Abstract
Stylosanthes guianensis is an excellent forage legume in subtropical and tropical regions with drought tolerance, but little is known about its drought tolerance mechanism. Dehydration responsive element binding proteins (DREBs) are responsive to abiotic stresses. A SgDREB2C was cloned from S. guianensis, while SgDREB2C protein was localized at nucleus. SgDREB2C transcript was induced by dehydration treatment. Transgenic Arabidopsis overexpressing SgDREB2C showed enhanced osmotic and drought tolerance with higher levels of relative germination rate, seedlings survival rate and Fv/Fm and lower levels of ion leakage compared with WT after osmotic and drought stress treatments. In addition, higher levels of superoxide dismutase (SOD) and ascorbate peroxidase (APX) activities and stress responsive gene (COR15A, COR47) transcripts were observed in transgenic Arabidopsis than in WT under drought stress. These results suggest that SgDREB2C regulated drought tolerance, which was associated with increased SOD and APX activities and stress-responsive gene expression under drought stress.
1. Introduction
Drought is one of the major abiotic stresses reducing crop productivity [1]. Thousands of genes are altered in expression in response to drought, leading to morphological, physiological and biochemical changes in plants [1,2]. Drought-responsive gene expression is regulated by dehydration response element binding (DREB) transcription factors that bind to the cis-dehydration response element in the promoter region of downstream genes. DREB members are classified into six subgroups, named A-1, A-2, A-3, A-4, A-5 and A-6 in Arabidopsis [3]. Among them, DREB1s/CBFs are major proteins involved in the regulation of cold acclimation [4,5,6,7], while DREB2s are involved in plant responses to drought, salinity and heat stress [8,9]. Expression of DREB2A and DREB2B from Arabidopsis plants is induced by dehydration and salt stress [10]. In addition, AtDREB2A and AtDREB2B have functional redundancy in response to heat stress [11]. Transgenic Arabidopsis plants overexpressing AtDREB2A show increased drought tolerance and heat tolerance [12], but only slight freezing tolerance [13]. Overexpression of OsDREB2A from rice results in enhanced drought and salt tolerance [14,15], while OsDREB2B expression leads to improved drought tolerance in transgenic rice [16]. Overexpression of ZmDREB2A from Zea mays improves heat and drought tolerance in transgenic Arabidopsis [8]. PcDREB2A-overexpressing Arabidopsis plants show increased tolerance to drought and osmotic stress [17]. Overexpression of EsDREB2B from Eremosparton songoricum leads to increased tolerance to osmotic stress, salt, cold and heat in transgenic tobacco [18].
DREB2C confers tolerance to multiple abiotic stresses in plants. Transcription of AtDREB2C is induced by oxidative stress [19], heat [20] and salt stress [21]. Constitutive expression of AtDREB2C results in improved thermotolerance and freezing tolerance in transgenic Arabidopsis plants [20,21]. It specifically activates HEAT SHOCK FACTOR A3 (HsfA3) and PHYTOCYSTATIN 4 (CYPS4) to regulate heat tolerance in Arabidopsis [22,23]. Overexpressing DREB2C enhances tolerance to oxidative stress in transgenic Arabidopsis by regulating HsfA3, which promotes APX2 expression [19]. In addition, DREB2C plays an important role in seed germination [24,25]. Transgenic Arabidopsis plants overexpressing AtDREB2C perform delayed germination and increase abscisic acid (ABA) content [25]. OsDREB2C expression in rice is induced by drought and salinity [26]. MsDREB2C from Malus sieversii is induced by salicylic acid (SA), jasmonic acid (JA), ABA, drought, salt, cold and heat [27]. Its overexpression leads to improved tolerance to drought, heat and cold in transgenic plants [28]. Compared to DREB2A and DREB2B, the role of DREB2C in regulation of drought tolerance was less investigated.
The antioxidant defense system protects plants against drought-induced oxidative damage [29]. It consists of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and non-enzymatic antioxidants such as ascorbic acid and reduced glutathione [30]. Higher levels of antioxidant enzyme activities are commonly observed in the drought-tolerant species compared with drought-sensitive ones [31,32]. Among four wheat genotypes, drought-resistant genotypes have higher SOD activities and transcript levels of Mn-SOD than those in drought-susceptible ones [32]. APX activity is increased in three different drought-tolerant alfalfa varieties, but the highest expression of MsAPX is shown in the drought-tolerant variety [29].
Stylosanthes guianensis (Aublet) Sw. is a tropical forage legume with drought tolerance that is commonly cultivated in tropical and subtropical areas, but it is sensitive to chilling [33,34]. ABA treatment leads to enhanced chilling tolerance as a result of increased antioxidant enzyme activities [35]. Higher antioxidant enzyme activities are maintained in the chilling-tolerant mutants than in the wild type during chilling stress [36]. 9-cis-EPOXYCAROTENOID DIOXYGENASE gene (SgNCED1) expression is induced in response to drought, salt and low temperature, which is associated with ABA accumulation under abiotic stress in S. guianensis [37]. Its overexpression results in promoted ABA synthesis and elevated drought tolerance in transgenic tobacco and stylo plants [33,34]. However, the investigations of drought tolerance in S. guianensis plants are limited. An SgDREB2C showing greatly induced expression by drought was observed in our unpublished transcriptome analysis, implying that it may play an important role in regulation of drought tolerance in S. guianensis. The objectives of this study were to identify the role of SgDREB2C in drought tolerance and provide a candidate gene for improvement of drought tolerance in forage legumes. Transgenic Arabidopsis overexpressing SgDREB2C was obtained and used for evaluation of drought tolerance. In addition, antioxidant enzyme activities and several stress-responsible genes’ expression in response to drought were detected.
2. Results
2.1. Characterization of SgDREB2C
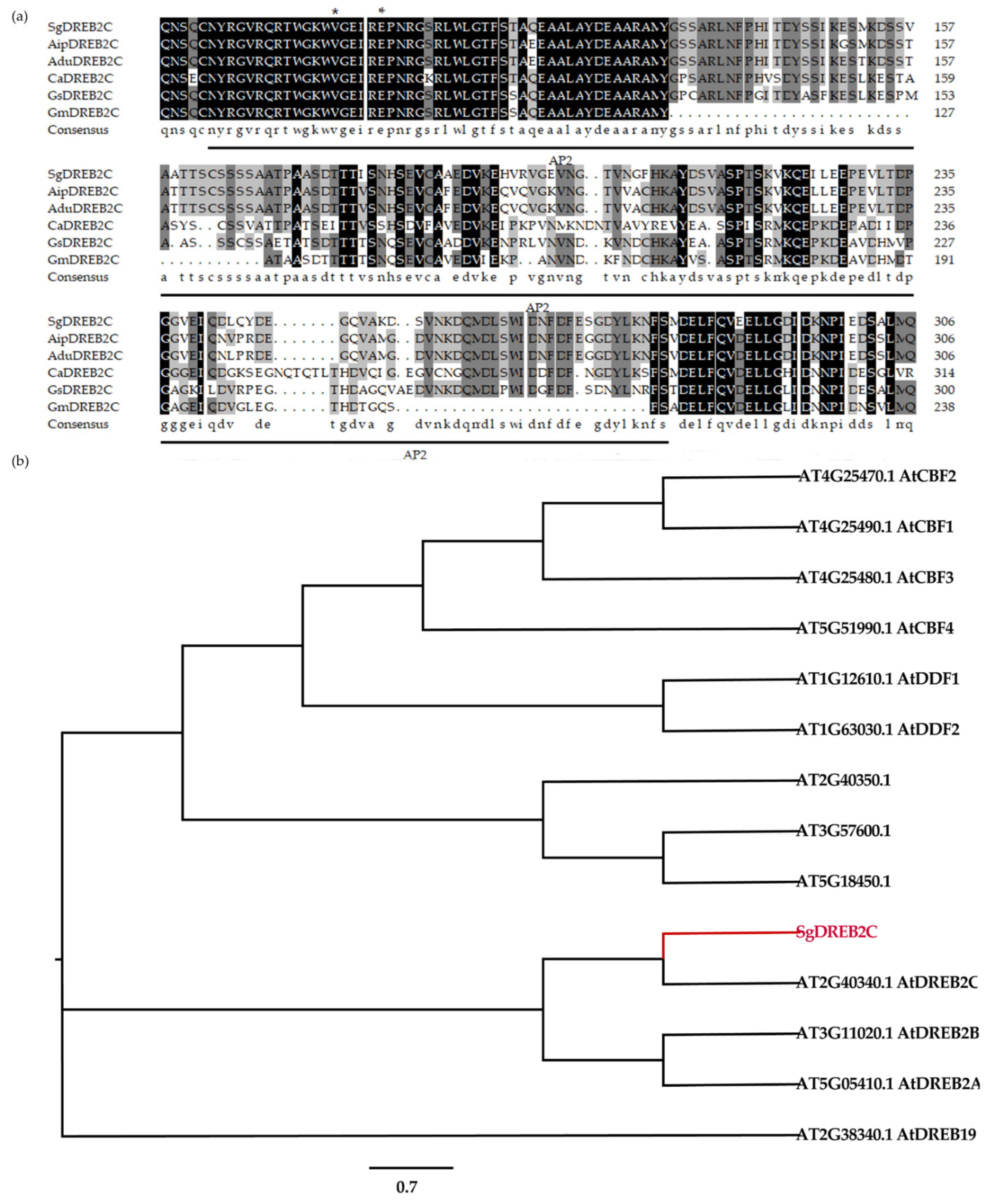
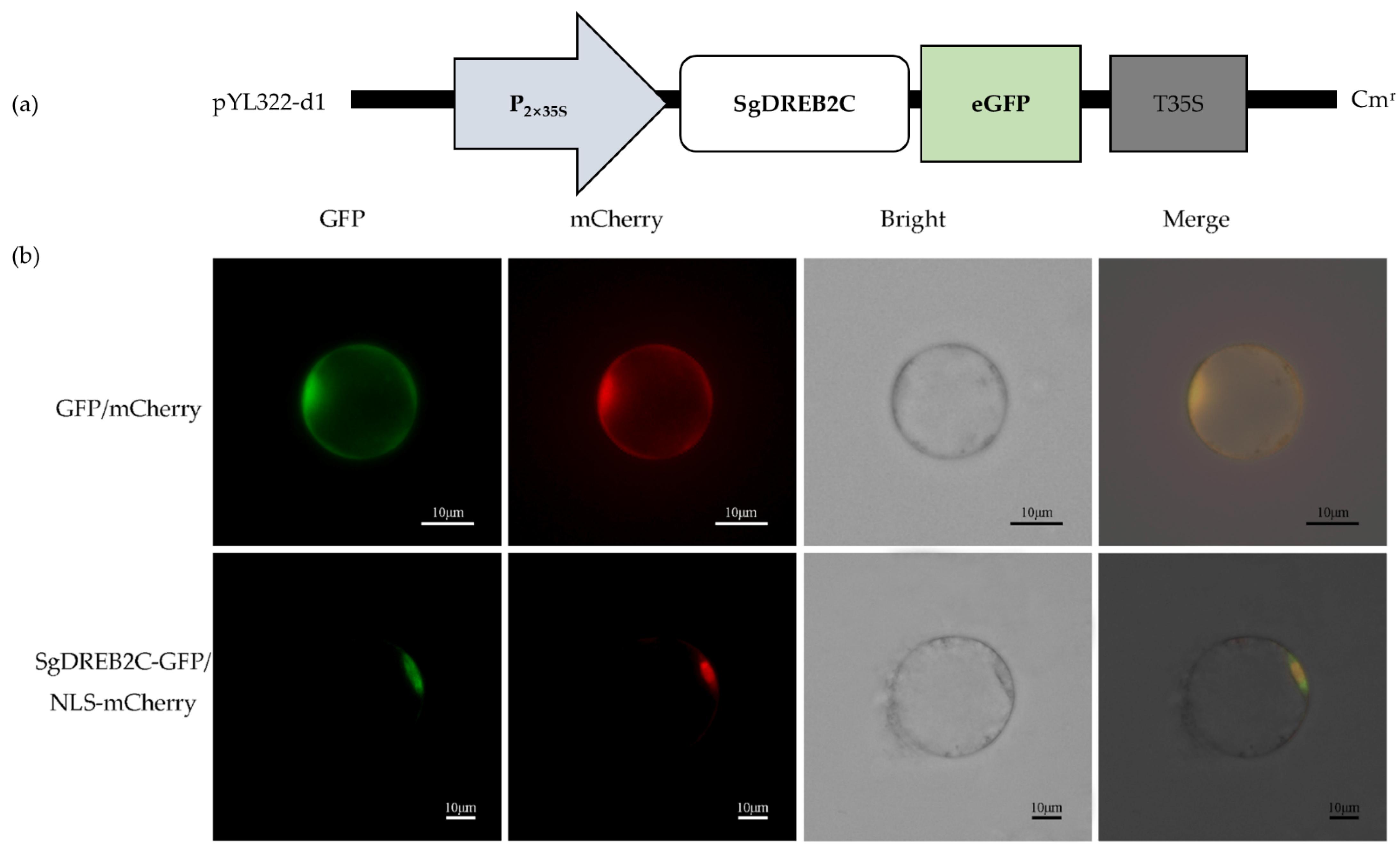
A DREB2C gene from S. guianensis (SgDREB2C) was cloned. It contains an open reading frame (ORF) of 1227 bp (GeneBank accession number OK663529) and encodes a deduced polypeptide of 408 amino acids with MW of 44.8 kDa and an isoelectric point at 4.94. Sequence alignment analysis showed that SgDREB2C was most homologous (85.7%) to AipDREB2C (XP_016167062.1) from peanut (Arachis ipaensis) in amino acids (Figure 1a). An AP2 DNA binding domain consisting of 64 amino acids (from 82 to 145 aa) was observed in SgDREB2C protein. Phylogenic tree analysis showed that SgDREB2C protein was similar to AtDREB2C (AT2G40340.1) among DREBs in Arabidopsis (Figure 1b). Subcellular localization analysis revealed fluorescence of GFP and mCherry in the whole cell, while the fluorescence of SgDREB2C-GFP and NLS-mCherry was observed only in nucleus (Figure 2), indicating that SgDREB2C is localized in nucleus.
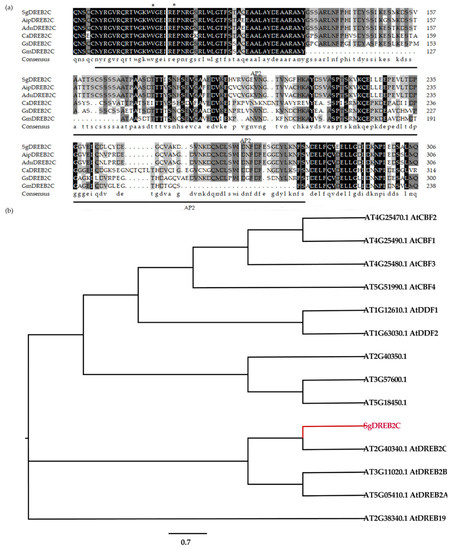
Figure 1.
Alignment of multiple sequences and phylogenetic relationships of SgDREB2C and DREBs from A. thaliana. (a) Multiple sequence alignment of SgDREB2C and DREBs from other plant species including AipDREB2C (XP_016167062.1) from Arachis ipaensis, AduDREB2C (XP_015973808.1) from Arachis duranensis, AhyDREB2C (XP_025609101.1) from Arachis hypogea, CaDREB2C (XP_004491005.1) from Cicer arietinum, GsDREB2C (XP_028199704.1) from Glycine soja, GmDREB2C (NP_001240005.1) from Glycine max and MtDREB2A (XP_003616701.1) from Medicago truncatula. The asterisks (*) represent the conserved amino acid. (b) Phylogenetic tree analysis of SgDREB2C with other DREBs from different subgroups in A. thaliana.
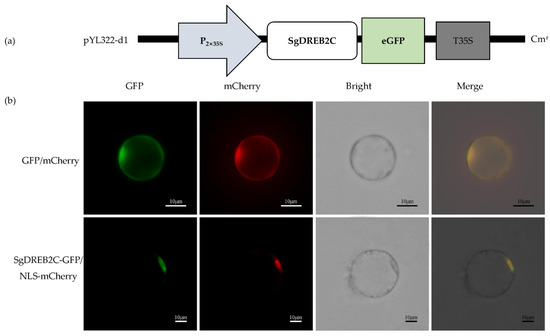
Figure 2.
Subcellular localization of SgDREB2C. (a) Schematic draft of p35GFP-SgDREB2C construct used for subcellular location assay; (b) Confocal images of rice protoplasts co-expressing SgDREB2C-GFP and NLS-mCherry or GFP and mCherry as control.
2.2. Analysis of Spatial Expression of SgDREB2C and Response to Dehydration
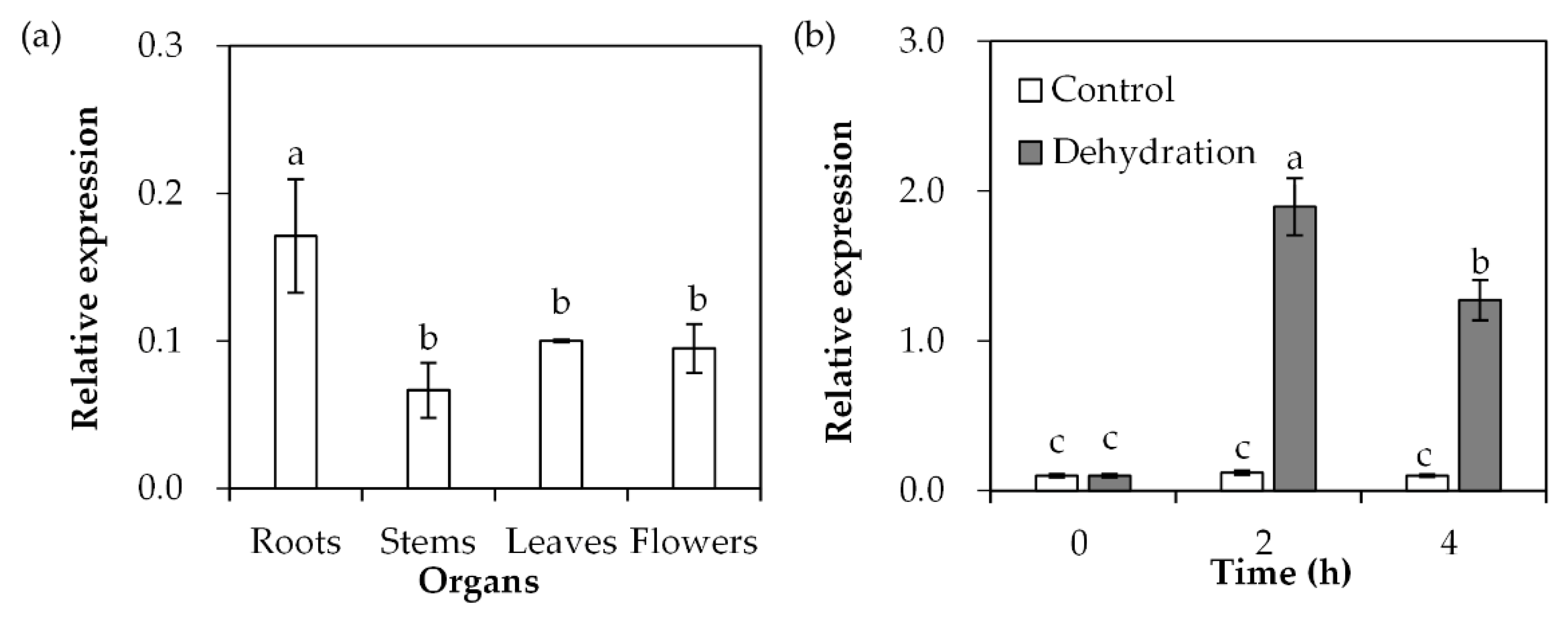
SgDREB2C transcript was detected in roots, stems, leaves and flowers of S. guianensis. The data showed that the highest transcript level was in roots compared with the other tissues (Figure 3a). After dehydration treatment, SgDREB2C transcript was induced and reached the maximum level at 2 h, with 16-fold higher expression than in the unstressed control (Figure 3b).
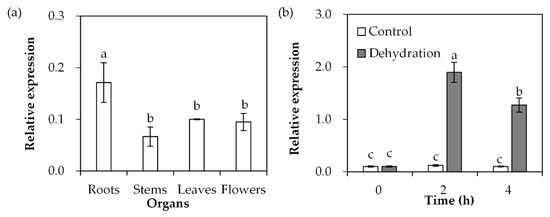
Figure 3.
Analysis of SgDREB2C transcript in different organs and in response to dehydration treatment. Roots, stems and leaves were sampled from 3-week-old S. guianensis seedlings (a). The leaves from 3-week-old seedlings were placed in a hood for dehydration treatment to isolate total RNA, which was used to determine the relative expression of SgDREB2C using qPCR (b), while ACTIN was used as reference gene. Means of three replicates and standard errors are presented; the same letter above the column indicates no significant difference at p < 0.05.
2.3. Analysis of Drought Tolerance in Transgenic Plants Overexpressing SgDREB2C
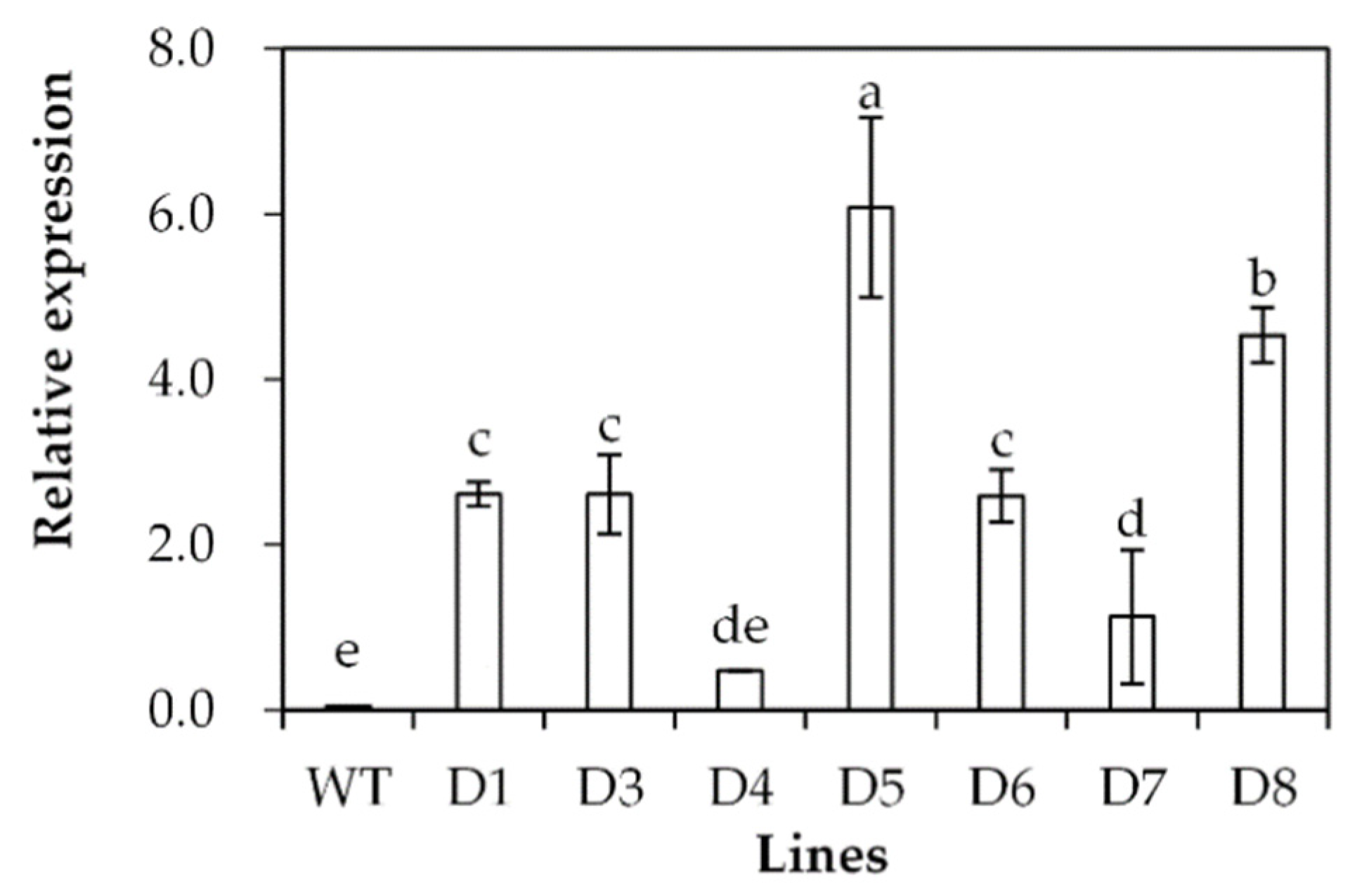
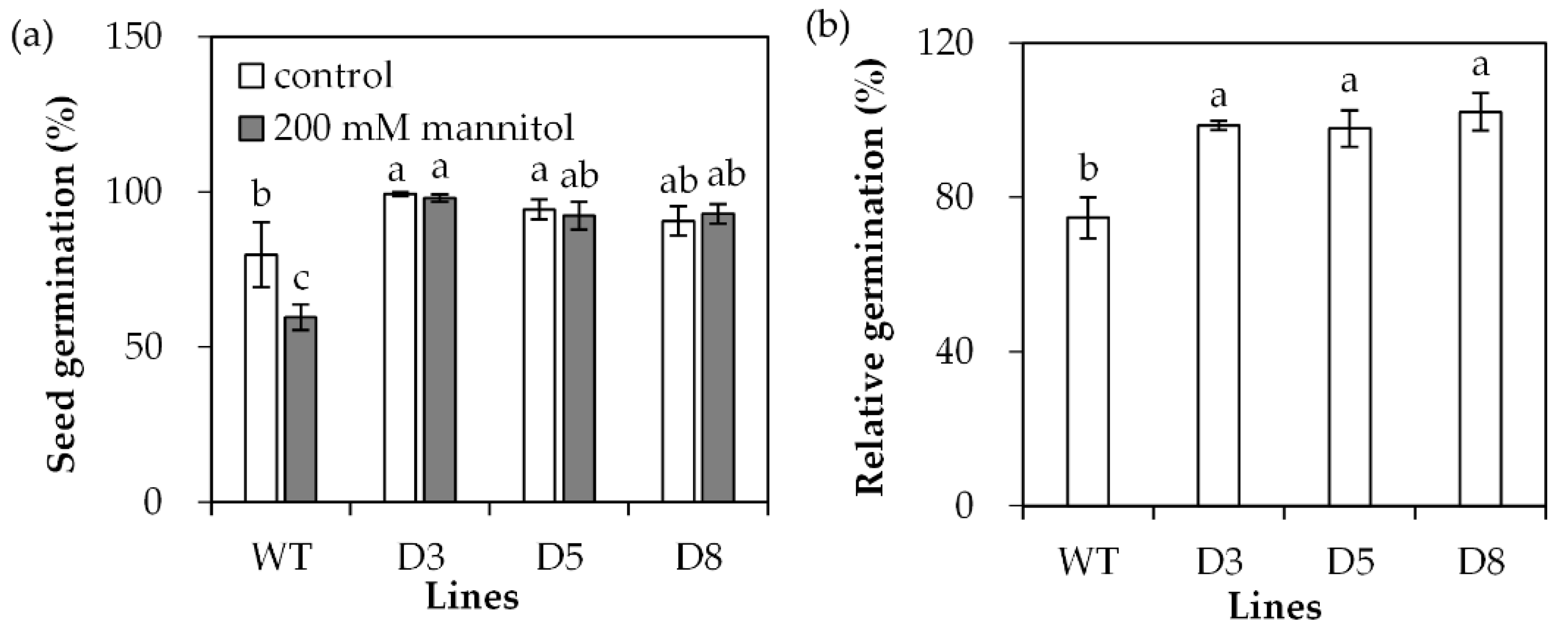
Transgenic Arabidopsis plants overexpressing SgDREB2C were generated. Seven homozygous lines were selected based on resistance to basta and harvested for further investigations. Three transgenic lines (D3, D5 and D8) with higher levels of SgDREB2C transcript were selected for evaluation of drought tolerance (Figure 4). The results showed that the seed germination rate was higher in transgenic plants than that in the wild type (WT) under control conditions. It was decreased significantly in WT plants but unaltered in transgenic plants on the MS medium containing 200 mM mannitol (Figure 5a), which led to higher relative germination rates in transgenic plants as compared with the WT plants (Figure 5b).
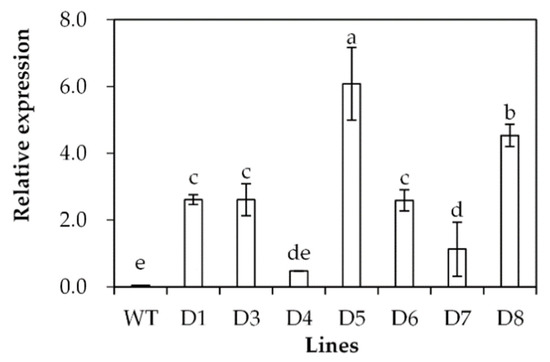
Figure 4.
Analysis of SgDREB2C transcript in transgenic plants in comparison with the wild type (WT). The relative expression of SgDREB2C was evaluated using qPCR, and ACTIN was used as reference gene. Means of three replicates and standard errors are presented; the same letter above the column indicates no significant difference at p < 0.05.
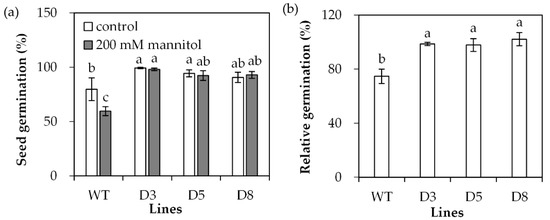
Figure 5.
Seed germination in transgenic plants in comparison with the wild type (WT) in response to osmotic stress. Sterilized seeds were placed on 1/2 MS medium containing 200 mM mannitol or without it as control for 4 days for determination of germination rate (a), and the data were used to calculate relative germination rate (b). Means of three replicates and standard errors are presented; the same letter above the column indicates no significant difference at p < 0.05.
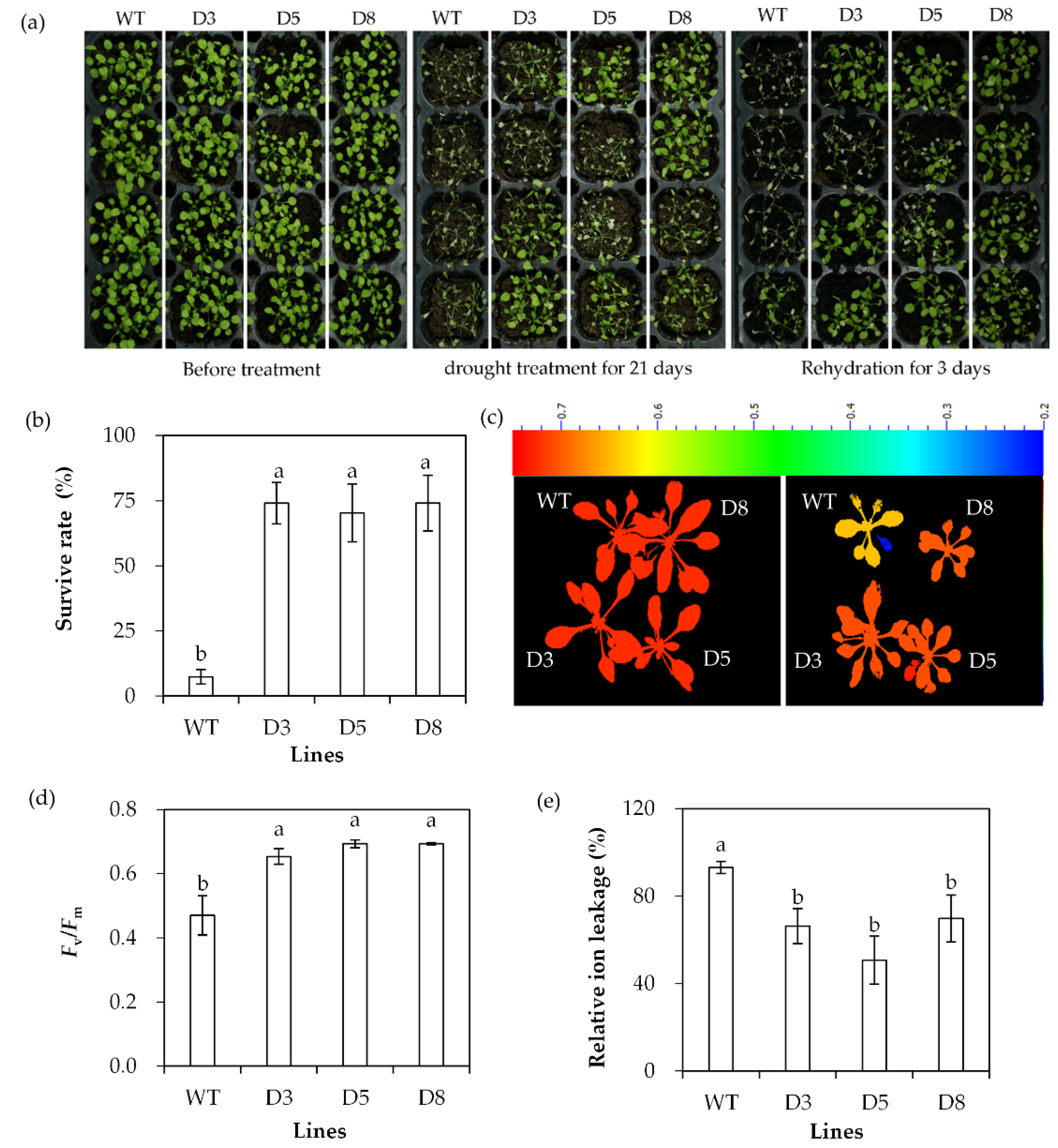
The drought tolerance of transgenic lines was evaluated based on survival rate, Fv/Fm and ion leakage after withholding irrigation. Most WT plants died with a survival rate of 7.4%, while transgenic lines had a survival rate of 70% to 74% after drought stress (Figure 6a,b). Fv/Fm was decreased and ion leakage was increased greatly after 20 d of withholding irrigation, while higher levels of Fv/Fm and lower levels of ion leakage were observed in transgenic plants than in WT plants (Figure 6c–e). The results indicated that transgenic plants had increased drought tolerance.
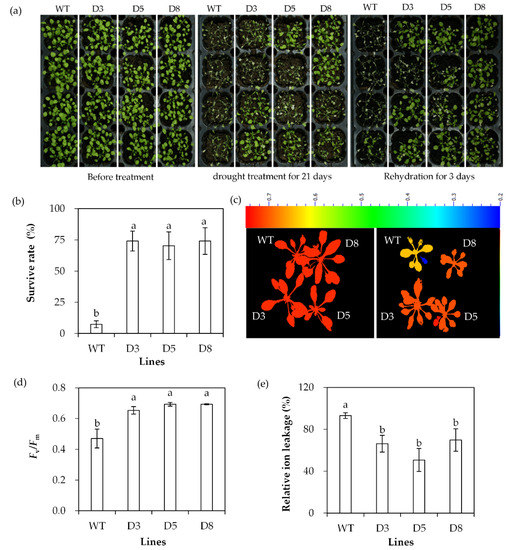
Figure 6.
Analysis of drought tolerance in transgenic plants in comparison with the wild type (WT). Two-week-old seedlings were withheld from irrigation for drought treatment. Photos were taken at the time points as indicated in the figure (a). Survival rate was determined after 21 days of drought, followed by 3 days of rewatering (b). Fv/Fm (c,d) and relative ion leakage (e) were determined after 14 days of drought. Means of three replicates and standard errors are presented; the same letter above the column indicates no significant difference at p < 0.05.
2.4. Analysis of Antioxidant Defense System and Stress-Responsive Genes
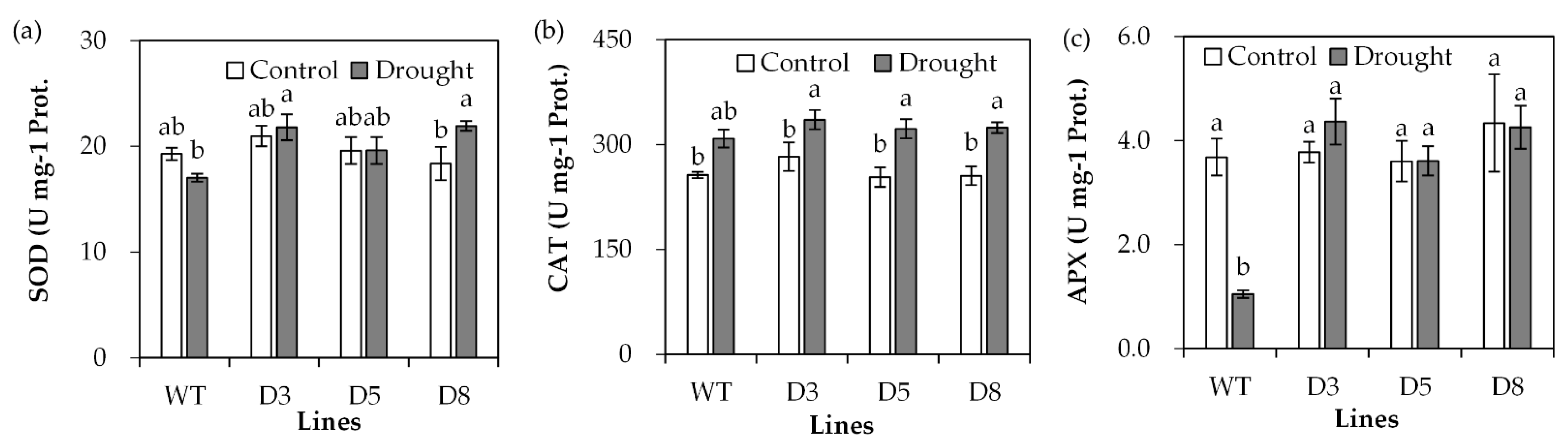
The antioxidant defense system protects plants against oxidative damage induced by drought. Antioxidant enzyme activities were determined. SOD activity showed no significant difference between transgenic plants and WT plants under control conditions, but higher activities were observed in transgenic plants than in WT plants under drought stress (Figure 7a). CAT activity showed no significant difference between transgenic lines and WT under either control or drought stress conditions (Figure 7b). There was no difference in APX activity between transgenic plants and WT plants under control conditions (Figure 7c). APX activity was greatly reduced in WT after drought treatment, while it was unaltered in transgenic lines after drought treatment (Figure 7c).
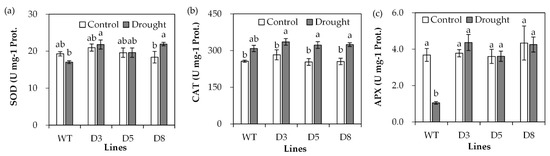
Figure 7.
Analysis of antioxidant enzyme activities in transgenic plants in comparison with the wild type (WT). Three-week old seedlings were withheld from irrigation for 14 days (drought) or continuously irrigated as control, and leaves were sampled for measurements of SOD (a), CAT (b) and APX (c) activities. Means of three replicates and standard errors are presented; the same letter above the column indicates no significant difference at p < 0.05.
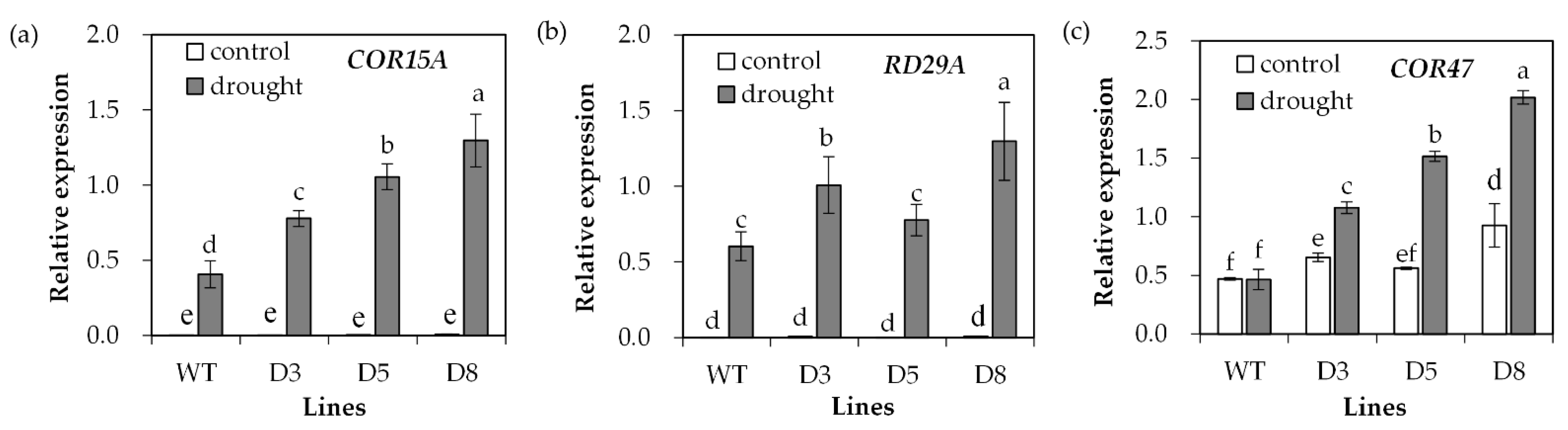
Transcript levels of stress-responsive genes were analyzed. COR15A and RD29A transcript levels showed no difference between transgenic plants and WT under control conditions, but they were greatly induced after drought treatment (Figure 8a,b). Higher levels of COR15A transcript were observed in transgenic plants under drought stress (Figure 8a), and transgenic lines D3 and D8 but not D5 had higher RD29A transcript levels than WT plants (Figure 8b). The COR47 transcript level was higher in transgenic lines D3 and D8 than in WT under control conditions. It was greatly induced in transgenic plants after drought treatment, but not altered in WT plants (Figure 8c).
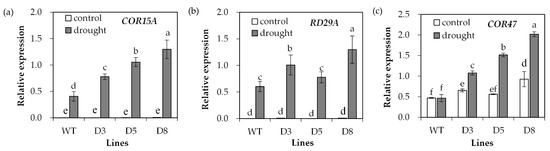
Figure 8.
Analysis of drought-responsive marker gene expression in response to drought. Two-week-old seedlings were withheld from irrigation for 14 days (drought) or continuously irrigated as control, and leaves were sampled for determination of the relative expression of drought-responsive genes including COR15A (a), RD29A (b) and COR47 (c). Means of three replicates and standard errors are presented; the same letter above the column indicates no significant difference at p < 0.05.
3. Discussion
SgDREB2C was cloned from S. guianensis with an AP2 DNA domain. SgDREB2C protein is located in nucleus, which is consistent with AtDREB2C [21] and MsDREB2C [27]. DREB2s are important transcriptional regulators in response to drought and salt stress [3,9,16]. In our study, the highest transcript level of SgDREB2C was observed in roots of S. guianensis, and SgDREB2C transcript was induced by dehydration. The results implied its potential role in the regulation of drought tolerance. The highest level of AtDREB2C transcript was observed in flowers [38], whereas that of MsDREB2C was observed in mature leaves [27]. The difference may be associated with gene function in plant species.
Transgenic plants overexpressing SgDREB2C showed enhanced osmotic stress and drought tolerance with higher germination rates, survival rates and Fv/Fm and lower levels of ion leakage compared with WT under drought conditions. The results suggested that SgDREB2C confers drought tolerance. Consistent with these findings, MsDREB2C was also significantly induced by drought, and its expression led to increased drought tolerance in transgenic Arabidopsis [27]. Photosynthesis is one of the major processes that is significantly affected by drought [39]. Fv/Fm reflects the photochemical activity of photosystem II (PSII). Drought causes the production of reactive oxygen species (ROS) that can damage PSII and further lead to decreased photosynthesis capability under drought stress [40]. In chickpea, the tolerant genotype has higher Fv/Fm than the sensitive one under drought stress [41]. In the present study, transgenic Arabidopsis plants maintained a survival rate that was associated with higher photosynthesis capability compared with that in WT under drought conditions.
The antioxidant system protects plants against oxidative damage by scavenging ROS under abiotic stress [42,43]. Higher activities of antioxidant enzymes are associated with drought tolerance in diverse plant species. PcDREB2A-overexpressing lines have lower ROS levels in transgenic plants under drought stress [17]. Higher activities of SOD and APX were maintained in transgenic Arabidopsis, and higher CAT activity was maintained in two lines of SgDREB2C-overexpressing transgenic plants compared with WT under drought. Nevertheless, the higher antioxidant enzyme activities are associated with increased drought tolerance in transgenic plants overexpressing SgDREB2C.
Stress-responsive genes are regulated by DREBs through binding to the cis-acting dehydration-responsive element/C-repeat (DRE/CRT) element in their promoter regions [3,21,28]. RD29A, COR47 and COR15A genes are responsive to drought in Arabidopsis [44]. The increased drought tolerance in VvNAC08-OE [45] and VyP5CR-OE transgenic lines [46] and ataf1-1, ataf1-2 mutants of A. thaliana [47] is partly associated with higher transcription levels of drought stress-responsive genes RD29A, COR47 and COR15A. Higher transcript levels of COR15A and COR47 were maintained in three SgDREB2C-overexpressing lines and higher levels of RD29A transcript were maintained in two lines compared with WT plants under drought stress. Similarly, the COR15A transcript was higher in transgenic Arabidopsis overexpressing AtDREB2C compared with its level in WT plants, although COR47 and RD29A levels were not affected [21]. Nevertheless, our results indicated that the higher transcript levels of drought-responsive gene expression are associated with the increased drought tolerance in SgDREB2C transgenic lines.
In summary, the role of SgDREB2C in the regulation of drought tolerance was identified. The SgDREB2C transcript was induced by drought, and its constitutive expression led to enhanced drought tolerance in transgenic plants. The increased drought tolerance was associated with the higher levels of stress-responsive gene transcripts and antioxidant enzyme activities compared with the wild type under drought stress.
4. Materials and Methods
4.1. Plant Growth and Dehydration Treatment
The germinated seeds of S. guianensis cv CIAT 184 were sown in 11 cm diameter plastic pots with a mixture of peat and perlite (3:1, v/v). The seedlings were grown in a greenhouse under natural light with temperatures from 25 to 30 °C for 3 weeks as previously described [36]. Roots, stems, and leaves were harvested for examination of tissue-specific expression, while the flowers from mature plants were harvested. For dehydration treatment, the second leaves from the tops of the seedlings were detached and placed in a laminar flow hood for 4 h for gradual dehydration, followed by immersion in liquid nitrogen for isolation of total RNA.
4.2. Cloning and Sequence Analysis of SgDREB2C
Total RNA was isolated from 0.1 g of S. guianensis leaves using TRI-Gene RNA Extraction Kit (GenStar Biosolutions Co., Ltd., Beijing, China). Two ug of total RNA was used for reverse transcription by using Evo M-MLV RT Kit with gDNA Clean (Accurate Biology, Changsha, China) in the presence of oligo (dT)18. The cDNA was used as a template to amplify the coding sequence of SgDREB2C using two primers (forward: 5′-ATGGGTGCTTATGATCAAGGTTCTA-3′ and reverse: 5′-CTCTAATCATCCTTCAACGTTTGCATTATTTCCCTTTTGGAT-3′), and 2×TSINGKE® Master Mix (Tsingke Biotechnology Co., Ltd., Beijing, China). The conserved domain of SgDREB2C protein was analyzed using SMART (http://smart.embl-heidelberg.de/), which was accessed on 20 April 2018. SgDREB2C and its homologous DREB2C sequences from other plants obtained from National Center for Biotechnology Information (NCBI) were aligned using DNAMAN software version 6.0. The phylogenic tree was generated using MEGA version 7.0.26 with the best model JTT+F+I+G4.
4.3. Subcellular Localization Assay
The coding sequence of SgDREB2C without termination codon was cloned into pYL322-eGFP-N and fused with eGFP. The recombinant vector (35S-SgDREB2C-GFP) and control vector of nucleic localization sequence (35S-NLS-mCherry) were transiently co-transformed into protoplast isolated from 2-week-old rice sheath using PEG-mediated transformation as previously described [48]. The fluorescence was observed by a laser scanning confocal microscope (Leica DM6B, Leica Microsystem CMS GmbH. Mannheim Germany).
4.4. Generation of Transgenic Arabidopsis
The coding sequence of SgDREB2C was cloned into pCAMBIA3301 driven by CaMV35S promoter. The recombinant vector was introduced into Agrobacterium tumefaciens strain EHA105, which was used to transform Arabidopsis by the floral dip method [49]. The sterilized seeds of transgenic Arabidopsis were placed on MS medium containing 50 mg/L basta to obtain positive transformants. Homozygous transgenic lines (T3) were identified.
4.5. Evaluation of Drought Tolerance
Fifty sterilized seeds of transgenic lines and wild type were placed on 1/2 MS medium containing 200 mM mannitol or without mannitol as control as previously described [50]. After 4 days, the germinated seeds were counted for calculation of germination rate. Each treatment contained three plates as replicates.
Seven-day-old seedlings grown on 1/2 MS medium were transferred to a 50-hole tray to grow for 7 d in a growth chamber at 22 °C under light of 200 μmol m−2 s−1. After the seedlings were fully irrigated, plants were withheld from irrigation so that the soil gradually became dry. When the wild type plants showed serious wilting at 21 d after withholding irrigation, ion leakage and maximal photochemical efficiency (Fv/Fm) of photosystem II were measured as previously described [51]. The plants were re-irrigated to allow 3 d of recovery, and surviving plants were counted for calculation of survival rate.
4.6. Measurements of Antioxidant Enzyme Activities
Fresh leaves (0.3 g) were ground in 2 mL of 50 mM ice-cold phosphate buffer (pH 7.8) containing 2% (w/v) PVP and 2 mM EDTA, followed by centrifugation at 14,000 rpm for 30 min at 4 °C. The supernatants were collected for measurements of superoxide dismutase (SOD) and catalase (CAT) activities as previously described [52]. For extraction of ascorbate peroxidase (APX), fresh leaves (0.3 g) were ground in 2 mL of 50 mM phosphate buffer (pH 7.0) containing 1 mM ascorbic acid and 1 mM ETDA, followed by centrifugation as above. APX activity was determined as previously described [52]. One unit of SOD activity was defined as the amount of enzyme required to lead to 50% inhibition of photochemical reduction of ρ-nitro blue tetrazolium chloride (NBT). One unit of CAT and APX activity was defined as the amount of enzyme required for catalyzing the conversion of H2O2 (extinction coefficient 0.0394 mM/cm) or ascorbic acid (AsA) (extinction coefficient 2.8 mM/cm) within 1 min [52].
4.7. Real-Time Quantitative PCR (qPCR)
Total RNA was extracted using TRI Gene reagent (GenStar Biosolutions Co., Ltd., Beijing, China), followed by synthesis of the first strand cDNA using Evo M-MLV Mix Kit (Accurate Biology, Changsha, Hunan, China). PCR was performed using 2 × SYBR® Green Premix Pro Taq HS Premix (Accurate Biology, Changsha, Hunan, China) on the Bio-Rad CFX Manager version 1.6 (Bio-Rad, Hercules, CA, USA). ACTIN was used as reference gene to normalized gene expression. qPCR primers are listed in Table S1. Each sample contained three biological replicates.
4.8. Statistic Analysis
All data for these experiments were processed using Excel version 2016 and SPSS software Version 19.0 (IBM, Chicago, IL, USA). The data were tested by one-way analysis of variance (ANOVA) based on least significant difference (LSD) (p < 0.05).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23073520/s1.
Author Contributions
Conceptualization, S.L.; performance of experiments, Y.H., L.X. and Z.S.; data analysis, Y.H.; writing—original draft preparation, Y.H.; writing—review and editing, S.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gong, Z.Z.; Xiong, L.M.; Shi, H.Z.; Yang, S.H.; Herrera-Estrella, L.R.; Xu, G.H.; Chao, D.Y.; Li, J.R.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci.China-Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.; Ghafoor, A.; Du, X. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells 2020, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Z.; Xie, S.; Si, T.; Li, Y.; Zhu, J.-K. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.X.; Ding, Y.L.; Shi, Y.T.; Zhang, X.Y.; Gong, Z.Z.; Yang, S.H. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016, 212, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Kidokoro, S.; Watanabe, K.; Ohori, T.; Moriwaki, T.; Maruyama, K.; Mizoi, J.; Htwe, N.M.P.S.; Fujita, Y.; Sekita, S.; Shinozaki, K.; et al. Soybean DREB1/CBF-type transcription factors function in heat and drought as well as cold stress-responsive gene expression. Plant J. 2015, 81, 505–518. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Chen, M.; Guo, J.K.; Wang, Y.X.; Min, D.H.; Jiang, Q.Y.; Ji, H.T.; Huang, C.Y.; Wei, W.; Xu, H.J.; et al. Overexpression of soybean DREB1 enhances drought stress tolerance of transgenic wheat in the field. J. Exp. Bot. 2020, 71, 1842–1857. [Google Scholar] [CrossRef]
- Qin, F.; Kakimoto, M.; Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Tran, L.S.P.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 2007, 50, 54–69. [Google Scholar] [CrossRef]
- Song, C.; Lee, J.; Kim, T.; Hong, J.C.; Lim, C.O. VOZ1, a transcriptional repressor of DREB2C, mediates heat stress responses in Arabidopsis. Planta 2018, 247, 1439–1448. [Google Scholar] [CrossRef]
- Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Seki, M.; Miura, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration- and high-salinity-responsive gene expression. Plant Mol. Biol. 2000, 42, 657–665. [Google Scholar] [CrossRef]
- Larkindale, J.; Vierling, E. Core genome responses involved in acclimation to high temperature. Plant Physiol. 2008, 146, 748. [Google Scholar] [CrossRef] [Green Version]
- Sakuma, Y.; Maruyama, K.; Qin, F.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell. 2006, 18, 1292–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-X.; Tang, Y.-J.; Ma, Q.-B.; Yang, C.-Y.; Mu, Y.-H.; Suo, H.-C.; Luo, L.-H.; Nian, H. OsDREB2A, a rice transcription factor, significantly affects salt tolerance in transgenic soybean. PLoS ONE 2013, 8, e83011. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Meng, X.-P.; Zhang, Y.; Xia, M.; Wang, X.-P. Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol. Lett. 2008, 30, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Wang, X.W.; Wu, Z.J.; Chen, M.; Chai, T.Y.; Wang, H. Overexpression of the Polygonum cuspidatum PcDREB2A gene encoding a DRE-Binding transcription factor enhances the drought tolerance of transgenic Arabidopsis thaliana. J. Plant. Biol. 2021, in press. [CrossRef]
- Li, X.S.; Zhang, D.Y.; Li, H.Y.; Wang, Y.C.; Zhang, Y.M.; Wood, A.J. EsDREB2B, a novel truncated DREB2-type transcription factor in the desert legume Eremosparton songoricum, enhances tolerance to multiple abiotic stresses in yeast and transgenic tobacco. BMC Plant Biol. 2014, 14, 44. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.E.; Lim, C.J.; Chen, H.; Je, J.; Song, C.; Lim, C.O. Overexpression of Arabidopsis dehydration-responsive element-binding protein 2C confers tolerance to oxidative stress. Mol. Cells 2012, 33, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.J.; Hwang, J.E.; Chen, H.; Hong, J.K.; Yang, K.A.; Choi, M.S.; Lee, K.O.; Chung, W.S.; Lee, S.Y.; Lim, C.O. Over-expression of the Arabidopsis DRE/CRT-binding transcription factor DREB2C enhances thermotolerance. Biochem. Biophys. Res. Commun. 2007, 362, 431–436. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kang, J.-Y.; Park, H.-J.; Kim, M.D.; Bae, M.S.; Choi, H.-I.; Kim, S.Y. DREB2C interacts with ABF2, a bZIP protein regulating abscisic acid-responsive gene expression, and its overexpression affects abscisic acid sensitivity. Plant Physiol. 2010, 153, 716–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Hwang, J.E.; Lim, C.J.; Kim, D.Y.; Lee, S.Y.; Lim, C.O. Arabidopsis DREB2C functions as a transcriptional activator of HsfA3 during the heat stress response. Biochem. Biophys. Res. Commun. 2010, 401, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Je, J.; Song, C.; Hwang, J.E.; Chung, W.S.; Lim, C.O. DREB2C acts as a transcriptional activator of the thermo tolerance-related phytocystatin 4 (AtCYS4) gene. Transgenic Res. 2014, 23, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Nguyen, T.T.; Baek, J.; Song, Y.H.; Hong, J.C.; Lim, C.O. DREB2 family transcription factors promote the expression of Phytocystatin1 in Arabidopsis thaliana during seed germination. J. Plant Biol. 2020, 64, 45–53. [Google Scholar] [CrossRef]
- Je, J.; Chen, H.; Song, C.; Lim, C.O. Arabidopsis DREB2C modulates ABA biosynthesis during germination. Biochem. Biophys. Res. Commun. 2014, 452, 91–98. [Google Scholar] [CrossRef]
- Herath, V. Small family, big impact: In silico analysis of DREB2 transcription factor family in rice. Comput. Biol. Chem. 2016, 65, 128–139. [Google Scholar] [CrossRef]
- Zhao, K.; Shen, X.J.; Yuan, H.Z.; Liu, Y.; Liao, X.; Wang, Q.; Liu, L.L.; Li, F.; Li, T.H. Isolation and characterization of dehydration-responsive element-binding factor 2C (MsDREB2C) from Malus sieversii Roem. Plant Cell Physiol. 2013, 54, 1415–1430. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Guo, X.W.; Liao, X.; Wang, Q.; Liu, D.; Li, T.H. Arabidopsis plants overexpressing the MsDREB2C exhibit increased susceptibility to alternaria mali infection. J. Plant Growth Regul. 2015, 34, 78–87. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, S.; Liu, Z.; Yang, F.; Yin, G. Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. J. Plant Physiol. 2019, 232, 226–240. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, P.; Arora, P.; Verma, V.; Khanna, K.; Saini, P.; Bhardwaj, R. Role and Regulation of ROS and Antioxidants as Signaling Molecules in Response to Abiotic Stresses. In Plant Signaling Molecules, 1st ed.; Khan, M.I., Reddy, P.S., Ferrante, A., Khan, N., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2019; pp. 141–156. [Google Scholar]
- Da Costa, M.; Huang, B.R. Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. J. Am. Soc. Hort. Sci. 2007, 132, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Sheoran, S.; Thakur, V.; Narwal, S.; Turan, R.; Mamrutha, H.M.; Singh, V.; Tiwari, V.; Sharma, I. Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat (Triticum aestivum L.) under drought. Appl. Biochem. Biotechnol. 2015, 177, 1282–1298. [Google Scholar] [CrossRef]
- Bao, G.; Zhuo, C.; Qian, C.; Xiao, T.; Guo, Z.; Lu, S. Co-expression of NCED and ALO improves vitamin C level and tolerance to drought and chilling in transgenic tobacco and stylo plants. Plant Biotechnol. J. 2016, 14, 206–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Lu, S.; Cai, J.; Guo, Z. Overexpressing SgNCED1 in tobacco increases ABA level, antioxidant enzyme activities, and stress tolerance. J. Plant Growth Regul. 2008, 27, 151–158. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, Z.; Liu, Z. Effects of abscisic acid on antioxidant systems of Stylosanthes guianensis (Aublet) Sw. under chilling stress. Crop Sci. 2005, 45, 599–605. [Google Scholar] [CrossRef]
- Lu, S.; Wang, X.; Guo, Z. Differential responses to chilling in Stylosanthes guianensis (Aublet) Sw. and its mutants. Agron. J. 2013, 105, 377–382. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Z. Cloning of a 9-cis-epoxycarotenoid dioxygenase gene (SgNCED1) from Stylosanthes guianensis and its expression in response to abiotic stresses. Plant Cell Rep. 2007, 26, 1383–1390. [Google Scholar] [CrossRef]
- Chen, H.; Je, J.; Song, C.; Hwang, J.E.; Lim, C.O. A proximal promoter region of Arabidopsis DREB2C confers tissue—Specific expression under heat stress. J. Integr. Plant Biol. 2012, 54, 640–651. [Google Scholar] [CrossRef]
- Ghotbi-Ravandi, A.A.; Shahbazi, M.; Shariati, M.; Mulo, P. Effects of mild and severe drought stress on photosynthetic efficiency in tolerant and susceptible barley (Hordeum vulgare L.) genotypes. J. Agron. Crop Sci. 2014, 200, 403–415. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Collin, A.; Sitko, K.; Janiak, A.; Kalaji, H.M.; Szarejko, I. Genetic and physiological dissection of photosynthesis in barley exposed to drought stress. Int. J. Mol. Sci. 2019, 20, 6341. [Google Scholar] [CrossRef] [Green Version]
- Saglam, A.; Terzi, R.; Demiralay, M. Effect of polyethylene glycol induced drought stress on photosynthesis in two chickpea genotypes with different drought tolerance. Acta Biol. Hung. 2014, 65, 178–188. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Huang, Y.; Khadr, A.; Wang, Y.-H.; Xu, Z.-S.; Xiong, A.-S. DcDREB1A, a DREB-binding transcription factor from Daucus carota, enhances drought tolerance in transgenic Arabidopsis thaliana and modulates lignin levels by regulating lignin-biosynthesis-related genes. Environ. Exp. Bot. 2020, 169, 103896. [Google Scholar] [CrossRef]
- Seki, M.; Narusaka, M.; Abe, H.; Kasuga, M.; Yamaguchi-Shinozaki, K.; Carninci, P.; Hayashizaki, Y.; Shinozaki, K. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell. 2001, 13, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Ju, Y.L.; Min, Z.; Yue, X.F.; Zhang, Y.L.; Zhang, J.X.; Zhang, Z.Q.; Fang, Y.L. Overexpression of grapevine VvNAC08 enhances drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 151, 214–222. [Google Scholar] [CrossRef]
- Chen, C.C.; Cui, X.Y.; Zhang, P.Y.; Wang, Z.; Zhang, J.X. Expression of the pyrroline-5-carboxylate reductase (P5CR) gene from the wild grapevine Vitis yeshanensis promotes drought resistance in transgenic Arabidopsis. Plant Physiol. Biochem. 2021, 168, 188–201. [Google Scholar] [CrossRef]
- Lu, P.L.; Chen, N.Z.; An, R.; Su, Z.; Qi, B.S.; Ren, F.; Chen, J.; Wang, X.C. A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis. Plant Mol. Biol. 2007, 63, 289–305. [Google Scholar] [CrossRef]
- Woo, J.W.; Kim, J.; Il Kwon, S.; Corvalan, C.; Cho, S.W.; Kim, H.; Kim, S.-G.; Kim, S.-T.; Choe, S.; Kim, J.-S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Veerappa, R.; Robert, D.; Slocum, R.D.; Siegenthaler, A.; Wang, J.; Clark, G.; Roux, S.J. Ectopic expression of a pea apyrase enhances root system architecture and drought survival in Arabidopsis and soybean. Plant Cell Environ. 2019, 42, 337–353. [Google Scholar] [CrossRef]
- Huang, S.; Chen, M.; Zhao, Y.; Wen, X.; Guo, Z.; Lu, S. CBL4-CIPK5 pathway confers salt but not drought and chilling tolerance by regulating ion homeostasis. Environ. Exp. Bot. 2020, 179, 104230. [Google Scholar] [CrossRef]
- Guo, Z.; Tan, J.; Zhuo, C.; Wang, C.; Xiang, B.; Wang, Z. Abscisic acid, H2O2 and nitric oxide interactions mediated cold-induced S-adenosylmethionine synthetase in Medicago sativa subsp. falcata that confers cold tolerance through up-regulating polyamine oxidation. Plant Biotechnol. J. 2014, 12, 601–612. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).