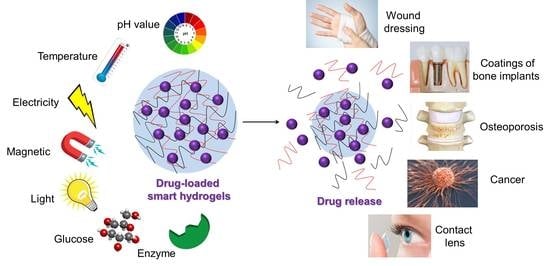
Smart Hydrogels for Advanced Drug Delivery Systems
Abstract
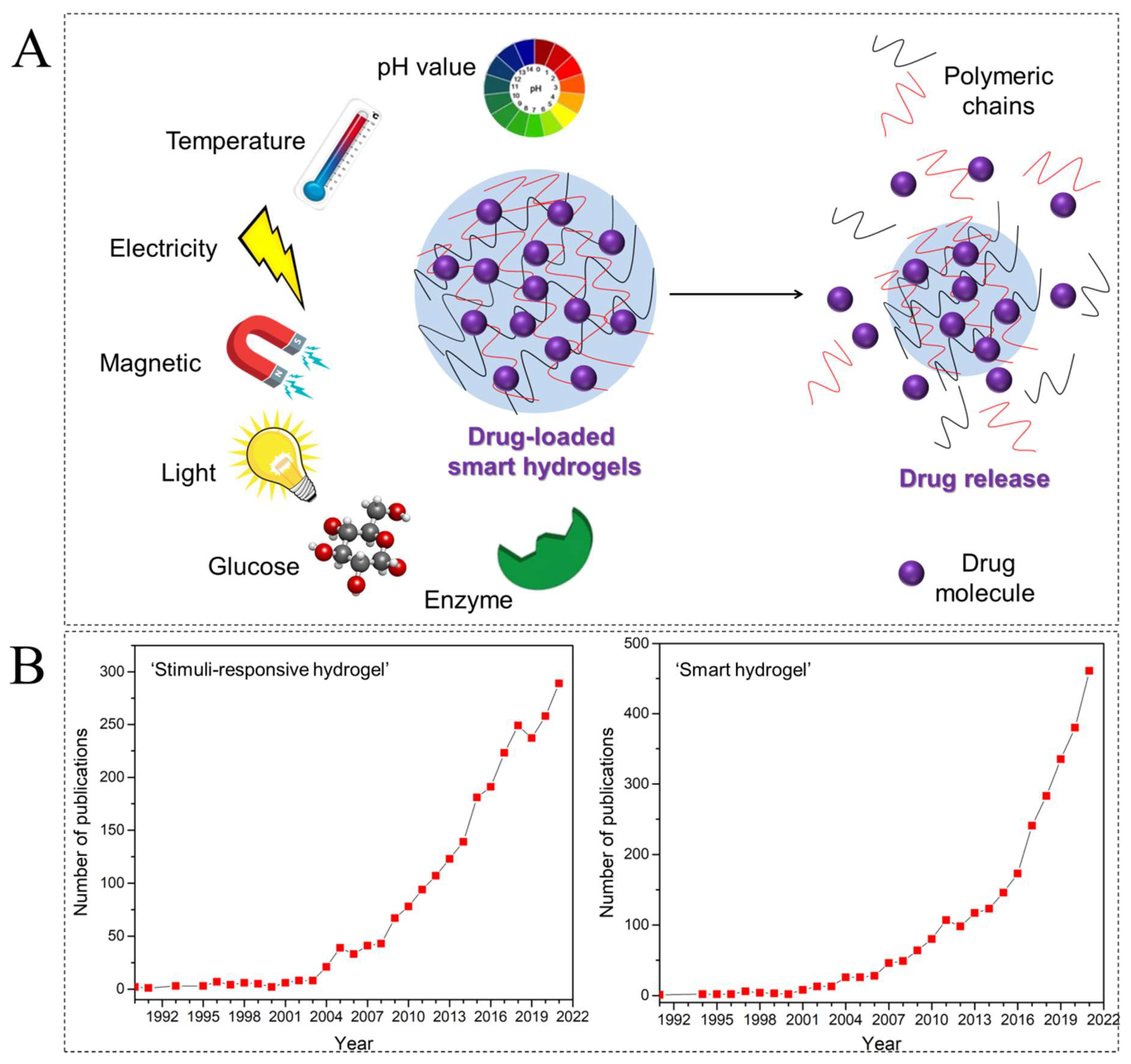
:1. Introduction
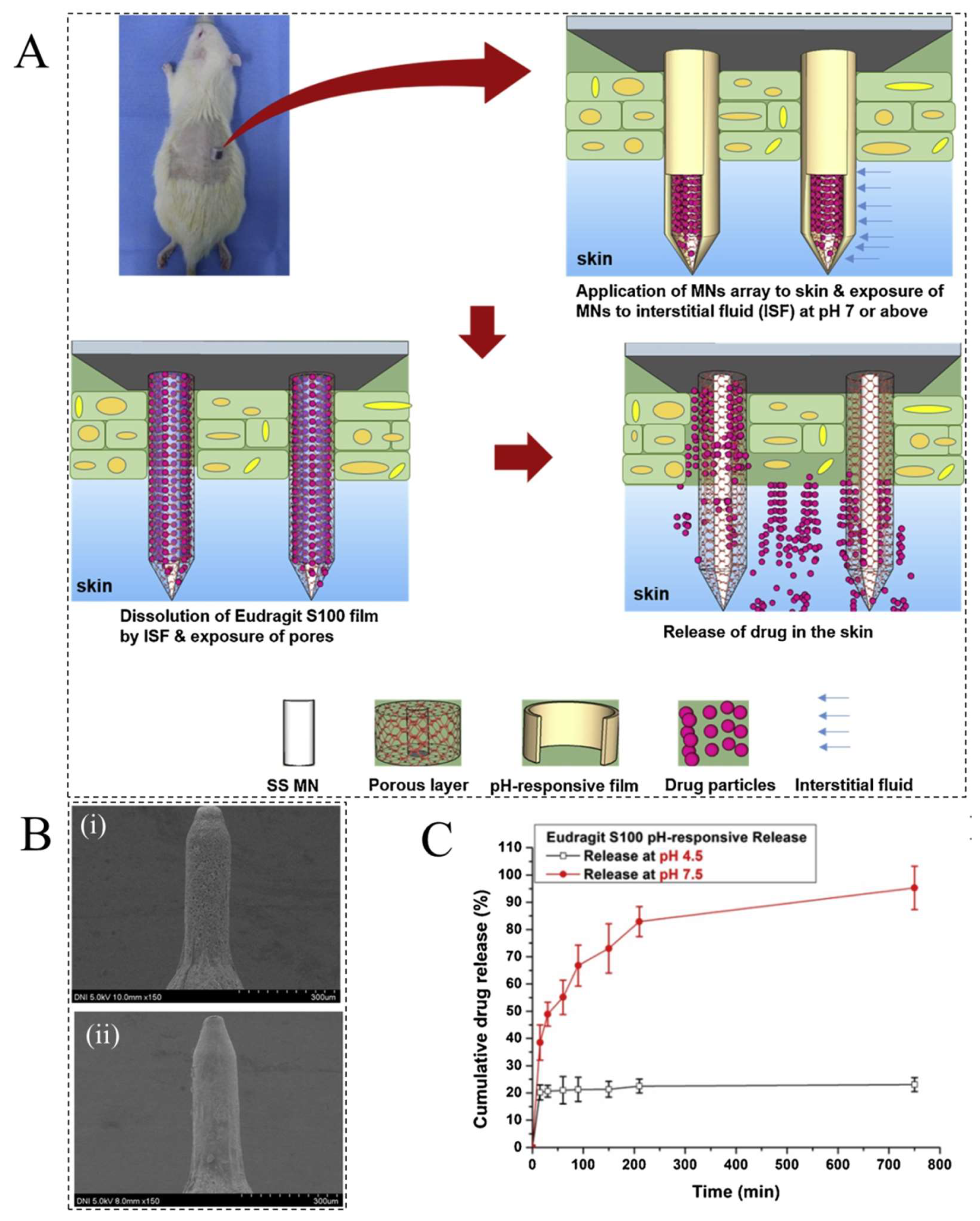
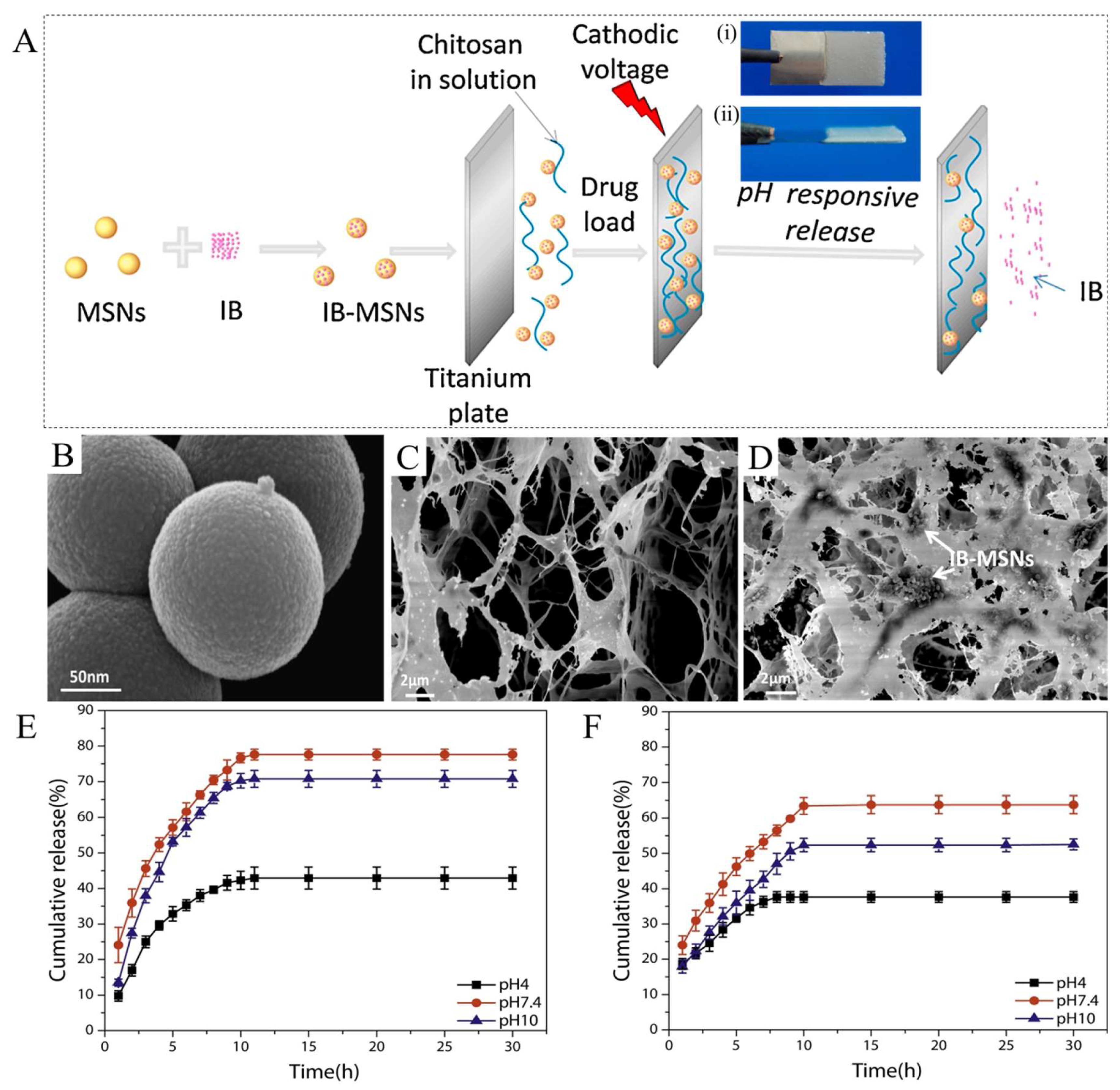
2. pH-Responsive Hydrogels (PRHs)
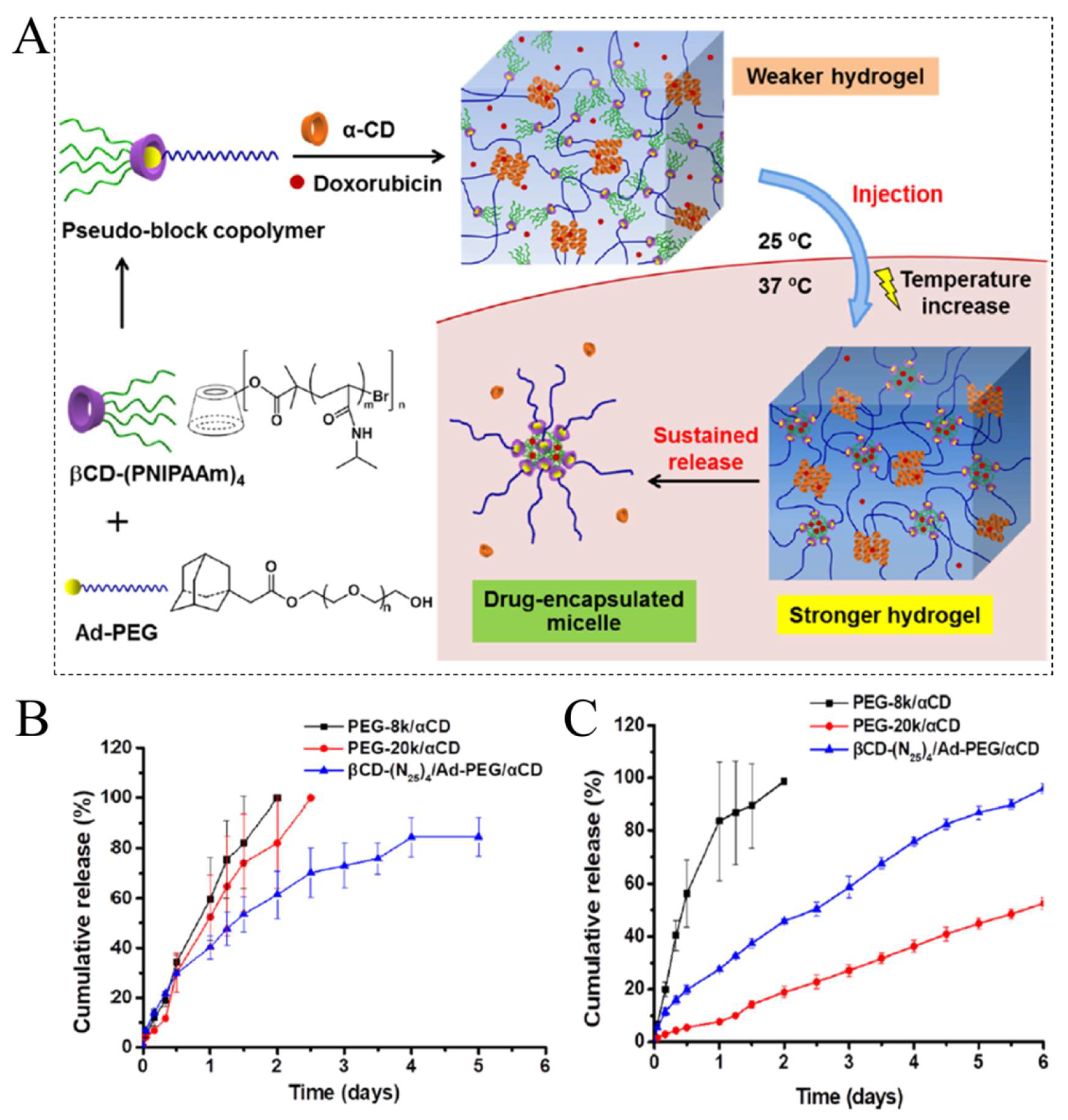
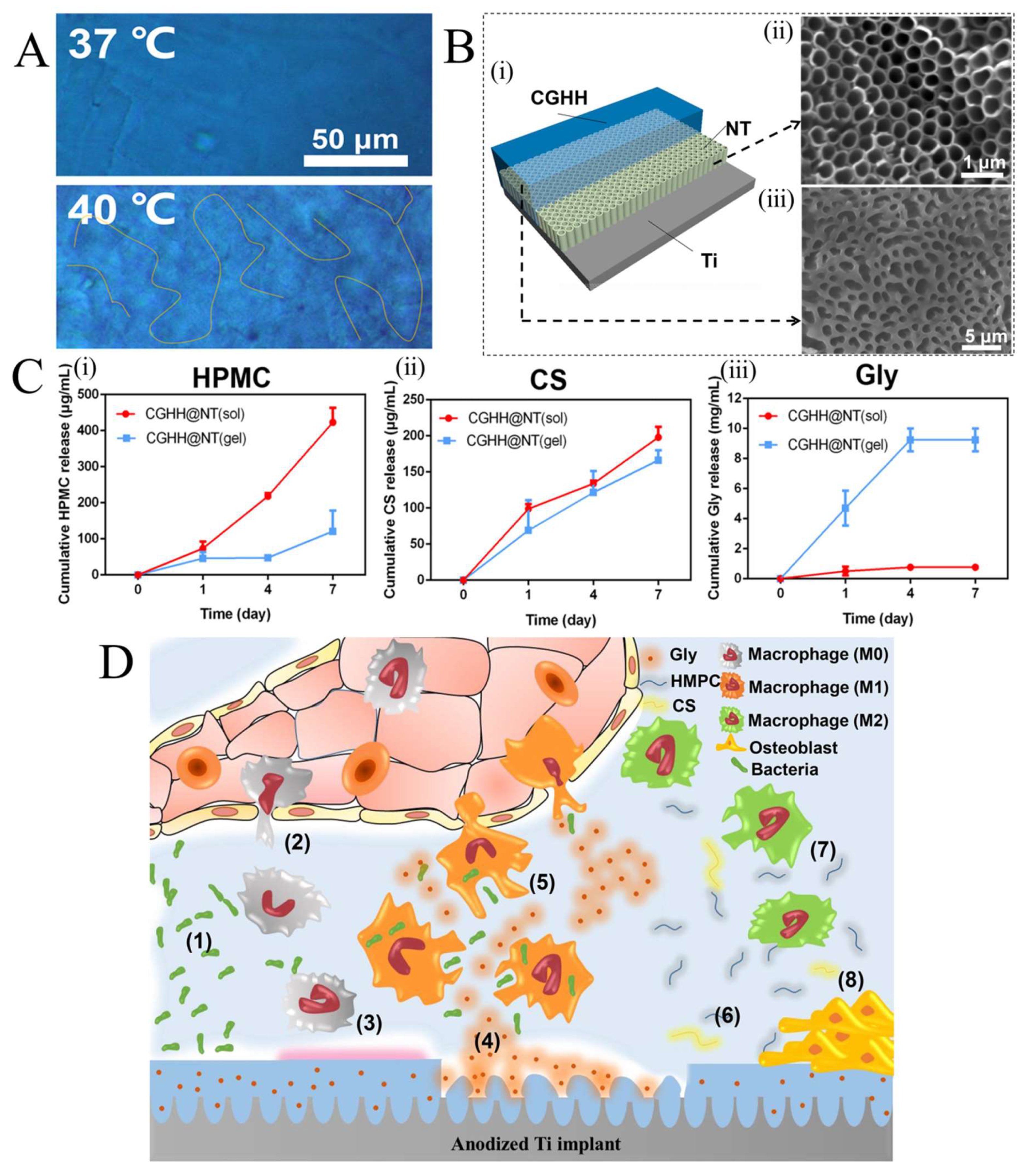
3. Temperature-Responsive Hydrogels (TRHs)
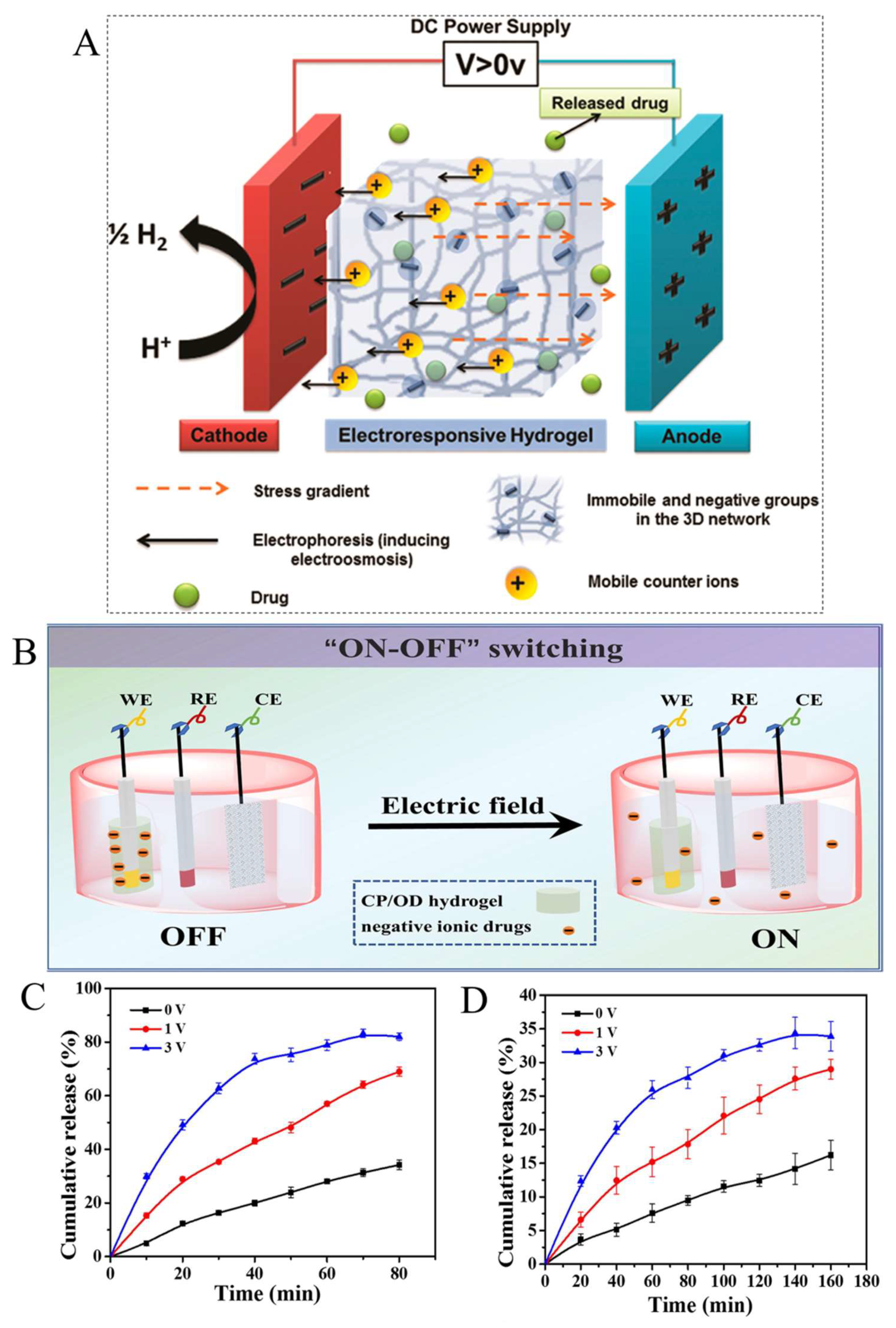
4. Electrically and Magnetically Responsive Hydrogels (E and MRHs)
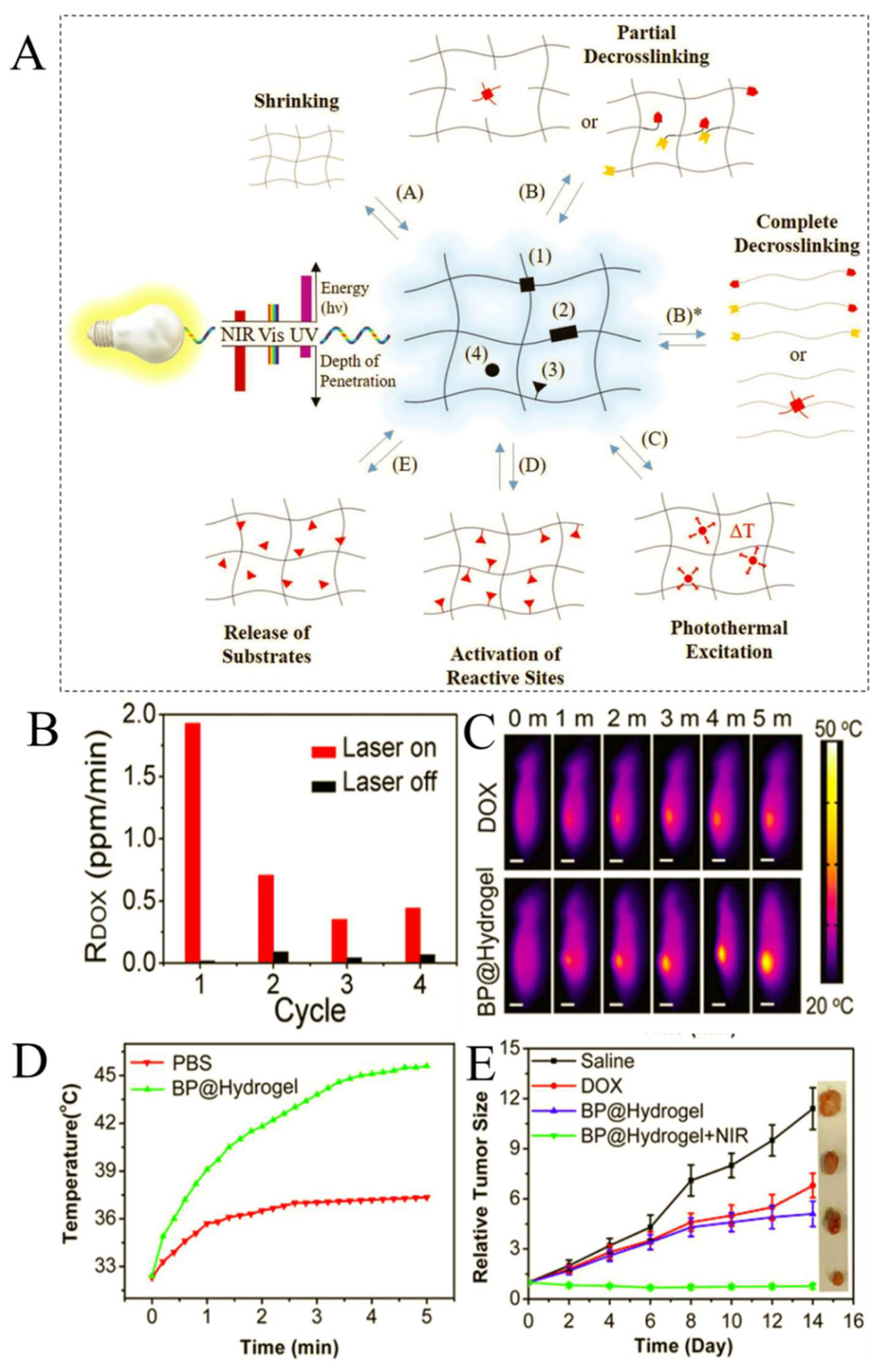
5. Light-Responsive Hydrogels (LRHs)
6. Biomolecule-Responsive Hydrogels
7. Conclusions, Challenges, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRHs | pH-responsive hydrogels |
| MNs | Stainless steel microneedles |
| ROS | Reactive oxygen species |
| DEX | Dexamethasone |
| PEG | Poly(ethylene glycol) |
| SEM | Scanning electron microscopy |
| Ti | Titanium |
| IB | Ibuprofen |
| MSNs | Mesoporous silica nanoparticles |
| TRHs | Temperature-responsive hydrogels |
| LCST | Lower critical solution temperature |
| UCST | Upper critical solution temperature |
| PDEAM | Poly(N,N-diethyl acrylamide) |
| PNIPAM | Poly (N-isopropylacrylamide) |
| PMVE | Poly(methylvinylether) |
| PVC | Poly(N-vinylcaprolactam) |
| β-CD | β-cyclodextrin |
| Ad-PEG | Adamantyl-terminated poly(ethylene glycol) |
| α-CD | α-cyclodextrin |
| DOX | Doxorubicin |
| HMPC | Hydroxypropyl methylcellulose |
| CS | Chitosan |
| Gly | Glycerin |
| CGHH | Hydroxypropyl methylcellulose/Chitosan/Glycerin composite |
| NT | Nanotube |
| BMP | Bone morphogenetic protein |
| G′ | Storage modulus |
| G″ | Loss modulus |
| ERHs | Electrically responsive hydrogels |
| CP | Chitosan-graft-polyaniline |
| OD | Oxidized dextran |
| MRHs | Magnetically responsive hydrogels |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
| LRHs | Light-responsive hydrogels |
| NIR | Near-infrared radiation |
| BPNSs | Black phosphorus nanosheets |
| GRHs | Glucose-responsive hydrogels |
| IVDD | Intervertebral disc degeneration |
| HA | Hyaluronic acid |
| Met | Metformin |
| ERHs | Enzyme-responsive hydrogels |
| HAase | Hyaluronidases |
| β-GUS | β-glucuronidase |
| CMS | Chymotrypsin |
| V8 | Glutamyl endonuclease |
| PDOP | Polydopamine |
| Ag NPs | Silver nanoparticles |
| PAH | Polyallylamine hydrochloride |
| PG | Poly(L-glutamic acid) |
| LBL | Layer by layer |
| PAR | Poly(arginine) |
| VEGFA | Vascular endothelial growth factor A |
| FDA | Food and Drug Administration |
| ERC | European Research Council |
References
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational applications of hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Gasik, M.; Gantar, A.; Novak, S. Viscoelastic behaviour of hydrogel-based composites for tissue engineering under mechanical load. Biomed. Mater. 2017, 12, 025004. [Google Scholar]
- Khan, F.; Tanaka, M. Designing smart biomaterials for tissue engineering. Int. J. Mol. Sci. 2018, 19, 17. [Google Scholar] [CrossRef] [Green Version]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Res. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef]
- Available online: https://www.scopus.com/ (accessed on 1 January 2022).
- Wang, X.; Yang, Y.; Shi, Y.; Jia, F. Smart hydrogels in tissue engineering and regenerative medicine. Front. Chem. 2020, 8, 245. [Google Scholar] [CrossRef]
- Merati, A.A.; Hemmatinejad, N.; Shakeri, M.; Bashari, A. Preparation, Classification, and Applications of Smart Hydrogels. Adv. Funct. Text. Polym. Fabr. Process. Appl. 2019, 337–364. [Google Scholar]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-responsive hydrogels for local post-surgical drug delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.D.; Nath, L.K.; Chakraborty, P. Recent progress in smart polymers: Behavior, mechanistic understanding and application. Polym.-Plast. Technol. Eng. 2018, 57, 945–957. [Google Scholar] [CrossRef]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Anis, A.; Kim, D.; Pal, K. Environment sensitive hydrogels for drug delivery applications. Eur. Polym. J. 2019, 120, 109220. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Ratemi, E. pH-responsive polymers for drug delivery applications. Stimuli Resp. Polym. Nanocarriers Drug Deliv. Appl. 2018, 1, 121–141. [Google Scholar]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Rafael, D.; Melendres, M.M.R.; Andrade, F.; Montero, S.; Martinez-Trucharte, F.; Vilar-Hernandez, M.; Durán-Lara, E.F.; Schwartz Jr, S.; Abasolo, I. Thermo-responsive hydrogels for cancer local therapy: Challenges and state-of-art. Int. J. Pharm. 2021, 606, 120954. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Xian, S.; Webber, M.J. Temperature-responsive supramolecular hydrogels. J. Mater. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors: A review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dai, Z.; Sheng, X.; Xia, D.; Shao, P.; Yang, L.; Luo, X. Conducting polymer hydrogels as a sustainable platform for advanced energy, biomedical and environmental applications. Sci. Total Environ. 2021, 786, 147430. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S. Electrically Conducting Hydrogels for Health care: Concept, Fabrication Methods, and Applications. Int. J. Bioprint. 2020, 6, 273. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-responsive hydrogels: From strategic design to biomedical applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Review on magnetic natural polymer constructed hydrogels as vehicles for drug delivery. Biomacromolecules 2020, 21, 2574–2594. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent advances on magnetic sensitive hydrogels in tissue engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: A review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- LeValley, P.J.; Sutherland, B.P.; Jaje, J.; Gibbs, S.; Jones, R.M.; Gala, R.P.; Kloxin, C.J.; Kiick, K.L.; Kloxin, A.M. On-Demand and Tunable Dual Wavelength Release of Antibodies Using Light-Responsive Hydrogels. ACS Appl. Bio Mater. 2020, 3, 6944–6958. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, L. Novel light-responsive hydrogels with antimicrobial and antifouling capabilities. Langmuir 2018, 35, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifzadeh, G.; Hosseinkhani, H. Biomolecule-responsive hydrogels in medicine. Adv. Healthc. Mater. 2017, 6, 1700801. [Google Scholar] [CrossRef] [PubMed]
- Ooi, H.W.; Hafeez, S.; Van Blitterswijk, C.A.; Moroni, L.; Baker, M.B. Hydrogels that listen to cells: A review of cell-responsive strategies in biomaterial design for tissue regeneration. Mater. Horiz. 2017, 4, 1020–1040. [Google Scholar] [CrossRef]
- Tang, W.; Chen, C. Hydrogel-based colloidal photonic crystal devices for glucose sensing. Polymers 2020, 12, 625. [Google Scholar] [CrossRef] [Green Version]
- Kopač, T.; Krajnc, M.; Ručigaj, A. A mathematical model for pH-responsive ionically crosslinked TEMPO nanocellulose hydrogel design in drug delivery systems. Int. J. Biol. Macromol. 2021, 168, 695–707. [Google Scholar] [CrossRef]
- Yin, Z.C.; Wang, Y.L.; Wang, K. A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 12–18. [Google Scholar] [CrossRef]
- Hu, Y.; Kim, Y.; Hong, I.; Kim, M.; Jung, S. Fabrication of Flexible pH-Responsive Agarose/Succinoglycan Hydrogels for Controlled Drug Release. Polymers 2021, 13, 2049. [Google Scholar] [CrossRef]
- Khan, H.; Chaudhary, J.P.; Meena, R. Anionic carboxymethylagarose-based pH-responsive smart superabsorbent hydrogels for controlled release of anticancer drug. Inter. J. Biol. Macromol. 2019, 124, 1220–1229. [Google Scholar] [CrossRef]
- Jommanee, N.; Chanthad, C.; Manokruang, K. Preparation of injectable hydrogels from temperature and pH responsive grafted chitosan with tuned gelation temperature suitable for tumor acidic environment. Carbohydr. Polym. 2018, 198, 486–494. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Yadollahie, M.; Habibzadeh, P. Metabolic Acidosis and Alkalosis. In Pathophysiologic Basis of Acid-Base Disorders; Springer: Singapore, 2021; pp. 95–115. [Google Scholar]
- Ullah, A.; Jang, M.; Khan, H.; Choi, H.J.; An, S.; Kim, D.; Kim, Y.R.; Kim, U.K.; Kim, G.M. Microneedle array with a pH-responsive polymer coating and its application in smart drug delivery for wound healing. Sens. Actuators B Chem. 2021, 345, 130441. [Google Scholar] [CrossRef]
- Zhou, G.; Groth, T. Host responses to biomaterials and anti-inflammatory design—A brief review. Macromol. Biosci. 2018, 18, 1800112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef] [Green Version]
- Győri, D.S.; Mócsai, A. Osteoclast signal transduction during bone metastasis formation. Front. Cell Dev. Biol. 2020, 8, 507. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Chao, Y.C.; Hsiao, M.H.; Chou, H.S.; Jheng, Y.H.; Yu, X.H.; Lee, N.; Yang, C.; Liu, D.M. Inhibition of Periodontitis Induction Using a Stimuli-Responsive Hydrogel Carrying Naringin. J. Periodontol. 2017, 88, 190–196. [Google Scholar] [CrossRef]
- Chauhan, N.; Gupta, P.; Arora, L.; Pal, D.; Singh, Y. Dexamethasone-loaded, injectable pullulan-poly (ethylene glycol) hydrogels for bone tissue regeneration in chronic inflammatory conditions. Mater. Sci. Eng. C 2021, 130, 112463. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Colloids Surf. B Biointerfaces 2014, 123, 657–663. [Google Scholar] [CrossRef]
- d’Eramo, L.; Chollet, B.; Leman, M.; Martwong, E.; Li, M.; Geisler, H.; Dupire, J.; Kerdraon, M.; Vergne, C.; Monti, F.; et al. Microfluidic actuators based on temperature-responsive hydrogels. Microsyst. Nanoeng. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Takata, K.; Takai, H.; Yoshizaki, Y.; Nagata, T.; Kawahara, K.; Yoshida, Y.; Kuzuya, A.; Ohya, Y. Peptide drug release behavior from biodegradable temperature-responsive injectable hydrogels exhibiting irreversible gelation. Gels 2017, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Chen, Y.; Deng, Y.; Ngai, T.; Wang, C. Dynamic supramolecular hydrogels: Regulating hydrogel properties through self-complementary quadruple hydrogen bonds and thermo-switch. ACS Macro Lett. 2017, 6, 641–646. [Google Scholar] [CrossRef]
- Li, Z.; Hao, B.; Tang, Y.; Li, H.; Lee, T.C.; Feng, A.; Zhang, L.; Thang, S.H. Effect of end-groups on sulfobetaine homopolymers with the tunable upper critical solution temperature (UCST). Eur. Polym. J. 2020, 132, 109704. [Google Scholar] [CrossRef]
- Takahashi, H.; Okano, T. Thermally-triggered fabrication of cell sheets for tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2019, 138, 276–292. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Z.; Zhu, J.; Wen, Y.; Zhao, F.; Lei, L.; Phan-Thien, N.; Khoo, B.C.; Li, J. Thermoresponsive hydrogel induced by dual supramolecular assemblies and its controlled release property for enhanced anticancer drug delivery. Biomacromolecules 2020, 21, 1516–1527. [Google Scholar] [CrossRef]
- Melo-Fonseca, F.; Gasik, M.; Madeira, S.; Silva, F.S.; Miranda, G. Surface characterization of titanium-based substrates for orthopaedic applications. Mater. Charact. 2021, 177, 111161. [Google Scholar] [CrossRef]
- Costa, M.M.; Lima, R.; Melo-Fonseca, F.; Bartolomeu, F.; Alves, N.; Miranda, A.; Gasik, M.; Silva, F.S.; Silva, N.A.; Miranda, G. Development of β-TCP-Ti6Al4V structures: Driving cellular response by modulating physical and chemical properties. Mater. Sci. Eng. C 2019, 98, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Komasa, S.; Hashimoto, Y.; Hontsu, S.; Okazaki, J. In vitro and in vivo osteogenic activity of titanium implants coated by pulsed laser deposition with a thin film of fluoridated hydroxyapatite. Inter. J. Mol. Sci. 2018, 19, 1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Chiesa, R.; Rimondini, L.; Gasik, M. Metallurgical gallium additions to titanium alloys demonstrate a strong time-increasing antibacterial activity without any cellular toxicity. ACS Biomater. Sci. Eng. 2019, 5, 2815–2820. [Google Scholar] [CrossRef] [Green Version]
- Kandi, V.; Vadakedath, S. Implant-associated infections: A review of the safety of cardiac implants. Cureus 2020, 12, 12. [Google Scholar] [CrossRef]
- Burr, D.B. Changes in bone matrix properties with aging. Bone 2019, 120, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Rimondini, L.; Gasik, M. Bacterial attachment and biofilm formation on biomaterials: The case of dental and orthopaedic implants. In Biomaterials and Immune Response; CRC Press: Boca Raton, FL, USA, 2018; pp. 87–120. [Google Scholar]
- van Hengel, I.A.; Tierolf, M.W.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Antibacterial titanium implants biofunctionalized by plasma electrolytic oxidation with silver, zinc, and copper: A systematic review. Inter. J. Mol. Sci. 2021, 22, 3800. [Google Scholar] [CrossRef] [PubMed]
- Gasik, M.; Braem, A.; Chaudhari, A.; Duyck, J.; Vleugels, J. Titanium implants with modified surfaces: Meta-analysis of in vivo osteointegration. Mater. Sci. Eng. C 2015, 49, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhang, L.; Wang, D.; Liu, X.; Li, H.; Liang, C.; Zhao, X. Thermo-sensitive hydrogel on anodized titanium surface to regulate immune response. Surf. Coat. Technol. 2021, 405, 126624. [Google Scholar] [CrossRef]
- Paidikondala, M.; Wang, S.; Hilborn, J.; Larsson, S.; Varghese, O.P. Impact of Hydrogel Cross-Linking Chemistry on the in Vitro and in Vivo Bioactivity of Recombinant Human Bone Morphogenetic Protein-2. ACS Appl. Bio Mater. 2019, 2, 2006–2012. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, Q.; Shi, Y.; Li, W.; Fu, Y.; Jin, J. Sintered porous Ti6Al4V scaffolds incorporated with recombinant human bone morphogenetic protein-2 microspheres and thermosensitive hydrogels can enhance bone regeneration. Rsc Adv. 2019, 9, 1541–1550. [Google Scholar] [CrossRef] [Green Version]
- Merino, S.; Martin, C.; Kostarelos, K.; Prato, M.; Vazquez, E. Nanocomposite hydrogels: 3D polymer-nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [Green Version]
- Sangsuriyonk, K.; Paradee, N.; Sirivat, A. Electrically, controlled release of anticancer drug 5-fluorouracil from carboxymethyl cellulose hydrogels. Int. J. Biol. Macromol. 2020, 165, 865–873. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, T.; Lei, Q.; He, J.; Ma, P.X.; Guo, B. Stimuli-responsive conductive nanocomposite hydrogels with high stretchability, self-healing, adhesiveness, and 3D printability for human motion sensing. ACS Appl. Mater. Interfaces 2019, 11, 6796–6808. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Margel, S. Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery. Polymers 2021, 13, 4259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Song, S.C. Multiple hyperthermia-mediated release of TRAIL/SPION nanocomplex from thermosensitive polymeric hydrogels for combination cancer therapy. Biomaterials 2017, 132, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, S.; Ma, J.; Da Ling, S.; Wang, Y.D.; Kong, T.T.; Xu, J.H. Fabrication of magnetic core/shell hydrogels via microfluidics for controlled drug delivery. Chem. Eng. Sci. 2021, 248, 117216. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Soni, S.; Sachdev, A.; Mishra, S. Near-infrared stimulated hydrogel patch for photothermal therapeutics and thermoresponsive drug delivery. J. Photochem. Photobiol. B Biol. 2020, 210, 111960. [Google Scholar] [CrossRef]
- Anugrah, D.S.B.; Ramesh, K.; Kim, M.; Hyun, K.; Lim, K.T. Near-infrared light-responsive alginate hydrogels based on diselenide-containing cross-linkage for on demand degradation and drug release. Carbohydr. Polym. 2019, 223, 115070. [Google Scholar] [CrossRef]
- He, Y.; Leng, J.; Li, K.; Xu, K.; Lin, C.; Yuan, Z.; Zhang, R.; Wang, D.; Tao, B.; Huang, T.J.; et al. A multifunctional hydrogel coating to direct fibroblast activation and infected wound healing via simultaneously controllable photobiomodulation and photodynamic therapies. Biomaterials 2021, 278, 121164. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B. Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Constantin, M.; Bucatariu, S.; Ascenzi, P.; Butnaru, M.; Fundueanu, G. Smart drug delivery system activated by specific biomolecules. Mater. Sci. Eng. C 2020, 108, 110466. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, W.; Xu, P.; Fu, X.; Yu, X.; Chen, L.; Leng, F.; Yu, C.; Yang, Z. Glucose-responsive multifunctional metal–organic drug-loaded hydrogel for diabetic wound healing. Acta Biomater. 2022, 140, 206–218. [Google Scholar] [CrossRef]
- Tong, M.Q.; Luo, L.Z.; Xue, P.P.; Han, Y.H.; Wang, L.F.; Zhuge, D.L.; Yao, Q.; Chen, B.; Zhao, Y.Z.; Xu, H.L. Glucose-responsive hydrogel enhances the preventive effect of insulin and liraglutide on diabetic nephropathy of rats. Acta Biomater. 2021, 122, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Yu, H.; Wang, L.; Chen, X.; Feng, J.; Li, C.; Xiong, W.; Zhang, Q. Glucose-responsive hydrogel-based microneedles containing phenylborate ester bonds and N-isopropylacrylamide moieties and their transdermal drug delivery properties. Eur. Polym. J. 2021, 148, 110348. [Google Scholar] [CrossRef]
- Walter, S.V.; Ennen-Roth, F.; Büning, D.; Denizer, D.; Ulbricht, M. Glucose-responsive polymeric hydrogel materials: From a novel technique for the measurement of glucose binding toward swelling pressure sensor applications. ACS Appl. Bio Mater. 2019, 2, 2464–2480. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.E.; Coleman, C.M. Impact of diabetes mellitus on bone health. Int. J. Mol. Sci. 2019, 2, 4873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Zhang, F.; Ma, J.; Ding, W. Intervertebral disc ageing and degeneration: The antiapoptotic effect of oestrogen. Ageing Res. Rev. 2020, 57, 100978. [Google Scholar] [CrossRef] [PubMed]
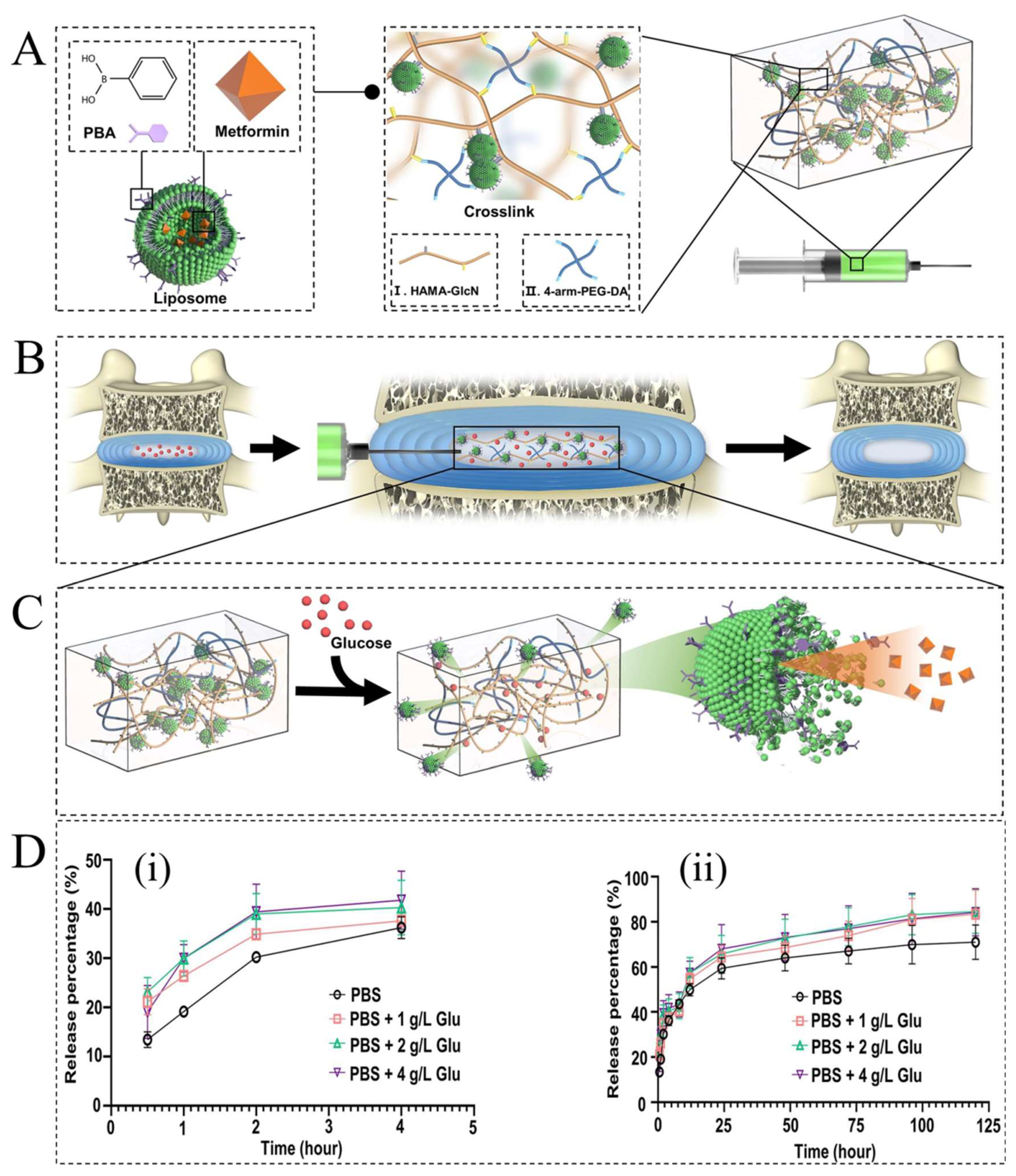
- Zheng, D.; Chen, W.; Ruan, H.; Cai, Z.; Chen, X.; Chen, T.; Zhang, Y.; Cui, W.; Chen, H.; Shen, H. Metformin-hydrogel with glucose responsiveness for chronic inflammatory suppression. Chem. Eng. J. 2022, 428, 131064. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, J.; Zhang, L.; Wang, L.; Xu, H.; Han, Y.; Jia, J.; Lu, Y.; Yu, R.; Liu, H. Injectable postoperative enzyme-responsive hydrogels for reversing temozolomide resistance and reducing local recurrence after glioma operation. Biomater. Sci. 2020, 8, 5306–5316. [Google Scholar] [CrossRef]
- Kim, S.; Cui, Z.K.; Koo, B.; Zheng, J.; Aghaloo, T.; Lee, M. Chitosan–lysozyme conjugates for enzyme-triggered hydrogel degradation in tissue engineering applications. ACS Appl. Mater. Interfaces 2018, 10, 41138–41145. [Google Scholar] [CrossRef]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Sun, L.; Jin, Y.; Ding, X.; Li, L.; Ji, J.; Chen, H. Construction of high drug loading and enzymatic degradable multilayer films for self-defense drug release and long-term biofilm inhibition. Biomacromolecules 2018, 19, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Tallet, L.; Gribova, V.; Ploux, L.; Vrana, N.E.; Lavalle, P. New smart antimicrobial hydrogels, nanomaterials, and coatings: Earlier action, more specific, better dosing? Adv. Healthc. Mater. 2021, 10, 2001199. [Google Scholar] [CrossRef] [PubMed]
- Pala, L.; Sirec, T.; Spitz, U. Modified enzyme substrates for the detection of bacteria: A review. Molecules 2020, 25, 3690. [Google Scholar] [CrossRef] [PubMed]
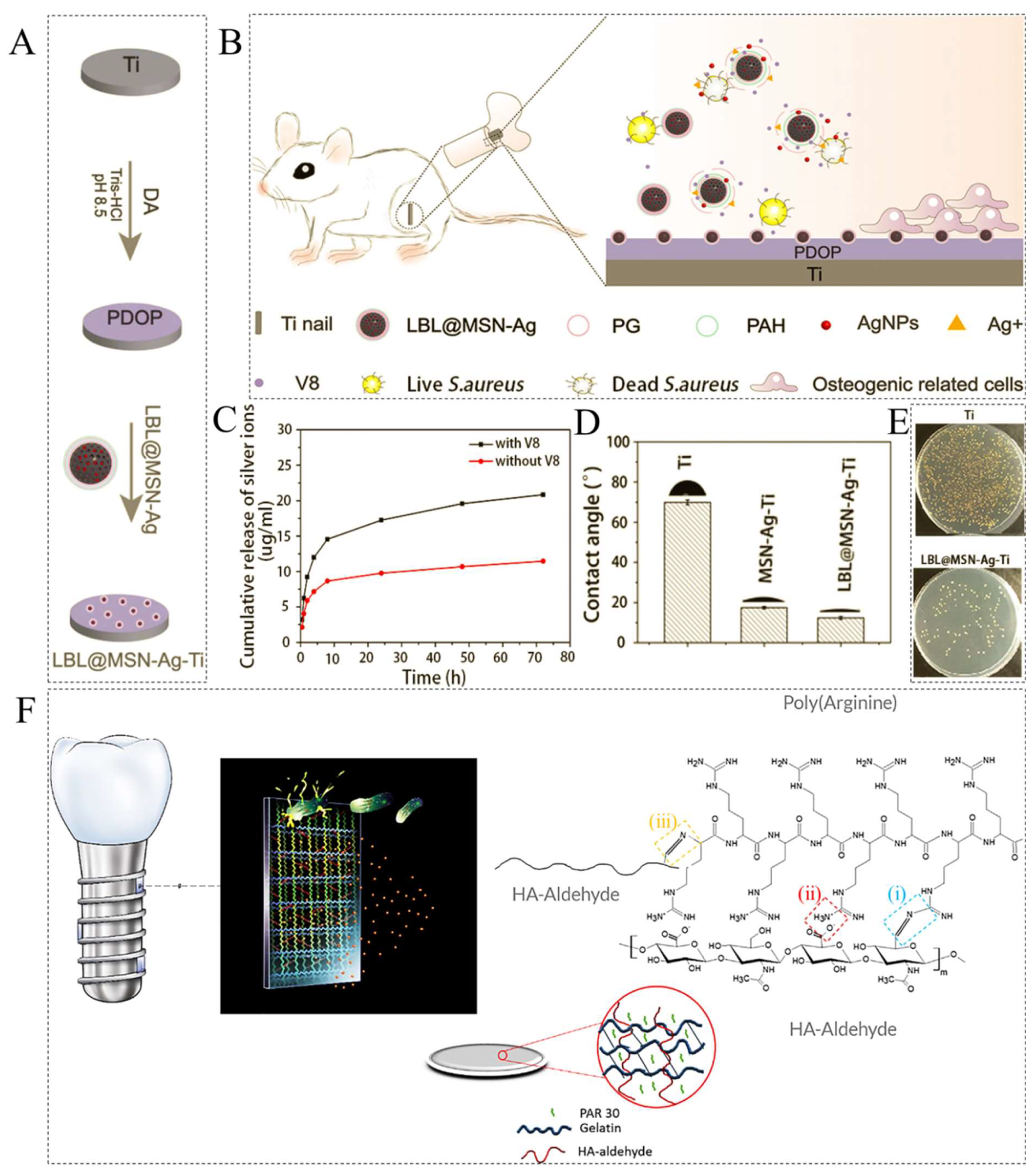
- Ding, Y.; Hao, Y.; Yuan, Z.; Tao, B.; Chen, M.; Lin, C.; Liu, P.; Cai, K. A dual-functional implant with an enzyme-responsive effect for bacterial infection therapy and tissue regeneration. Biomater. Sci. 2020, 8, 1840–1854. [Google Scholar] [CrossRef]
- Singh, V.; Phukan, U.J. Interaction of host and Staphylococcus aureus protease-system regulates virulence and pathogenicity. Med. Microbiol. Immunol. 2019, 208, 585–607. [Google Scholar] [CrossRef]
- Ahmed, W.; Zhai, Z.; Gao, C. Adaptive antibacterial biomaterial surfaces and their applications. Mater. Today Bio 2019, 2, 100017. [Google Scholar] [CrossRef]
- Gu, J.; Feng, L.; Yan, S.; Yin, J. In Situ Biomineralized Phosphorylated Poly (l-Glutamic Acid)/Sodium Alginate Injectable Hydrogels. J. Macromol. Sci. Part B 2021, 61, 160–172. [Google Scholar] [CrossRef]
- Shi, M.; Mo, W.; Qi, H.; Ni, Y.; Wang, R.; Shen, K.; Zhang, F.; Jiang, S.; Zhang, X.; Chen, L.; et al. Oxygen Ion Implantation Improving Cell Adhesion on Titanium Surfaces through Increased Attraction of Fibronectin PHSRN Domain. Adv. Healthc. Mater. 2022, 2101983. [Google Scholar] [CrossRef]
- Knopf-Marques, H.; Barthes, J.; Lachaal, S.; Mutschler, A.; Muller, C.; Dufour, F.; Rabineau, M.; Courtial, E.J.; Bystroňová, J.; Marquette, C.; et al. Multifunctional polymeric implant coatings based on gelatin, hyaluronic acid derivative and chain length-controlled poly (arginine). Mater. Sci. Eng. C 2019, 104, 109898. [Google Scholar] [CrossRef]
- Muller, C.; Berber, E.; Lutzweiler, G.; Ersen, O.; Bahri, M.; Lavalle, P.; Ball, V.; Vrana, N.E.; Barthes, J. Polyarginine Decorated Polydopamine Nanoparticles with Antimicrobial Properties for Functionalization of Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 982. [Google Scholar] [CrossRef]
- Takeno, H.; Aoki, Y.; Kimura, K. Effects of silica and clay nanoparticles on the mechanical properties of poly (vinyl alcohol) nanocomposite hydrogels. Colloids Surf. Physicochem. Eng. Asp. 2021, 630, 127592. [Google Scholar] [CrossRef]
- Shabsigh, A.; Kleinmann, N.; Smith, A.B.; Scherr, D.; Seltzer, E.; Schoenberg, M.; Lerner, S.P. Pharmacokinetics of UGN-101, a mitomycin-containing reverse thermal gel instilled via retrograde catheter for the treatment of low-grade upper tract urothelial carcinoma. Cancer Chemother. Pharmacol. 2021, 87, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cordis.europa.eu/project/id/945602 (accessed on 1 January 2022).
| Type of Hydrogels | Examples | Key Features | Properties | Applications | References |
|---|---|---|---|---|---|
| pH-responsive | Chitosan, guar gum succinate, kappa-carrageenan, PEI, PAM, PAA, PDEAEMA, PDMAEMA, PEAAc, pHEMA, PMAA, PPAA, and PVA | pH variation results in swelling/deswelling behavior due to the changes in hydrophobicity of the polymeric chains and increase in electrostatic repulsion between chains | Biocompatibility, sustained release of incorporated drugs, increased hydrophilicity, and swelling, strong electrostatic interactions, and stability | Drug delivery, Sensing, inflammation responsive hydrogels, wound and skin healing. | [19,20] |
| Temperature responsive | Poloxamer, Pluronic, PAA, PNIPA, PNVCL grafted with PEO, TMC crosslinked with PEG, glycerophosphate, and methoxy poly(ethylene glycol)-poly(pyrrolidone-co-lactide) | Temperature variation disturbs the equilibrium exists between hydrophobic and hydrophilic segments of the polymeric chain and increase the sol-gel transformation rate | Unique physical properties similar to the extracellular matrix, easy functionalization with drug molecules, controlled degradation | Drug delivery, intraocular lenses, tissue engineering. | [21,22,23] |
| Electric field responsive | PPy nanoparticles loaded in PLGA, PEG hydrogels, Agarose, calcium alginate, carbomer, chondroitin sulphate, hyaluronic acid, partially hydrolyzed PAM, PDMA, and xanthan gum | Upon the application of an electric field, deswelling or bending takes place, based on the shape and position of the gel relative to the electrodes. | Biocompatibility, minimal invasiveness, controlled release of the cargo depending on the strength or the duration of applied electric field | Drug delivery, creams and suspensions as emulsion stabilizer, in cosmetics as thickener and stabilizer, buccal delivery. | [24,25,26] |
| Magnetic field responsive | Alginate-xanthan cross-linked with Ca2+ magnetic nanoparticles, Hemicellulose crosslinked with GGM, hemicellulose hydrogels with magnetic iron oxide (Fe3O4), methacrylate chondroitin sulfate with magnetic nanoparticles, PNIPA, and xanthan-bovine serum albumin-magnetic nanoparticles | Application of heating, mechanical deformation, or external magnetic field to magnetic nanoparticles, such as nanoparticles of magnetite, maghemite, and ferrite | Swelling behavior responsive to temperature too, some of them dispose of anisotropic properties, successful absorption and controlled release of drugs | Drug delivery, sensing, microfluidics, tissue engineering. | [27,28,29] |
| Light responsive | Poly [2-((4,5-dimethoxy-2-nitrobenzyl) oxy)-N-(2-(methacryloyloxy)ethyl)-N,N-dimethyl-2-oxoethan-1-aminium, HPMC, Carbopol hydrogels containing diclofenac-sodium chitosan microspheres, Azo benzene-pHEMA, azo benzene-bovine albumin, triphenylmethane leuco derivatives, and trisodium salt of copper chlorophyllin-PNIPAM23 | External stimulus of either visible or UV light initiates sol-gel transformation | Control release, reversible and irreversible, spatiotemporal control over functional groups, reasonable strengthens according to application. | Drug delivery, optical delivery, microfluidics, self-sterilization and self-cleaning. | [30,31,32,33] |
| Biomolecules responsive | Insulin, phenylborate derivative 4-(1,6-dioxo-2,5-diaza-7-oxamyl) phenylboronic acid in combination with PNIPA, and poly(2-hydroxyethyl methacrylate-co-N,N-dimethylaminoethyl methacrylate) in combination with glucose oxidase | Changes in biomolecule concentration and pH in hydrogel as a self-regulated, can expand the polyelectrolytes resulting in swelling/deswelling behavior. | Enzyme responsive, achieves molecular recognition, high affinity, and specificity, controlled release, biocompatibility. | Drug delivery, insulin-delivery system, cell culture, sensing, tissue engineering. | [34,35,36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. https://doi.org/10.3390/ijms23073665
Bordbar-Khiabani A, Gasik M. Smart Hydrogels for Advanced Drug Delivery Systems. International Journal of Molecular Sciences. 2022; 23(7):3665. https://doi.org/10.3390/ijms23073665
Chicago/Turabian StyleBordbar-Khiabani, Aydin, and Michael Gasik. 2022. "Smart Hydrogels for Advanced Drug Delivery Systems" International Journal of Molecular Sciences 23, no. 7: 3665. https://doi.org/10.3390/ijms23073665
APA StyleBordbar-Khiabani, A., & Gasik, M. (2022). Smart Hydrogels for Advanced Drug Delivery Systems. International Journal of Molecular Sciences, 23(7), 3665. https://doi.org/10.3390/ijms23073665