In Vitro and In Vivo Effects of the Urokinase Plasminogen Activator Inhibitor WX-340 on Anaplastic Thyroid Cancer Cell Lines
Abstract
:1. Introduction
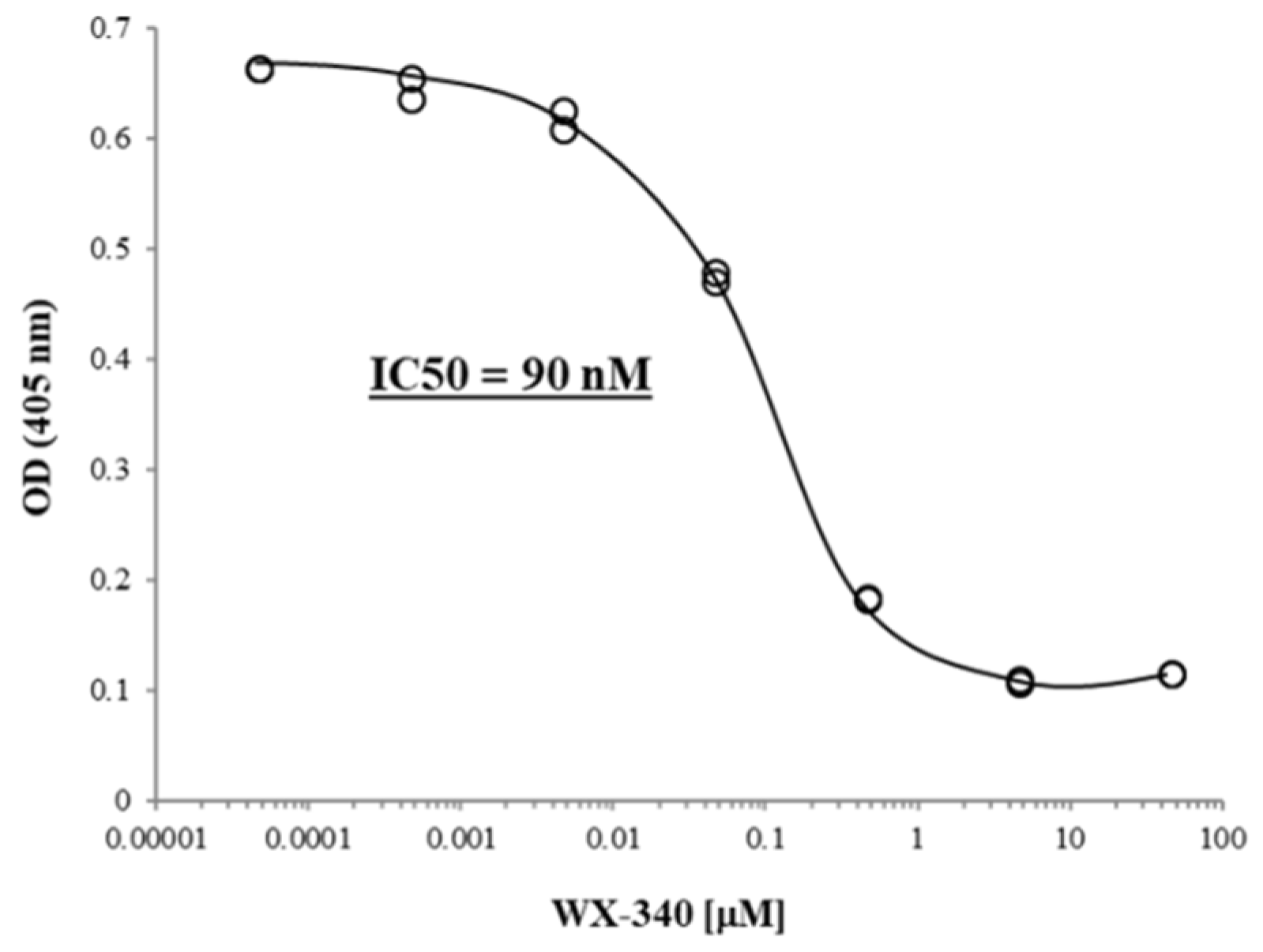
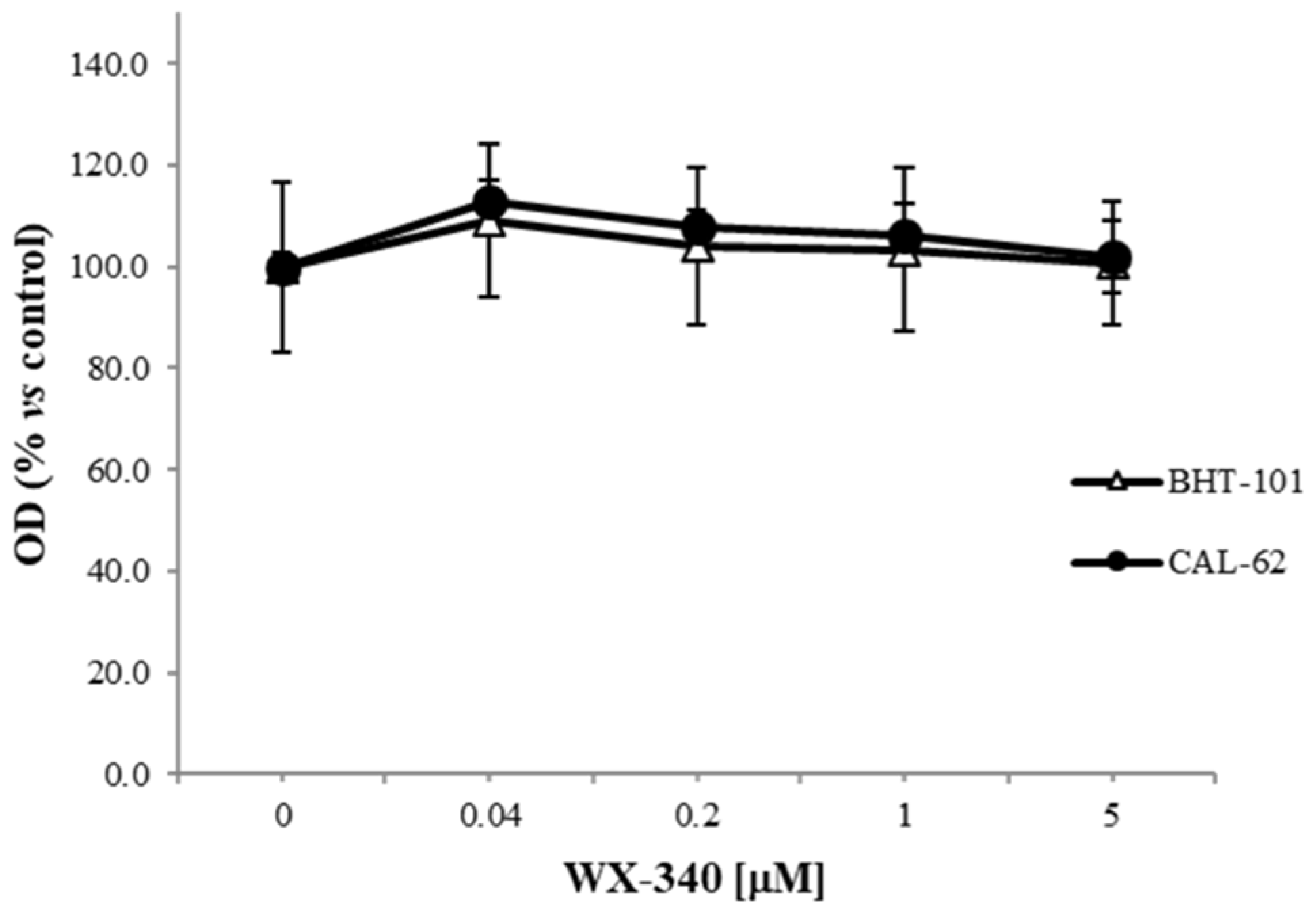
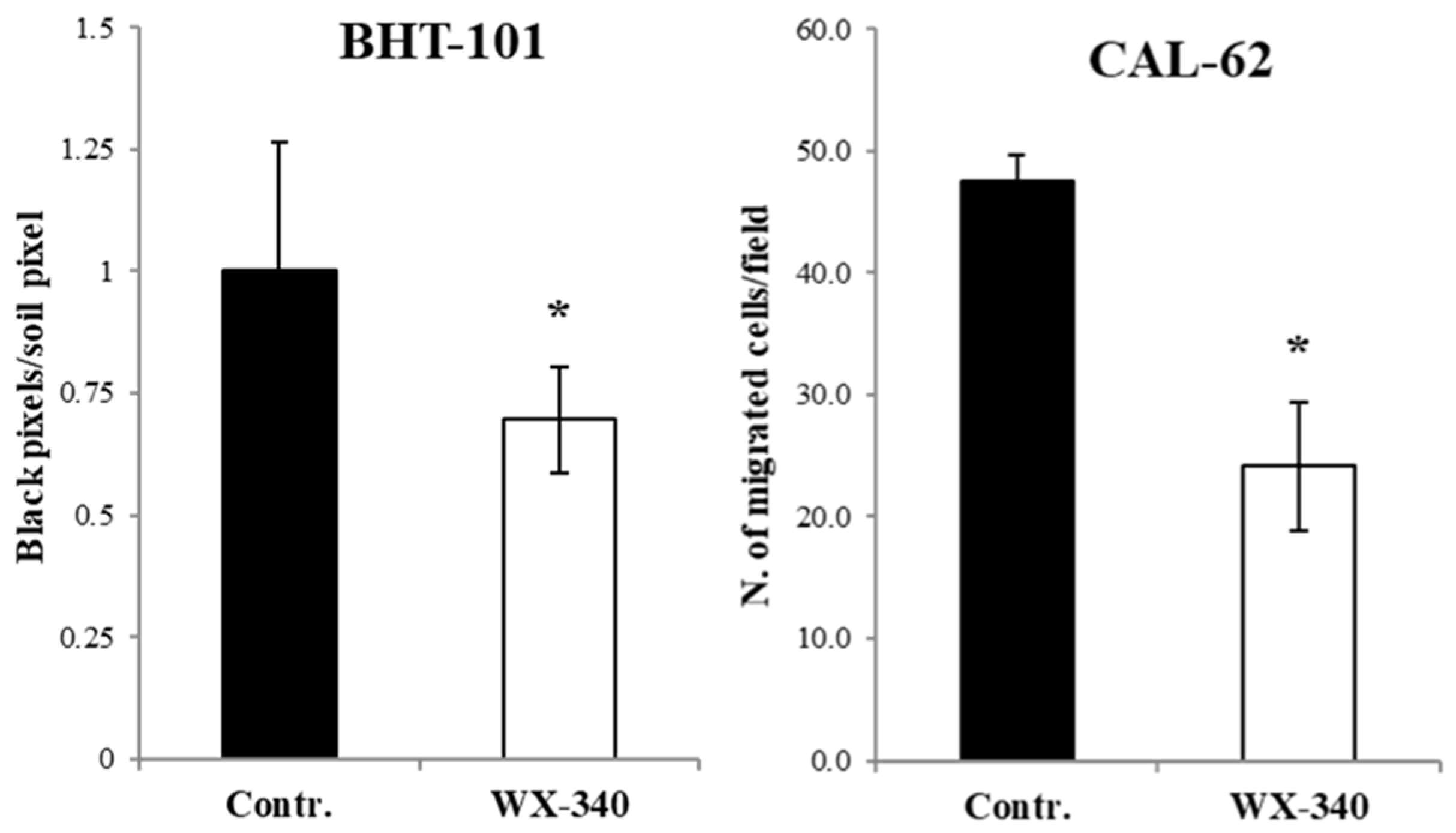
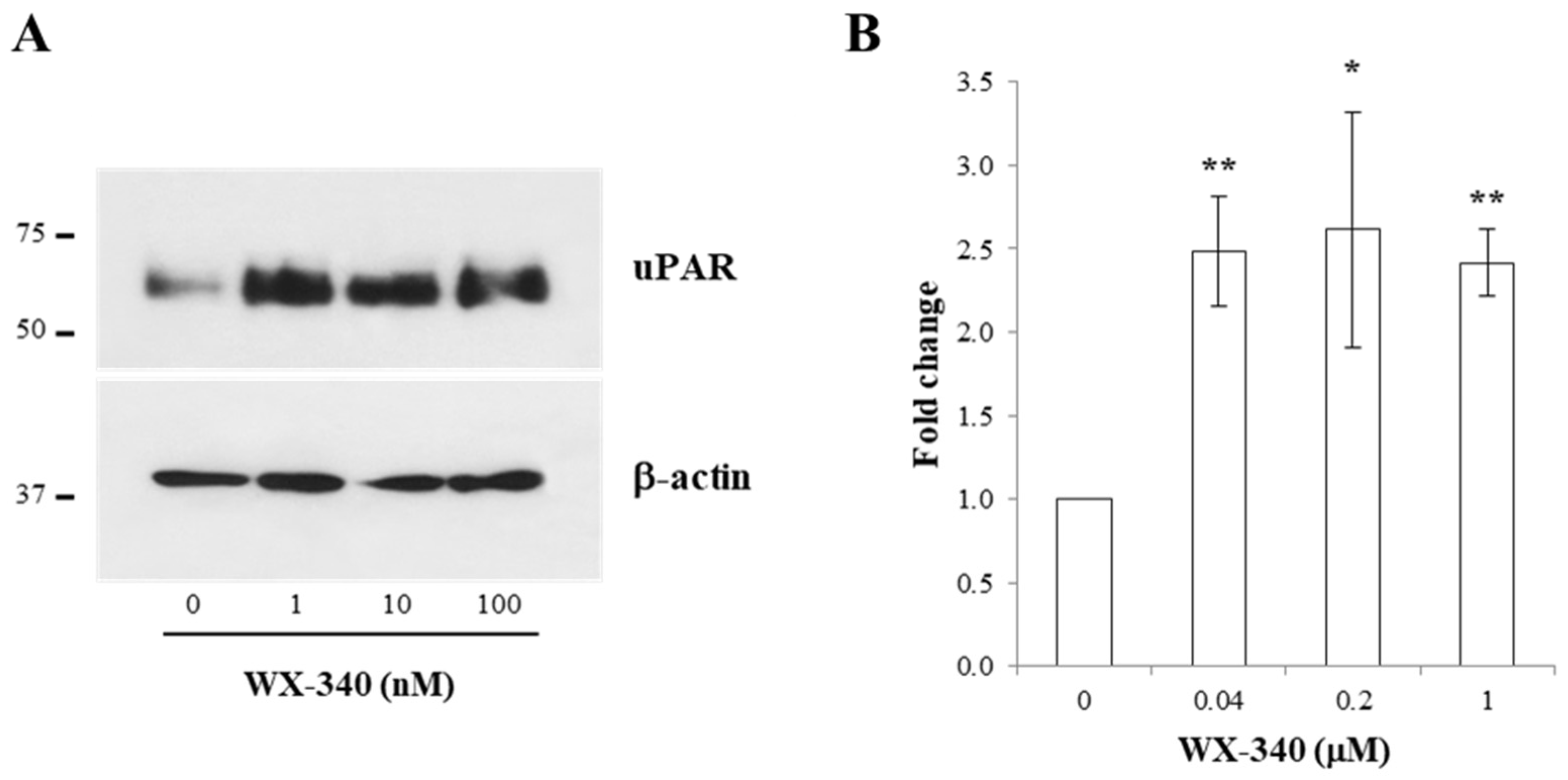
2. Results
3. Discussion
4. Materials and Methods
4.1. uPA Inhibitor
4.2. Cell Cultures
4.3. Proliferation Assay
4.4. Adhesion Assay
4.5. Invasion Assay
4.6. Western Blotting
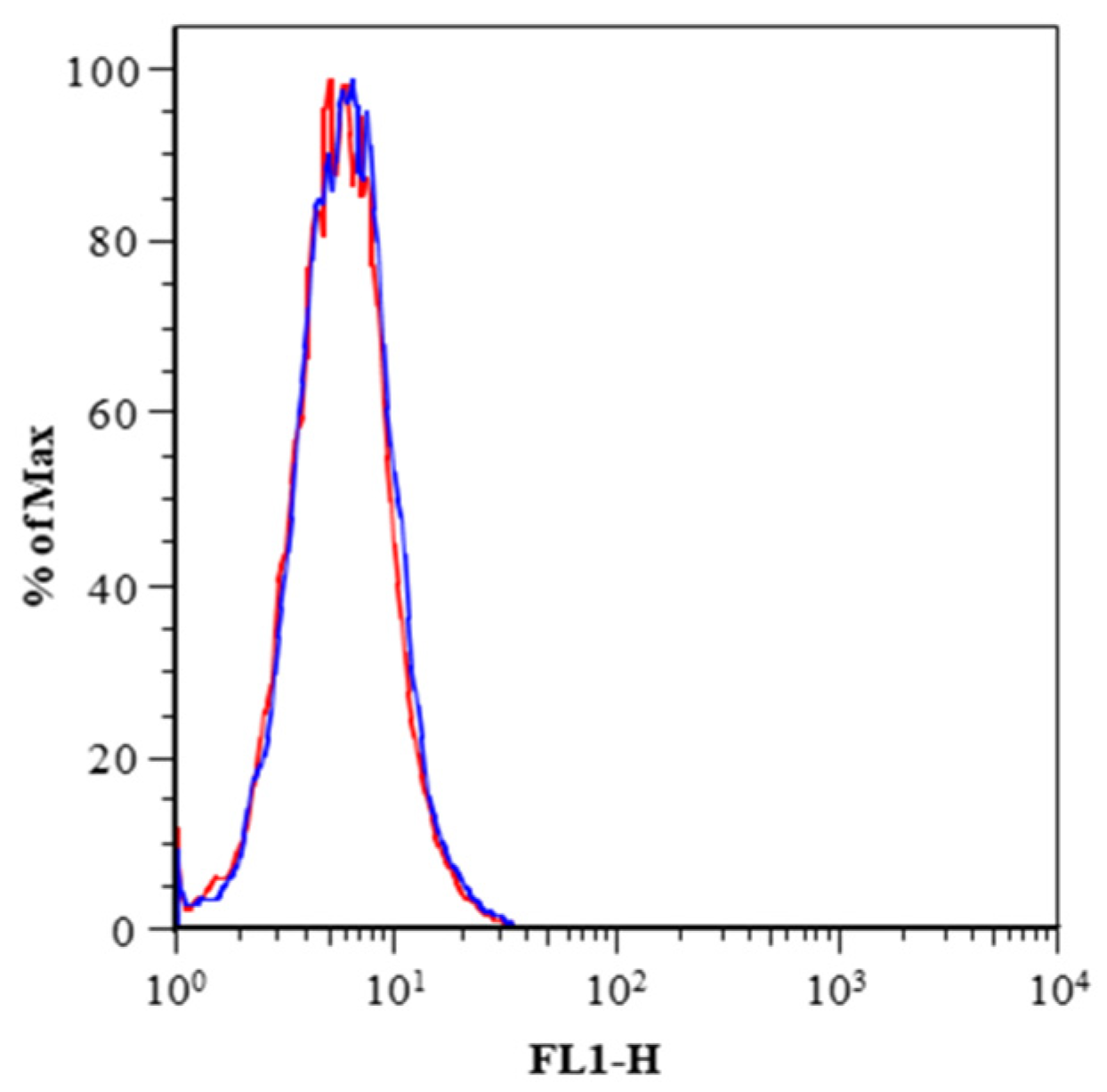
4.7. Flow Cytometric Analysis
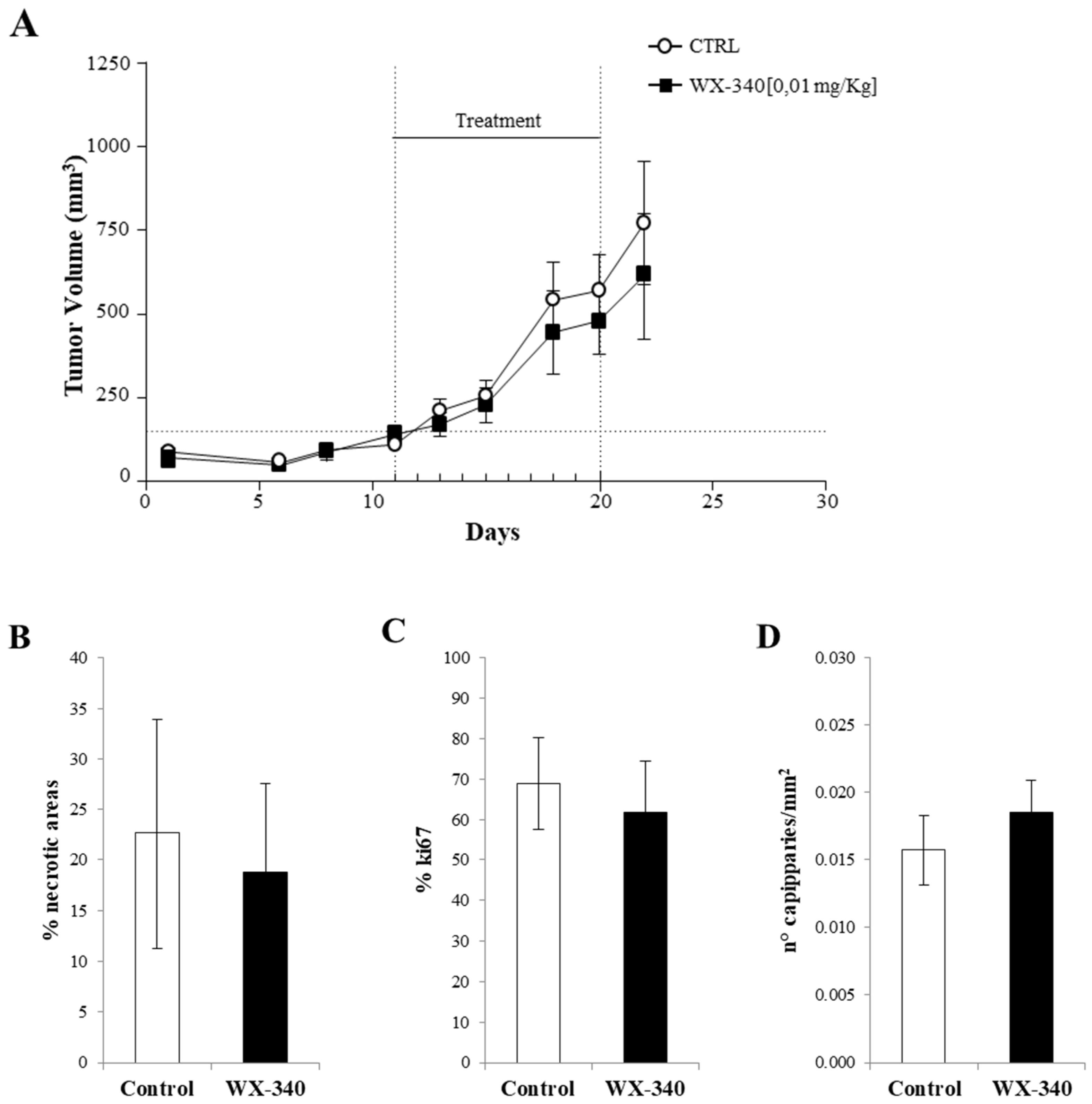
4.8. In Vivo Experiments
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Breast, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review; 1975–2012; National Cancer Institute: Bethesda, MD, USA, 2021. Available online: http://seer.cancer.gov/csr/1975_2018/ (accessed on 25 February 2022).
- Grodski, S.; Brown, T.; Sidhu, S.; Gill, A.; Robinson, B.; Learoyd, D.; Sywak, M.; Reeve, T.; Delbridge, L. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery 2008, 144, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Ulisse, S.; Graziano, F.M.; Marzullo, A.; Ruggieri, M.; Calvanese, A.; Piccirilli, F.; Cavaliere, R.; Fumarola, A.; D’Armiento, M. Trend in thyroid carcinoma size, age at diagnosis, and histology in a retrospective study of 500 cases diagnosed over 20 years. Thyroid 2006, 16, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Ulisse, S.; Bosco, D.; Nardi, F.; Nesca, A.; D’Armiento, E.; Guglielmino, V.; De Vito, C.; Sorrenti, S.; Pironi, D.; Tartaglia, F.; et al. Thyroid Imaging Reporting and Data System Score Combined with the New Italian Classification for Thyroid Cytology Improves the Clinical Management of Indeterminate Nodules. Int. J. Endocrinol. 2017, 2017, 9692304. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Ruffilli, I.; Elia, G.; Ragusa, F.; Paparo, S.R.; Ulisse, S.; Baldini, E.; Giannini, R.; Miccoli, P.; et al. Molecular testing in the diagnosis of differentiated thyroid carcinomas. Gland Surg. 2018, 7, S19–S29. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Biddinger, P.W.; Thompson, L.D.R. Diagnostic Pathology and Molecular Genetics of the Thyroid; Volters Kluver & Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- DeLellis, R.A.; Lloyd, R.; Heitz, P.U.; Heng, C. (Eds.) World Health Organization Classification of Tumors, Pathology & Genetics—Tumors of Endocrine Organs; IARC Press: Lyon, France, 2004. [Google Scholar]
- Nikiforov, Y.E. Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocr. Pathol. 2004, 15, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.L.; Tometsko, M.; LiVolsi, V.A.; Swalsky, P.; Finkelstein, S.D.; Barnes, E.L. Molecular evidence of anaplastic transformation in coexisting well-differentiated and anaplastic carcinomas of the thyroid. Am. J. Surg. Pathol. 2003, 27, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Eloy, C.; Ferreira, L.; Salgado, C.; Soares, P.; Sobrinho-Simões, M. Poorly differentiated and undifferentiated thyroid carcinomas. Turk. Patoloji Derg. 2015, 31, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Ulisse, S.; Baldini, E.; Lauro, A.; Pironi, D.; Tripodi, D.; Lori, E.; Ferent, I.C.; Amabile, M.I.; Catania, A.; Di Matteo, F.M.; et al. Papillary thyroid cancer prognosis: An evolving field. Cancers 2021, 13, 5567. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimpasic, T.; Ghossein, R.; Carlson, D.L.; Nixon, I.; Palmer, F.L.; Shaha, A.R.; Patel, S.G.; Tuttle, R.M.; Shah, J.P.; Ganly, I. Outcomes in patients with poorly differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2014, 99, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Romesser, P.B.; Sherman, E.J.; Shaha, A.R.; Lian, M.; Wong, R.J.; Sabra, M.; Rao, S.S.; Fagin, J.A.; Tuttle, R.M.; Lee, N.Y. External beam radiotherapy with or without concurrent chemiotherapy in advanced or recurrent non-anaplastic non-medullary thyroid cancer. J. Surg. Oncol. 2014, 110, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, P.; Ruffilli, I.; Elia, G.; Ragusa, F.; Ulisse, S.; Baldini, E.; Miccoli, M.; Materazzi, G.; Antonelli, A.; Ferrari, S.M. Novel treatment options for anaplastic thyroid cancer. Expert. Rev. Endocrinol. Metab. 2017, 12, 279–288. [Google Scholar] [CrossRef]
- Keutegen, X.M.; Sadowski, S.M.; Kebebew, E. Management of anaplastic thyroid cancer. Gland Surg. 2015, 4, 44–51. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Sorrenti, S.; D’Armiento, M. The urokinase plasminogen activator system: A target for anti-cancer therapy. Curr. Cancer Drug Target 2009, 9, 32–71. [Google Scholar] [CrossRef] [PubMed]
- Ulisse, S.; Baldini, E.; Toller, M.; Marchioni, E.; Giacomelli, L.; De Antoni, E.; Ferretti, E.; Marzullo, A.; Graziano, F.M.; Trimboli, P.; et al. Differential expression of the components of the plasminogen activating system in human thyroid tumor derived cell lines and papillary carcinomas. Eur. J. Cancer 2006, 42, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Buergy, D.; Weber, T.; Maurer, G.D.; Mudduluru, G.; Medved, F.; Leupold, J.H.; Brauckhoff, M.; Post, S.; Dralle, H.; Allgayer, H. Urokinase receptor, MMP-1 and MMP-9 are markers to differentiate prognosis, adenoma and carcinoma in thyroid malignancies. Int. J. Cancer 2009, 125, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Ulisse, S.; Baldini, E.; Sorrenti, S.; Barollo, S.; Gnessi, L.; Catania, A.; Pellizzo, M.R.; Nardi, F.; Mian, C.; De Antoni, E.; et al. High expression of the urokinase plasminogen activator and its cognate receptor associates with advanced stages and reduced disease-free interval in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Ulisse, S.; Baldini, E.; Sorrenti, S.; Barollo, S.; Prinzi, N.; Catania, A.; Nesca, A.; Gnessi, L.; Pelizzo, M.R.; Mian, C.; et al. In papillary thyroid carcinoma BRAFV600E is associated with increased expression of the urokinase plasminogen activator and its cognate receptor, but not with disease-free interval. Clin. Endocrinol. 2012, 77, 780–786. [Google Scholar] [CrossRef]
- Baldini, E.; Sorrenti, S.; D’Armiento, E.; Di Matteo, F.M.; Catania, A.; Ulisse, S. The urokinase plasminogen activating system in thyroid cancer: Clinical implications. G. Chir. 2012, 33, 305–310. [Google Scholar] [PubMed]
- Horvatić Herceg, G.; Herceg, D.; Kralik, M.; Bence-Zigman, Z.; Tomić-Brzac, H.; Kulić, A. Urokinase-Type Plasminogen Activator and its Inhibitor in Thyroid Neoplasms: A Cytosol Study. Wien. Klin. Wochenschr. 2006, 118, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Horvatić Herceg, G.; Herceg, D.; Kralik, M.; Kulic, A.; Bence-Zigman, Z.; Tomic-Brzac, H.; Bracic, I.; Kusacic-Kuna, S.; Prgomet, D. Urokinase plasminogen activator and its inhibitor type-1 as prognostic factors in differentiated thyroid carcinoma patients. Otolaryngol. Head Neck Surg. 2013, 149, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.F.; Roque, L.; Krug, T.; Leite, V. Poorly differentiated and anaplastic thyroid carcinomas: Chromosomal and oligo-array profile of five new cell lines. Br. J. Cancer 2007, 96, 1237–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinal-Enríquez, J.; Muñoz-Montero, S.; Imaz-Rosshandler, I.; Huerta-Verde, A.; Mejía, C.; Hernández-Lemus, E. Genome-wide expression analysis suggests a crucial role of dysregulation of matrix metalloproteinases pathway in undifferentiated thyroid carcinoma. BMC Genom. 2015, 16, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowicki, T.S.; Kummer, N.T.; Iacob, C.; Suslina, N.; Schaefer, S.; Schantz, S.; Shin, E.; Moscatello, A.L.; Tiwari, R.K.; Geliebter, J. Inhibition of uPAR and uPA reduces invasion in papillary thyroid carcinoma cells. Laryngoscope 2010, 120, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, T.S.; Zhao, H.; Darzynkiewicz, Z.; Moscatello, A.; Shin, E.; Schantz, S.; Tiwari, R.K.; Gleliebter, J. Downregulation of uPAR inhibits migration, invasion, proliferation FAK/PI3K/Akt signaling and induces senescence in papillary thyroid carcinoma cells. Cell Cycle 2011, 10, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masucci, M.T.; Minopoli, M.; Di Carluccio, G.; Motti, M.L.; Carriero, M.V. Therapeutic strategies targeting urokinase and its receptor in cancer. Cancers 2022, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ou, Y.; Li, Y.; Xiao, R.; Shu, M.; Zhou, Y.; Xie, J.; He, S.; Qiu, P.; Yan, G. A small-molecule triptolide suppresses angiogenesis and invasion of human anaplastic thyroid carcinoma cells via down-regulation of the nuclear factor-kappa B pathway. Mol. Pharmacol. 2009, 75, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellelli, R.; Castellone, M.D.; Garcia-Rostan, G.; Ugolini, C.; Nucera, C.; Sadow, P.M.; Nappi, T.C.; Salerno, P.; Cantisani, M.C.; Basolo, F.; et al. FOXM1 is a molecular determinant of the mitogenic and invasive phenotype of anaplastic thyroid carcinoma. Endocr. Relat. Cancer 2012, 19, 695–710. [Google Scholar] [CrossRef] [Green Version]
- Miyagawa, Y.; Araki, K.; Yamashita, T.; Tanaka, S.; Tanaka, Y.; Tomifuji, M.; Ueda, Y.; Yonemitsu, Y.; Shimada, H.; Shiotani, A. Induction of cell fusion/apoptosis in anaplastic thyroid carcinoma in orthotopic mouse model by urokinase-specific oncolytic Sendai virus. Head Neck 2019, 41, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.; Toller, M.; Graziano, F.M.; Russo, F.P.; Pepe, M.; Biordi, L.; Marchioni, E.; Curcio, F.; Ulisse, S.; Ambesi-Impiombato, F.S.; et al. Expression of matrix metalloproteinases and their specific inhibitors in normal and different human thyroid tumor cell lines. Thyroid 2004, 14, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cang, S.; Li, G.; Su, Y.; Zhang, H.; Wang, L.; Yang, J.; Shi, X.; Qin, G.; Yuan, H. Integrated analysis of transcriptome data revealed MMP3 and MMP13 as critical genes in anaplastic thyroid cancer progression. J. Cell Physiol. 2019, 234, 22260–22271. [Google Scholar] [CrossRef]
- Yoshida, T.; Suganuma, N.; Sato, S.; Toda, S.; Nakayama, H.; Masudo, K.; Okubo, Y.; Hayashi, H.; Yokose, T.; Koshikawa, N.; et al. Membrane type 1 matrix metalloproteinase regulates anaplastic thyroid carcinoma cell growth and invasion into the collagen matrix. Biochem. Biophys. Res. Commun. 2020, 529, 1195–1200. [Google Scholar] [CrossRef]
- Blasi, F.; Sidenius, N. The urokinase receptor: Focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 2010, 584, 1923–1930. [Google Scholar] [CrossRef] [Green Version]
- Resnati, M.; Pallavicini, I.; Wang, J.M.; Oppenheim, J.; Serhan, C.N.; Romano, M.; Blasi, F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc. Natl. Acad Sci. USA 2002, 99, 1359–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweinitz, A.; Steinmetzer, T.; Banke, I.J.; Arlt, M.J.; Stürzebecher, A.; Schuster, O.; Geissler, A.; Giersiefen, H.; Zeslawska, E.; Jacob, U.; et al. Design of novel and selective inhibitors of urokinase-type plasminogen activator with improved pharmacokinetic properties for use as antimetastatic agents. J. Biol. Chem. 2004, 279, 33613–33622. [Google Scholar] [CrossRef] [Green Version]
- Setyono-Han, B.; Stürzebecher, J.; Schmalix, W.A.; Muehlenweg, B.; Sieuwerts, A.M.; Timmermans, M.; Magdolen, V.; Schmitt, M.; Klijn, J.G.; Foekens, J.A. Suppression of rat breast cancer metastasis and reduction of primary tumour growth by the small synthetic urokinase inhibitor WX-UK1. Thromb. Haemost. 2005, 93, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; Ebert, M.P.; Laubender, R.P.; Bevan, P.; Mala, C.; Boeck, S. Phase II randomised proof-of-concept study of the urokinase inhibitor upamostat (WX-671) in combination with gemcitabine compared with gemcitabine alone in patients with non-resectable, locally advanced pancreatic cancer. Br. J. Cancer 2013, 108, 766–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setyono-Han, B.; Schneider, A.; Timmermans, M.; Sieuwerts, A.; Blasi, F.; Schmalix, W.; Foekens, J. Anti-tumor and anti-metastatic activity of WX-340 a highly specific uPA-inhibitor in the rat BN-472 mammary carcinoma model. Cancer Res. 2007, 67 (Suppl. 9), 5615, Proceedings of the 98th AACR Annual Meeting, Los Angeles, CA, USA, 14–18 April 2007; American Association for Cancer Research: Philadelphia, PA, USA. Available online: https://cancerres.aacrjournals.org/content/67/9_Supplement/5615.article-info (accessed on 20 February 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldini, E.; Presutti, D.; Favoriti, P.; Santini, S.; Papoff, G.; Tuccilli, C.; Carletti, R.; Di Gioia, C.; Lori, E.; Ferent, I.C.; et al. In Vitro and In Vivo Effects of the Urokinase Plasminogen Activator Inhibitor WX-340 on Anaplastic Thyroid Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 3724. https://doi.org/10.3390/ijms23073724
Baldini E, Presutti D, Favoriti P, Santini S, Papoff G, Tuccilli C, Carletti R, Di Gioia C, Lori E, Ferent IC, et al. In Vitro and In Vivo Effects of the Urokinase Plasminogen Activator Inhibitor WX-340 on Anaplastic Thyroid Cancer Cell Lines. International Journal of Molecular Sciences. 2022; 23(7):3724. https://doi.org/10.3390/ijms23073724
Chicago/Turabian StyleBaldini, Enke, Dario Presutti, Pasqualino Favoriti, Simonetta Santini, Giuliana Papoff, Chiara Tuccilli, Raffaella Carletti, Cira Di Gioia, Eleonora Lori, Iulia Catalina Ferent, and et al. 2022. "In Vitro and In Vivo Effects of the Urokinase Plasminogen Activator Inhibitor WX-340 on Anaplastic Thyroid Cancer Cell Lines" International Journal of Molecular Sciences 23, no. 7: 3724. https://doi.org/10.3390/ijms23073724





