef] [PubMed]
- South, P.F.; Cavanagh, A.P.; Liu, H.W.; Ort, D.R. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 2019, 363, eaat9077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roell, M.S.; Schada von Borzykowski, L.; Westhoff, P.; Plett, A.; Paczia, N.; Claus, P.; Urte, S.; Erb, T.J.; Weber, A.P.M. A synthetic C4 shuttle via the beta-hydroxyaspartate cycle in C3 plants. Proc. Natl. Acad. Sci. USA 2021, 118, e2022307118. [Google Scholar] [CrossRef]
- Han, X.; Chang, X.; Zhang, Z.; Chen, H.; He, H.; Zhong, B.; Deng, X.W. Origin and evolution of core components responsible for monitoring light environment changes during plant terrestrialization. Mol. Plant 2019, 12, 847–862. [Google Scholar] [CrossRef]
- Marondedze, C.; Liu, X.; Huang, S.; Wong, C.; Zhou, X.; Pan, X.; An, H.; Xu, N.; Tian, X.; Wong, A. Towards a tailored indoor horticulture: A functional genomics guided phenotypic approach. Hortic. Res. 2018, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Liang, L.; Freed, E.F.; Gill, R.T. Directed evolution of CRISPR/Cas systems for precise gene editing. Trends Biotechnol. 2021, 39, 262–273. [Google Scholar] [CrossRef]
- Desta, Z.A.; Ortiz, R. Genomic selection: Genome-wide prediction in plant improvement. Trends Plant Sci. 2014, 19, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.; Collins, J.; Romesburg, F. The future is synthetic biology. Cell 2018, 175, 895–897. [Google Scholar]
- Fernie, A.R.; Yan, J. De novo domestication: An alternative route toward new crops for the future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Duan, X.; Wang, P.; Li, X.; Yuan, X.; Wang, Z.; Wan, L.; Yang, G.; Hong, D. Comprehensive Speed Breeding: A high-throughput and rapid generation system for long-day crops. Plant Biotechnol. J. 2021, 20, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suarez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef] [Green Version]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Xie, G.; Yang, Q.; Ren, M. Biotechnological development of plants for space agriculture. Nat. Commun. 2021, 12, 5998. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Cheng, H.; Hou, C.; Ren, M. Integration of Light and Auxin Signaling in Shade Plants: From Mechanisms to Opportunities in Urban Agriculture. Int. J. Mol. Sci. 2022, 23, 3422. https://doi.org/10.3390/ijms23073422
Xie X, Cheng H, Hou C, Ren M. Integration of Light and Auxin Signaling in Shade Plants: From Mechanisms to Opportunities in Urban Agriculture. International Journal of Molecular Sciences. 2022; 23(7):3422. https://doi.org/10.3390/ijms23073422
Chicago/Turabian StyleXie, Xiulan, Hao Cheng, Chenyang Hou, and Maozhi Ren. 2022. "Integration of Light and Auxin Signaling in Shade Plants: From Mechanisms to Opportunities in Urban Agriculture" International Journal of Molecular Sciences 23, no. 7: 3422. https://doi.org/10.3390/ijms23073422





