New and Emerging Targeted Therapies for Hidradenitis Suppurativa
Abstract
:1. Introduction
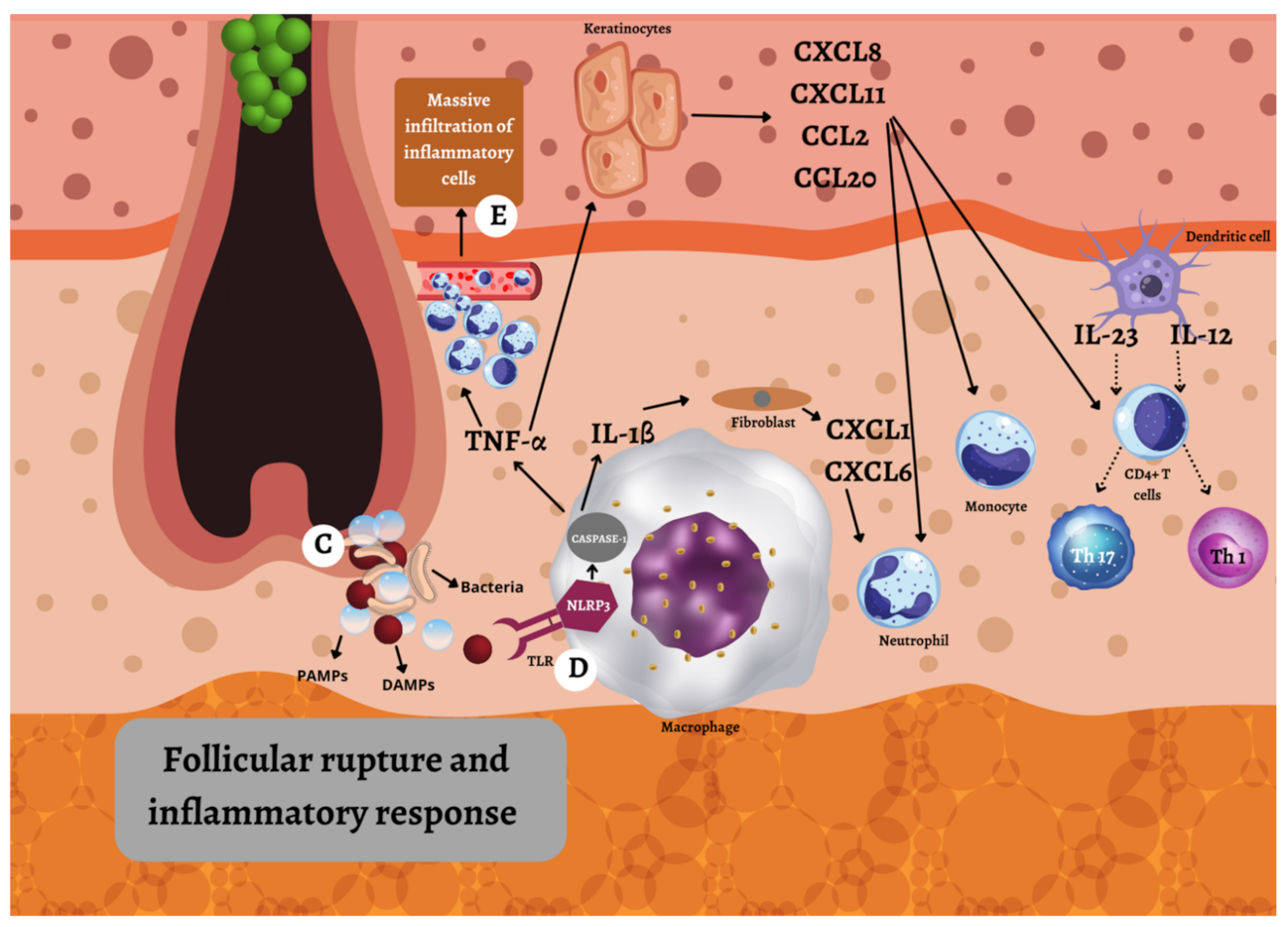
2. Pathogenesis
2.1. Genetic Factors
2.2. Environmental Factors
2.3. Immunologic Factors
3. Treatment
3.1. Topical Therapy
3.2. Systemic Therapy
3.3. Surgical Therapy
3.4. Adjuvant Therapy
4. Biologic Therapy
4.1. Anti-TNF-α Agents
4.1.1. Adalimumab
4.1.2. Infliximab
4.1.3. Etanercept
4.1.4. Golimumab
4.1.5. Certolizumab Pegol
4.2. Anti-IL-17 Agents
4.2.1. Secukinumab
4.2.2. Brodalumab
4.2.3. Bimekizumab
4.2.4. Ixekizumab
4.2.5. CJM112
4.3. Anti-IL-12/23 Agents
Ustekinumab
4.4. Anti-IL-23 Agents
4.4.1. Guselkumab
4.4.2. Risankizumab
4.4.3. Tildrakizumab
4.5. Anti- IL-1 Agents
4.5.1. Anakinra
4.5.2. Bermekimab
4.5.3. Canakinumab
5. Other Immunomodulatory Therapy
5.1. Phosphodiesterase-4 (PDE-4) Inhibitor
Apremilast
5.2. Complement C5a Inhibitors
5.2.1. Vilobelimab (IFX-1)
5.2.2. Avacopan
5.3. Inhibitors of Janus Kinase (JAK) Family
5.3.1. Janus Kinase (JAK) Inhibitors
5.3.2. IL-1 Receptor-Associated Kinase 4 (IRAK4) Inhibitors
5.3.3. Tyrosine Kinase 2 (TYK2) Inhibitors
5.3.4. Tyrosine Kinase 2 (TYK2) Inhibitors/Janus Kinase 1 (JAK1) Inhibitors
5.4. CD-20 Inhibitor
Rituximab
5.5. CD-40 Inhibitor
Iscalimab (CFZ533)
5.6. Anti IL-36 Agents
5.6.1. Spesolimab
5.6.2. Imsidolimab (ANB019)
5.7. Leukotriene A4 (LTA4) Inhibitor
LYS 006
5.8. Inhibitor of Chemokines That Bind to CXCR1 and CXCR2 Receptors
LY 3041658
5.9. Anti-Granulocyte Colony-Stimulating Factor (G-CSF) Agent
CSL 324
6. Discussion
7. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sabat, R.; Jemec, G.B.E.; Matusiak, Ł.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis Suppurativa. Nat. Rev. Dis. Primer 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Martorell, A.; García-Martínez, F.J.; Jiménez-Gallo, D.; Pascual, J.C.; Pereyra-Rodriguez, J.; Salgado, L.; Vilarrasa, E. An Update on Hidradenitis Suppurativa (Part I): Epidemiology, Clinical Aspects, and Definition of Disease Severity. Actas Dermosifiliogr. 2015, 106, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Lavian, J.; Lin, G.; Strunk, A.; Alloo, A. Incidence of Hidradenitis Suppurativa in the United States: A Sex- and Age-Adjusted Population Analysis. J. Am. Acad. Dermatol. 2017, 77, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Megna, M.; Timoshchuk, E.A.; Patruno, C.; Balato, N.; Fabbrocini, G.; Monfrecola, G. Hidradenitis Suppurativa: From Pathogenesis to Diagnosis and Treatment. Clin. Cosmet. Investig. Dermatol. 2017, 10, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad Kamil, M.A.; Mohd Affandi, A. Hidradenitis Suppurativa in Kuala Lumpur, Malaysia: A 7-Year Retrospective Review. Dermatol. Res. Pract. 2018, 2018, 2017959. [Google Scholar] [CrossRef] [Green Version]
- Buimer, M.G.; Wobbes, T.; Klinkenbijl, J.H.G. Hidradenitis Suppurativa. Br. J. Surg. 2009, 96, 350–360. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Damiani, G.; Orenstein, L.A.V.; Hamzavi, I.; Jemec, G.B. Hidradenitis Suppurativa: An Update on Epidemiology, Phenotypes, Diagnosis, Pathogenesis, Comorbidities and Quality of Life. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, 50–61. [Google Scholar] [CrossRef]
- Rambhatla, P.V.; Lim, H.W.; Hamzavi, I. A Systematic Review of Treatments for Hidradenitis Suppurativa. Arch. Dermatol. 2012, 148, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Van der Zee, H.H.; Jemec, G.B.E. New Insights into the Diagnosis of Hidradenitis Suppurativa: Clinical Presentations and Phenotypes. J. Am. Acad. Dermatol. 2015, 73, S23–S26. [Google Scholar] [CrossRef]
- Kimball, A.B.; Sobell, J.M.; Zouboulis, C.C.; Gu, Y.; Williams, D.A.; Sundaram, M.; Teixeira, H.D.; Jemec, G.B.E. HiSCR (Hidradenitis Suppurativa Clinical Response): A Novel Clinical Endpoint to Evaluate Therapeutic Outcomes in Patients with Hidradenitis Suppurativa from the Placebo-Controlled Portion of a Phase 2 Adalimumab Study. J. Eur. Acad. Dermatol. Venereol. JEADV 2016, 30, 989–994. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and Validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a Novel Dynamic Scoring System to Assess HS Severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, V.Y.; Hsiao, J.L.; Lowes, M.; Hamzavi, I. A Comprehensive Guide to Hidradenitis Suppurativa—EBook; Elsevier Health Sciences: Philadelphia, PA, USA, 2021; ISBN 978-0-323-77725-4. [Google Scholar]
- Lim, S.Y.D.; Oon, H.H. Systematic Review of Immunomodulatory Therapies for Hidradenitis Suppurativa. Biol. Targets Ther. 2019, 13, 53–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pink, A.; Simpson, M.; Desai, N.; Smith, C.; Hills, A.; Mortimer, P.; Trembath, R.; Barker, J. Mutations in the Gamma-Secretase Genes NCSTN, PSENEN and PSEN1 Underlie Rare Forms of Hidradenitis Suppurativa (Acne Inversa). Br. J. Dermatol. 2012, 166, e32. [Google Scholar]
- Jfri, A.H.; O’Brien, E.A.; Litvinov, I.V.; Alavi, A.; Netchiporouk, E. Hidradenitis Suppurativa: Comprehensive Review of Predisposing Genetic Mutations and Changes. J. Cutan. Med. Surg. 2019, 23, 519–527. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Wang, B. γ-Secretase Genetics of Hidradenitis Suppurativa: A Systematic Literature Review. Dermatology 2021, 237, 698–704. [Google Scholar] [CrossRef]
- Melnik, B.C.; Plewig, G. Impaired Notch-MKP-1 Signalling in Hidradenitis Suppurativa: An Approach to Pathogenesis by Evidence from Translational Biology. Exp. Dermatol. 2013, 22, 172–177. [Google Scholar] [CrossRef]
- Rice, A.S.; Cook, C. Dowling Degos Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tilley, A.E.; Harvey, B.-G.; Heguy, A.; Hackett, N.R.; Wang, R.; O’Connor, T.P.; Crystal, R.G. Down-Regulation of the Notch Pathway in Human Airway Epithelium in Association with Smoking and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2009, 179, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Bi, P.; Kuang, S. Notch Signaling as a Novel Regulator of Metabolism. Trends Endocrinol. Metab. TEM 2015, 26, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Jemec, G.B.E. Clinical Practice. Hidradenitis Suppurativa. N. Engl. J. Med. 2012, 366, 158–164. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in Health and Disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Strowig, T.; Flavell, R.A. Inflammasomes: Far beyond Inflammation. Nat. Immunol. 2012, 13, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.P.; Cassel, S.L. Inflammasome Mediated Autoinflammatory Disorders. Postgrad. Med. 2010, 122, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Hodak, E.; Atzmony, L.; Pavlovsky, L.; Comaneshter, D.; Cohen, A.D. Hidradenitis Suppurativa Is Associated with Familial Mediterranean Fever—A Population-Based Study. J. Investig. Dermatol. 2017, 137, 2019–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzano, A.V.; Trevisan, V.; Gattorno, M.; Ceccherini, I.; De Simone, C.; Crosti, C. Pyogenic Arthritis, Pyoderma Gangrenosum, Acne, and Hidradenitis Suppurativa (PAPASH): A New Autoinflammatory Syndrome Associated with a Novel Mutation of the PSTPIP1 Gene. JAMA Dermatol. 2013, 149, 762–764. [Google Scholar] [CrossRef]
- Calderón-Castrat, X.; Bancalari-Díaz, D.; Román-Curto, C.; Romo-Melgar, A.; Amorós-Cerdán, D.; Alcaraz-Mas, L.A.; Fernández-López, E.; Cañueto, J. PSTPIP1 Gene Mutation in a Pyoderma Gangrenosum, Acne and Suppurative Hidradenitis (PASH) Syndrome. Br. J. Dermatol. 2016, 175, 194–198. [Google Scholar] [CrossRef]
- Boer, J.; Nazary, M.; Riis, P.T. The Role of Mechanical Stress in Hidradenitis Suppurativa. Dermatol. Clin. 2016, 34, 37–43. [Google Scholar] [CrossRef]
- Ergun, T. Hidradenitis Suppurativa and the Metabolic Syndrome. Clin. Dermatol. 2018, 36, 41–47. [Google Scholar] [CrossRef]
- Pescitelli, L.; Ricceri, F.; Prignano, F. Hidradenitis Suppurativa and Associated Diseases. G. Ital. Dermatol. E Venereol. Organo Uff. Soc. Ital. Dermatol. E Sifilogr. 2018, 153, 8–17. [Google Scholar] [CrossRef]
- Bui, T.-L.; Silva-Hirschberg, C.; Torres, J.; Armstrong, A.W. Hidradenitis Suppurativa and Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2018, 78, 395–402. [Google Scholar] [CrossRef]
- Silfvast-Kaiser, A.; Youssef, R.; Paek, S.Y. Diet in Hidradenitis Suppurativa: A Review of Published and Lay Literature. Int. J. Dermatol. 2019, 58, 1225–1230. [Google Scholar] [CrossRef]
- Bukvić Mokos, Z.; Miše, J.; Balić, A.; Marinović, B. Understanding the Relationship Between Smoking and Hidradenitis Suppurativa. Acta Dermatovenerol. Croat. ADC 2020, 28, 9–13. [Google Scholar] [PubMed]
- Langan, E.A.; Recke, A.; Bokor-Billmann, T.; Billmann, F.; Kahle, B.K.; Zillikens, D. The Role of the Cutaneous Microbiome in Hidradenitis Suppurativa—Light at the End of the Microbiological Tunnel. Int. J. Mol. Sci. 2020, 21, 1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.-B.; Yang, C.-C.; Tsai, S.-J. Hidradenitis Suppurativa: Disease Pathophysiology and Sex Hormones. Chin. J. Physiol. 2021, 64, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Cartron, A.; Driscoll, M.S. Comorbidities of Hidradenitis Suppurativa: A Review of the Literature. Int. J. Womens Dermatol. 2019, 5, 330–334. [Google Scholar] [CrossRef]
- Phan, K.; Loya, A.; Ramachandran, V.; Smith, S.D. Hidradenitis Suppurativa and Risk of Suicide—Systematic Review and Meta-Analysis. J. Dermatol. Treat. 2020, 31, 615–616. [Google Scholar] [CrossRef]
- Vossen, A.R.J.V.; van der Zee, H.H.; Prens, E.P. Hidradenitis Suppurativa: A Systematic Review Integrating Inflammatory Pathways Into a Cohesive Pathogenic Model. Front. Immunol. 2018, 9, 2965. [Google Scholar] [CrossRef] [Green Version]
- Amat-Samaranch, V.; Agut-Busquet, E.; Vilarrasa, E.; Puig, L. New Perspectives on the Treatment of Hidradenitis Suppurativa. Ther. Adv. Chronic Dis. 2021, 12, 204062232110559. [Google Scholar] [CrossRef]
- Giang, J.; Seelen, M.A.J.; van Doorn, M.B.A.; Rissmann, R.; Prens, E.P.; Damman, J. Complement Activation in Inflammatory Skin Diseases. Front. Immunol. 2018, 9, 639. [Google Scholar] [CrossRef]
- Del Duca, E.; Morelli, P.; Bennardo, L.; Di Raimondo, C.; Nisticò, S.P. Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa. Int. J. Mol. Sci. 2020, 21, 8436. [Google Scholar] [CrossRef]
- Liu, T.; Li, S.; Ying, S.; Tang, S.; Ding, Y.; Li, Y.; Qiao, J.; Fang, H. The IL-23/IL-17 Pathway in Inflammatory Skin Diseases: From Bench to Bedside. Front. Immunol. 2020, 11, 594735. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of Effector CD4 T Cell Populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, A.; McNish, S.; Shanmugam, V.K. Interferon-Gamma (IFN-γ) Is Elevated in Wound Exudate from Hidradenitis Suppurativa. Immunol. Investig. 2017, 46, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldburg, S.R.; Strober, B.E.; Payette, M.J. Hidradenitis Suppurativa: Epidemiology, Clinical Presentation, and Pathogenesis. J. Am. Acad. Dermatol. 2020, 82, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, M.; Clark, A.K.; Lee, D.E.; Shi, V.Y. Targeted Treatments for Hidradenitis Suppurativa: A Review of the Current Literature and Ongoing Clinical Trials. J. Dermatol. Treat. 2018, 29, 441–449. [Google Scholar] [CrossRef]
- Balato, A.; Caiazzo, G.; Annunziata, M.C.; Marasca, C.; Scala, E.; Cacciapuoti, S.; Fabbrocini, G. Anti-TNF-α Therapy Modulates MTORC1 Signalling in Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, e43–e45. [Google Scholar] [CrossRef]
- Souwer, Y.; Groot Kormelink, T.; Taanman-Kueter, E.W.; Muller, F.J.; van Capel, T.M.M.; Varga, D.V.; Bar-Ephraim, Y.E.; Teunissen, M.B.M.; van Ham, S.M.; Kuijpers, T.W.; et al. Human TH17 Cell Development Requires Processing of Dendritic Cell-Derived CXCL8 by Neutrophil Elastase. J. Allergy Clin. Immunol. 2018, 141, 2286–2289.e5. [Google Scholar] [CrossRef] [Green Version]
- Matusiak, Ł.; Szczęch, J.; Bieniek, A.; Nowicka-Suszko, D.; Szepietowski, J.C. Increased Interleukin (IL)-17 Serum Levels in Patients with Hidradenitis Suppurativa: Implications for Treatment with Anti-IL-17 Agents. J. Am. Acad. Dermatol. 2017, 76, 670–675. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Moran, B.; Petrasca, A.; Smith, C.M. IL-17 in Inflammatory Skin Diseases Psoriasis and Hidradenitis Suppurativa. Clin. Exp. Immunol. 2020, 201, 121–134. [Google Scholar] [CrossRef]
- Jiang, S.W.; Whitley, M.J.; Mariottoni, P.; Jaleel, T.; MacLeod, A.S. Hidradenitis Suppurativa: Host-Microbe and Immune Pathogenesis Underlie Important Future Directions. JID Innov. Skin Sci. Mol. Popul. Health 2021, 1, 100001. [Google Scholar] [CrossRef]
- Whiteside, S.K.; Snook, J.P.; Williams, M.A.; Weis, J.J. Bystander T Cells: A Balancing Act of Friends and Foes. Trends Immunol. 2018, 39, 1021–1035. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Desai, N.; Emtestam, L.; Hunger, R.E.; Ioannides, D.; Juhász, I.; Lapins, J.; Matusiak, L.; Prens, E.P.; Revuz, J.; et al. European S1 Guideline for the Treatment of Hidradenitis Suppurativa/Acne Inversa. J. Eur. Acad. Dermatol. Venereol. JEADV 2015, 29, 619–644. [Google Scholar] [CrossRef]
- Gulliver, W.; Zouboulis, C.C.; Prens, E.; Jemec, G.B.E.; Tzellos, T. Evidence-Based Approach to the Treatment of Hidradenitis Suppurativa/Acne Inversa, Based on the European Guidelines for Hidradenitis Suppurativa. Rev. Endocr. Metab. Disord. 2016, 17, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemec, G.B.; Wendelboe, P. Topical Clindamycin versus Systemic Tetracycline in the Treatment of Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 1998, 39, 971–974. [Google Scholar] [CrossRef]
- Kirby, J.S.; Milton, S. Exploratory Trial of Ruxolitinib 1.5% Cream for the Treatment of Early Stage Hidradenitis Suppurativa. Cinical Trial Registration NCT04414514, ClinicalTrials.gov; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04414514 (accessed on 6 February 2022).
- Alikhan, A.; Sayed, C.; Alavi, A.; Alhusayen, R.; Brassard, A.; Burkhart, C.; Crowell, K.; Eisen, D.B.; Gottlieb, A.B.; Hamzavi, I.; et al. North American Clinical Management Guidelines for Hidradenitis Suppurativa: A Publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, Evaluation, and the Use of Complementary and Procedural Management. J. Am. Acad. Dermatol. 2019, 81, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Brajac, I.; Puizina Ivić, N.; Bukvić Mokos, Z.; Bolanča, Ž.; Vukšić Polić, M.; Žic, R.; Martić, K.; Mijatović, D.; Budi, S. Smjernice za Dijagnostiku i Liječenje Gnojnog Hidradnitisa (Hidradenitis Suppurativa) Liječ. Vjesn. 2017, 139, 247–253. [Google Scholar]
- Sánchez, A.R.; Rogers, R.S.; Sheridan, P.J. Tetracycline and Other Tetracycline-Derivative Staining of the Teeth and Oral Cavity. Int. J. Dermatol. 2004, 43, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Join-Lambert, O.; Coignard, H.; Jais, J.-P.; Guet-Revillet, H.; Poirée, S.; Fraitag, S.; Jullien, V.; Ribadeau-Dumas, F.; Thèze, J.; Le Guern, A.-S.; et al. Efficacy of Rifampin-Moxifloxacin-Metronidazole Combination Therapy in Hidradenitis Suppurativa. Dermatol. Basel Switz. 2011, 222, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, C.M.; Charlie, A.M.; Leslie, K.S. Hidradenitis Suppurativa: A Guide for the Practicing Physician. Mayo Clin. Proc. 2015, 90, 1679–1693. [Google Scholar] [CrossRef] [Green Version]
- Hughes, R.; Kelly, G.; Sweeny, C.; Lally, A.; Kirby, B. The Medical and Laser Management of Hidradenitis Suppurativa. Am. J. Clin. Dermatol. 2015, 16, 111–123. [Google Scholar] [CrossRef]
- Revuz, J.E.; Canoui-Poitrine, F.; Wolkenstein, P.; Viallette, C.; Gabison, G.; Pouget, F.; Poli, F.; Faye, O.; Roujeau, J.C.; Bonnelye, G.; et al. Prevalence and Factors Associated with Hidradenitis Suppurativa: Results from Two Case-Control Studies. J. Am. Acad. Dermatol. 2008, 59, 596–601. [Google Scholar] [CrossRef]
- Ellis, C.R.; Azmat, C.E. Adalimumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kim, E.S.; Garnock-Jones, K.P.; Keam, S.J. Adalimumab: A Review in Hidradenitis Suppurativa. Am. J. Clin. Dermatol. 2016, 17, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Goldburg, S.R.; Strober, B.E.; Payette, M.J. Hidradenitis Suppurativa: Current and Emerging Treatments. J. Am. Acad. Dermatol. 2020, 82, 1061–1082. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Okun, M.M.; Williams, D.A.; Gottlieb, A.B.; Papp, K.A.; Zouboulis, C.C.; Armstrong, A.W.; Kerdel, F.; Gold, M.H.; Forman, S.B.; et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N. Engl. J. Med. 2016, 375, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Rosales Santillan, M.; Morss, P.C.; Porter, M.L.; Kimball, A.B. Biologic Therapies for the Treatment of Hidradenitis Suppurativa. Expert Opin. Biol. Ther. 2020, 20, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Okun, M.M.; Prens, E.P.; Gniadecki, R.; Foley, P.A.; Lynde, C.; Weisman, J.; Gu, Y.; Williams, D.A.; Jemec, G.B.E. Long-Term Adalimumab Efficacy in Patients with Moderate-to-Severe Hidradenitis Suppurativa/Acne Inversa: 3-Year Results of a Phase 3 Open-Label Extension Study. J. Am. Acad. Dermatol. 2019, 80, 60–69.e2. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, V.; Pathmarajah, P.; Peterknecht, E.; Qazi, E.; Barlow, R.; Muralidharan, V.; Abdullah, A.; McDonald, B.; Bewley, A. Real Life Data on the Biopsychosocial Effects of Adalimumab in the Management of Hidradenitis Suppurativa: A Multicenter Cross Sectional Analysis and Consideration of a Multisystem Monitoring Approach to Follow Up. Dermatol. Ther. 2021, 34, e14643. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Vogelzang, E.H.; Lambert, J.; Wolbink, G.; Cheifetz, A.S. Therapeutic Drug Monitoring with Biologic Agents in Immune Mediated Inflammatory Diseases. Expert Rev. Clin. Immunol. 2019, 15, 837–848. [Google Scholar] [CrossRef]
- Abdalla, T.; Mansour, M.; Bouazzi, D.; Lowes, M.A.; Jemec, G.B.E.; Alavi, A. Therapeutic Drug Monitoring in Patients with Suboptimal Response to Adalimumab for Hidradenitis Suppurativa: A Retrospective Case Series. Am. J. Clin. Dermatol. 2021, 22, 275–283. [Google Scholar] [CrossRef]
- Marzano, A.V.; Genovese, G.; Casazza, G.; Moltrasio, C.; Dapavo, P.; Micali, G.; Sirna, R.; Gisondi, P.; Patrizi, A.; Dini, V.; et al. Evidence for a “window of Opportunity” in Hidradenitis Suppurativa Treated with Adalimumab: A Retrospective, Real-Life Multicentre Cohort Study. Br. J. Dermatol. 2021, 184, 133–140. [Google Scholar] [CrossRef]
- Ponikowska, M.; Matusiak, L.; Szepietowski, J.C. Current Systemic Treatment Strategies for Hidradenitis Suppurativa. Expert Opin. Orphan Drugs 2017, 5, 241–251. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Adalimumab for the Treatment of Hidradenitis Suppurativa/Acne Inversa. Expert Rev. Clin. Immunol. 2016, 12, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Delobeau, M.; Abdou, A.; Puzenat, E.; Deveza, E.; Biver-Dalle, C.; van de Laak, A.; Roche-Kubler, B.; Vuitton, L.; Koch, S.; Wendling, D.; et al. Observational Case Series on Adalimumab-Induced Paradoxical Hidradenitis Suppurativa. J. Dermatol. Treat. 2016, 27, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.; Sobell, J.M.; Leonardi, C.L.; Lynde, C.W.; Karunaratne, M.; Valdecantos, W.C.; Hendrickson, B.A. Safety of Adalimumab Dosed Every Week and Every Other Week: Focus on Patients with Hidradenitis Suppurativa or Psoriasis. Am. J. Clin. Dermatol. 2018, 19, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricceri, F.; Rosi, E.; Di Cesare, A.; Pescitelli, L.; Fastame, M.T.; Prignano, F. Clinical Experience with Adalimumab Biosimilar Imraldi in Hidradenitis Suppurativa. Dermatol. Ther. 2020, 33, e14387. [Google Scholar] [CrossRef]
- Fatima, R.; Bittar, K.; Aziz, M. Infliximab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Grant, A.; Gonzalez, T.; Montgomery, M.O.; Cardenas, V.; Kerdel, F.A. Infliximab Therapy for Patients with Moderate to Severe Hidradenitis Suppurativa: A Randomized, Double-Blind, Placebo-Controlled Crossover Trial. J. Am. Acad. Dermatol. 2010, 62, 205–217. [Google Scholar] [CrossRef]
- Mekkes, J.R.; Bos, J.D. Long-Term Efficacy of a Single Course of Infliximab in Hidradenitis Suppurativa. Br. J. Dermatol. 2008, 158, 370–374. [Google Scholar] [CrossRef]
- Oskardmay, A.N.; Miles, J.A.; Sayed, C.J. Determining the Optimal Dose of Infliximab for Treatment of Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2019, 81, 702–708. [Google Scholar] [CrossRef]
- Ghias, M.H.; Johnston, A.D.; Kutner, A.J.; Micheletti, R.G.; Hosgood, H.D.; Cohen, S.R. High-Dose, High-Frequency Infliximab: A Novel Treatment Paradigm for Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2020, 82, 1094–1101. [Google Scholar] [CrossRef]
- Lozeron, P.; Denier, C.; Lacroix, C.; Adams, D. Long-Term Course of Demyelinating Neuropathies Occurring during Tumor Necrosis Factor-Alpha-Blocker Therapy. Arch. Neurol. 2009, 66, 490–497. [Google Scholar] [CrossRef]
- Vossen, M.G.; Gattringer, K.B.; Khalifeh, N.; Koreny, M.; Spertini, V.; Mallouhi, A.; Willeit, M.; Volc-Platzer, B.; Asboth, F.; Graninger, W.; et al. Gemella Morbillorum Bacteremia after Anti-Tumor Necrosis Factor Alpha as Acne Inversa Therapy. J. Clin. Microbiol. 2012, 50, 1109–1112. [Google Scholar] [CrossRef] [Green Version]
- Scheinfeld, N. A Case of a Patient with Stage III Familial Hidradenitis Suppurativa Treated with 3 Courses of Infliximab and Died of Metastatic Squamous Cell Carcinoma. Dermatol. Online J. 2014, 20, 17. [Google Scholar] [CrossRef]
- Prens, L.M.; Bouwman, K.; Aarts, P.; Arends, S.; van Straalen, K.R.; Dudink, K.; Horváth, B.; Prens, E.P. Adalimumab and Infliximab Survival in Patients with Hidradenitis Suppurativa: A Daily Practice Cohort Study. Br. J. Dermatol. 2021, 185, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Westerkam, L.L.; Tackett, K.J.; Sayed, C.J. Comparing the Effectiveness and Safety Associated With Infliximab vs Infliximab-Abda Therapy for Patients with Hidradenitis Suppurativa. JAMA Dermatol. 2021, 157, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Gerriets, V. Etanercept. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Adams, D.R.; Yankura, J.A.; Fogelberg, A.C.; Anderson, B.E. Treatment of Hidradenitis Suppurativa with Etanercept Injection. Arch. Dermatol. 2010, 146, 501–504. [Google Scholar] [CrossRef]
- Padda, I.S.; Bhatt, R.; Parmar, M. Golimumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- van der Zee, H.H.; Prens, E.P. Failure of Anti-Interleukin-1 Therapy in Severe Hidradenitis Suppurativa: A Case Report. Dermatol. Basel Switz. 2013, 226, 97–100. [Google Scholar] [CrossRef]
- Tursi, A. Concomitant Hidradenitis Suppurativa and Pyostomatitis Vegetans in Silent Ulcerative Colitis Successfully Treated with Golimumab. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2016, 48, 1511–1512. [Google Scholar] [CrossRef]
- Goel, N.; Stephens, S. Certolizumab Pegol. mAbs 2010, 2, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Wohlmuth-Wieser, I.; Alhusayen, R. Treatment of Hidradenitis Suppurativa with Certolizumab Pegol during Pregnancy. Int. J. Dermatol. 2021, 60, e140–e141. [Google Scholar] [CrossRef]
- Holm, J.G.; Jørgensen, A.-H.R.; Yao, Y.; Thomsen, S.F. Certolizumab Pegol for Hidradenitis Suppurativa: Case Report and Literature Review. Dermatol. Ther. 2020, 33, e14494. [Google Scholar] [CrossRef]
- Esme, P.; Akoglu, G.; Caliskan, E. Rapid Response to Certolizumab Pegol in Hidradenitis Suppurativa: A Case Report. Skin Appendage Disord. 2021, 7, 58–61. [Google Scholar] [CrossRef]
- Sand, F.L.; Thomsen, S.F. Off-Label Use of TNF-Alpha Inhibitors in a Dermatological University Department: Retrospective Evaluation of 118 Patients. Dermatol. Ther. 2015, 28, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Aboobacker, S.; Kurn, H.; Al Aboud, A.M. Secukinumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Prussick, L.; Rothstein, B.; Joshipura, D.; Saraiya, A.; Turkowski, Y.; Abdat, R.; Alomran, A.; Zancanaro, P.; Kachuk, C.; Dumont, N.; et al. Open-Label, Investigator-Initiated, Single-Site Exploratory Trial Evaluating Secukinumab, an Anti-Interleukin-17A Monoclonal Antibody, for Patients with Moderate-to-Severe Hidradenitis Suppurativa. Br. J. Dermatol. 2019, 181, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Casseres, R.G.; Prussick, L.; Zancanaro, P.; Rothstein, B.; Joshipura, D.; Saraiya, A.; Turkowski, Y.; Au, S.C.; Alomran, A.; Abdat, R.; et al. Secukinumab in the Treatment of Moderate to Severe Hidradenitis Suppurativa: Results of an Open-Label Trial. J. Am. Acad. Dermatol. 2020, 82, 1524–1526. [Google Scholar] [CrossRef] [PubMed]
- Reguiaï, Z.; Fougerousse, A.C.; Maccari, F.; Bécherel, P.A. Effectiveness of Secukinumab in Hidradenitis Suppurativa: An Open Study (20 Cases). J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, e750–e751. [Google Scholar] [CrossRef] [PubMed]
- Fauny, M.; Moulin, D.; D’Amico, F.; Netter, P.; Petitpain, N.; Arnone, D.; Jouzeau, J.-Y.; Loeuille, D.; Peyrin-Biroulet, L. Paradoxical Gastrointestinal Effects of Interleukin-17 Blockers. Ann. Rheum. Dis. 2020, 79, 1132–1138. [Google Scholar] [CrossRef]
- Shalom, G.; Freud, T.; Ben Yakov, G.; Khoury, R.; Dreiher, J.; Vardy, D.A.; Comaneshter, D.; Cohen, A.D. Hidradenitis Suppurativa and Inflammatory Bowel Disease: A Cross-Sectional Study of 3207 Patients. J. Investig. Dermatol. 2016, 136, 1716–1718. [Google Scholar] [CrossRef]
- Ribero, S.; Ramondetta, A.; Fabbrocini, G.; Bettoli, V.; Potenza, C.; Chiricozzi, A.; Licciardello, M.; Marzano, A.V.; Bianchi, L.; Rozzo, G.; et al. Effectiveness of Secukinumab in the Treatment of Moderate-Severe Hidradenitis Suppurativa: Results from an Italian Multicentric Retrospective Study in a Real-Life Setting. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, e441–e442. [Google Scholar] [CrossRef]
- Marasca, C.; Megna, M.; Balato, A.; Balato, N.; Napolitano, M.; Fabbrocini, G. Secukinumab and Hidradenitis Suppurativa: Friends or Foes? JAAD Case Rep. 2019, 5, 184–187. [Google Scholar] [CrossRef] [Green Version]
- Novartis Pharmaceuticals. A Randomized, Double-Blind, Multi-Center Study Assessing Short (16 Weeks) and Long-Term Efficacy (up to 1 Year), Safety, and Tolerability of 2 Subcutaneous Secukinumab Dose Regimens in Adult Patients with Moderate to Severe Hidradenitis Suppurativa (SUNSHINE). Clinical Trial Registration NCT03713619, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03713619 (accessed on 30 January 2022).
- Novartis Pharmaceuticals. A Randomized, Double-Blind, Multicenter Study Assessing Short (16 Weeks) and Long-Term Efficacy (up to 1 Year), Safety, and Tolerability of 2 Subcutaneous Secukinumab Dose Regimens in Adult Patients with Moderate to Severe Hidradenitis Suppurativa (SUNRISE). Clinical Trial Registration NCT03713632, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03713632 (accessed on 20 January 2022).
- Novartis Pharmaceuticals. A Multicenter, Double-Blind, Randomized Withdrawal Extension Study of Subcutaneous Secukinumab to Demonstrate Long-Term Efficacy, Safety and Tolerability in Subjects with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT04179175, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04179175 (accessed on 30 January 2022).
- Golbari, N.M.; Basehore, B.M.; Zito, P.M. Brodalumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Frew, J.W.; Navrazhina, K.; Grand, D.; Sullivan-Whalen, M.; Gilleaudeau, P.; Garcet, S.; Ungar, J.; Krueger, J.G. The Effect of Subcutaneous Brodalumab on Clinical Disease Activity in Hidradenitis Suppurativa: An Open-Label Cohort Study. J. Am. Acad. Dermatol. 2020, 83, 1341–1348. [Google Scholar] [CrossRef]
- Frew, J.W.; Navrazhina, K.; Sullivan-Whalen, M.; Gilleaudeau, P.; Garcet, S.; Krueger, J.G. Weekly Administration of Brodalumab in Hidradenitis Suppurativa: An Open-Label Cohort Study. Br. J. Dermatol. 2021, 184, 350–352. [Google Scholar] [CrossRef]
- Renert-Yuval, Y. A Small Pilot Study to Develop Biomarkers of Weekly Brodalumab Administration in Hidradenitis Suppurativa patients. Clinical Trial Registration NCT04979520, ClinicalTrials.gov; 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04979520 (accessed on 13 February 2022).
- Adams, R.; Maroof, A.; Baker, T.; Lawson, A.D.G.; Oliver, R.; Paveley, R.; Rapecki, S.; Shaw, S.; Vajjah, P.; West, S.; et al. Bimekizumab, a Novel Humanized IgG1 Antibody That Neutralizes Both IL-17A and IL-17F. Front. Immunol. 2020, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Glatt, S.; Jemec, G.B.E.; Forman, S.; Sayed, C.; Schmieder, G.; Weisman, J.; Rolleri, R.; Seegobin, S.; Baeten, D.; Ionescu, L.; et al. Efficacy and Safety of Bimekizumab in Moderate to Severe Hidradenitis Suppurativa: A Phase 2, Double-Blind, Placebo-Controlled Randomized Clinical Trial. JAMA Dermatol. 2021, 157, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- UCB Biopharma SRL. A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study Evaluating the Efficacy and Safety of Bimekizumab in Study Participants with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT04242446, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04242446 (accessed on 10 February 2022).
- UCB Biopharma SRL. A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study Evaluating the Efficacy and Safety of Bimekizumab in Study Participants with Moderate to Severe Hidradenitis Suppurativa. Clinical trial Registration NCT04242498, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04242498 (accessed on 10 February 2022).
- UCB Biopharma SRL. A Phase 3, Open-Label, Parallel Group, Multicenter, Extension Study Evaluating the Long-Term Treatment of Bimekizumab in Study Participants with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT04901195, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04901195 (accessed on 10 February 2022).
- Preuss, C.V.; Quick, J. Ixekizumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Odorici, G.; Pellacani, G.; Conti, A. Ixekizumab in Hidradenitis Suppurativa in a Psoriatic Patient. G. Ital. Dermatol. E Venereol. Organo Uff. Soc. Ital. Dermatol. E Sifilogr. 2020, 155, 788–789. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Ruggiero, A.; Di Guida, A.; Patrì, A.; Fabbrocini, G.; Marasca, C. Ixekizumab: An Efficacious Treatment for Both Psoriasis and Hidradenitis Suppurativa. Dermatol. Ther. 2020, 33, e13756. [Google Scholar] [CrossRef]
- Reardon, K.; Levin, J.; Levin, C. Severe Hidradenitis Suppurativa with Herpes Simplex Virus 1 Superinfection and Clinical Responsiveness to Ixekizumab. JAAD Case Rep. 2021, 9, 7–8. [Google Scholar] [CrossRef]
- Kaul, M.; Jarvis, P.; Rozenberg, I.; Kolbinger, F.; Di Padova, F.; Calonder, C.; Espie, P.; Rondeau, J.M.; Cebe, R.; Huber, T.; et al. First-in-Human Study Demonstrating the Safety and Clinical Efficacy of Novel Anti-IL-17A Monoclonal Antibody CJM112 in Moderate to Severe Plaque Psoriasis. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, 1143–1151. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. A Randomized, Double-Blind, Placebo Controlled, Multiple Dose Study to Evaluate the Clinical Efficacy, Safety, Tolerability, Dose Relation, Pharmacokinetics and Pharmacodynamics of CJM112 in Moderate to Severe Chronic Hidradenitis Suppurativa Patients. Clinical Trial Registration NCT02421172, ClinicalTrials.gov.; 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02421172 (accessed on 13 February 2022).
- Colquhoun, M.; Kemp, A.K. Ustekinumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Blok, J.L.; Li, K.; Brodmerkel, C.; Horvátovich, P.; Jonkman, M.F.; Horváth, B. Ustekinumab in Hidradenitis Suppurativa: Clinical Results and a Search for Potential Biomarkers in Serum. Br. J. Dermatol. 2016, 174, 839–846. [Google Scholar] [CrossRef]
- Romaní, J.; Vilarrasa, E.; Martorell, A.; Fuertes, I.; Ciudad, C.; Molina-Leyva, A. Ustekinumab with Intravenous Infusion: Results in Hidradenitis Suppurativa. Dermatol. Basel Switz. 2020, 236, 21–24. [Google Scholar] [CrossRef]
- Sánchez-Martínez, E.M.; García-Ruiz, R.; Moneva-Léniz, L.M.; Mateu-Puchades, A. Effectiveness and Safety of Ustekinumab in Patients with Hidradenitis Suppurativa Using Intravenous Induction. Dermatol. Ther. 2020, 33, e14054. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Pozo-Román, T.; Sánchez-Velicia, L.; Vega-Gutiérrez, J.; Arias-Santiago, S.; Molina-Leyva, A. Ustekinumab in the Treatment of Patients with Hidradenitis Suppurativa: Multicenter Case Series and Systematic Review. J. Dermatol. Treat. 2022, 33, 348–353. [Google Scholar] [CrossRef]
- Valenzuela-Ubiña, S.; Jiménez-Gallo, D.; Villegas-Romero, I.; Rodríguez-Mateos, M.E.; Linares-Barrios, M. Effectiveness of Ustekinumab for Moderate-to-Severe Hidradenitis Suppurativa: A Case Series. J. Dermatol. Treat. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Megna, M.; Balato, A.; Raimondo, A.; Balato, N. Guselkumab for the Treatment of Psoriasis. Expert Opin. Biol. Ther. 2018, 18, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Kearney, N.; Byrne, N.; Kirby, B.; Hughes, R. Successful Use of Guselkumab in the Treatment of Severe Hidradenitis Suppurativa. Clin. Exp. Dermatol. 2020, 45, 618–619. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M.; Podda, M. Guselkumab in the Treatment of Severe Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, e140–e141. [Google Scholar] [CrossRef] [PubMed]
- Casseres, R.G.; Kahn, J.S.; Her, M.J.; Rosmarin, D. Guselkumab in the Treatment of Hidradenitis Suppurativa: A Retrospective Chart Review. J. Am. Acad. Dermatol. 2019, 81, 265–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, H.S.; Villa, N.M.; Shi, V.Y.; Hsiao, J.L. Guselkumab in the Treatment of Concomitant Hidradenitis Suppurativa, Psoriasis, and Crohn’s Disease. J. Dermatol. Treat. 2021, 32, 261–263. [Google Scholar] [CrossRef]
- Jørgensen, A.-H.R.; Holm, J.G.; Thomsen, S.F. Guselkumab for Hidradenitis Suppurativa in a Patient with Concomitant Crohn’s Disease: Report and Systematic Literature Review of Effectiveness and Safety. Clin. Case Rep. 2020, 8, 2874–2877. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Martinez-Lopez, A.; Salvador-Rodriguez, L.; Arias-Santiago, S.; Molina-Leyva, A. The Use of Guselkumab 100 Mg Every 4 Weeks on Patients with Hidradenitis Suppurativa and a Literature Review. Dermatol. Ther. 2020, 33, e13456. [Google Scholar] [CrossRef]
- Janssen Research & Development, LLC. A Phase 2, Multicenter, Randomized, Placebo-Controlled, Double-Blind, Proof-of-Concept Study to Evaluate Guselkumab for the Treatment of Subjects with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT0368924, ClinicalTrials.gov.; 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03628924 (accessed on 23 February 2022).
- Haugh, I.M.; Preston, A.K.; Kivelevitch, D.N.; Menter, A.M. Risankizumab: An Anti-IL-23 Antibody for the Treatment of Psoriasis. Drug Des. Devel. Ther. 2018, 12, 3879–3883. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, L.E.; Keiserman, M.; Papp, K.; McCasland, L.; White, D.; Lu, W.; Wang, Z.; Soliman, A.M.; Eldred, A.; Barcomb, L.; et al. Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 24-Week Results from the Randomised, Double-Blind, Phase 3 KEEPsAKE 1 Trial. Ann. Rheum. Dis. 2022, 81, 225–231. [Google Scholar] [CrossRef]
- Marques, E.; Arenberger, P.; Smetanová, A.; Gkalpakiotis, S.; Zimová, D.; Arenbergerová, M. Successful Treatment of Recalcitrant Hidradenitis Suppurativa with Risankizumab after Failure of Anti-Tumour Necrosis Factor Alpha. Br. J. Dermatol. 2021, 184, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Caposiena Caro, R.D.; Pensa, C.; Lambiase, S.; Candi, E.; Bianchi, L. Risankizumab Effectiveness in a Recalcitrant Case of Hidradenitis Suppurativa after Anti-TNF and Anti-Interleukin-17 Failures. Dermatol. Ther. 2021, 34, e15116. [Google Scholar] [CrossRef] [PubMed]
- Licata, G.; Gambardella, A.; Buononato, D.; De Rosa, A.; Calabrese, G.; Pellerone, S.; Argenziano, G. A Case of Moderate Hidradenitis Suppurativa and Psoriasis Successfully Treated with Risankizumab. Int. J. Dermatol. 2021, 61, e126–e129. [Google Scholar] [CrossRef]
- AbbVie. A Phase 2, Multicenter, Randomized, Placebo-Controlled, Double-Blind Study to Evaluate the Safety and Efficacy of Risankizumab in Adult Subjects with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT03926169, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03926169 (accessed on 13 February 2022).
- Sinclair, R.; Thirthar Palanivelu, V. Tildrakizumab for the Treatment of Psoriasis. Expert Rev. Clin. Immunol. 2019, 15, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Kok, Y.; Nicolopoulos, J.; Howard, A.; Varigos, G.; Kern, J.; Dolianitis, C. Tildrakizumab in the Treatment of Moderate-to-Severe Hidradenitis Suppurativa. Australas. J. Dermatol. 2020, 61, e488–e490. [Google Scholar] [CrossRef]
- Kok, Y.; Nicolopoulos, J.; Dolianitis, C. Tildrakizumab as a Potential Long-Term Therapeutic Agent for Severe Hidradenitis Suppurativa: A 15 Months Experience of an Australian Institution. Australas. J. Dermatol. 2021, 62, e313–e316. [Google Scholar] [CrossRef]
- Tegtmeyer, K.; Atassi, G.; Zhao, J.; Maloney, N.J.; Lio, P.A. Off-Label Studies on Anakinra in Dermatology: A Review. J. Dermatol. Treat. 2022, 33, 73–86. [Google Scholar] [CrossRef]
- Mendonça, L.O.; Grossi, A.; Caroli, F.; de Oliveira, R.A.; Kalil, J.; Castro, F.F.M.; Pontillo, A.; Ceccherini, I.; Barros, M.A.M.T.; Gattorno, M. A Case Report of a Novel Compound Heterozygous Mutation in a Brazilian Patient with Deficiency of Interleukin-1 Receptor Antagonist (DIRA). Pediatr. Rheumatol. 2020, 18, 67. [Google Scholar] [CrossRef]
- Tzanetakou, V.; Kanni, T.; Giatrakou, S.; Katoulis, A.; Papadavid, E.; Netea, M.G.; Dinarello, C.A.; van der Meer, J.W.M.; Rigopoulos, D.; Giamarellos-Bourboulis, E.J. Safety and Efficacy of Anakinra in Severe Hidradenitis Suppurativa: A Randomized Clinical Trial. JAMA Dermatol. 2016, 152, 52–59. [Google Scholar] [CrossRef]
- Leslie, K.S.; Tripathi, S.V.; Nguyen, T.V.; Pauli, M.; Rosenblum, M.D. An Open-Label Study of Anakinra for the Treatment of Moderate to Severe Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2014, 70, 243–251. [Google Scholar] [CrossRef]
- Zarchi, K.; Dufour, D.N.; Jemec, G.B.E. Successful Treatment of Severe Hidradenitis Suppurativa with Anakinra. JAMA Dermatol. 2013, 149, 1192–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, V.; Alikhan, A. Failure of Anakinra in a Case of Severe Hidradenitis Suppurativa. J. Drugs Dermatol. JDD 2016, 15, 772–774. [Google Scholar] [PubMed]
- Menis, D.; Maroñas-Jiménez, L.; Delgado-Marquez, A.M.; Postigo-Llorente, C.; Vanaclocha-Sebastián, F. Two Cases of Severe Hidradenitis Suppurativa with Failure of Anakinra Therapy. Br. J. Dermatol. 2015, 172, 810–811. [Google Scholar] [CrossRef]
- Kurzrock, R.; Hickish, T.; Wyrwicz, L.; Saunders, M.; Wu, Q.; Stecher, M.; Mohanty, P.; Dinarello, C.A.; Simard, J. Interleukin-1 Receptor Antagonist Levels Predict Favorable Outcome after Bermekimab, a First-in-Class True Human Interleukin-1α Antibody, in a Phase III Randomized Study of Advanced Colorectal Cancer. Oncoimmunology 2018, 8, 1551651. [Google Scholar] [CrossRef] [PubMed]
- Kanni, T.; Argyropoulou, M.; Spyridopoulos, T.; Pistiki, A.; Stecher, M.; Dinarello, C.A.; Simard, J.; Giamarellos-Bourboulis, E.J. MABp1 Targeting IL-1α for Moderate to Severe Hidradenitis Suppurativa Not Eligible for Adalimumab: A Randomized Study. J. Investig. Dermatol. 2018, 138, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Kanni, T.; Argyropoulou, M.; Dinarello, C.A.; Simard, J.; Giamarellos-Bourboulis, E.J. MABp1 Targeting Interleukin-1α in Hidradenitis Suppurativa Ineligible for Adalimumab Treatment: Results of the Open-Label Extension Period. Clin. Exp. Dermatol. 2021, 46, 162–163. [Google Scholar] [CrossRef]
- Gottlieb, A.; Natsis, N.E.; Kerdel, F.; Forman, S.; Gonzalez, E.; Jimenez, G.; Hernandez, L.; Kaffenberger, J.; Guido, G.; Lucas, K.; et al. A Phase II Open-Label Study of Bermekimab in Patients with Hidradenitis Suppurativa Shows Resolution of Inflammatory Lesions and Pain. J. Investig. Dermatol. 2020, 140, 1538–1545.e2. [Google Scholar] [CrossRef]
- Janssen Research & Development, LLC. A Phase 2a/2b, Multicenter, Randomized, Placebo and Active Comparator-Controlled, Double-Blind, Dose-Ranging Study to Evaluate the Safety and Efficacy of Bermekimab (JNJ-77474462) for the Treatment of Subjects with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT04988308, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04988308 (accessed on 14 February 2022).
- Dhimolea, E. Canakinumab. mAbs 2010, 2, 3–13. [Google Scholar] [CrossRef]
- Houriet, C.; Seyed Jafari, S.M.; Thomi, R.; Schlapbach, C.; Borradori, L.; Yawalkar, N.; Hunger, R.E. Canakinumab for Severe Hidradenitis Suppurativa: Preliminary Experience in 2 Cases. JAMA Dermatol. 2017, 153, 1195–1197. [Google Scholar] [CrossRef]
- Jaeger, T.; Andres, C.; Grosber, M.; Zirbs, M.; Hein, R.; Ring, J.; Traidl-Hoffmann, C. Pyoderma Gangrenosum and Concomitant Hidradenitis Suppurativa--Rapid Response to Canakinumab (Anti-IL-1β). Eur. J. Dermatol. EJD 2013, 23, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Tekin, B.; Salman, A.; Ergun, T. Hidradenitis Suppurativa Unresponsive to Canakinumab Treatment: A Case Report. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.Z.; Ro, T.; Jolly, P.; Sayed, C.J. Non-Response to Interleukin-1 Antagonist Canakinumab in Two Patients with Refractory Pyoderma Gangrenosum and Hidradenitis Suppurativa. J. Clin. Aesthetic Dermatol. 2017, 10, 36–38. [Google Scholar]
- Vossen, A.R.; van Doorn, M.B.; van der Zee, H.H.; Prens, E.P. Apremilast for Moderate Hidradenitis Suppurativa: Results of a Randomized Controlled Trial. J. Am. Acad. Dermatol. 2019, 80, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Aarts, P.; Vossen, A.R.J.V.; van der Zee, H.H.; Prens, E.P.; van Straalen, K.R. Long-Term Treatment with Apremilast in Hidradenitis Suppurativa: A 2-Year Follow-up of Initial Responders. J. Am. Acad. Dermatol. 2021, 85, 258–260. [Google Scholar] [CrossRef]
- Weber, P.; Seyed Jafari, S.M.; Yawalkar, N.; Hunger, R.E. Apremilast in the Treatment of Moderate to Severe Hidradenitis Suppurativa: A Case Series of 9 Patients. J. Am. Acad. Dermatol. 2017, 76, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Giamarellos-Bourboulis, E.J.; Argyropoulou, M.; Kanni, T.; Spyridopoulos, T.; Otto, I.; Zenker, O.; Guo, R.; Riedemann, N.C. Clinical Efficacy of Complement C5a Inhibition by IFX-1 in Hidradenitis Suppurativa: An Open-Label Single-Arm Trial in Patients Not Eligible for Adalimumab. Br. J. Dermatol. 2020, 183, 176–178. [Google Scholar] [CrossRef]
- InflaRx GmbH. A Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study to Determine Efficacy and Safety of IFX-1 in Subjects with Moderate to Severe Hidradenitis Suppurativa. In Clinical Trial Registration NCT03487276, ClinicalTrials.gov.; 2021. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03487276 (accessed on 16 February 2022).
- Lee, A. Avacopan: First Approval. Drugs 2022, 82, 79–85. [Google Scholar] [CrossRef]
- ChemoCentryx. A Randomized, Double-Blind, Placebo-Controlled, Parallel Group, Phase 2 Study to Evaluate the Safety and Efficacy of Avacopan in Subjects with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT03852472, ClinicalTrials.gov.; 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03852472 (accessed on 16 February 2022).
- Aarts, P.; Dudink, K.; Vossen, A.R.J.V.; van Straalen, K.R.; Ardon, C.B.; Prens, E.P.; van der Zee, H.H. Clinical Implementation of Biologics and Small Molecules in the Treatment of Hidradenitis Suppurativa. Drugs 2021, 81, 1397–1410. [Google Scholar] [CrossRef]
- Incyte Corporation. A Phase 2, Dose-Escalation, Placebo-Controlled Study of INCB054707 in Participants with Hidradenitis Suppurativa. Clinical Trial Registration NCT03569371, ClinicalTrials.gov.; 2021. Available online: https://clinicaltrials.gov/show/NCT03569371 (accessed on 16 February 2022).
- Incyte Corporation. A Phase 2, Open-Label, Single-Arm Study of the Safety of INCB054707 in Participants with Hidradenitis Suppurativa. Clinical Trial Registration NCT03607487, ClinicalTrials.gov.; 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03607487 (accessed on 16 February 2022).
- Incyte Corporation. A Phase 2, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study of the Efficacy and Safety of INCB054707 in Participants with Hidradenitis Suppurativa. Clinical Trial Registration NCT04476043, ClinicalTrials.gov.; 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04476043 (accessed on 16 February 2022).
- AbbVie. A Phase 2, Multicenter, Randomized, Placebo-Controlled, Double-Blind Study to Evaluate Upadacitinib in Adult Subjects with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT04430855, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04430855 (accessed on 16 February 2022).
- Savage, K.T.; Santillan, M.R.; Flood, K.S.; Charrow, A.; Porter, M.L.; Kimball, A.B. Tofacitinib Shows Benefit in Conjunction with Other Therapies in Recalcitrant Hidradenitis Suppurativa Patients. JAAD Case Rep. 2020, 6, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Kymera Therapeutics, Inc. A Phase 1 Randomized, Placebo-Controlled, Single and Multiple Ascending Dose Trial to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Orally Administered KT-474 in Healthy Adult Volunteers and Patients with Atopic Dermatitis (AD) or Hidradenitis Suppurativa (HS). Clinical Trial Registration NCT04772885, ClinicalTrials.gov.; 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04772885 (accessed on 16 February 2022).
- Pfizer. A Phase 2A, Multicenter, Randomized, Double-Blind, Placebo-Controlled, 16-Week Study Evaluating the Safety and Efficacy of PF-06650833, PF-06700841,and PF-06826647 in Adults with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT04092452, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04092452 (accessed on 16 February 2022).
- Hanif, N.; Anwer, F. Rituximab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Takahashi, K.; Yanagi, T.; Kitamura, S.; Hata, H.; Imafuku, K.; Iwami, D.; Hotta, K.; Morita, K.; Shinohara, N.; Shimizu, H. Successful Treatment of Hidradenitis Suppurativa with Rituximab for a Patient with Idiopathic Carpotarsal Osteolysis and Chronic Active Antibody-Mediated Rejection. J. Dermatol. 2018, 45, e116–e117. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. A Randomized, Subject and Investigator Blinded, Placebo-Controlled and Multi-Center Platform Study, to Assess Efficacy and Safety of Different Investigational Drugs in Patients with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT03827798, ClinicalTrials.gov.; 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03827798 (accessed on 16 February 2022).
- Elias, M.; Zhao, S.; Le, H.T.; Wang, J.; Neurath, M.F.; Neufert, C.; Fiocchi, C.; Rieder, F. IL-36 in Chronic Inflammation and Fibrosis—Bridging the Gap? J. Clin. Investig. 2021, 131, 144336. [Google Scholar] [CrossRef] [PubMed]
- Boehringer Ingelheim. Randomized, Double-Blind, Placebo-Controlled, Study of Spesolimab in Patients with Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT04762277, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04762277 (accessed on 16 February 2022).
- AnaptysBio, Inc. A Phase 2, Randomized, Double-Blind, Placebo Controlled Study to Evaluate the Efficacy and Safety of Imsidolimab (ANB019) in the Treatment of Subjects with Hidradenitis Suppurativa. Clinical Trial Registration NCT04856930, ClinicalTrials.gov.; 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04856930 (accessed on 16 February 2022).
- Eli Lilly and Company. A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 2 Study to Evaluate the Efficacy and Safety of LY3041658 in Adults with Moderate-to-Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT04493502, ClinicalTrials.gov.; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04493502 (accessed on 16 February 2022).
- CSL Behring. A Multicenter, Open-Label, 2-Regimen, Repeat-Dose Study to Assess the Safety and Pharmacokinetics of Intravenous CSL324 in Subjects with Hidradenitis Suppurativa and Palmoplantar Pustulosis. Clinical Trial Registration NCT03972280, ClinicalTrials.gov.; 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03972280 (accessed on 16 February 2022).
- Lu, J.D.; Milakovic, M.; Piguet, V.; Alavi, A. Antidrug Antibodies to Tumour Necrosis Factor Inhibitors in Hidradenitis Suppurativa: A Systematic Review. Br. J. Dermatol. 2021, 184, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Strand, V.; Balsa, A.; Al-Saleh, J.; Barile-Fabris, L.; Horiuchi, T.; Takeuchi, T.; Lula, S.; Hawes, C.; Kola, B.; Marshall, L. Immunogenicity of Biologics in Chronic Inflammatory Diseases: A Systematic Review. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2017, 31, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Cludts, I.; Spinelli, F.R.; Morello, F.; Hockley, J.; Valesini, G.; Wadhwa, M. Anti-Therapeutic Antibodies and Their Clinical Impact in Patients Treated with the TNF Antagonist Adalimumab. Cytokine 2017, 96, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lipa, K.; Zając, N.; Witkowski, G.; Ciechanowicz, P.; Wiszniewski, K.; Szymańska, E.; Walecka, I. Hidradenitis Suppurativa—Biologic Therapy and Other Available Treatment Options. Adv. Dermatol. Allergol. Dermatol. Alergol. 2022, 38. [Google Scholar] [CrossRef]
- Holcomb, Z.E.; Porter, M.L.; Kimball, A.B. A Safety Review of Biologic Therapies for the Management of Hidradenitis Suppurativa and Unmet Needs. Expert Opin. Drug Saf. 2021, 20, 1147–1161. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Gulliver, W.; Ingram, J.; Kirby, B.; Giamarellos-Bourboulis, E.J.; Podda, M.; Tzellos, T.; Jemec, G.B.E. Endpoints of Clinical Trials for Hidradenitis Suppurativa: Proceedings of a Round-Table Session. Exp. Dermatol. 2020, 29 (Suppl. S1), 67–72. [Google Scholar] [CrossRef]
- Faivre, C.; Villani, A.P.; Aubin, F.; Lipsker, D.; Bottaro, M.; Cohen, J.-D.; Durupt, F.; Jeudy, G.; Sbidian, E.; Toussirot, E.; et al. Hidradenitis Suppurativa (HS): An Unrecognized Paradoxical Effect of Biologic Agents (BA) Used in Chronic Inflammatory Diseases. J. Am. Acad. Dermatol. 2016, 74, 1153–1159. [Google Scholar] [CrossRef]
- AbbVie. A Phase 4, Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Assess the Safety and Efficacy of Adalimumab Used in Conjunction with Surgery in Subjects With Moderate to Severe Hidradenitis Suppurativa. Clinical Trial Registration NCT02808975, ClinicalTrials.gov.; 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT02808975 (accessed on 16 February 2022).
- Der Sarkissian, S.; Hessam, S.; Kirby, J.S.; Lowes, M.A.; Mintoff, D.; Naik, H.B.; Ring, H.C.; Suyien, N.C.; Frew, J.W. Identification of Biomarkers and Critical Evaluation of Biomarker Validation in Hidradenitis Suppurativa: A Systematic Review. JAMA Dermatol. 2022, 158, 300–313. [Google Scholar] [CrossRef]


| Hurley stage I | single or multiple isolated abscesses without scars and sinus tracts (68% of patients) |
| Hurley stage II | recurrent abscesses with single or multiple scars and sinus tracts; widely separated lesions (28% of patients) |
| Hurley stage III | multiple lesions, extensive scars, and sinus tracts involving the entire region (4% of patients) |
| Mild HS | 3 or less than 3 points |
| Moderate HS | 4–10 points |
| Severe HS | 11 or more than 11 points |
| IHS4 points = number of nodules × 1 + number of abscesses × 2 + number of draining fistulas × 4 | |
| Biologic Drug | Structure | Studies | Dosage Regimen | Efficacy |
|---|---|---|---|---|
| ADALIMUMAB | Human recombinant IgG1 monoclonal antibody | Phase III RCT (PIONEER I) (n = 307) [64] | week 0–160 mg sc week 2–80 mg sc from week 4–40 mg sc weekly | 41.8% of patients treated with ADA achieved HiSCR after week 12 vs. 26.0% of patients treated with placebo |
| Phase III RCT (PIONEER II) (n = 326) [64] | week 0–160 mg sc week 2–80 mg sc from week 4–40 mg sc weekly | 58.9% of patients treated with ADA achieved HiSCR after week 12 vs. 27.6% of patients treated with placebo | ||
| Phase III OLE of PIONEER I and II (n = 88) [69] | every week–40 mg sc | 62.5% of patients achieved HiSCR at week 36, and 52.3% of patients achieved HiSCR at week 168 | ||
| Retrospective real-life cohort study (n = 101) [70] | week 0–160 mg sc week 2–80 mg sc from week 4–40 mg sc weekly | 77% of patients showed improving in HS-PGA | ||
| Retrospective real-life cohort study (n = 389) [73] | week 0–160 mg sc week 2–80 mg sc from week 4–40 mg sc weekly | 43.7% of patients achieved HiSCR at week 16, and 53.9% of patients achieved HiSCR at week 52 | ||
| ADALIMUMAB BIOSIMILAR SB5 | Human recombinant IgG1 monoclonal antibody | Retrospective observational study (n = 11) [78] | week 0–160 mg sc week 2–80 mg sc from week 4–40 mg sc weekly | 54.5% of patients achieved HiSCR at week 36 |
| INFLIXIMAB | Chimeric human/mouse IgG1 monoclonal antibody | Phase II RCT (n = 38) [80] | weeks 0, 2, 4, 6, 14, 22–5 mg/kg iv | 26.7% of patients treated with IFX had 50% or greater decrease in HSSI vs. 5% of patients treated with placebo |
| Prospective cohort study (n = 42) [83] | weeks 0, 2, 6–7.5 mg/kg iv from week 10–7.5 mg/kg or 10 mg/kg iv every 4 weeks | 70.8% of patients treated with IFX 7.5 mg/kg achieved HS-PGA 0–2 at week 12; 50% of patients treated with IFX 10 mg/kg achieved HS-PGA 0–2 at week 12 | ||
| INFLIXIMAB BIOSIMILAR (IFX-abda) | Chimeric human/mouse IgG1 monoclonal antibody | Retrospective cohort study (n = 34) [88] | weeks 0, 2, 6–10 mg/kg iv from week 10–10 mg/kg iv every 4/8 weeks | 71% of patients treated with IFX-abda achieved HiSCR vs. 60% of patients treated with IFX |
| ETANERCEPT | Human recombinant TNF-receptor p75 Fc-IgG1 fusion protein | Phase II RCT (n = 20) [90] | every week–2 × 50 mg sc | There was no statistically significant difference in HS-PGA at weeks 12 or 24 between treatment and placebo groups |
| GOLIMUMAB | Human recombinant IgG1 monoclonal antibody | Case report [92] Case report [93] | every 4 weeks–50 mg sc; week 0–200 mg sc from week 4–100 mg sc every 4 weeks | HS-PGA deteriorated from 5 to 8; Remission of HS after 2 months |
| CERTOLIZUMAB PEGOL | Pegylated humanized monoclonal antigen- binding fragment (Fab’) of IgG | Case reports (n = 3) [95,96,97] Retrospective study (n = 2) [98] | every 2 weeks–400 mg sc; every 2 weeks–200 mg sc | Good disease control in 3 case reports; No efficacy |
| Biologic Drug | Structure | Studies | Dosage Regimen | Efficacy |
|---|---|---|---|---|
| SECUKINUMAB | Human IgG1κ monoclonal antibody | Open-label trial (n = 9) [100] | weeks 0, 1, 2, 3, 4–300 sc mg from week 8–300 mg sc every 4 weeks | 78% of patients achieved HiSCR at week 24 |
| Open-label trial (n = 20) [101] | weeks 0, 1, 2, 3, 4–300 mg sc from week 6/8–300 mg sc every 2/4 weeks | 70% of patients achieved HiSCR at week 24 | ||
| Retrospective cohort study (n = 20) [102] | weeks 0, 1, 2, 3, 4–300 mg sc from week 8–300 mg sc every 4 weeks | 75% of patients achieved HiSCR by week 16 | ||
| Phase III RCTs (NCT03713619, NCT03713632, NCT04179175) [107,108,109] | Ongoing | |||
| BRODALUMAB | Human IgG2 monoclonal antibody | Open-label trial (n = 10) [111] | weeks 0, 1, 2–210 mg sc from week 4–210 mg sc every 2 weeks | 100% of patients achieved HiSCR |
| Open-label trial (n = 10) [112] | every week–210 mg sc | 100% of patients achieved HiSCR | ||
| BIMEKIZUMAB | Humanized IgG1κ monoclonal antibody | Phase II RCT (n = 90) [115] | week 0–640 mg sc from week 2–320 mg sc every 2 weeks | 57.3% of patients achieved HiSCR at week 12 vs. 26.1% of patients treated with placebo |
| Phase III RCTs (NCT04242446, NCT04242498, NCT04901195) [116,117,118] | Ongoing | |||
| IXEKIZUMAB | Humanized IgG4κ monoclonal antibody | Case reports (n = 3) [120,121,122] | week 0–160 mg sc weeks 2, 4, 6, 8, 10, 12–80 mg sc from week 16–80 mg sc every 4 weeks | Good disease control |
| CJM112 | Human IgG1κ monoclonal antibody | Phase II RCT (NCT02421172) (n = 66) [124] | weeks 0, 1, 2, 3, 4–300 sc mg from week 6–300 mg sc every 2 weeks | HS-PGA response rate with CJM112 32.5% vs. 12.5% with placebo |
| Biologic Drug | Structure | Studies | Dosage Regimen | Efficacy |
|---|---|---|---|---|
| USTEKINUMAB | Human IgG1κ monoclonal antibody | Phase II open- label trial (n = 17) [126] | weeks 0, 4, 16, 28–45 mg sc if under 90 kg and 90 mg if over 90 kg | 47% of patients achieved HiSCR at week 40 |
| Multicentre retrospective study (n = 14) [127] | week 0—iv infusion adjusted by weight (≤55 kg, 260 mg; 55–85 kg, 390 mg; ≥85 kg, 520 mg) from week 8–90 mg sc every 8 weeks | 50% of patients achieved HiSCR at week 16 | ||
| Prospective study (n = 6) [128] | week 0—iv infusion adjusted by weight (≤55 kg, 260 mg; 55–85 kg, 390 mg; ≥85 kg, 520 mg) from week 8–90 mg sc every 8 weeks | 50% of patients achieved HiSCR at week 12 | ||
| Case series (n = 10) [129] | every 8/12 weeks– 90 mg sc | 70% of patients showed improvement in HS-PGA score | ||
| Case series (n = 10) [130] | every 8 weeks–90 mg sc | 90% of patients achieved HiSCR |
| Biologic Drug | Structure | Studies | Dosage Regimen | Efficacy |
|---|---|---|---|---|
| GUSELKUMAB | Human IgG1κ monoclonal antibody | A phase II RCT (NCT03628924) (n = 184) [138] | weeks 0, 4, 8, 12–200 mg sc; weeks 0, 4, 8–1200 mg iv week 12–200 mg sc | 50.8% of participants achieved HiSCR at week 16; 45% of participants achieved HiSCR at week 16 |
| Case series (n = 4) [137] | week 0–100 mg sc from week 4–100 mg every 4 weeks | Variable results | ||
| Case reports (n = 14) [132,133,134,135,136] | week 0–100 mg sc from week 4–100 mg every 8 weeks | Variable results | ||
| RISANKIZUMAB | Human IgG1κ monoclonal antibody | Case reports (n = 4) [141,142,143] | weeks 0, 4–150 mg sc from week 16–150 mg every 12 weeks | Positive results |
| A phase II RCT (NCT03926169) (n = 243) | ongoing | |||
| TILDRAKIZUMAB | Humanized IgG1κ monoclonal antibody | Case series (n = 4) [146,147] | weeks 0, 4–100 mg sc from week 8–200 mg every 4 weeks | 100% of patients achieved HiSCR at week 8 |
| Biologic Drug | Structure | Studies | Dosage Regimen | Efficacy |
|---|---|---|---|---|
| ANAKINRA | Human recombinant IL-1 receptor (IL-1R) monoclonal antibody | Phase II RCT (n = 20) [150] | 100 mg sc g.d./12 weeks | 78% of patients treated with anakinra achieved HiSCR vs. 30% of patients treated with placebo |
| Case series (n = 6) [151] | 100 mg sc g.d./8 weeks | A rebound of the disease 8 weeks after the end of the treatment | ||
| Case reports (n = 4) [92,153,154] | 100 mg sc g.d. | Failure | ||
| BERMEKIMAB | Human recombinant IgG1κ monoclonal antibody | A phase II RCT (NCT02643654) (n = 20) [156] | every 2 weeks–7.5 mg/kg iv | 60% of patients treated with bermekimab achieved HiSCR vs. 10% of patients treated with placebo |
| OLE of NCT02643654 (n = 8) [157] | every 2 weeks–7.5 mg/kg iv | 75% of patients achieved HiSCR at week 12 | ||
| Open-label trial (n = 42) [158] | every week–400 mg sc | 61% of patients naive to anti-TNF therapy and 63% having failed anti-TNF therapy achieved HiSCR at week 12 | ||
| Phase II RCT (NCT04988308) (n = 290) [159] | ongoing | |||
| CANAKINUMAB | Human recombinant IgG1κ monoclonal antibody | Case reports (n = 6) [161,162,163,164] | every week/4 weeks/8 weeks–150 mg sc | Variable results |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markota Čagalj, A.; Marinović, B.; Bukvić Mokos, Z. New and Emerging Targeted Therapies for Hidradenitis Suppurativa. Int. J. Mol. Sci. 2022, 23, 3753. https://doi.org/10.3390/ijms23073753
Markota Čagalj A, Marinović B, Bukvić Mokos Z. New and Emerging Targeted Therapies for Hidradenitis Suppurativa. International Journal of Molecular Sciences. 2022; 23(7):3753. https://doi.org/10.3390/ijms23073753
Chicago/Turabian StyleMarkota Čagalj, Adela, Branka Marinović, and Zrinka Bukvić Mokos. 2022. "New and Emerging Targeted Therapies for Hidradenitis Suppurativa" International Journal of Molecular Sciences 23, no. 7: 3753. https://doi.org/10.3390/ijms23073753
APA StyleMarkota Čagalj, A., Marinović, B., & Bukvić Mokos, Z. (2022). New and Emerging Targeted Therapies for Hidradenitis Suppurativa. International Journal of Molecular Sciences, 23(7), 3753. https://doi.org/10.3390/ijms23073753






