Types and Functions of Mitoribosome-Specific Ribosomal Proteins across Eukaryotes
Abstract
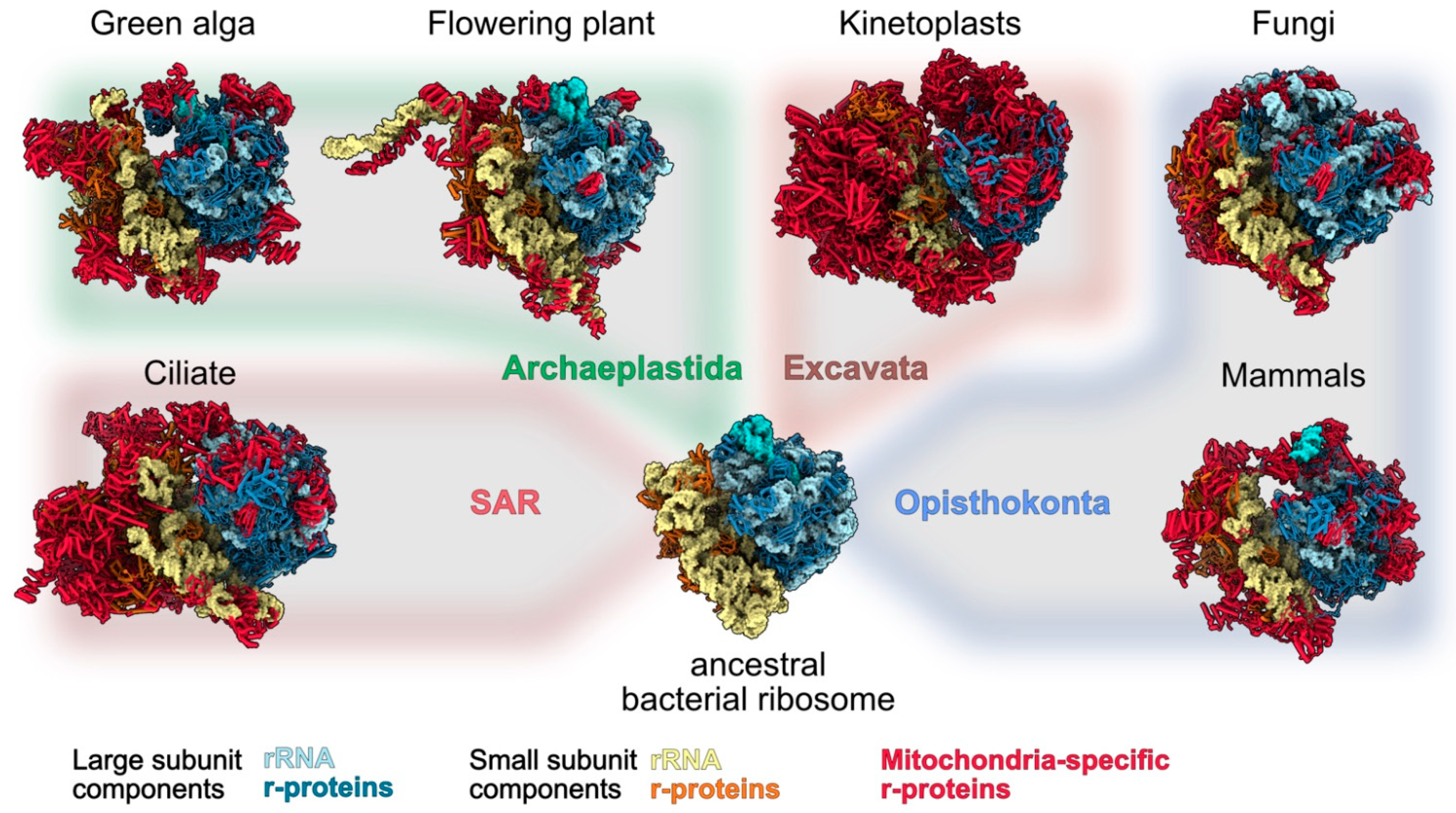
:1. Introduction
2. Technologies That Allowed to Identify Mitoribosome-Specific Proteins
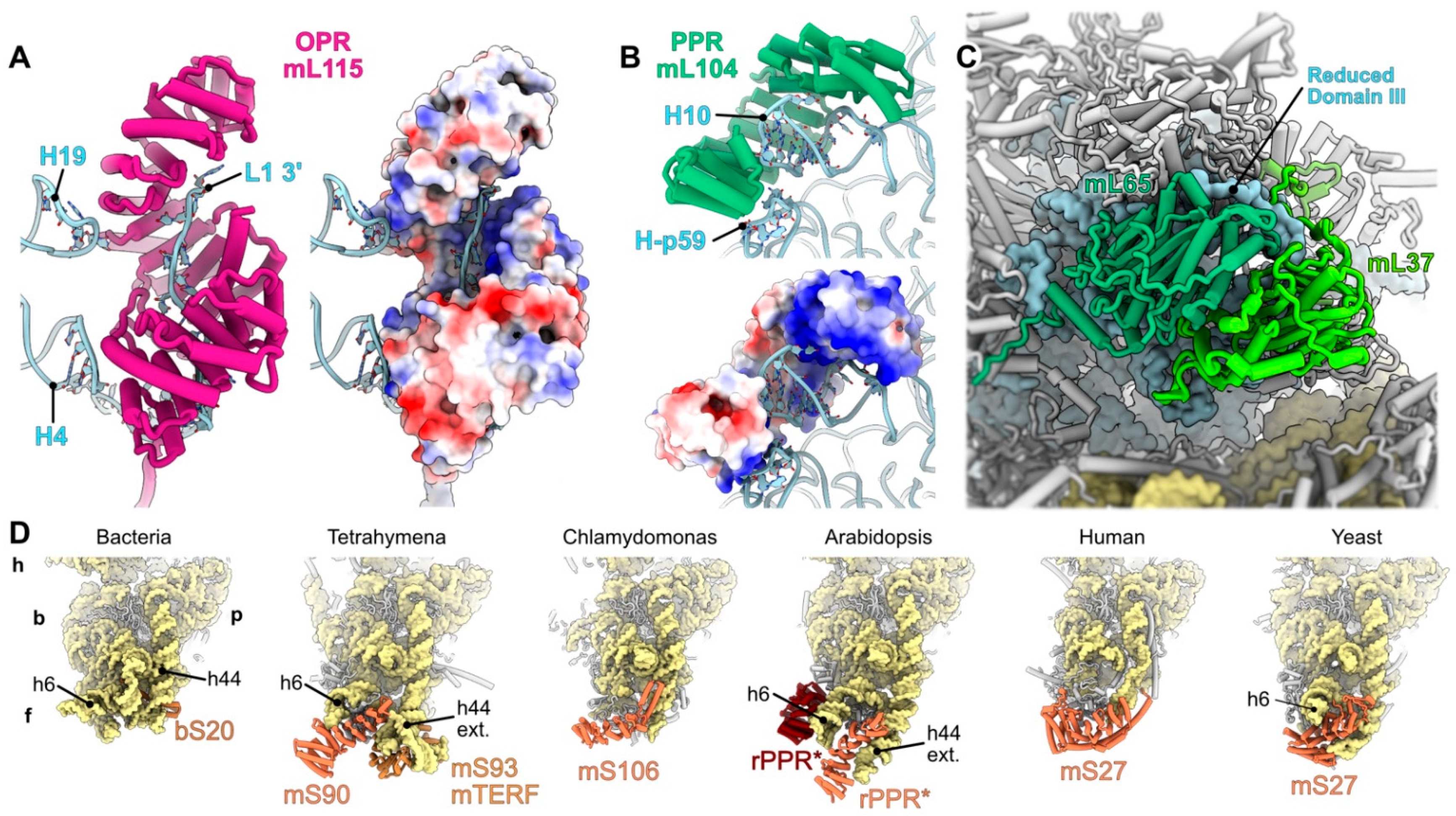
3. Prominence of Helical Repeat Proteins in Mitochondrial Gene Expression
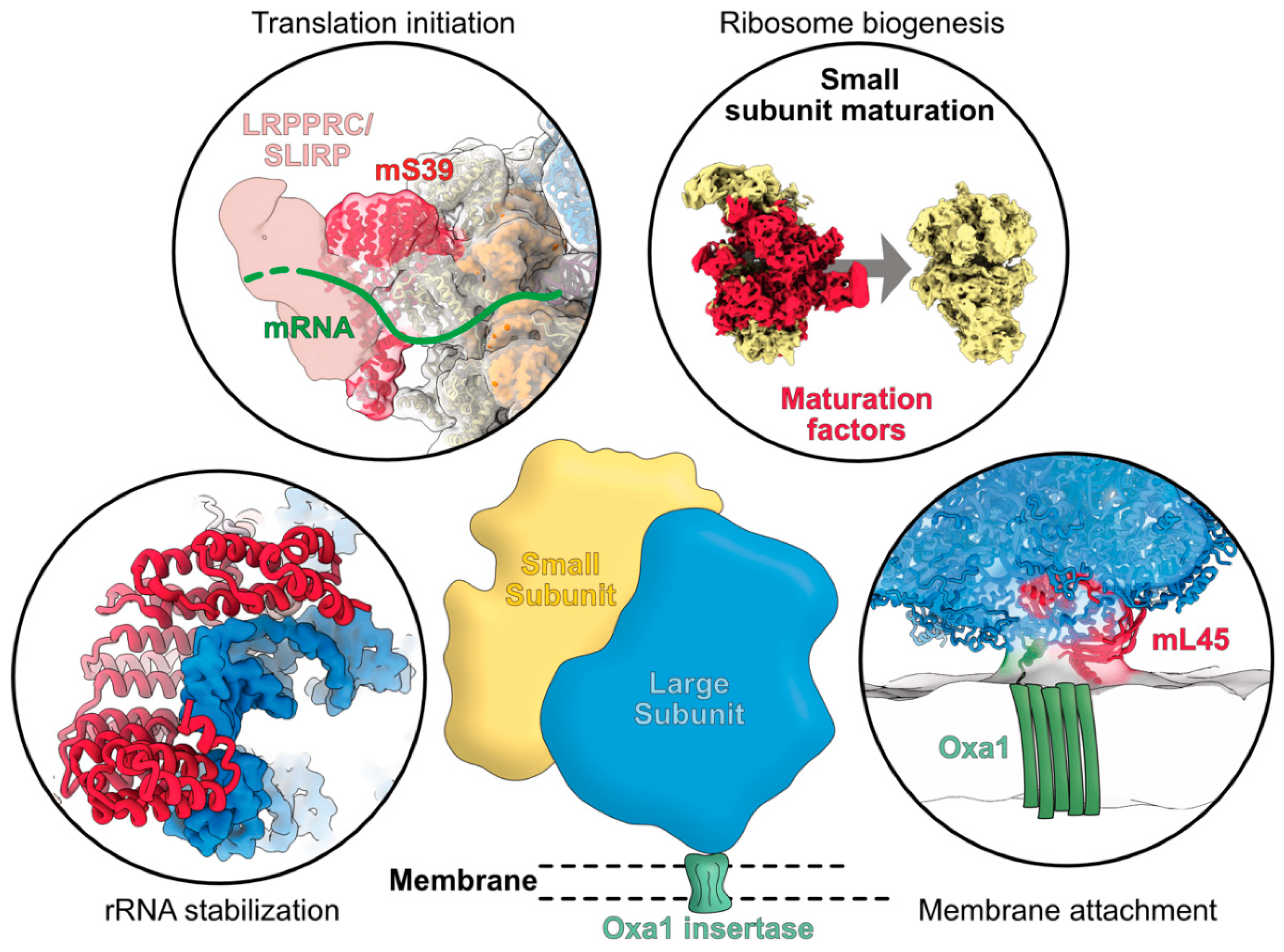
4. Functions and Modes of Action of Mitoribosome-Specific Proteins
4.1. rRNA Stabilization Mediated by Mitoribosome-Specific Proteins
4.2. Mitochondrial Specific mRNA Recruitment Processes
4.3. Involvement of Mitoribosome Specific Proteins for Protein Binding
4.4. Specific Processes for Mitoribosome Attachment to Membranes
4.5. Functions of Mitoribosome Specific Proteins for Ribosome Assembly
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melnikov, S.; Ben-Shem, A.; de Loubresse, N.G.; Jenner, L.; Yusupova, G.; Yusupov, M. One core, two shells: Bacterial and eukaryotic ribosomes. Nat. Struct. Mol. Biol. 2012, 19, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Schmeing, T.M.; Ramakrishnan, V. What recent ribosome structures have revealed about the mechanism of translation. Nature 2009, 461, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Londei, P.; Ferreira-Cerca, S. Ribosome Biogenesis in Archaea. Front. Microbiol. 2021, 12, 686977. [Google Scholar] [CrossRef] [PubMed]
- Khatter, H.; Myasnikov, A.G.; Natchiar, S.K.; Klaholz, B.P. Structure of the human 80S ribosome. Nature 2015, 520, 640–645. [Google Scholar] [CrossRef]
- Waltz, F.; Giegé, P. Striking Diversity of Mitochondria-Specific Translation Processes across Eukaryotes. Trends Biochem. Sci. 2020, 45, 149–162. [Google Scholar] [CrossRef]
- Kummer, E.; Ban, N. Mechanisms and regulation of protein synthesis in mitochondria. Nat. Rev. Mol. Cell Biol. 2021, 22, 307–325. [Google Scholar] [CrossRef]
- Eme, L.; Spang, A.; Lombard, J.; Stairs, C.W.; Ettema, T.J.G. Archaea and the origin of eukaryotes. Nat. Rev. Microbiol. 2017, 15, 711–723. [Google Scholar] [CrossRef]
- Martijn, J.; Vosseberg, J.; Guy, L.; Offre, P.; Ettema, T.J.G. Deep mitochondrial origin outside the sampled alphaproteobacteria. Nature 2018, 557, 101–105. [Google Scholar] [CrossRef]
- Muñoz-Gómez, S.A.; Susko, E.; Williamson, K.; Eme, L.; Slamovits, C.H.; Moreira, D.; López-García, P.; Roger, A.J. Site-and-branch-heterogeneous analyses of an expanded dataset favour mitochondria as sister to known Alphaproteobacteria. Nat. Ecol. Evol. 2022, 6, 253–262. [Google Scholar] [CrossRef]
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef] [Green Version]
- Dorrell, R.G.; Howe, C.J. What makes a chloroplast? Reconstructing the establishment of photosynthetic symbioses. J. Cell Sci. 2012, 125, 1865–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkholm, P.; Harish, A.; Hagström, E.; Ernst, A.M.; Andersson, S.G.E. Mitochondrial genomes are retained by selective constraints on protein targeting. Proc. Natl. Acad. Sci. USA 2015, 112, 10154–10161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.F. Why chloroplasts and mitochondria retain their own genomes and genetic systems: Colocation for redox regulation of gene expression. Proc. Natl. Acad. Sci. USA 2015, 112, 10231–10238. [Google Scholar] [CrossRef] [Green Version]
- Zoschke, R.; Bock, R. Chloroplast Translation: Structural and Functional Organization, Operational Control, and Regulation. Plant Cell 2018, 30, 745–770. [Google Scholar] [CrossRef] [Green Version]
- Bieri, P.; Leibundgut, M.; Saurer, M.; Boehringer, D.; Ban, N. The complete structure of the chloroplast 70S ribosome in complex with translation factor pY. EMBO J. 2017, 36, 475–486. [Google Scholar] [CrossRef]
- Boerema, A.P.; Aibara, S.; Paul, B.; Tobiasson, V.; Kimanius, D.; Forsberg, B.O.; Wallden, K.; Lindahl, E.; Amunts, A. Structure of the chloroplast ribosome with chl-RRF and hibernation-promoting factor. Nat. Plants 2018, 4, 212–217. [Google Scholar] [CrossRef]
- Graf, M.; Arenz, S.; Huter, P.; Dönhöfer, A.; Nováček, J.; Wilson, D.N. Cryo-EM structure of the spinach chloroplast ribosome reveals the location of plastid-specific ribosomal proteins and extensions. Nucleic Acids Res. 2016, 45, gkw1272. [Google Scholar] [CrossRef]
- Clemons, W.M.; May, J.L.C.; Wimberly, B.T.; McCutcheon, J.P.; Capel, M.S.; Ramakrishnan, V. Structure of a bacterial 30S ribosomal subunit at 5.5 Å resolution. Nature 1999, 400, 833–840. [Google Scholar] [CrossRef]
- Ben-Shem, A.; de Loubresse, N.G.; Melnikov, S.; Jenner, L.; Yusupova, G.; Yusupov, M. The Structure of the Eukaryotic Ribosome at 3.0 A Resolution. Science 2011, 334, 1524–1529. [Google Scholar] [CrossRef] [Green Version]
- Greber, B.J.; Bieri, P.; Leibundgut, M.; Leitner, A.; Aebersold, R.; Boehringer, D.; Ban, N. The complete structure of the 55S mammalian mitochondrial ribosome. Science 2015, 348, 303–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amunts, A.; Brown, A.; Toots, J.; Scheres, S.H.W.; Ramakrishnan, V. The structure of the human mitochondrial ribosome. Science 2015, 348, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, N.; Brown, A.; Amunts, A.; Ramakrishnan, V. The structure of the yeast mitochondrial ribosome. Science 2017, 355, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramrath, D.J.F.; Niemann, M.; Leibundgut, M.; Bieri, P.; Prange, C.; Horn, E.K.; Leitner, A.; Boehringer, D.; Schneider, A.; Ban, N. Evolutionary shift toward protein-based architecture in trypanosomal mitochondrial ribosomes. Science 2018, 362, eaau7735. [Google Scholar] [CrossRef]
- Waltz, F.; Soufari, H.; Bochler, A.; Giegé, P.; Hashem, Y. Cryo-EM structure of the RNA-rich plant mitochondrial ribosome. Nat. Plants 2020, 6, 377–383. [Google Scholar] [CrossRef]
- Waltz, F.; Nguyen, T.; Arrivé, M.; Bochler, A.; Chicher, J.; Hammann, P.; Kuhn, L.; Quadrado, M.; Mireau, H.; Hashem, Y.; et al. Small is big in Arabidopsis mitochondrial ribosome. Nat. Plants 2019, 5, 106–117. [Google Scholar] [CrossRef]
- Waltz, F.; Salinas-Giegé, T.; Englmeier, R.; Meichel, H.; Soufari, H.; Kuhn, L.; Pfeffer, S.; Förster, F.; Engel, B.D.; Giegé, P.; et al. How to build a ribosome from RNA fragments in Chlamydomonas mitochondria. Nat. Commun. 2021, 12, 7176. [Google Scholar] [CrossRef]
- Tobiasson, V.; Amunts, A. Ciliate mitoribosome illuminates evolutionary steps of mitochondrial translation. eLife 2020, 9, e59264. [Google Scholar] [CrossRef]
- Bieri, P.; Greber, B.J.; Ban, N. High-resolution structures of mitochondrial ribosomes and their functional implications. Curr. Opin. Struct. Biol. 2018, 49, 44–53. [Google Scholar] [CrossRef]
- Valach, M.; Alcazar, J.A.G.; Sarrasin, M.; Lang, B.F.; Gray, M.W.; Burger, G. An Unexpectedly Complex Mitoribosome in Andalucia godoyi, a Protist with the Most Bacteria-like Mitochondrial Genome. Mol. Biol. Evol. 2021, 38, 788–804. [Google Scholar] [CrossRef]
- Mager, J. Chloramphenicol and chlortetracycline inhibition of amino acid incorporation into proteins in a cell-free system from Tetrahymena pyriformis. Biochim. Biophys. Acta 1960, 38, 150–152. [Google Scholar] [CrossRef]
- Rendi, R. On the occurrence of intramitochondrial ribonucleoprotein particles. Exp. Cell Res. 1959, 17, 585–587. [Google Scholar] [CrossRef]
- Palade, G.E. A small particulate component of the cytoplasm. J. Cell Biol. 1955, 1, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, P.; Grivell, L.A. Mitochondrial ribosomes. FEBS Lett. 1971, 13, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.E.; Hessler, R.A.; Denslow, N.D.; Edwards, J.S.; O’Brien, T.W. Protein composition of the bovine mitochondrial ribosome. J. Biol. Chem. 1982, 257, 8788–8794. [Google Scholar] [CrossRef]
- Koc, E.C.; Burkhart, W.; Blackburn, K.; Moyer, M.B.; Schlatzer, D.M.; Moseley, A.; Spremulli, L.L. The Large Subunit of the Mammalian Mitochondrial Ribosome. J. Biol. Chem. 2001, 276, 43958–43969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koc, E.C.; Burkhart, W.; Blackburn, K.; Moseley, A.; Koc, H.; Spremulli, L.L. A Proteomics Approach to the Identification of Mammalian Mitochondrial Small Subunit Ribosomal Proteins. J. Biol. Chem. 2000, 275, 32585–32591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.R.; Koc, E.C.; Datta, P.P.; Booth, T.M.; Spremulli, L.L.; Agrawal, R.K. Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell 2003, 115, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Bonen, L.; Calixte, S. Comparative Analysis of Bacterial-Origin Genes for Plant Mitochondrial Ribosomal Proteins. Mol. Biol. Evol. 2006, 23, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Gan, X.; Arita, K.; Isono, S.; Kitakawa, M.; Yoshino, K.; Yonezawa, K.; Kato, A.; Inoue, H.; Isono, K. Identification and comparative analysis of the large subunit mitochondrial ribosomal proteins of Neurospora crassa. FEMS Microbiol. Lett. 2006, 254, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.R.; Booth, T.M.; Simpson, L.; Maslov, D.A.; Agrawal, R.K. Structure of a mitochondrial ribosome with minimal RNA. Proc. Natl. Acad. Sci. USA 2009, 106, 9637–9642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslov, D.A.; Sharma, M.R.; Butler, E.; Falick, A.M.; Gingery, M.; Agrawal, R.K.; Spremulli, L.L.; Simpson, L. Isolation and characterization of mitochondrial ribosomes and ribosomal subunits from Leishmania tarentolae. Mol. Biochem. Parasitol. 2006, 148, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Rugen, N.; Straube, H.; Franken, L.E.; Braun, H.-P.; Eubel, H. Complexome profiling reveals association of PPR proteins with ribosomes in the mitochondria of plants. Mol. Cell. Proteom. 2019, 18, 1345–1362. [Google Scholar] [CrossRef]
- Smirnov, A.; Förstner, K.U.; Holmqvist, E.; Otto, A.; Günster, R.; Becher, D.; Reinhardt, R.; Vogel, J. Grad-seq guides the discovery of ProQ as a major small RNA-binding protein. Proc. Natl. Acad. Sci. USA 2016, 113, 11591–11596. [Google Scholar] [CrossRef] [Green Version]
- Gerovac, M.; Vogel, J.; Smirnov, A. The World of Stable Ribonucleoproteins and Its Mapping with Grad-Seq and Related Approaches. Front. Mol. Biosci. 2021, 8, 186. [Google Scholar] [CrossRef]
- O’Reilly, F.J.; Rappsilber, J. Cross-linking mass spectrometry: Methods and applications in structural, molecular and systems biology. Nat. Struct. Mol. Biol. 2018, 25, 1000–1008. [Google Scholar] [CrossRef] [Green Version]
- Kühlbrandt, W. The Resolution Revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef]
- Amunts, A.; Brown, A.; Bai, X.C.; Llacer, J.L.; Hussain, T.; Emsley, P.; Long, F.; Murshudov, G.; Scheres, S.H.W.; Ramakrishnan, V. Structure of the Yeast Mitochondrial Large Ribosomal Subunit. Science 2014, 343, 1485–1489. [Google Scholar] [CrossRef] [Green Version]
- Greber, B.J.; Boehringer, D.; Leitner, A.; Bieri, P.; Voigts-Hoffmann, F.; Erzberger, J.P.; Leibundgut, M.; Aebersold, R.; Ban, N. Architecture of the large subunit of the mammalian mitochondrial ribosome. Nature 2013, 505, 515–519. [Google Scholar] [CrossRef]
- Hammani, K.; Bonnard, G.; Bouchoucha, A.; Gobert, A.; Pinker, F.; Salinas, T.; Giegé, P. Helical repeats modular proteins are major players for organelle gene expression. Biochimie 2014, 100, 141–150. [Google Scholar] [CrossRef]
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.; Rojas, M.; Fujii, S.; Yap, A.; Chong, Y.S.; Bond, C.S.; Small, I. A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins. PLoS Genet. 2012, 8, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Pinker, F.; Fuchsbauer, O.; Gutmann, B.; Boutin, R.; Roblin, P.; Sauter, C.; Giegé, P. Structural insights into protein-only RNase P complexed with tRNA. Nat. Commun. 2013, 4, 1353. [Google Scholar] [CrossRef] [PubMed]
- Pinker, F.; Schelcher, C.; Fernandez-Millan, P.; Gobert, A.; Birck, C.; Thureau, A.; Roblin, P.; Giegé, P.; Sauter, C. Biophysical analysis of Arabidopsis protein-only RNase P alone and in complex with tRNA provides a refined model of tRNA binding. J. Biol. Chem. 2017, 292, 13904–13913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teramoto, T.; Kaitany, K.J.; Kakuta, Y.; Kimura, M.; Fierke, C.A.; Hall, T.M.T. Pentatricopeptide repeats of protein-only RNase P use a distinct mode to recognize conserved bases and structural elements of pre-tRNA. Nucleic Acids Res. 2020, 48, 11815–11826. [Google Scholar] [CrossRef]
- Preker, P.J.; Keller, W. The HAT helix, a repetitive motif implicated in RNA processing. Trends Biochem. Sci. 1998, 23, 15–16. [Google Scholar] [CrossRef]
- Hammani, K.; Cook, W.B.; Barkan, A. RNA binding and RNA remodeling activities of the half-a-tetratricopeptide (HAT) protein HCF107 underlie its effects on gene expression. Proc. Natl. Acad. Sci. USA 2012, 109, 5651–5656. [Google Scholar] [CrossRef] [Green Version]
- Roberti, M.; Polosa, P.L.; Bruni, F.; Manzari, C.; Deceglie, S.; Gadaleta, M.N.; Cantatore, P. The MTERF family proteins: Mitochondrial transcription regulators and beyond. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Spåhr, H.; Habermann, B.; Gustafsson, C.M.; Larsson, N.G.; Hallberg, B.M. Structure of the human MTERF4-NSUN4 protein complex that regulates mitochondrial ribosome biogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 15253–15258. [Google Scholar] [CrossRef] [Green Version]
- Méteignier, L.-V.; Ghandour, R.; Zimmerman, A.; Kuhn, L.; Meurer, J.; Zoschke, R.; Hammani, K. Arabidopsis mTERF9 protein promotes chloroplast ribosomal assembly and translation by establishing ribonucleoprotein interactions in vivo. Nucleic Acids Res. 2021, 49, 1114–1132. [Google Scholar] [CrossRef]
- Ozawa, S.-I.; Cavaiuolo, M.; Jarrige, D.; Kuras, R.; Rutgers, M.; Eberhard, S.; Drapier, D.; Wollman, F.-A.; Choquet, Y. The OPR Protein MTHI1 Controls the Expression of Two Different Subunits of ATP Synthase CFo in Chlamydomonas reinhardtii. Plant Cell 2020, 32, 1179–1203. [Google Scholar] [CrossRef] [PubMed]
- Viola, S.; Cavaiuolo, M.; Drapier, D.; Eberhard, S.; Vallon, O.; Wollman, F.; Choquet, Y. MDA 1, a nucleus-encoded factor involved in the stabilization and processing of the atpA transcript in the chloroplast of Chlamydomonas. Plant J. 2019, 98, tpj.14300. [Google Scholar] [CrossRef]
- Kleinknecht, L.; Wang, F.; Stübe, R.; Philippar, K.; Nickelsen, J.; Bohne, A.-V. RAP, the Sole Octotricopeptide Repeat Protein in Arabidopsis, Is Required for Chloroplast 16S rRNA Maturation. Plant Cell 2014, 26, 777–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillebrand, A.; Matz, J.M.; Almendinger, M.; Müller, K.; Matuschewski, K.; Schmitz-Linneweber, C. Identification of clustered organellar short (cos) RNAs and of a conserved family of organellar RNA-binding proteins, the heptatricopeptide repeat proteins, in the malaria parasite. Nucleic Acids Res. 2018, 46, 10417–10431. [Google Scholar] [CrossRef]
- Filipovska, A.; Rackham, O. Modular recognition of nucleic acids by PUF, TALE and PPR proteins. Mol. Biosyst. 2012, 8, 699–708. [Google Scholar] [CrossRef]
- Rubinson, E.H.; Eichman, B.F. Nucleic acid recognition by tandem helical repeats. Curr. Opin. Struct. Biol. 2012, 22, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Delannoy, E.; Stanley, W.A.; Bond, C.S.; Small, I.D. Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem. Soc. Trans. 2007, 35, 1643–1647. [Google Scholar] [CrossRef] [Green Version]
- Edwards, T.A.; Pyle, S.E.; Wharton, R.P.; Aggarwal, A.K. Structure of Pumilio reveals similarity between RNA and peptide binding motifs. Cell 2001, 105, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Nikolay, R.; van den Bruck, D.; Achenbach, J.; Nierhaus, K.H. Ribosomal Proteins: Role in Ribosomal Functions. In eLS; Wiley: Hoboken, NJ, USA, 2015; pp. 1–12. [Google Scholar]
- Itoh, Y.; Naschberger, A.; Mortezaei, N.; Herrmann, J.M.; Amunts, A. Analysis of translating mitoribosome reveals functional characteristics of translation in mitochondria of fungi. Nat. Commun. 2020, 11, 5187. [Google Scholar] [CrossRef]
- Rodnina, M.V. Translation in Prokaryotes. Cold Spring Harb. Perspect. Biol. 2018, 10, a032664. [Google Scholar] [CrossRef]
- Shine, J.; Dalgarno, L. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: Complementarity to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. USA 1974, 71, 1342–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geissmann, T.; Marzi, S.; Romby, P. The role of mRNA structure in translational control in bacteria. RNA Biol. 2009, 6, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Duval, M.; Korepanov, A.; Fuchsbauer, O.; Fechter, P.; Haller, A.; Fabbretti, A.; Choulier, L.; Micura, R.; Klaholz, B.P.; Romby, P.; et al. Escherichia coli ribosomal protein S1 unfolds structured mRNAs onto the ribosome for active translation initiation. PLoS Biol. 2013, 11, e1001731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, K.; Green, R.; Buskirk, A.R. Translational initiation in E. Coli occurs at the correct sites genome-wide in the absence of mrna-rrna base-pairing. eLife 2020, 9, e55002. [Google Scholar] [CrossRef] [PubMed]
- Unseld, M.; Marienfeld, J.R.; Brandt, P.; Brennicke, A. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat. Genet. 1997, 15, 57–61. [Google Scholar] [CrossRef]
- Waltz, F.; Corre, N.; Hashem, Y.; Giegé, P. Specificities of the plant mitochondrial translation apparatus. Mitochondrion 2020, 53, 30–37. [Google Scholar] [CrossRef]
- Burger, G.; Gray, M.W.; Forget, L.; Lang, B.F. Strikingly Bacteria-Like and Gene-Rich Mitochondrial Genomes throughout Jakobid Protists. Genome Biol. Evol. 2013, 5, 418–438. [Google Scholar] [CrossRef] [Green Version]
- Temperley, R.J.; Wydro, M.; Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M. Human mitochondrial mRNAs—Like members of all families, similar but different. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1081–1085. [Google Scholar] [CrossRef] [Green Version]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef]
- Montoya, J.; Ojala, D.; Attardi, G. Distinctive features of the 5′-terminal sequences of the human mitochondrial mRNAs. Nature 1981, 290, 465–470. [Google Scholar] [CrossRef]
- Kummer, E.; Leibundgut, M.; Rackham, O.; Lee, R.G.; Boehringer, D.; Filipovska, A.; Ban, N. Unique features of mammalian mitochondrial translation initiation revealed by cryo-EM. Nature 2018, 560, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Aibara, S.; Singh, V.; Modelska, A.; Amunts, A. Structural basis of mitochondrial translation. eLife 2020, 9, e58362. [Google Scholar] [CrossRef] [PubMed]
- Baggio, F.; Bratic, A.; Mourier, A.; Kauppila, T.E.S.; Tain, L.S.; Kukat, C.; Habermann, B.; Partridge, L.; Larsson, N.-G. Drosophila melanogaster LRPPRC2 is involved in coordination of mitochondrial translation. Nucleic Acids Res. 2014, 42, 13920–13938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzzenente, B.; Metodiev, M.D.; Wredenberg, A.; Bratic, A.; Park, C.B.; Cámara, Y.; Milenkovic, D.; Zickermann, V.; Wibom, R.; Hultenby, K.; et al. LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J. 2012, 31, 443–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siira, S.J.; Spåhr, H.; Shearwood, A.-M.J.; Ruzzenente, B.; Larsson, N.-G.; Rackham, O.; Filipovska, A. LRPPRC-mediated folding of the mitochondrial transcriptome. Nat. Commun. 2017, 8, 1532. [Google Scholar] [CrossRef] [Green Version]
- Lagouge, M.; Mourier, A.; Lee, H.J.; Spåhr, H.; Wai, T.; Kukat, C.; Ramos, E.S.; Motori, E.; Busch, J.D.; Siira, S.; et al. SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from Degradation. PLoS Genet. 2015, 11, e1005423. [Google Scholar] [CrossRef]
- Weraarpachai, W.; Antonicka, H.; Sasarman, F.; Seeger, J.; Schrank, B.; Kolesar, J.E.; Lochmüller, H.; Chevrette, M.; Kaufman, B.A.; Horvath, R.; et al. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat. Genet. 2009, 41, 833–837. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Woellhaf, M.W.; Bonnefoy, N. Control of protein synthesis in yeast mitochondria: The concept of translational activators. Biochim. Biophys. Acta 2013, 1833, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Derbikova, K.S.; Levitsky, S.A.; Chicherin, I.V.; Vinogradova, E.N.; Kamenski, P.A. Activation of Yeast Mitochondrial Translation: Who Is in Charge? Biochemistry 2018, 83, 87–97. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Planchard, N.; Dahan, J.; Arnal, N.; Balzergue, S.; Benamar, A.; Bertin, P.; Brunaud, V.; Dargel-Graffin, C.; Macherel, D.; et al. A Case of Gene Fragmentation in Plant Mitochondria Fixed by the Selection of a Compensatory Restorer of Fertility-Like PPR Gene. Mol. Biol. Evol. 2021, 38, 3445–3458. [Google Scholar] [CrossRef]
- Salinas-Giegé, T.; Cavaiuolo, M.; Cognat, V.; Ubrig, E.; Remacle, C.; Duchêne, A.-M.; Vallon, O.; Maréchal-Drouard, L. Polycytidylation of mitochondrial mRNAs in Chlamydomonas reinhardtii. Nucleic Acids Res. 2017, 45, 12963–12973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahoon, A.B.; Qureshi, A.A. Leaderless mRNAs are circularized in Chlamydomonas reinhardtii mitochondria. Curr. Genet. 2018, 64, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Feagin, J.E.; Harrell, M.I.; Lee, J.C.; Coe, K.J.; Sands, B.H.; Cannone, J.J.; Tami, G.; Schnare, M.N.; Gutell, R.R. The Fragmented Mitochondrial Ribosomal RNAs of Plasmodium falciparum. PLoS ONE 2012, 7, e38320. [Google Scholar] [CrossRef]
- Soufari, H.; Waltz, F.; Parrot, C.; Durrieu-Gaillard, S.; Bochler, A.; Kuhn, L.; Sissler, M.; Hashem, Y. Structure of the mature kinetoplastids mitoribosome and insights into its large subunit biogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 29851–29861. [Google Scholar] [CrossRef] [PubMed]
- Noller, H.F.; Lancaster, L.; Zhou, J.; Mohan, S. The ribosome moves: RNA mechanics and translocation. Nat. Struct. Mol. Biol. 2017, 24, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Trabuco, L.G.; Schreiner, E.; Eargle, J.; Cornish, P.; Ha, T.; Luthey-Schulten, Z.; Schulten, K. The Role of L1 Stalk–tRNA Interaction in the Ribosome Elongation Cycle. J. Mol. Biol. 2010, 402, 741–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaskolowski, M.; Ramrath, D.J.F.; Bieri, P.; Niemann, M.; Mattei, S.; Calderaro, S.; Leibundgut, M.; Horn, E.K.; Boehringer, D.; Schneider, A.; et al. Structural Insights into the Mechanism of Mitoribosomal Large Subunit Biogenesis. Mol. Cell 2020, 79, 629–644. [Google Scholar] [CrossRef]
- Tobiasson, V.; Rej Gahura, O.; Aibara, S.; Baradaran, R.; Ikov, A.; Amunts, A. Interconnected assembly factors regulate the biogenesis of mitoribosomal large subunit. EMBO J. 2021, 40, e106292. [Google Scholar] [CrossRef]
- Ratje, A.H.; Loerke, J.; Mikolajka, A.; Brünner, M.; Hildebrand, P.W.; Starosta, A.L.; Dönhöfer, A.; Connell, S.R.; Fucini, P.; Mielke, T.; et al. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature 2010, 468, 713–716. [Google Scholar] [CrossRef] [Green Version]
- Ott, M.; Amunts, A.; Brown, A. Organization and Regulation of Mitochondrial Protein Synthesis. Annu. Rev. Biochem. 2016, 85, 77–101. [Google Scholar] [CrossRef]
- Keil, M.; Bareth, B.; Woellhaf, M.W.; Peleh, V.; Prestele, M.; Rehling, P.; Herrmann, J.M. Oxa1-Ribosome Complexes Coordinate the Assembly of Cytochrome c Oxidase in Mitochondria. J. Biol. Chem. 2012, 287, 34484–34493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, R.A. Insertion of proteins into the inner membrane of mitochondria: The role of the Oxa1 complex. Biochim. Biophys. Acta—Mol. Cell Res. 2002, 1592, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Krüger, V.; Deckers, M.; Hildenbeutel, M.; van der Laan, M.; Hellmers, M.; Dreker, C.; Preuss, M.; Herrmann, J.M.; Rehling, P.; Wagner, R.; et al. The Mitochondrial Oxidase Assembly Protein1 (Oxa1) Insertase Forms a Membrane Pore in Lipid Bilayers. J. Biol. Chem. 2012, 287, 33314–33326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiller, S.B.; Höpker, J.; Oeljeklaus, S.; Schütze, C.; Schrempp, S.G.; Vent-Schmidt, J.; Horvath, S.E.; Frazier, A.E.; Gebert, N.; van der Laan, M.; et al. Mitochondrial OXA Translocase Plays a Major Role in Biogenesis of Inner-Membrane Proteins. Cell Metab. 2016, 23, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Dalbey, R.E. Inserting membrane proteins: The YidC/Oxa1/Alb3 machinery in bacteria, mitochondria, and chloroplasts. Biochim. Biophys. Acta—Biomembr. 2011, 1808, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Hennon, S.W.; Soman, R.; Zhu, L.; Dalbey, R.E. YidC/Alb3/Oxa1 Family of Insertases. J. Biol. Chem. 2015, 290, 14866–14874. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.E.; Elmore, K.B.; Tripathy, A.; Koc, H.; Koc, E.C.; Spremulli, L.L. Properties of the C-terminal Tail of Human Mitochondrial Inner Membrane Protein Oxa1L and Its Interactions with Mammalian Mitochondrial Ribosomes. J. Biol. Chem. 2010, 285, 28353–28362. [Google Scholar] [CrossRef] [Green Version]
- Jia, L. Yeast Oxa1 interacts with mitochondrial ribosomes: The importance of the C-terminal region of Oxa1. EMBO J. 2003, 22, 6438–6447. [Google Scholar] [CrossRef]
- Szyrach, G.; Ott, M.; Bonnefoy, N.; Neupert, W.; Herrmann, J.M. Ribosome binding to the Oxa1 complex facilitates co-translational protein insertion in mitochondria. EMBO J. 2003, 22, 6448–6457. [Google Scholar] [CrossRef]
- Hildenbeutel, M.; Theis, M.; Geier, M.; Haferkamp, I.; Neuhaus, H.E.; Herrmann, J.M.; Ott, M. The Membrane Insertase Oxa1 Is Required for Efficient Import of Carrier Proteins into Mitochondria. J. Mol. Biol. 2012, 423, 590–599. [Google Scholar] [CrossRef]
- Itoh, Y.; Andréll, J.; Choi, A.; Richter, U.; Maiti, P.; Best, R.B.; Barrientos, A.; Battersby, B.J.; Amunts, A. Mechanism of membrane-tethered mitochondrial protein synthesis. Science 2021, 371, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Prestele, M.; Bauerschmitt, H.; Funes, S.; Bonnefoy, N.; Herrmann, J.M. Mba1, a membrane-associated ribosome receptor in mitochondria. EMBO J. 2006, 25, 1603–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, S.; Woellhaf, M.W.; Herrmann, J.M.; Förster, F. Organization of the mitochondrial translation machinery studied in situ by cryoelectron tomography. Nat. Commun. 2015, 6, 6019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englmeier, R.; Pfeffer, S.; Förster, F. Structure of the Human Mitochondrial Ribosome Studied In Situ by Cryoelectron Tomography. Structure 2017, 25, 1574–1581.e2. [Google Scholar] [CrossRef] [Green Version]
- Benz, M.; Soll, J.; Ankele, E. Arabidopsis thaliana Oxa proteins locate to mitochondria and fulfill essential roles during embryo development. Planta 2013, 237, 573–588. [Google Scholar] [CrossRef]
- Kressler, D.; Hurt, E.; Baßler, J. A Puzzle of Life: Crafting Ribosomal Subunits. Trends Biochem. Sci. 2017, 42, 640–654. [Google Scholar] [CrossRef] [Green Version]
- Erdmann, P.S.; Hou, Z.; Klumpe, S.; Khavnekar, S.; Beck, F.; Wilfling, F.; Plitzko, J.M.; Baumeister, W. In situ cryo-electron tomography reveals gradient organization of ribosome biogenesis in intact nucleoli. Nat. Commun. 2021, 12, 5364. [Google Scholar] [CrossRef]
- Klinge, S.; Woolford, J.L. Ribosome assembly coming into focus. Nat. Rev. Mol. Cell Biol. 2019, 20, 116–131. [Google Scholar] [CrossRef]
- Kargas, V.; Castro-Hartmann, P.; Escudero-Urquijo, N.; Dent, K.; Hilcenko, C.; Sailer, C.; Zisser, G.; Marques-Carvalho, M.J.; Pellegrino, S.; Wawiórka, L.; et al. Mechanism of completion of peptidyltransferase centre assembly in eukaryotes. eLife 2019, 8, e44904. [Google Scholar] [CrossRef]
- Ameismeier, M.; Zemp, I.; van den Heuvel, J.; Thoms, M.; Berninghausen, O.; Kutay, U.; Beckmann, R. Structural basis for the final steps of human 40S ribosome maturation. Nature 2020, 587, 683–687. [Google Scholar] [CrossRef]
- Cheng, J.; Lau, B.; La Venuta, G.; Ameismeier, M.; Berninghausen, O.; Hurt, E.; Beckmann, R. 90S pre-ribosome transformation into the primordial 40S subunit. Science 2020, 369, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.; Cheng, J.; Flemming, D.; La Venuta, G.; Berninghausen, O.; Beckmann, R.; Hurt, E. Structure of the Maturing 90S Pre-ribosome in Association with the RNA Exosome. Mol. Cell 2021, 81, 293–303.e4. [Google Scholar] [CrossRef] [PubMed]
- Nikolay, R.; Hilal, T.; Qin, B.; Mielke, T.; Bürger, J.; Loerke, J.; Textoris-Taube, K.; Nierhaus, K.H.; Spahn, C.M.T. Structural Visualization of the Formation and Activation of the 50S Ribosomal Subunit during In Vitro Reconstitution. Mol. Cell 2018, 70, 881–893.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolay, R.; Hilal, T.; Schmidt, S.; Qin, B.; Schwefel, D.; Vieira-Vieira, C.H.; Mielke, T.; Bürger, J.; Loerke, J.; Amikura, K.; et al. Snapshots of native pre-50S ribosomes reveal a biogenesis factor network and evolutionary specialization. Mol. Cell 2021, 81, 1200–1215.e9. [Google Scholar] [CrossRef]
- Sissler, M.; Hashem, Y. Mitoribosome assembly comes into view. Nat. Struct. Mol. Biol. 2021, 28, 631–633. [Google Scholar] [CrossRef]
- Cipullo, M.; Gesé, G.V.; Khawaja, A.; Hällberg, B.M.; Rorbach, J. Structural basis for late maturation steps of the human mitoribosomal large subunit. Nat. Commun. 2021, 12, 3673. [Google Scholar] [CrossRef]
- Hillen, H.S.; Lavdovskaia, E.; Nadler, F.; Hanitsch, E.; Linden, A.; Bohnsack, K.E.; Urlaub, H.; Richter-Dennerlein, R. Structural basis of GTPase-mediated mitochondrial ribosome biogenesis and recycling. Nat. Commun. 2021, 12, 3672. [Google Scholar] [CrossRef]
- Cheng, J.; Berninghausen, O.; Beckmann, R. A distinct assembly pathway of the human 39S late pre-mitoribosome. Nat. Commun. 2021, 12, 4544. [Google Scholar] [CrossRef]
- Lenarčič, T.; Jaskolowski, M.; Leibundgut, M.; Scaiola, A.; Schönhut, T.; Saurer, M.; Lee, R.G.; Rackham, O.; Filipovska, A.; Ban, N. Stepwise maturation of the peptidyl transferase region of human mitoribosomes. Nat. Commun. 2021, 12, 3671. [Google Scholar] [CrossRef]
- Chandrasekaran, V.; Desai, N.; Burton, N.O.; Yang, H.; Price, J.; Miska, E.A.; Ramakrishnan, V. Visualizing formation of the active site in the mitochondrial ribosome. eLife 2021, 10, e68806. [Google Scholar] [CrossRef]
- Saurer, M.; Ramrath, D.J.F.; Niemann, M.; Calderaro, S.; Prange, C.; Mattei, S.; Scaiola, A.; Leitner, A.; Bieri, P.; Horn, E.K.; et al. Mitoribosomal small subunit biogenesis in trypanosomes involves an extensive assembly machinery. Science 2019, 365, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Lenarčič, T.; Niemann, M.; Ramrath, D.J.F.; Calderaro, S.; Flügel, T.; Saurer, M.; Leibundgut, M.; Boehringer, D.; Prange, C.; Horn, E.K.; et al. Mitoribosomal small subunit maturation involves formation of initiation-like complexes. Proc. Natl. Acad. Sci. USA 2022, 119, e2114710118. [Google Scholar] [CrossRef] [PubMed]
- Rorbach, J.; Boesch, P.; Gammage, P.A.; Nicholls, T.J.J.; Pearce, S.F.; Patel, D.; Hauser, A.; Perocchi, F.; Minczuk, M. MRM2 and MRM3 are involved in biogenesis of the large subunit of the mitochondrial ribosome. Mol. Biol. Cell 2014, 25, 2542–2555. [Google Scholar] [CrossRef]
- De Silva, D.; Tu, Y.-T.; Amunts, A.; Fontanesi, F.; Barrientos, A. Mitochondrial ribosome assembly in health and disease. Cell Cycle 2015, 14, 2226–2250. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.; Smith, E.; Barrientos, A. Yeast Mitoribosome Large Subunit Assembly Proceeds by Hierarchical Incorporation of Protein Clusters and Modules on the Inner Membrane. Cell Metab. 2018, 27, 645–656.e7. [Google Scholar] [CrossRef] [Green Version]
- Van Vranken, J.G.; Nowinski, S.M.; Clowers, K.J.; Jeong, M.-Y.; Ouyang, Y.; Berg, J.A.; Gygi, J.P.; Gygi, S.P.; Winge, D.R.; Rutter, J. ACP Acylation Is an Acetyl-CoA-Dependent Modification Required for Electron Transport Chain Assembly. Mol. Cell 2018, 71, 567–580.e4. [Google Scholar] [CrossRef] [Green Version]
- Aphasizheva, I.; Maslov, D.; Wang, X.; Huang, L.; Aphasizhev, R. Pentatricopeptide Repeat Proteins Stimulate mRNA Adenylation/Uridylation to Activate Mitochondrial Translation in Trypanosomes. Mol. Cell 2011, 42, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Porcelli, A.M.; Ghelli, A.; Zanna, C.; Pinton, P.; Rizzuto, R.; Rugolo, M. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem. Biophys. Res. Commun. 2005, 326, 799–804. [Google Scholar] [CrossRef]
- Pecoraro, A.; Pagano, M.; Russo, G.; Russo, A. Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins. Int. J. Mol. Sci. 2021, 22, 5496. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaltsoyiannes, V.; Corre, N.; Waltz, F.; Giegé, P. Types and Functions of Mitoribosome-Specific Ribosomal Proteins across Eukaryotes. Int. J. Mol. Sci. 2022, 23, 3474. https://doi.org/10.3390/ijms23073474
Scaltsoyiannes V, Corre N, Waltz F, Giegé P. Types and Functions of Mitoribosome-Specific Ribosomal Proteins across Eukaryotes. International Journal of Molecular Sciences. 2022; 23(7):3474. https://doi.org/10.3390/ijms23073474
Chicago/Turabian StyleScaltsoyiannes, Vassilis, Nicolas Corre, Florent Waltz, and Philippe Giegé. 2022. "Types and Functions of Mitoribosome-Specific Ribosomal Proteins across Eukaryotes" International Journal of Molecular Sciences 23, no. 7: 3474. https://doi.org/10.3390/ijms23073474
APA StyleScaltsoyiannes, V., Corre, N., Waltz, F., & Giegé, P. (2022). Types and Functions of Mitoribosome-Specific Ribosomal Proteins across Eukaryotes. International Journal of Molecular Sciences, 23(7), 3474. https://doi.org/10.3390/ijms23073474






