The Metabolic Impact of Two Different Parenteral Nutrition Lipid Emulsions in Children after Hematopoietic Stem Cell Transplantation: A Lipidomics Investigation
Abstract
:1. Introduction
2. Results
2.1. General Characteristics of the Patients
2.2. Lipidomics Results
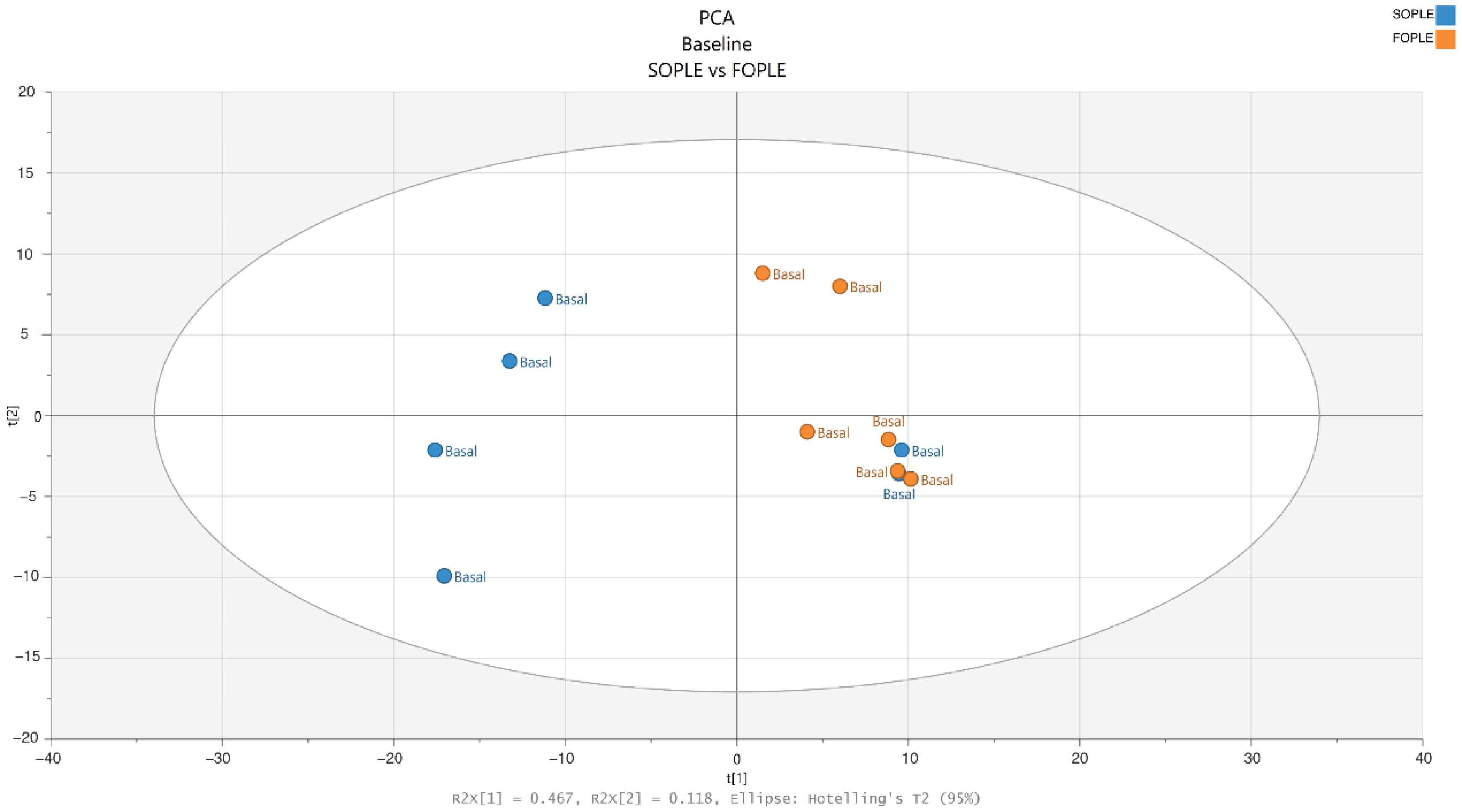
2.2.1. Differences at Baseline in the Lipidomics Profile
2.2.2. Differences after the Intervention in the Lipidomics Profile
2.3. Differences after the Intervention in the General Biochemistry, Inflammatory Biomarkers, and FAs Profiles in Erythrocytes
2.4. Data Integration Analysis for Biomarker Discovery Using Latent Components (DIABLO)
3. Discussion
Strengths and Limitations of the Current Study
4. Materials and Methods
4.1. Subjects
4.2. Inclusion and Exclusion Criteria
4.3. Parenteral Nutrition
4.3.1. Soybean Formula (SOPLE, 20 g of Purified SO per 100 mL)
4.3.2. FO-Containing Emulsion (FOPLE, 200 mg/mL (20%) of Triacylglycerols)
4.4. Sampling
4.5. Analysis of FA Profiles in Plasma and Erythrocytes
4.6. Reverse Phase-Ultra Performance Liquid Chromatography-Fourier Transformation Mass Spectrometry (RP-UPLC-FTMS) Lipidomics
4.6.1. Plasma Sample Preparation for RP-UPLC-FTMS Lipidomics
4.6.2. Data Acquisition
4.6.3. Data Preprocessing
4.6.4. Feature-Clustering
4.7. Multivariate Statistical Analysis
4.8. Univariate Statistical Analysis
T-Test
4.9. Pathway Analysis with Mummichog and Gene Set Enrichment Analysis (GSEA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhry, M.; Ali, N. Reduced-Intensity Conditioning Hematopoietic Stem Cell Transplantation: Looking Forward to an International Consensus. Blood Res. 2015, 50, 69–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, K.H.; Evans, V.; Yap, J. Indications and Patterns of Use for Parenteral Nutrition in Pediatric Oncology. J. Parenter. Enter. Nutr. 2020, 44, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.C.; Hirani, S.P.; Needle, J.J. Nutritional and Post-Transplantation Outcomes of Enteral versus Parenteral Nutrition in Pediatric Hematopoietic Stem Cell Transplantation: A Systematic Review of Randomized and Nonrandomized Studies. Biol. Blood Marrow Transpl. 2019, 25, e252–e259. [Google Scholar] [CrossRef] [PubMed]
- McMillen, K.K.; Coghlin-Dickson, T.; Adintori, P.A. Optimization of Nutrition Support Practices Early After Hematopoietic Cell Transplantation. Bone Marrow Transpl. 2021, 56, 314–326. [Google Scholar] [CrossRef]
- Jonas, C.R.; Puckett, A.B.; Jones, D.P.; Griffith, D.P.; Szeszycki, E.E.; Bergman, G.F.; Furr, C.E.; Tyre, C.; Carlson, J.L.; Galloway, J.R.; et al. Plasma Antioxidant Status after High-Dose Chemotherapy: A Randomized Trial of Parenteral Nutrition in Bone Marrow Transplantation Patients. Am. J. Clin. Nutr. 2000, 72, 181–189. [Google Scholar] [CrossRef]
- Murray, S.M.; Pindoria, S. Nutrition Support for Bone Marrow Transplant Patients. Cochrane Database Syst. Rev. 2017, 2017, CD002920. [Google Scholar] [CrossRef]
- Woods, T.; Tariman, J.; Lee, Y. Enteral and Parenteral Nutrition: An Integrative Literature Review on Nutrition in Pediatric Recipients of Hematopoietic Stem Cell Transplantation. Clin. J. Oncol. Nurs. 2019, 23, 351–354. [Google Scholar] [CrossRef]
- Nava, T.; Ansari, M.; Dalle, J.H.; de Heredia, C.D.; Güngör, T.; Trigoso, E.; Falkenberg, U.; Bertaina, A.; Gibson, B.; Jarisch, A.; et al. Supportive Care during Pediatric Hematopoietic Stem Cell Transplantation: Beyond Infectious Diseases. A Report from Workshops on Supportive Care of the Pediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2020, 55, 1126–1136. [Google Scholar] [CrossRef]
- Ifversen, M.; Meisel, R.; Sedlacek, P.; Kalwak, K.; Sisinni, L.; Hutt, D.; Lehrnbecher, T.; Balduzzi, A.; Diesch, T.; Jarisch, A.; et al. Supportive Care During Pediatric Hematopoietic Stem Cell Transplantation: Prevention of Infections. A Report from Workshops on Supportive Care of the Paediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT). Front. Pediatr. 2021, 9, 1126–1136. [Google Scholar] [CrossRef]
- Aoyama, T.; Imataki, O.; Mori, K.; Yoshitsugu, K.; Fukaya, M.; Okamura, I.; Enami, T.; Tatara, R.; Ikeda, T.; Okamura-Shiki, I. Nutritional risk in allogeneic stem cell transplantation: Rationale for a tailored nutritional pathway. Ann. Hematol. 2017, 96, 617–625. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Conversano, L.; Torelli, G.F.; Arcese, W.; Capria, S.; Cangiano, C.; Falcone, C.; Fanelli, F.R. Clinical and metabolic effects of different parenteral nutrition regimens in patients undergoing allogeneic bone marrow transplantation. Transplantation 1998, 66, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-L.; Chiang, C.-L.; Wang, P.-N. Parenteral nutrition support after bone marrow transplantation: Comparison of total and partial parenteral nutrition during the early posttransplantation period. Nutrition 2001, 17, 773–775. [Google Scholar] [CrossRef]
- Ren, T.; Cong, L.; Wang, Y.; Tang, Y.; Tian, B.; Lin, X.; Zhang, Y.; Tang, X. Lipid emulsions in parenteral nutrition: Current applications and future developments. Expert Opin. Drug Deliv. 2013, 10, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Langlois, P.L.; Dhaliwal, R.; Lemieux, M.; Heyland, D.K. Intravenous fish oil lipid emulsions in critically ill patients: An updated systematic review and meta-analysis. Crit. Care 2015, 19, 167. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Yao, X.; Zeng, R.; Sun, R.; Tian, H.; Shi, C.; Li, L.; Tian, J.; Yang, K. Safety and efficacy of a new parenteral lipid emulsion (SMOF) for surgical patients: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Li, N.-N.; Zhou, Y.; Qin, X.-P.; Chen, Y.; He, D.; Feng, J.-Y.; Wu, X.-T. Does intravenous fish oil benefit patients post-surgery? A meta-analysis of randomised controlled trials. Clin. Nutr. 2014, 33, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Baena-Gómez, M.; Aguilar, M.D.L.T.; Mesa, M.; Llorente-Cantarero, F.; Navero, J.P.; Gil-Campos, M. Effects of Parenteral Nutrition Formulas on Plasma Lipid Profile in Children with Bone Marrow Transplantation. Ann. Nutr. Metab. 2013, 63, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Baena-Gómez, M.A.; De La Torre Aguilar, M.J.; Mesa, M.D.; Navero, J.L.P.; Gil-Campos, M. Changes in Antioxidant Defense System Using Different Lipid Emulsions in Parenteral Nutrition in Children after Hematopoietic Stem Cell Transplantation. Nutrients 2015, 7, 7242–7255. [Google Scholar] [CrossRef] [Green Version]
- Baena-Gómez, M.A.; De La Torre-Aguilar, M.J.; Aguilera-García, C.M.; Olza, J.; Pérez-Navero, J.L.; Gil-Campos, M. Inflammatory Response Using Different Lipid Parenteral Nutrition Formulas in Children After Hematopoietic Stem Cell Transplantation. Nutr. Cancer 2016, 68, 804–810. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef] [Green Version]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Reikvam, H.; Grønningsæter, I.-S.; Mosevoll, K.A.; Lindås, R.; Hatfield, K.; Bruserud, Ø. Patients with Treatment-Requiring Chronic Graft versus Host Disease after Allogeneic Stem Cell Transplantation Have Altered Metabolic Profiles due to the Disease and Immunosuppressive Therapy: Potential Implication for Biomarkers. Front. Immunol. 2018, 8, 1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Huang, A.; Chen, Q.; Chen, X.; Fei, Y.; Zhao, X.; Zhang, W.; Hong, Z.; Zhu, Z.; Yang, J.; et al. A distinct glycerophospholipid metabolism signature of acute graft versus host disease with predictive value. JCI Insight 2019, 5, e129494. [Google Scholar] [CrossRef] [PubMed]
- Michonneau, D.; Latis, E.; Curis, E.; Dubouchet, L.; Ramamoorthy, S.; Ingram, B.; De Latour, R.P.; Robin, M.; De Fontbrune, F.S.; Chevret, S.; et al. Metabolomics analysis of human acute graft-versus-host disease reveals changes in host and microbiota-derived metabolites. Nat. Commun. 2019, 10, 5695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contaifer, J.D.; Roberts, C.H.; Kumar, N.G.; Natarajan, R.; Fisher, B.J.; Leslie, K.; Reed, J.; Toor, A.A.; Wijesinghe, D.S. A Preliminary Investigation towards the Risk Stratification of Allogeneic Stem Cell Recipients with Respect to the Potential for Development of GVHD via Their Pre-Transplant Plasma Lipid and Metabolic Signature. Cancers 2019, 11, 1051. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.L.; Farhadfar, N.; Starkweather, A.; Garrett, T.J.; Yao, Y.; Wingard, J.R.; Mahmud, I.; Menzies, V.; Patel, P.; Alabasi, K.M.; et al. Global Metabolomics in Allogeneic Hematopoietic Cell Transplantation Recipients Discordant for Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transpl. 2020, 26, 1803–1810. [Google Scholar] [CrossRef]
- Li, S.; Park, Y.; Duraisingham, S.; Strobel, F.H.; Khan, N.; Soltow, Q.A.; Jones, D.P.; Pulendran, B. Predicting Network Activity from High Throughput Metabolomics. PLoS Comput. Biol. 2013, 9, e1003123. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Tenenhaus, A.; Philippe, C.; Guillemot, V.; Le Cao, K.-A.; Grill, J.; Frouin, V. Variable selection for generalized canonical correlation analysis. Biostatistics 2014, 15, 569–583. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Shannon, C.P.; Gautier, B.; Rohart, F.; Vacher, M.; Tebbutt, S.J.; Lê Cao, K.-A. DIABLO: An integrative approach for identifying key molecular drivers from multi-omics assays. Bioinformatics 2019, 35, 3055–3062. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, I.; Cao, K.-A.L.; Davis, M.J.; Déjean, S. Visualising associations between paired ‘omics’ data sets. BioData Min. 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.X.; Wan, J.B.; He, C. Concise Review: Regulation of Stem Cell Proliferation and Differentiation by Essential Fatty Acids and Their Metabolites. Stem Cells 2014, 32, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Dahate, P.; Raghuwanshi, S.; Sharma, D.S.; Kovuru, N.; Gutti, U.; Yarla, N.S.; Gutti, R.K.; Narasaiah, K. Current Updates on Role of Lipids in Hematopoiesis. Infect. Disord. Drug Targets 2018, 18, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.S.; Al-Sharea, A.; Dragoljevic, D.; Murphy, A.J. Hand of FATe: Lipid Metabolism in Hematopoietic Stem Cells. Curr. Opin. Lipidol. 2018, 29, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, C.; Gregurich, M.A.; Hockenberry, M. Lipid Profiles of Pediatric Hematopoietic Stem Cell Transplant Survivors. J. Pediatr. Oncol. Nurs. 2012, 29, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Bis, G.; Szlasa, W.; Sondaj, K.; Zendran, I.; Mielcarek-Siedziuk, M.; Barg, E. Lipid Complications after Hematopoietic Stem Cell Transplantation (HSCT) in Pediatric Patients. Nutrients 2020, 12, 2500. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Torrinhas, R.S.; Jacintho, T.M. New Parenteral Lipid Emulsions for Clinical Use. J. Parenter. Enter. Nutr. 2006, 30, 351–367. [Google Scholar] [CrossRef] [Green Version]
- Goulet, O.J.; Cai, W.; Seo, J.M. Lipid Emulsion Use in Pediatric Patients Requiring Long-Term Parenteral Nutrition. J. Parenter. Enter. Nutr. 2020, 44, S55–S67. [Google Scholar] [CrossRef]
- Finn, K.L.; Chung, M.; Rothpletz-Puglia, P.; Byham-Gray, L. Impact of Providing a Combination Lipid Emulsion Compared with a Standard Soybean Oil Lipid Emulsion in Children Receiving Parenteral Nutrition. J. Parenter. Enter. Nutr. 2015, 39, 656–667. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, V.; Malviya, M.N.; Soll, R. Lipid emulsions for parenterally fed term and late preterm infants. Cochrane Database Syst. Rev. 2019, 6, CD013171. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Lamazière, A.; Nader, E.A.; Talbotec, C.; Wolf, C.; Lambe, C. Erythrocyte fatty acid membrane composition in children on long-term parenteral nutrition enriched with ω-3 fatty acids. Am. J. Clin. Nutr. 2022, 115, 422–431. [Google Scholar] [CrossRef] [PubMed]
- van Hoogevest, P.; Wendel, A. The Use of Natural and Synthetic Phospholipids as Pharmaceutical Excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pironi, L.; Guidetti, M.; Verrastro, O.; Iacona, C.; Agostini, F.; Pazzeschi, C.; Sasdelli, A.S.; Melchiorre, M.; Ferreri, C. Functional lipidomics in patients on home parenteral nutrition: Effect of lipid emulsions. World J. Gastroenterol. 2017, 23, 4604–4614. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.S. Measurement of Red Cell Lifespan and Aging. Transfus. Med. Hemother. 2012, 39, 302–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabukusta, B.; Nettebrock, N.T.; Kol, M.; Hilderink, A.; Tafesse, F.G.; Holthuis, J.C.M. Ceramide phosphoethanolamine synthase SMSr is a target of caspase-6 during apoptotic cell death. Biosci. Rep. 2017, 37, BSR20170867. [Google Scholar] [CrossRef] [Green Version]
- Schütze, S.; Potthoff, K.; Machleidt, T.; Berkovic, D.; Wiegmann, K.; Krönke, M. TNF activates NF-κB by phosphatidylcholine-specific phospholipase C-induced “Acidic” sphingomyelin breakdown. Cell 1992, 71, 765–776. [Google Scholar] [CrossRef]
- Aureli, M.; Murdica, V.; Loberto, N.; Samarani, M.; Prinetti, A.; Bassi, R.; Sonnino, S. Exploring the link between ceramide and ionizing radiation. Glycoconj. J. 2014, 31, 449–459. [Google Scholar] [CrossRef]
- Kallunki, T.; Olsen, O.D.; Jäättelä, M. Cancer-associated lysosomal changes: Friends or foes? Oncogene 2013, 32, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Skoczen, S.; Tomasik, P.J.; Fijorek, K.; Strojny, W.; Wieczorek, A.; Balwierz, W.; Sztefko, K.; Siedlar, M. Concentrations of adipokines in children before and after hematopoietic stem cell transplantation. Pediatr. Hematol. Oncol. 2016, 33, 21–38. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, J.; Li, T.; Ji, Z.; Xin, Y.; Zhang, S.; Qin, F.; Zhao, L. Integrative metabolic profile of myelodysplastic syndrome based on UHPLC–MS. Biomed. Chromatogr. 2021, 35, e5136. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Gil-Campos, M.; Larqué, E.; Ramírez-Tortosa, M.C.; Linde, J.; Villada, I.; Cañete, R.; Gil, A. Changes in plasma fatty acid composition after intake of a standardised breakfast in prepubertal obese children. Br. J. Nutr. 2007, 99, 909–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Gromski, P.S.; Xu, Y.; Kotze, H.L.; Correa, E.; Ellis, D.I.; Armitage, E.G.; Turner, M.L.; Goodacre, R. Influence of Missing Values Substitutes on Multivariate Analysis of Metabolomics Data. Metabolites 2014, 4, 433–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klåvus, A.; Kokla, M.; Noerman, S.; Koistinen, V.M.; Tuomainen, M.; Zarei, I.; Meuronen, T.; Häkkinen, M.R.; Rummukainen, S.; Babu, A.F.; et al. “Notame”: Workflow for Non-Targeted LC–MS Metabolic Profiling. Metabolites 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- González-Ruiz, V.; Schvartz, D.; Sandström, J.; Pezzatti, J.; Jeanneret, F.; Tonoli, D.; Boccard, J.; Monnet-Tschudi, F.; Sanchez, J.-C.; Rudaz, S. An Integrative Multi-Omics Workflow to Address Multifactorial Toxicology Experiments. Metabolites 2019, 9, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccard, J.; Rudaz, S. Exploring Omics data from designed experiments using analysis of variance multiblock Orthogonal Partial Least Squares. Anal. Chim. Acta 2016, 920, 18–28. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]



| SOPLE | FOPLE | ||
|---|---|---|---|
| Sex (male/female) | 2/2 | 2/4 | |
| Age (months) | 101.5 (8–180) | 90.5 (31–132) | |
| Pathology | Hematologic diseases | 4 | 4 |
| Solid tumors | 2 | ||
| Type of HSCT | Allogeneic | 4 | 4 |
| Autologous | 2 | ||
| GVHD | 3 | 2 | |
| VOD | 0 | 0 | |
| Time of engraftment | PMN: PMN > 500/mm3 | 15.5 (14–21) | 13 (11–20) |
| Platelets > 20,000 | 17.5 (15–24) | 15 (12–68) | |
| Total days of PN | 13 (11–25) | 16 (9–24) | |
| Days of hospitalization | 34 (31–37) | 31 (29–43) |
| Effect Name | RSS | RSR | RSR p-Value | R2Y p-Value | Tp1 | Tp2 | Tp3 | To1 |
|---|---|---|---|---|---|---|---|---|
| Treatment | 0.08 | 1.159 | 0.03 | 0.03 | 0.046 | 0.042 | 0.825 | 0.239 |
| Time | 0.081 | 1.098 | 0.81 | 0.01 | 0.049 | 0.869 | 0.058 | 0.252 |
| Treatment x Time | 0.085 | 1.196 | 0.05 | 0.01 | 0.851 | 0.041 | 0.053 | 0.232 |
| Residuals | 0.753 | 1 | NA | NA | 0.054 | 0.049 | 0.064 | 0.277 |
| SOPLE (n = 4) | FOPLE (n = 6) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal | Final | Basal | Final | |||||||||
| Median | Min | Max | Median | Min | Max | Median | Min | Max | Median | Min | Max | |
| Glucose (mg/dL) | 90.5 | 63.0 | 103.0 | 94.0 | 77.0 | 208.0 | 94.5 | 68.0 | 103.0 | 91.5 | 82 | 120 |
| Urea (mg/dL) | 19.0 | 13.0 | 27.0 | 26.0 | 24.0 | 55.0 | 17.0 | 10.0 | 21.0 | 30.0 | 12 | 49 |
| Creatinine (mg/dL) | 0.40 | 0.30 | 0.53 | 0.43 | 0.35 | 0.68 | 0.48 | 0.35 | 0.55 | 0.42 | 0.33 | 0.52 |
| AST (U/L) | 44.0 | 19.0 | 114.0 | 41.5 | 15.0 | 81.0 | 36.0 | 17.0 | 82.0 | 32.5 | 25 | 71 |
| ALT (U/L) | 44.5 | 14.0 | 228.0 | 57.0 | 18.0 | 99.0 | 45.5 | 10.0 | 127.0 | 34.0 | 16 | 59 |
| GGT (U/L) | 19.0 | 9.0 | 46.0 | 36.0 | 19.0 | 161.0 | 28.5 | 17.0 | 77.0 | 102.0 a | 59 | 267 |
| ALP (U/L) | 105.5 | 87.0 | 220.0 | 144.5 | 117.0 | 332.0 | 155.5 | 102.0 | 182.0 | 160.5 | 106 | 332 |
| ApoA (mg/dL) | 98.5 | 71.0 | 105.0 | 56.0 a | 50.0 | 77.0 | 98.5 | 60.0 | 124.0 | 65.0 | 54 | 77 |
| ApoB (mg/dL) | 73.0 | 42.0 | 89.0 | 127.0 a | 53.0 | 145.0 | 97.5 | 54.0 | 205.0 | 120.5 | 88 | 222 |
| Total cholesterol (mg/dL) | 145 | 112 | 173 | 214 | 97 | 232 | 189 | 123 | 292 | 209 | 176 | 366 |
| HDL (mg/dL) | 35.0 | 26.0 | 42.0 | 15.0 a | 12.0 | 23.0 | 32.0 | 15.0 | 45.0 | 18.5 | 13 | 24 |
| LDL (mg/dL) | 13.5 | 9.0 | 19.0 | 110.5 | 49.0 | 181.0 | 15.5 | 0.0 | 38.0 | 131.0 a | 109 | 163 |
| Triacylglycerols (mg/dL) | 135 | 79 | 176 | 308 | 153 | 418 | 107 | 91 | 445 | 299 | 161 | 588 |
| Bilirubin (mg/dL) | 0.80 | 0.30 | 1.40 | 0.70 | 0.10 | 1.90 | 0.45 | 0.30 | 0.90 | 1.00 a | 0.5 | 1.7 |
| SOPLE (n = 4) | FOPLE (n = 6) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal | Final | Basal | Final | |||||||||
| Fatty Acids, % Relative to Total FAs 1 | Median | Min | Max | Median | Min | Max | Median | Min | Max | Median | Min | Max |
| Myristic acid (C14:0) | 0.80 | 0.44 | 1.76 | 0.66 | 0.49 | 1.10 | 0.53 | 0.36 | 3.03 | 0.63 | 0.37 | 0.96 |
| Palmitic acid (C16:0) | 24.70 | 23.60 | 26.10 | 24.10 | 22.10 | 25.0 | 23.30 | 22.70 | 28.30 | 23.75 | 22.60 | 25.50 |
| Palmitoleic acid (C16:1) | 0.49 | 0.37 | 0.70 | 0.19 | 0.00 | 0.63 | 0.39 | 0.00 | 1.18 | 0.25 | 0.00 | 0.83 |
| Margaric acid (C17:0) | 0.39 | 0.33 | 0.60 | 0.87 a | 0.45 | 0.98 | 0.39 | 0.00 | 1.27 | 0.16 | 0.00 | 0.90 |
| Estearic acid (C18:0) | 15.85 | 15.70 | 17.00 | 15.80 | 15.30 | 20.3 | 15.95 | 13.00 | 17.30 | 15.70 | 14.90 | 17.70 |
| Oleic acid (C18:1n-9c) | 15.35 | 13.00 | 20.50 | 15.30 | 14.40 | 16.2 | 14.85 | 13.70 | 25.20 | 15.15 | 14.60 | 17.10 |
| Vaccenic acid (C18:1n-7) | 0.99 | 0.50 | 1.17 | 1.09 | 0.92 | 1.25 | 1.11 | 0.95 | 1.49 | 1.11 | 1.01 | 1.21 |
| Linoleic acid (C18:2ω-6) | 7.90 | 7.70 | 8.50 | 8.85 | 7.70 | 10.5 | 9.30 | 5.40 | 9.50 | 8.15 | 7.30 | 9.90 |
| Arachidic acid (C20:0) | 0.42 | 0.00 | 0.48 | 0.23 | 0.00 | 0.50 | 0.00 | 0.00 | 0.53 | 0.43 | 0.00 | 0.50 |
| Linolenic acid (C18:3ω-3) | 0.16 | 0.00 | 0.41 | 0.00 | 0.00 | 0.33 | 0.00 | 0.00 | 0.40 | 0.00 | 0.00 | 0.00 |
| Behenic acid (C22:0) | 1.63 | 1.21 | 1.86 | 1.59 | 1.54 | 1.72 | 1.90 | 1.17 | 3.97 | 1.72 | 1.55 | 1.94 |
| Dihomo-γ-linolenic acid (C20:3ω-6) | 1.78 | 1.69 | 1.95 | 1.69 | 1.51 | 1.93 | 1.41 | 0.00 | 2.39 | 1.33 | 1.16 | 2.21 |
| Dihomo-α-linolenic (C20:3ω-3) | 0.61 | 0.57 | 0.73 | 0.75 | 0.62 | 1.52 | 0.54 | 0.42 | 0.85 | 0.66 | 0.38 | 1.10 |
| Arachidonic acid (C20:4ω-6) | 14.80 | 10.30 | 17.60 | 14.45 | 13.10 | 15.8 | 14.65 | 9.70 | 16.40 | 13.65 | 12.80 | 15.10 |
| Eicosapentanoic acid (C20:5ω-3) | 0.00 | 0.00 | 0.34 | 0.00 | 0.00 | 0.57 | 0.35 | 0.00 | 0.46 | 1.51 a | 0.46 | 2.12 |
| Lingnoceric (24:0) | 4.65 | 3.63 | 5.07 | 4.44 | 4.36 | 4.89 | 4.59 | 2.88 | 5.25 | 4.50 | 4.15 | 4.70 |
| Nervonic acid (24:1n9) | 3.76 | 3.06 | 5.14 | 3.69 | 2.93 | 4.47 | 3.47 | 2.08 | 6.11 | 3.25 | 2.95 | 3.88 |
| Docosapentanoic acid (C22:5ω-3) | 1.33 | 1.28 | 1.78 | 1.43 | 1.24 | 1.53 | 1.48 | 0.92 | 1.94 | 1.84 | 1.38 | 2.41 |
| Docosahexaenoic acid (C22:6ω-3) | 3.56 | 2.44 | 4.10 | 3.73 | 2.37 | 4.04 | 4.05 | 2.74 | 4.93 | 4.43 | 4.07 | 6.15 |
| SFA | 48.80 | 47.20 | 49.90 | 48.55 | 47.10 | 49.9 | 47.90 | 46.30 | 49.00 | 47.05 | 46.10 | 48.50 |
| UFA | 51.20 | 50.10 | 52.80 | 51.45 | 50.10 | 52.90 | 52.10 | 51.00 | 53.70 | 52.95 | 51.50 | 53.90 |
| MUFA | 20.65 | 19.50 | 24.70 | 20.15 | 19.40 | 21.60 | 19.80 | 18.70 | 29.90 | 19.65 | 19.50 | 22.10 |
| SFA/MUFA ratio | 2.35 | 2.00 | 2.50 | 2.45 | 2.20 | 2.50 | 2.40 | 1.60 | 2.60 | 2.40 | 2.20 | 2.50 |
| DUFA | 7.90 | 7.70 | 8.50 | 8.85 | 7.70 | 10.5 | 9.30 | 5.40 | 9.50 | 8.15 | 7.30 | 9.90 |
| MUFA/DUFA ratio | 2.65 | 2.50 | 2.90 | 2.30 | 1.90 | 2.8 | 2.30 | 2.00 | 5.60 | 2.40 | 2.10 | 2.80 |
| PUFA | 30.50 | 26.60 | 32.10 | 31.20 | 30.30 | 31.9 | 32.40 | 21.40 | 32.80 | 32.70 | 30.20 | 34.00 |
| MUFA/PUFA ratio | 0.65 | 0.60 | 0.90 | 0.70 | 0.60 | 0.70 | 0.60 | 0.60 | 1.40 | 0.60 | 0.60 | 0.70 |
| PUFA ω-6 | 24.40 | 20.80 | 27.10 | 25.20 | 24.50 | 25.6 | 25.35 | 16.00 | 25.90 | 23.45 | 22.90 | 25.80 |
| PUFA ω-3 | 5.80 | 4.40 | 6.90 | 6.15 | 5.10 | 6.60 | 6.65 | 4.70 | 7.80 | 8.70 a | 7.10 | 10.70 |
| PUFA ω-6 >18 C | 16.55 | 12.30 | 19.40 | 16.25 | 14.70 | 17.5 | 16.35 | 10.70 | 17.80 | 15.35 | 14.00 | 16.60 |
| PUFA ω-3 >18 C | 5.45 | 4.40 | 6.90 | 5.95 | 5.10 | 6.60 | 6.55 | 4.70 | 7.80 | 8.70 a | 7.10 | 10.70 |
| UI | 2.65 | 2.40 | 3.00 | 2.70 | 2.60 | 2.90 | 2.85 | 2.30 | 3.10 | 3.00 | 2.80 | 3.30 |
| Ratio ω-6/ω-3 | 3.84 | 3.62 | 6.13 | 4.14 | 3.70 | 4.91 | 3.58 | 2.99 | 5.49 | 2.68 | 2.19 | 3.35 |
| Index ω-3 | 3.56 | 2.44 | 4.44 | 3.87 | 2.37 | 4.34 | 4.44 | 2.74 | 5.36 | 6.14 a | 4.67 | 7.70 |
| Delta9 desaturase | 0.97 | 0.76 | 1.30 | 0.99 | 0.71 | 1.02 | 0.92 | 0.82 | 1.94 | 0.99 | 0.83 | 1.11 |
| Delta6 desaturase | 0.23 | 0.22 | 0.23 | 0.18 | 0.17 | 0.25 | 0.18 | 0.00 | 0.26 | 0.17 | 0.12 | 0.27 |
| Delta5 desaturase | 0.12 | 0.10 | 0.19 | 0.12 | 0.10 | 0.13 | 0.10 | 0.00 | 0.17 | 0.10 | 0.08 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel-Huerta, O.D.; de la Torre-Aguilar, M.J.; Mesa, M.D.; Flores-Rojas, K.; Pérez-Navero, J.L.; Baena-Gómez, M.A.; Gil, A.; Gil-Campos, M. The Metabolic Impact of Two Different Parenteral Nutrition Lipid Emulsions in Children after Hematopoietic Stem Cell Transplantation: A Lipidomics Investigation. Int. J. Mol. Sci. 2022, 23, 3667. https://doi.org/10.3390/ijms23073667
Rangel-Huerta OD, de la Torre-Aguilar MJ, Mesa MD, Flores-Rojas K, Pérez-Navero JL, Baena-Gómez MA, Gil A, Gil-Campos M. The Metabolic Impact of Two Different Parenteral Nutrition Lipid Emulsions in Children after Hematopoietic Stem Cell Transplantation: A Lipidomics Investigation. International Journal of Molecular Sciences. 2022; 23(7):3667. https://doi.org/10.3390/ijms23073667
Chicago/Turabian StyleRangel-Huerta, Oscar Daniel, María José de la Torre-Aguilar, María Dolores Mesa, Katherine Flores-Rojas, Juan Luis Pérez-Navero, María Auxiliadora Baena-Gómez, Angel Gil, and Mercedes Gil-Campos. 2022. "The Metabolic Impact of Two Different Parenteral Nutrition Lipid Emulsions in Children after Hematopoietic Stem Cell Transplantation: A Lipidomics Investigation" International Journal of Molecular Sciences 23, no. 7: 3667. https://doi.org/10.3390/ijms23073667
APA StyleRangel-Huerta, O. D., de la Torre-Aguilar, M. J., Mesa, M. D., Flores-Rojas, K., Pérez-Navero, J. L., Baena-Gómez, M. A., Gil, A., & Gil-Campos, M. (2022). The Metabolic Impact of Two Different Parenteral Nutrition Lipid Emulsions in Children after Hematopoietic Stem Cell Transplantation: A Lipidomics Investigation. International Journal of Molecular Sciences, 23(7), 3667. https://doi.org/10.3390/ijms23073667







