Role of Induced Programmed Cell Death in the Chemopreventive Potential of Apigenin
Abstract
1. Introduction
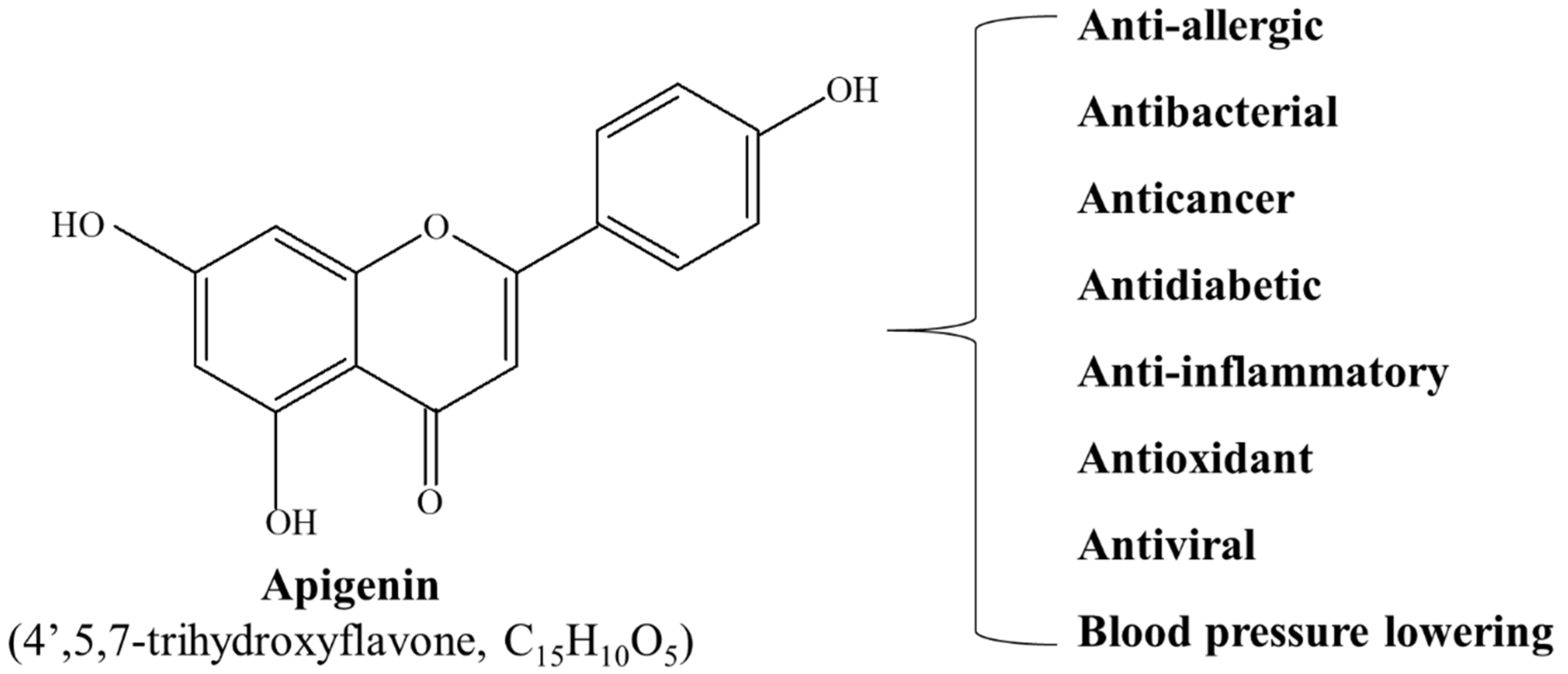
2. Apigenin
3. Physiological Functions of Apigenin
4. Apigenin in Cancer Therapy
5. Effect of Apigenin on Apoptosis
5.1. Apoptosis
5.2. Types of Apoptosis
5.2.1. Intrinsic (Mitochondrial) Pathway
5.2.2. Extrinsic (Death Receptor) Pathway
5.3. Induction of Apoptosis by Apigenin
5.3.1. Effect of Apigenin on Caspase-Mediated Apoptosis
5.3.2. Effect of Apigenin on Tumor Suppressor p53-Dependent Apoptosis
5.3.3. Effect of Apigenin on Tumor Suppressor p53-Independent Apoptosis
6. Effect of Apigenin on Autophagy
6.1. Autophagy
6.2. Types of Autophagy
6.3. Induction of Autophagy by Apigenin
| Cancer/Cell Lines | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|
| Breast | |||
| T47D and MDA-MB-231 | LC3-I, LC3-II | [75] | |
| Cevical | |||
| HeLa | GRP78 | [169] | |
| Colon | |||
| HCT116 | LC3-II | Wnt, c-Myc, Axin2, cyclin D1, β-catenin, p-AKT, p70S6, p-p70, S6, 4EBP1, p-4EBP1 | [89,170] |
| SW480 | LC3-II | Wnt | [170] |
| HT-29 | Beclin-1, LC3-II | p62, p-mTOR, p-PI3K, p-AKT | [92] |
| Gastric | |||
| AGS and SNU-638 | Atg5, Beclin1, LC3-II AMPKα ULK1, GRP78, p-PERK, p-eIF2α ATF4, CHOP, GRP78, CD63 | p62, p-mTOR, Ezh2 | [168] |
| Liver | |||
| HepG2 and HepG2 xenograft | LC3-I, LC3-II, Atg5, Beclin1, LC3-II/I ratio, AMPK | SQSTM1/p62, p-PI3K, p-AKT, p-mTOR, p-mTOR/mTOR ratio, NQO2 | [111,171,172,173] |
| Hep3B | LC3-II, Atg7, ROS | [115] | |
| SMMC-7721 and SK-HEP-1 | LC3B-II, ULK1 | p62 | [174] |
| Leukemia | |||
| TF-1 | LC3-II, Atg5, Atg12, LMWPTP | p-Src, p-JAK2,p-STAT3, p-STAT5, p-SHP2, p-mTOR, p-p70S6K | [106] |
| Lung | |||
| H1975 | LC3-II | p-EGFR, Kras, c-Myc, HIF-1α, p-AMPKα | [175] |
| Multiple myeloma | |||
| NCI-H929 | Beclin1, LC3B-II | [176] | |
| Neuroblastoma | |||
| SH-SY5Y | LC3-II, p-AKT, mTOR | Beclin 1, TLR-4, Myd88 | [177] |
| Pancreatic | |||
| PANC-1 | LC3-I, LC3-II, p-AKT | p62, NRF2, SOD, CATALASE, HSP90, p-4EBP1 | [178] |
| PaCa-44 | LC3-I, LC3-II, p62, NRF2, SOD, catalase, HSP90, 4EBP1, p-AKT | [178] | |
| Renal | |||
| ACHN and OS-RC-2 | Beclin1, LC3-II, p-AMPKα, p-JNK | Ki-67, PCNA, p62, p-PI3K, p-AKT, p-mTOR | [179] |
| Skin | |||
| COLO-16 and HEK | ATM, ATR, UPR, BiP, IRE1α, PERK, Atg, LC3-I, LC3-II | [180] | |
| Thyroid | |||
| BCPAP | Beclin1, LC3-I, LC3-II, Nrf2, HO-1 | p62 | [181] |
7. Effect of Apigenin on Necroptosis
7.1. Necroptosis
7.2. Necroptosis in Cancer
7.3. Induction of Necroptosis by Apigenin
8. Effect of Apigenin on Ferroptosis
8.1. Ferroptosis
8.2. Ferroptosis and Cancer
8.3. Induction of Ferroptosis by Apigenin
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, S.; Higurashi, T.; Komiya, Y.; Arimoto, J.; Horita, N.; Kaneko, T.; Iwasaki, M.; Nakagama, H.; Nakajima, A. Chemoprevention of colorectal cancer: Past, present, and future. Cancer Sci. 2019, 110, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Sung, B.; Chung, H.Y.; Kim, N.D. Role of apigenin in cancer prevention via the induction of apoptosis and autophagy. J. Cancer Prev. 2016, 21, 216–226. [Google Scholar] [CrossRef]
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005, 591, 8–15. [Google Scholar] [CrossRef]
- Khanna, D.; Sethi, G.; Ahn, K.S.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Aggarwal, A.; Aggarwal, B.B. Natural products as a gold mine for arthritis treatment. Curr. Opin. Pharmacol. 2007, 7, 344–351. [Google Scholar] [CrossRef]
- Liu, C.; Ho, P.C.; Wong, F.C.; Sethi, G.; Wang, L.Z.; Goh, B.C. Garcinol: Current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef]
- Ajaikumar, K.B.; Asheef, M.; Babu, B.H.; Padikkala, J. The inhibition of gastric mucosal injury by Punicagranatum L. (pomegranate) methanolic extract. J. Ethnopharmacol. 2005, 96, 171–176. [Google Scholar] [CrossRef]
- Nair, A.S.; Shishodia, S.; Ahn, K.S.; Kunnumakkara, A.B.; Sethi, G.; Aggarwal, B.B. Deguelin, an Akt inhibitor, suppresses IkappaBalpha kinase activation leading to suppression of NF-kappaB-regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. J. Immunol. 2006, 177, 5612–5622. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Tyagi, A.K.; Kunnumakkara, A.B.; Aggarwal, B.B. Neem (Azadirachta indica): An indian traditional panacea with modern molecular basis. Phytomedicine 2017, 34, 14–20. [Google Scholar] [CrossRef]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: A randomized, double-blind, placebo-controlled, two-dose, three-arm, and parallel-group study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Jacob, J.; Varma, K.; Jude, S.; Amalraj, A.; Arundhathy, C.A.; George, R.; Sreeraj, T.R.; Divya, C.; Kunnumakkara, A.B.; et al. Comparative oral absorption of curcumin in a natural turmeric matrix with two other curcumin formulations: An open-label parallel-arm study. Phytother. Res. 2017, 31, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015, 240, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef]
- Roy, N.K.; Deka, A.; Bordoloi, D.; Mishra, S.; Kumar, A.P.; Sethi, G.; Kunnumakkara, A.B. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. 2016, 377, 74–86. [Google Scholar] [CrossRef]
- Monisha, J.; Padmavathi, G.; Roy, N.K.; Deka, A.; Bordoloi, D.; Anip, A.; Kunnumakkara, A.B. NF-κB blockers gifted by mother nature: Prospectives in cancer cell chemosensitization. Curr. Pharm. Des. 2016, 22, 4173–4200. [Google Scholar] [CrossRef]
- Sailo, B.L.; Banik, K.; Girisa, S.; Bordoloi, D.; Fan, L.; Halim, C.E.; Wang, H.; Kumar, A.P.; Zheng, D.; Mao, X.; et al. FBXW7 in cancer: What has been unraveled thus far? Cancers 2019, 11, 246. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef]
- Henamayee, S.; Banik, K.; Sailo, B.L.; Shabnam, B.; Harsha, C.; Srilakshmi, S.; Vgm, N.; Baek, S.H.; Ahn, K.S.; Kunnumakkara, A.B. Therapeutic emergence of rhein as a potential anticancer drug: A review of its molecular targets and anticancer properties. Molecules 2020, 25, 2278. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019, 144, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Sailo, B.L.; Roy, N.K.; Thakur, K.K.; Banik, K.; Shakibaei, M.; Gupta, S.C.; Aggarwal, B.B. Cancer drug development: The missing links. Exp. Biol. Med. 2019, 244, 663–689. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Shabnam, B.; Monisha, J.; Fan, L.; Halim, C.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Kunnumakkara, A.B. Potential of zerumbone as an anti-cancer agent. Molecules 2019, 24, 734. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef]
- Pápay, Z.E.; Kósa, A.; Böddi, B.; Merchant, Z.; Saleem, I.Y.; Zariwala, M.G.; Klebovich, I.; Somavarapu, S.; Antal, I. Study on the pulmonary delivery system of apigenin-loaded albumin nanocarriers with antioxidant activity. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 274–288. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Bakhoda, M.R.; Bahmanpour, Z.; Ilkhani, K.; Zarrabi, A.; Makvandi, P.; Khan, H.; Mazaheri, S.; Darvish, M.; Mirzaei, H. Apigenin as tumor suppressor in cancers: Biotherapeutic activity, nanodelivery, and mechanisms with emphasis on pancreatic cancer. Front. Chem. 2020, 8, 829. [Google Scholar] [CrossRef]
- Wang, Y.C.; Huang, K.M. In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem. Toxicol. 2013, 53, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Gao, T.; Huang, Y.; Xue, J.; Xie, M.L. Apigenin ameliorates hypertension-induced cardiac hypertrophy and down-regulates cardiac hypoxia inducible factor-lα in rats. Food Funct. 2016, 7, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Ozçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Pamunuwa, G.; Karunaratne, D.N.; Waisundara, V.Y. Antidiabetic properties, bioactive constituents, and other therapeutic effects of Scoparia dulcis. Evid.-Based Complement. Alternat. Med. 2016, 2016, 8243215. [Google Scholar] [CrossRef]
- Li, R.R.; Pang, L.L.; Du, Q.; Shi, Y.; Dai, W.J.; Yin, K.S. Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma. Immunopharmacol. Immunotoxicol. 2010, 32, 364–370. [Google Scholar] [CrossRef]
- Birt, D.F.; Walker, B.; Tibbels, M.G.; Bresnick, E. Anti-mutagenesis and anti-promotion by apigenin, robinetin and indole-3-carbinol. Carcinogenesis 1986, 7, 959–963. [Google Scholar] [CrossRef]
- Farhood, B.; Goradel, N.H.; Mortezaee, K.; Khanlarkhani, N.; Najafi, M.; Sahebkar, A. Melatonin and cancer: From the promotion of genomic stability to use in cancer treatment. J. Cell. Physiol. 2019, 234, 5613–5627. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M.; Farhood, B.; Ahmadi, A.; Potes, Y.; Shabeeb, D.; Musa, A.E. Modulation of apoptosis by melatonin for improving cancer treatment efficiency: An updated review. Life Sci. 2019, 228, 228–241. [Google Scholar] [CrossRef]
- Woo, S.M.; Seo, S.U.; Kubatka, P.; Min, K.J.; Kwon, T.K. Honokiol enhances TRAIL-Mediated Apoptosis through STAMBPL1-induced survivin and c-FLIP degradation. Biomolecules 2019, 9, 838. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M.; Farhood, B.; Ahmadi, A.; Shabeeb, D.; Musa, A.E. NF-κB targeting for overcoming tumor resistance and normal tissues toxicity. J. Cell. Physiol. 2019, 234, 17187–17204. [Google Scholar] [CrossRef]
- Mortezaee, K.; Potes, Y.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019, 238, 116960. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Salehi, E.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Najafi, M.; Farhood, B.; Rosengren, R.J.; Sahebkar, A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019, 234, 12537–12550. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Cheki, M.; Rezapoor, S.; Geraily, G.; Motevaseli, E.; Carnovale, C.; Clementi, E.; Shirazi, A. Metformin: Prevention of genomic instability and cancer: A review. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 827, 1–8. [Google Scholar] [CrossRef]
- Najafi, M.; Goradel, N.H.; Farhood, B.; Salehi, E.; Solhjoo, S.; Toolee, H.; Kharazinejad, E.; Mortezaee, K. Tumor microenvironment: Interactions and therapy. J. Cell. Physiol. 2019, 234, 5700–5721. [Google Scholar] [CrossRef]
- Najafi, M.; Hashemi Goradel, N.; Farhood, B.; Salehi, E.; Nashtaei, M.S.; Khanlarkhani, N.; Khezri, Z.; Majidpoor, J.; Abouzaripour, M.; Habibi, M.; et al. Macrophage polarity in cancer: A review. J. Cell. Biochem. 2019, 120, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, S.; Song, Y.U.; Yao, J.; Huang, K.; Zhu, X. Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol. Lett. 2016, 11, 3075–3080. [Google Scholar] [CrossRef]
- Huang, C.; Wei, Y.X.; Shen, M.C.; Tu, Y.H.; Wang, C.C.; Huang, H.C. Chrysin, Abundant in morinda citrifolia fruit water-EtOAc extracts, combined with apigenin synergistically induced apoptosis and inhibited migration in human breast and liver cancer cells. J. Agric. Food Chem. 2016, 64, 4235–4245. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, G.; Oh, T.I.; Kim, B.M.; Shim, D.W.; Lee, K.H.; Kim, Y.J.; Lim, B.O.; Lim, J.H. Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int. J. Oncol. 2016, 48, 399–408. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Cheng, W.; Ni, J.; Zhang, Y.; Lin, J.; Song, Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol. Rep. 2017, 37, 2277–2285. [Google Scholar] [CrossRef]
- Gupta, S.; Afaq, F.; Mukhtar, H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene 2002, 21, 3727–3738. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol. Oncol. 2017, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, H.; Arango, D.; Nicholas, C.; Duarte, S.; Nuovo, G.J.; He, W.; Voss, O.H.; Gonzalez-Mejia, M.E.; Guttridge, D.C.; Grotewold, E.; et al. Dietary apigenin exerts immune-regulatory activity in vivo by reducing NF-κB activity, halting leukocyte infiltration and restoring normal metabolic function. Int. J. Mol. Sci. 2016, 17, 323. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Brenner, D.; Mak, T.W. Mitochondrial cell death effectors. Curr. Opin. Cell Biol. 2009, 21, 871–877. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Suhaili, S.H.; Karimian, H.; Stellato, M.; Lee, T.H.; Aguilar, M.I. Mitochondrial outer membrane permeabilization: A focus on the role of mitochondrial membrane structural organization. Biophys. Rev. 2017, 9, 443–457. [Google Scholar] [CrossRef]
- Elena-Real, C.A.; Díaz-Quintana, A.; González-Arzola, K.; Velázquez-Campoy, A.; Orzáez, M.; López-Rivas, A.; Gil-Caballero, S.; De la Rosa, M.; Díaz-Moreno, I. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3ε-dependent Apaf-1 inhibition. Cell Death Dis. 2018, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in cell death, inflammation, and disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.C.; Johnson, D.E. Signaling by cell surface death receptors: Alterations in head and neck cancer. Adv. Biol. Regul. 2018, 67, 170–178. [Google Scholar] [CrossRef]
- O’ Reilly, E.; Tirincsi, A.; Logue, S.E.; Szegezdi, E. The Janus face of death receptor signaling during tumor immunoediting. Front. Immunol. 2016, 7, 446. [Google Scholar] [CrossRef]
- Erekat, N.S. Apoptosis and its therapeutic implications in neurodegenerative diseases. Clin. Anat. 2022, 35, 65–78. [Google Scholar] [CrossRef]
- Erekat, N.S. Cerebellar upregulation of cell surface death receptor-mediated apoptotic factors in harmaline-induced tremor: An immunohistochemistry study. J. Cell Death 2018, 11, 1179066018809091. [Google Scholar] [CrossRef]
- Zhu, Y.; Mao, Y.; Chen, H.; Lin, Y.; Hu, Z.; Wu, J.; Xu, X.; Xu, X.; Qin, J.; Xie, L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell Int. 2013, 13, 54. [Google Scholar] [CrossRef]
- Shi, M.D.; Shiao, C.K.; Lee, Y.C.; Shih, Y.W. Apigenin, a dietary flavonoid, inhibits proliferation of human bladder cancer T-24 cells via blocking cell cycle progression and inducing apoptosis. Cancer Cell Int. 2015, 15, 33. [Google Scholar] [CrossRef]
- Kilani-Jaziri, S.; Frachet, V.; Bhouri, W.; Ghedira, K.; Chekir-Ghedira, L.; Ronot, X. Flavones inhibit the proliferation of human tumor cancer cell lines by inducing apoptosis. Drug Chem. Toxicol. 2012, 35, 1–10. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. Apigenin causes G(2)/M arrest associated with the modulation of p21(Cip1) and Cdc2 and activates p53-dependent apoptosis pathway in human breast cancer SK-BR-3 cells. J. Nutr. Biochem. 2009, 20, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Way, T.D.; Kao, M.C.; Lin, J.K. Degradation of HER2/neu by apigenin induces apoptosis through cytochrome c release and caspase-3 activation in HER2/neu-overexpressing breast cancer cells. FEBS Lett. 2005, 579, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Woo, J.K.; Jang, B.H.; Go, H.; Shin, Y.C.; Ko, S.G. Apigenin induces caspase-dependent apoptosis by inhibiting signal transducer and activator of transcription 3 signaling in HER2-overexpressing SKBR3 breast cancer cells. Mol. Med. Rep. 2015, 12, 2977–2984. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Ju, J.H.; Jang, K.; Shin, I. Induction of apoptotic cell death by phytoestrogens by up-regulating the levels of phospho-p53 and p21 in normal and malignant estrogen receptor α-negative breast cells. Nutr. Res. 2011, 31, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, B.; Cao, W.; Zhang, W.; Zhang, F.; Zhao, H.; Meng, R.; Zhang, L.; Niu, R.; Hao, X.; et al. Autophagy inhibition enhances apigenin-induced apoptosis in human breast cancer cells. Chin. J. Cancer Res. 2013, 25, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Landis-Piwowar, K.R.; Chen, M.S.; Dou, Q.P. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007, 9, R80. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, C.-Y.; Lee, K.-R.; Lin, H.-J.; Chen, T.-H.; Wan, L. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer 2015, 15, 958. [Google Scholar] [CrossRef]
- Seo, H.S.; Choi, H.S.; Kim, S.R.; Choi, Y.K.; Woo, S.M.; Shin, I.; Woo, J.K.; Park, S.Y.; Shin, Y.C.; Ko, S.G. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012, 366, 319–334. [Google Scholar] [CrossRef]
- Bai, H.; Jin, H.; Yang, F.; Zhu, H.; Cai, J. Apigenin induced MCF-7 cell apoptosis-associated reactive oxygen species. Scanning 2014, 36, 622–631. [Google Scholar] [CrossRef]
- Seo, H.-S.; Jo, J.K.; Ku, J.M.; Choi, H.-S.; Choi, Y.K.; Woo, J.-K.; Kim, H.i.; Kang, S.-y.; Lee, K.m.; Nam, K.W.; et al. Induction of caspase-dependent extrinsic apoptosis by apigenin through inhibition of signal transducer and activator of transcription 3 (STAT3) signalling in HER2-overexpressing BT-474 breast cancer cells. Biosci. Rep. 2015, 35, e00276. [Google Scholar] [CrossRef]
- Liu, R.; Ji, P.; Liu, B.; Qiao, H.; Wang, X.; Zhou, L.; Deng, T.; Ba, Y. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis. Oncol. Lett. 2017, 13, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Woo, J.K.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Induction of caspase-dependent apoptosis by apigenin by inhibiting STAT3 signaling in HER2-overexpressing MDA-MB-453 breast cancer cells. Anticancer Res. 2014, 34, 2869–2882. [Google Scholar] [PubMed]
- Choi, E.J.; Kim, G.H. Apigenin induces apoptosis through a mitochondria/caspase-pathway in human breast cancer MDA-MB-453 Cells. J. Clin. Biochem. Nutr. 2009, 44, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.W.; Chiang, L.C.; Lin, C.C. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci. 2005, 76, 1367–1379. [Google Scholar] [CrossRef]
- Souza, R.P.; Bonfim-Mendonça, P.d.S.; Gimenes, F.; Ratti, B.A.; Kaplum, V.; Bruschi, M.L.; Nakamura, C.V.; Silva, S.O.; Maria-Engler, S.S.; Consolaro, M.E.L. Oxidative stress triggered by apigenin induces apoptosis in a comprehensive panel of human cervical cancer-derived cell lines. Oxid. Med. Cell. Longev. 2017, 2017, 1512745. [Google Scholar] [CrossRef]
- Xu, Y.; Xin, Y.; Diao, Y.; Lu, C.; Fu, J.; Luo, L.; Yin, Z. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS ONE 2011, 6, e29169. [Google Scholar] [CrossRef]
- Shao, H.; Jing, K.; Mahmoud, E.; Huang, H.; Fang, X.; Yu, C. Apigenin sensitizes colon cancer cells to antitumor activity of ABT-263. Mol. Cancer Ther. 2013, 12, 2640–2650. [Google Scholar] [CrossRef]
- Zhong, Y.; Krisanapun, C.; Lee, S.H.; Nualsanit, T.; Sams, C.; Peungvicha, P.; Baek, S.J. Molecular targets of apigenin in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur. J. Cancer 2010, 46, 3365–3374. [Google Scholar] [CrossRef]
- Lee, Y.; Sung, B.; Kang, Y.J.; Kim, D.H.; Jang, J.Y.; Hwang, S.Y.; Kim, M.; Lim, H.S.; Yoon, J.H.; Chung, H.Y.; et al. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 2014, 44, 1599–1606. [Google Scholar] [CrossRef]
- Maeda, Y.; Takahashi, H.; Nakai, N.; Yanagita, T.; Ando, N.; Okubo, T.; Saito, K.; Shiga, K.; Hirokawa, T.; Hara, M.; et al. Apigenin induces apoptosis by suppressing Bcl-xl and Mcl-1 simultaneously via signal transducer and activator of transcription 3 signaling in colon cancer. Int. J. Oncol. 2018, 52, 1661–1673. [Google Scholar] [CrossRef]
- Wang, W.; Heideman, L.; Chung, C.S.; Pelling, J.C.; Koehler, K.J.; Birt, D.F. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol. Carcinog. 2000, 28, 102–110. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Yu, X.; Wang, X.; Zhu, X.; Xu, X. Apigenin inhibits in vitro and in vivo tumorigenesis in cisplatin-resistant colon cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. J. BUON 2019, 24, 488–493. [Google Scholar] [PubMed]
- Turktekin, M.; Konac, E.; Onen, H.I.; Alp, E.; Yilmaz, A.; Menevse, S. Evaluation of the effects of the flavonoid apigenin on apoptotic pathway gene expression on the colon cancer cell line (HT29). J. Med. Food 2011, 14, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, X.H.; Wang, Z.J. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol. In Vitro 2009, 23, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-G.; Wang, L.; Liu, W.-J.; Wang, J.-F.; Zhao, E.-J.; Zhou, F.-M.; Ji, X.-B.; Wang, L.-H.; Xia, Z.-K.; Wang, W.; et al. Apigenin inhibits IL-6 transcription and suppresses esophageal carcinogenesis. Front. Pharmacol. 2019, 10, 1002. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Li, Z.; Liu, C.; Yin, L. The apoptotic effect of apigenin on human gastric carcinoma cells through mitochondrial signal pathway. Tumour Biol. 2014, 35, 7719–7726. [Google Scholar] [CrossRef]
- Stump, T.A.; Santee, B.N.; Williams, L.P.; Kunze, R.A.; Heinze, C.E.; Huseman, E.D.; Gryka, R.J.; Simpson, D.S.; Amos, S. The antiproliferative and apoptotic effects of apigenin on glioblastoma cells. J. Pharm. Pharmacol. 2017, 69, 907–916. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 2010, 116, 164–176. [Google Scholar] [CrossRef]
- Chan, L.P.; Chou, T.H.; Ding, H.Y.; Chen, P.R.; Chiang, F.Y.; Kuo, P.L.; Liang, C.H. Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim. Biophys. Acta 2012, 1820, 1081–1091. [Google Scholar] [CrossRef]
- Das, S.; Das, J.; Samadder, A.; Boujedaini, N.; Khuda-Bukhsh, A.R. Apigenin-induced apoptosis in A375 and A549 cells through selective action and dysfunction of mitochondria. Exp. Biol. Med. 2012, 237, 1433–1448. [Google Scholar] [CrossRef]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Strategic formulation of apigenin-loaded PLGA nanoparticles for intracellular trafficking, DNA targeting and improved therapeutic effects in skin melanoma in vitro. Toxicol. Lett. 2013, 223, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Arango, D.; Parihar, A.; Villamena, F.A.; Wang, L.; Freitas, M.A.; Grotewold, E.; Doseff, A.I. Apigenin induces DNA damage through the PKCδ-dependent activation of ATM and H2AX causing down-regulation of genes involved in cell cycle control and DNA repair. Biochem. Pharmacol. 2012, 84, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Vargo, M.A.; Voss, O.H.; Poustka, F.; Cardounel, A.J.; Grotewold, E.; Doseff, A.I. Apigenin-induced-apoptosis is mediated by the activation of PKCdelta and caspases in leukemia cells. Biochem. Pharmacol. 2006, 72, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Budhraja, A.; Gao, N.; Zhang, Z.; Son, Y.O.; Cheng, S.; Wang, X.; Ding, S.; Hitron, A.; Chen, G.; Luo, J.; et al. Apigenin induces apoptosis in human leukemia cells and exhibits anti-leukemic activity in vivo. Mol. Cancer Ther. 2012, 11, 132–142. [Google Scholar] [CrossRef]
- Jayasooriya, R.G.; Kang, S.H.; Kang, C.H.; Choi, Y.H.; Moon, D.O.; Hyun, J.W.; Chang, W.Y.; Kim, G.Y. Apigenin decreases cell viability and telomerase activity in human leukemia cell lines. Food Chem. Toxicol. 2012, 50, 2605–2611. [Google Scholar] [CrossRef]
- Ruela-de-Sousa, R.R.; Fuhler, G.M.; Blom, N.; Ferreira, C.V.; Aoyama, H.; Peppelenbosch, M.P. Cytotoxicity of apigenin on leukemia cell lines: Implications for prevention and therapy. Cell Death Dis. 2010, 1, e19. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.R.; Milacic, V.; Dou, Q.P. Relationship between the methylation status of dietary flavonoids and their growth-inhibitory and apoptosis-inducing activities in human cancer cells. J. Cell. Biochem. 2008, 105, 514–523. [Google Scholar] [CrossRef]
- Kim, B.R.; Jeon, Y.K.; Nam, M.J. A mechanism of apigenin-induced apoptosis is potentially related to anti-angiogenesis and anti-migration in human hepatocellular carcinoma cells. Food Chem. Toxicol. 2011, 49, 1626–1632. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, A.K. Apigenin Sensitizes Huh-7 Human Hepatocellular Carcinoma Cells to TRAIL-induced Apoptosis. Biomol. Ther. 2012, 20, 62–67. [Google Scholar] [CrossRef]
- Şirin, N.; Elmas, L.; Seçme, M.; Dodurga, Y. Investigation of possible effects of apigenin, sorafenib and combined applications on apoptosis and cell cycle in hepatocellular cancer cells. Gene 2020, 737, 144428. [Google Scholar] [CrossRef]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Yu, J.S.; Yang, M.; Kim, A.K. Sub-toxic dose of apigenin sensitizes HepG2 cells to TRAIL through ERK-dependent up-regulation of TRAIL receptor DR5. Mol. Cells 2013, 35, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Valdameri, G.; Trombetta-Lima, M.; Worfel, P.R.; Pires, A.R.; Martinez, G.R.; Noleto, G.R.; Cadena, S.M.; Sogayar, M.C.; Winnischofer, S.M.; Rocha, M.E. Involvement of catalase in the apoptotic mechanism induced by apigenin in HepG2 human hepatoma cells. Chem. Biol. Interact. 2011, 193, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.H.; Sultana, S. Apigenin induces apoptosis in Hep G2 cells: Possible role of TNF-α and IFN-γ. Toxicology 2006, 217, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Molagoda, I.M.N.; Choi, Y.H.; Park, C.; Moon, D.O.; Kim, G.Y. Apigenin promotes TRAIL-mediated apoptosis regardless of ROS generation. Food Chem. Toxicol. 2018, 111, 623–630. [Google Scholar] [CrossRef]
- Hu, X.Y.; Liang, J.Y.; Guo, X.J.; Liu, L.; Guo, Y.B. 5-Fluorouracil combined with apigenin enhances anticancer activity through mitochondrial membrane potential (ΔΨm)-mediated apoptosis in hepatocellular carcinoma. Clin. Exp. Pharmacol. Physiol. 2015, 42, 146–153. [Google Scholar] [CrossRef]
- Gao, A.M.; Ke, Z.P.; Wang, J.N.; Yang, J.Y.; Chen, S.Y.; Chen, H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis 2013, 34, 1806–1814. [Google Scholar] [CrossRef]
- Kim, K.C.; Choi, E.H.; Lee, C. Axl receptor tyrosine kinase is a novel target of apigenin for the inhibition of cell proliferation. Int. J. Mol. Med. 2014, 34, 592–598. [Google Scholar] [CrossRef]
- Lu, H.F.; Chie, Y.J.; Yang, M.S.; Lee, C.S.; Fu, J.J.; Yang, J.S.; Tan, T.W.; Wu, S.H.; Ma, Y.S.; Ip, S.W.; et al. Apigenin induces caspase-dependent apoptosis in human lung cancer A549 cells through Bax- and Bcl-2-triggered mitochondrial pathway. Int. J. Oncol. 2010, 36, 1477–1484. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Zha, D.; Cai, F.; Zhang, W.; He, Y.; Huang, Q.; Zhuang, H.; Hua, Z.C. Apigenin potentiates TRAIL therapy of non-small cell lung cancer via upregulating DR4/DR5 expression in a p53-dependent manner. Sci. Rep. 2016, 6, 35468. [Google Scholar] [CrossRef]
- Pan, X.; Yang, Z.; Yang, Z.; Zhou, S.; Zhang, H.; Zang, L. Effect of apigenin on proliferation and apoptosis of human lung cancer NCI-H460 cells. Nan Fang Yi Ke Da Xue Xue Bao 2013, 33, 1137–1140. [Google Scholar] [PubMed]
- Lu, H.F.; Chie, Y.J.; Yang, M.S.; Lu, K.W.; Fu, J.J.; Yang, J.S.; Chen, H.Y.; Hsia, T.C.; Ma, C.Y.; Ip, S.W.; et al. Apigenin induces apoptosis in human lung cancer H460 cells through caspase- and mitochondria-dependent pathways. Hum. Exp. Toxicol. 2011, 30, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yu, M.; Shi, N.; Zhou, Y.; Li, F.; Li, X.; Huang, X.; Jin, J. Apigenin and Abivertinib, a novel BTK inhibitor synergize to inhibit diffuse large B-cell lymphoma in vivo and vitro. J. Cancer 2020, 11, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, L.; Benvenuto, M.; Mattera, R.; Di Stefano, E.; Zago, E.; Taffera, G.; Tresoldi, I.; Giganti, M.G.; Frajese, G.V.; Berardi, G.; et al. In vitro and in vivo anti-tumoral effects of the flavonoid apigenin in malignant mesothelioma. Front. Pharmacol. 2017, 8, 373. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Zhu, H.Y.; Zhang, X.H.; Du, Z.Y.; Xu, Y.J.; Yu, X.D. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the trinity of CK2, Cdc37 and Hsp90. Mol. Cancer 2011, 10, 104. [Google Scholar] [CrossRef]
- Hossain, M.M.; Banik, N.L.; Ray, S.K. N-Myc knockdown and apigenin treatment controlled growth of malignant neuroblastoma cells having N-Myc amplification. Gene 2013, 529, 27–36. [Google Scholar] [CrossRef][Green Version]
- Chakrabarti, M.; Banik, N.L.; Ray, S.K. miR-138 overexpression is more powerful than hTERT knockdown to potentiate apigenin for apoptosis in neuroblastoma in vitro and in vivo. Exp. Cell Res. 2013, 319, 1575–1585. [Google Scholar] [CrossRef]
- Mohan, N.; Ai, W.; Chakrabarti, M.; Banik, N.L.; Ray, S.K. KLF4 overexpression and apigenin treatment down regulated anti-apoptotic Bcl-2 proteins and matrix metalloproteinases to control growth of human malignant neuroblastoma SK-N-DZ and IMR-32 cells. Mol. Oncol. 2013, 7, 464–474. [Google Scholar] [CrossRef]
- Karmakar, S.; Davis, K.A.; Choudhury, S.R.; Deeconda, A.; Banik, N.L.; Ray, S.K. Bcl-2 inhibitor and apigenin worked synergistically in human malignant neuroblastoma cell lines and increased apoptosis with activation of extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 2009, 388, 705–710. [Google Scholar] [CrossRef]
- Torkin, R.; Lavoie, J.F.; Kaplan, D.R.; Yeger, H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol. Cancer Ther. 2005, 4, 1–11. [Google Scholar] [CrossRef]
- Lin, C.C.; Chuang, Y.J.; Yu, C.C.; Yang, J.S.; Lu, C.C.; Chiang, J.H.; Lin, J.P.; Tang, N.Y.; Huang, A.C.; Chung, J.G. Apigenin induces apoptosis through mitochondrial dysfunction in U-2 OS human osteosarcoma cells and inhibits osteosarcoma xenograft tumor growth in vivo. J. Agric. Food Chem. 2012, 60, 11395–11402. [Google Scholar] [CrossRef] [PubMed]
- Ittiudomrak, T.; Puthong, S.; Roytrakul, S.; Chanchao, C. α-Mangostin and apigenin induced cell cycle arrest and programmed cell death in SKOV-3 ovarian cancer cells. Toxicol. Res 2019, 35, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.K.; Jaiswar, S.P.; Dwivedi, A.; Goyal, S.; Dwivedi, V.N.; Pathak, A.K.; Kumar, V.; Sankhwar, P.L.; Ray, R.S. Synergistic effect of graphene oxide coated nanotised apigenin with paclitaxel (GO-NA/PTX): A ROS dependent mitochondrial mediated apoptosis in ovarian cancer. Anticancer Agents Med. Chem. 2017, 17, 1721–1732. [Google Scholar] [CrossRef]
- King, J.C.; Lu, Q.Y.; Li, G.; Moro, A.; Takahashi, H.; Chen, M.; Go, V.L.; Reber, H.A.; Eibl, G.; Hines, O.J. Evidence for activation of mutated p53 by apigenin in human pancreatic cancer. Biochim. Biophys. Acta 2012, 1823, 593–604. [Google Scholar] [CrossRef]
- Johnson, J.L.; de Mejia, E.G. Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK-3β/NF-κB signaling cascade. Mol. Nutr. Food Res. 2013, 57, 2112–2127. [Google Scholar] [CrossRef]
- Johnson, J.L.; Gonzalez de Mejia, E. Interactions between dietary flavonoids apigenin or luteolin and chemotherapeutic drugs to potentiate anti-proliferative effect on human pancreatic cancer cells, in vitro. Food Chem. Toxicol. 2013, 60, 83–91. [Google Scholar] [CrossRef]
- Hamacher, R.; Saur, D.; Fritsch, R.; Reichert, M.; Schmid, R.M.; Schneider, G. Casein kinase II inhibition induces apoptosis in pancreatic cancer cells. Oncol. Rep. 2007, 18, 695–701. [Google Scholar] [CrossRef]
- Granato, M.; Gilardini Montani, M.S.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J. Exp. Clin. Cancer Res. 2017, 36, 167. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic. Biol. Med. 2008, 44, 1833–1845. [Google Scholar] [CrossRef]
- Shukla, S.; Kanwal, R.; Shankar, E.; Datt, M.; Chance, M.R.; Fu, P.; MacLennan, G.T.; Gupta, S. Apigenin blocks IKKα activation and suppresses prostate cancer progression. Oncotarget 2015, 6, 31216–31232. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Kaur, P.; Shukla, S.; Abbas, A.; Fu, P.; Gupta, S. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: In vitro and in vivo study. Mol. Carcinog. 2012, 51, 952–962. [Google Scholar] [CrossRef]
- Shukla, S.; Fu, P.; Gupta, S. Apigenin induces apoptosis by targeting inhibitor of apoptosis proteins and Ku70-Bax interaction in prostate cancer. Apoptosis 2014, 19, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin suppresses insulin-like growth factor I receptor signaling in human prostate cancer: An in vitro and in vivo study. Mol. Carcinog. 2009, 48, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.; O’Neill, A.; Spengler, B.; Christoffel, V.; Fitzpatrick, J.M.; Watson, R.W. Apigenin drives the production of reactive oxygen species and initiates a mitochondrial mediated cell death pathway in prostate epithelial cells. Prostate 2005, 63, 131–142. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, V.; Verma, V.; Pandey, D.; Yadav, S.K.; Maikhuri, J.P.; Gupta, G. Apigenin manipulates the ubiquitin-proteasome system to rescue estrogen receptor-β from degradation and induce apoptosis in prostate cancer cells. Eur. J. Nutr. 2015, 54, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Kim, B.S.; Chun, S.Y.; Park, Y.K.; Kang, K.S.; Kwon, T.G. Apoptotic effects of genistein, biochanin-A and apigenin on LNCaP and PC-3 cells by p21 through transcriptional inhibition of polo-like kinase-1. J. Korean Med. Sci. 2011, 26, 1489–1494. [Google Scholar] [CrossRef]
- Oishi, M.; Iizumi, Y.; Taniguchi, T.; Goi, W.; Miki, T.; Sakai, T. Apigenin sensitizes prostate cancer cells to Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS ONE 2013, 8, e55922. [Google Scholar] [CrossRef]
- Meng, S.; Zhu, Y.; Li, J.F.; Wang, X.; Liang, Z.; Li, S.Q.; Xu, X.; Chen, H.; Liu, B.; Zheng, X.Y.; et al. Apigenin inhibits renal cell carcinoma cell proliferation. Oncotarget 2017, 8, 19834–19842. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. Apigenin induces c-Myc-mediated apoptosis in FRO anaplastic thyroid carcinoma cells. Mol. Cell. Endocrinol. 2013, 369, 130–139. [Google Scholar] [CrossRef]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta 2020, 1867, 118688. [Google Scholar] [CrossRef] [PubMed]
- Shendge, A.K.; Chaudhuri, D.; Basu, T.; Mandal, N. A natural flavonoid, apigenin isolated from Clerodendrum viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis in MCF-7 cells through the regulation of p53 and caspase-cascade pathway. Clin. Trans. Oncol. 2021, 23, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Shukla, S.; Gupta, S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: An in vitro and in vivo study. Carcinogenesis 2008, 29, 2210–2217. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Efficacy of PLGA-loaded apigenin nanoparticles in Benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: Mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 2013, 62, 670–680. [Google Scholar] [CrossRef]
- Hibino, E.; Hiroaki, H. Potential of rescue and reactivation of tumor suppressor p53 for cancer therapy. Biophys. Rev. 2022, 14, 267–275. [Google Scholar] [CrossRef]
- Ou, A.; Zhao, X.; Lu, Z. The potential roles of p53 signaling reactivation in pancreatic cancer therapy. Biochim. Biophys. Acta 2022, 1877, 188662. [Google Scholar] [CrossRef]
- Feng, J. The p53 pathway related genes predict the prognosis of colon cancer. Int. J. Gen. Med. 2022, 15, 169–177. [Google Scholar] [CrossRef]
- Hazari, Y.; Bravo-San Pedro, J.M.; Hetz, C.; Galluzzi, L.; Kroemer, G. Autophagy in hepatic adaptation to stress. J. Hepatol. 2020, 72, 183–196. [Google Scholar] [CrossRef]
- Yang, Y.; Klionsky, D.J. Autophagy and disease: Unanswered questions. Cell Death Differ. 2020, 27, 858–871. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kroemer, G. Transient autophagy inhibition precipitates oncogenesis: A red flag for pharmacological autophagy inhibitors? Trends Cell Biol. 2020, 30, 339–340. [Google Scholar] [CrossRef]
- Nazim, U.M.; Yin, H.; Park, S.Y. Downregulation of c-FLIP and upregulation of DR-5 by cantharidin sensitizes TRAIL-mediated apoptosis in prostate cancer cells via autophagy flux. Int. J. Mol. Med. 2020, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Ahmadi, Z.; Roomiani, S.; Katebi, M. Autophagy, anoikis, ferroptosis, necroptosis, and endoplasmic reticulum stress: Potential applications in melanoma therapy. J. Cell. Physiol. 2019, 234, 19471–19479. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Cicero, A.F.G.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell. Physiol. 2019, 234, 5643–5654. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Codogno, P. The mechanism and physiological function of macroautophagy. J. Innate Immun. 2013, 5, 427–433. [Google Scholar] [CrossRef]
- Matsui, C.; Yuliandari, P.; Deng, L.; Abe, T.; Shoji, I. The role of chaperone-mediated autophagy in hepatitis C virus-induced pathogenesis. Front. Cell. Infect. Microbiol. 2021, 11, 796664. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, A.; Sequeida, A.; Rodríguez, C.; Budini, M. Chapter 27—Chaperone-mediated autophagy—mechanisms and disease role. In Autophagy in Health and Disease, 2nd ed.; Rothermel, B.A., Diwan, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 399–412. [Google Scholar]
- Salmani, J.M.M.; Zhang, X.-P.; Jacob, J.A.; Chen, B.-A. Apigenin’s anticancer properties and molecular mechanisms of action: Recent advances and future prospectives. Chin. J. Nat. Med. 2017, 15, 321–329. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, H.G. Apigenin induces autophagy and cell death by targeting EZH2 under hypoxia conditions in gastric cancer cells. Int. J. Mol. Sci. 2021, 22, 13455. [Google Scholar] [CrossRef]
- Kayacan, S.; Yilancioglu, K.; Akdemir, A.S.; Kaya-Dagistanli, F.; Melikoglu, G.; Ozturk, M. Synergistic effect of apigenin and curcumin on apoptosis, paraptosis and autophagy-related cell death in HeLa cells. Anticancer Res. 2021, 41, 1271–1282. [Google Scholar] [CrossRef]
- Lin, C.M.; Chen, H.H.; Lin, C.A.; Wu, H.C.; Sheu, J.J.; Chen, H.J. Apigenin-induced lysosomal degradation of β-catenin in Wnt/β-catenin signaling. Sci. Rep. 2017, 7, 372. [Google Scholar] [CrossRef]
- Lu, J.; Meng, Z.; Chen, Y.; Yu, L.; Gao, B.; Zheng, Y.; Guan, S. Apigenin induced autophagy and stimulated autophagic lipid degradation. Food Funct. 2020, 11, 9208–9215. [Google Scholar] [CrossRef]
- Janda, E.; Martino, C.; Riillo, C.; Parafati, M.; Lascala, A.; Mollace, V.; Boutin, J.A. Apigenin and luteolin regulate autophagy by targeting NRH-Quinone oxidoreductase 2 in liver cells. Antioxidants 2021, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Lascala, A.; Martino, C.; Parafati, M.; Salerno, R.; Oliverio, M.; Pellegrino, D.; Mollace, V.; Janda, E. Analysis of proautophagic activities of Citrus flavonoids in liver cells reveals the superiority of a natural polyphenol mixture over pure flavones. J. Nutr. Biochem. 2018, 58, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xie, H.; Zhang, Z.L.; Li, Z.X.; Shi, L.; Wu, K.Y.; Zhou, Y.; Tian, Z.; Zhang, Y.; Zhou, W.; et al. Apigenin regulates the migration, invasion, and autophagy of hepatocellular carcinoma cells by downregulating YAP. Neoplasma 2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, D.; Liao, X.; Zhang, Y.; Xiao, J.; Chen, W.; Liu, Q.; Chen, Y.; Li, D.; Zhu, L.; et al. Apigenin combined with gefitinib blocks autophagy flux and induces apoptotic cell death through inhibition of HIF-1α, c-Myc, p-EGFR, and glucose metabolism in EGFR L858R+T790M-mutated H1975 Cells. Front. Pharmacol. 2019, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Adham, A.N.; Abdelfatah, S.; Naqishbandi, A.M.; Mahmoud, N.; Efferth, T. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine 2021, 80, 153371. [Google Scholar] [CrossRef]
- Mohan, N.; Banik, N.L.; Ray, S.K. Combination of N-(4-hydroxyphenyl) retinamide and apigenin suppressed starvation-induced autophagy and promoted apoptosis in malignant neuroblastoma cells. Neurosci. Lett. 2011, 502, 24–29. [Google Scholar] [CrossRef][Green Version]
- Gilardini Montani, M.S.; Cecere, N.; Granato, M.; Romeo, M.A.; Falcinelli, L.; Ciciarelli, U.; D’Orazi, G.; Faggioni, A.; Cirone, M. Mutant p53, stabilized by its interplay with HSP90, activates a positive feed-back loop between NRF2 and p62 that induces chemo-resistance to apigenin in pancreatic cancer cells. Cancers 2019, 11, 703. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Q.; Li, H.; Yang, B.; Wang, M. Vitexin suppresses renal cell carcinoma by regulating mTOR pathways. Transl. Androl. Urol. 2020, 9, 1700–1711. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Xu, S.; Chen, H.; Chen, X.; Gu, H. Apigenin restores impairment of autophagy and downregulation of unfolded protein response regulatory proteins in keratinocytes exposed to ultraviolet B radiation. J. Photochem. Photobiol. B 2019, 194, 84–95. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, X.; Gao, Y.; Zheng, J.; Xu, Q.; Sun, Y.; Guan, H.; Yu, H.; Sun, Z. Apigenin induces autophagic cell death in human papillary thyroid carcinoma BCPAP cells. Food Funct. 2015, 6, 3464–3472. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ofengeim, D.; Yuan, J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat. Rev. Mol. Cell Biol. 2013, 14, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.A.; Qin, Z.; Stewart, A.F.R.; Chen, H.H. Dabrafenib, an inhibitor of RIP3 kinase-dependent necroptosis, reduces ischemic brain injury. Neural Regen. Res. 2018, 13, 252–256. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Chan, F.K.; Kroemer, G. Necroptosis: Mechanisms and relevance to disease. Annu. Rev. Pathol. 2017, 12, 103–130. [Google Scholar] [CrossRef] [PubMed]
- Hadifar, S.; Behrouzi, A.; Fateh, A.; Khatami, S.; Rahimi Jamnani, F.; Siadat, S.D.; Vaziri, F. Comparative study of interruption of signaling pathways in lung epithelial cell by two different Mycobacterium tuberculosis lineages. J. Cell. Physiol. 2019, 234, 4739–4753. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef]
- Strilic, B.; Yang, L.; Albarrán-Juárez, J.; Wachsmuth, L.; Han, K.; Müller, U.C.; Pasparakis, M.; Offermanns, S. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 2016, 536, 215–218. [Google Scholar] [CrossRef]
- Seifert, L.; Werba, G.; Tiwari, S.; Giao Ly, N.N.; Alothman, S.; Alqunaibit, D.; Avanzi, A.; Barilla, R.; Daley, D.; Greco, S.H.; et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 2016, 532, 245–249. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef]
- Grootjans, S.; Vanden Berghe, T.; Vandenabeele, P. Initiation and execution mechanisms of necroptosis: An overview. Cell Death Differ. 2017, 24, 1184–1195. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, K.S.; Nam, H.S.; Cho, M.K.; Lee, S.H. Apigenin causes necroptosis by inducing ROS accumulation, mitochondrial dysfunction, and ATP depletion in malignant mesothelioma cells. Korean J. Physiol. Pharmacol. 2020, 24, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Warkad, M.S.; Kim, C.H.; Kang, B.G.; Park, S.H.; Jung, J.S.; Feng, J.H.; Inci, G.; Kim, S.C.; Suh, H.W.; Lim, S.S.; et al. Metformin-induced ROS upregulation as amplified by apigenin causes profound anticancer activity while sparing normal cells. Sci. Rep. 2021, 11, 14002. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- DeHart, D.N.; Fang, D.; Heslop, K.; Li, L.; Lemasters, J.J.; Maldonado, E.N. Opening of voltage dependent anion channels promotes reactive oxygen species generation, mitochondrial dysfunction and cell death in cancer cells. Biochem. Pharmacol. 2018, 148, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of mitochondria in ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef]
- Lu, B.; Chen, X.B.; Ying, M.D.; He, Q.J.; Cao, J.; Yang, B. The role of ferroptosis in cancer development and treatment response. Front. Pharmacol. 2017, 8, 992. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Trujillo-Alonso, V.; Pratt, E.C.; Zong, H.; Lara-Martinez, A.; Kaittanis, C.; Rabie, M.O.; Longo, V.; Becker, M.W.; Roboz, G.J.; Grimm, J.; et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat. Nanotechnol. 2019, 14, 616–622. [Google Scholar] [CrossRef]
- Guo, J.; Xu, B.; Han, Q.; Zhou, H.; Xia, Y.; Gong, C.; Dai, X.; Li, Z.; Wu, G. Ferroptosis: A novel anti-tumor action for cisplatin. Cancer Res. Treat. 2018, 50, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Kasukabe, T.; Kumakura, S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 2018, 52, 1011–1022. [Google Scholar] [CrossRef]
- Ma, S.; Henson, E.S.; Chen, Y.; Gibson, S.B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016, 7, e2307. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, B.; Zhou, L.; Zhang, Z.; Shen, Y.; Lv, H.; AlQudsy, L.H.H.; Shang, P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020, 483, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Yuan, J.; Liu, Y.; Qin, Y.; Wang, X.; Gu, J.; Chen, G.; Zhang, B.; Liu, H.K.; Zhao, J.; et al. Epileptic brain fluorescent imaging reveals apigenin can relieve the myeloperoxidase-mediated oxidative stress and inhibit ferroptosis. Proc. Natl. Acad. Sci. USA 2020, 117, 10155–10164. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Rong, G.; Liu, Y.; Huang, W.; He, D.; Lu, R. Delivery of apigenin-loaded magnetic Fe(2)O(3)/Fe(3)O(4)@mSiO(2) nanocomposites to A549 cells and their antitumor mechanism. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111719. [Google Scholar] [CrossRef] [PubMed]
- Adham, N.A.; Hegazy, M.E.F.; Naqishbandi, A.M.; Efferth, T. Induction of apoptosis, autophagy and ferroptosis by Thymus vulgaris and Arctium lappa extract in leukemia and multiple myeloma cell lines. Molecules 2020, 25, 5016. [Google Scholar] [CrossRef]
| Cancer/Cell Lines | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|
| Bladder | |||
| T24 | PARP cleavage, caspase-3, -7 and -9 cleavage, Bax, Bak, Bad, p–p53, p53, p21, p27, Cyt c (cytosol) | p-Akt, PDK, PI3K, Bcl-2, Bcl-xL, cyclin A, B1, and E, CDK2, Cdc2, Cdc25c, Bcl-xL, Mcl-1, Cyt c (mitochondrial) | [68,69] |
| RT112 | PARP cleavage | [70] | |
| Breast | |||
| SK-BR-3 | p53, p21, Bax, Cyt c, caspase-8 and -3, PARP, DFF45 cleavage, p27 | cyclin A, B, D, and E, CDK1, p-JAK, p-STAT3, VEGF, cyclin D1 and D3, CDK4 | [71,72,73] |
| MDA-MB-231 and MDA-MB-231 xenograft | p-p53 (Ser-15), p21, Bax, PARP cleavage, IκBα, caspase-3 and -7, FOXO3a, p27, Cyt c | Bcl-xL, cyclin B1, Bcl-2, PI3K, PKB/AKT | [74,75,76,77] |
| MCF-7 | p53, p-p53 (Ser-15), p21, caspase-8 and PARP cleavage, ROS, Cyt c, caspase-3, DFF45 cleavage, p27, FOXO3a | p-MDM2, p-JAK1, p-STAT3, NF-κB/p65, p-IκBα, cyclin D1 and D3, CDK4, PI3K, PKB/AKT | [72,77,78,79,80] |
| BT-474 | caspase-8 and -3, PARP, Cyt c, DFF45 cleavage, p27 | p-JAK1, p-JAK2, p-STAT3, VEGF, HIF-1α, cyclin D1 and D3, CDK4 | [72,81] |
| Hs578T | FOXO3a, p21, p27, PARP, Cyt c release | PI3K, PKB/AKT | [77] |
| MDA-MB-453 | caspase-3, -6, -7, -8, and PARP cleavage, Cyt c release, DFF45 cleavage, p27 | procaspase-9, p-JAK2, p-STAT3 | [72,82,83] |
| T47D | caspase-3 and PARP cleavage, Bax | Bcl-2, Bcl-xL | [75] |
| HBL-100 | Cyt c, caspase-3, DFF45 cleavage, p27 | cyclin D1 and D3, CDK4 | [72] |
| Cervical | |||
| HeLa | p53, p21, caspase-2 and -3, Fas, mitochondrial redox impairment, PARP, ROS, AIF, Endo G, Cyt c | Bcl-2, MMP, superoxide dismutase | [84,85,86] |
| SiHa, CaSki, and C-33A | mitochondrial redox impairment, ROS | MMP | [85] |
| Colon | |||
| HCT116 | p21, p53, NAG-1, Bim-EL, Bim-L, PARP cleavage | cyclin B1, Cdc2, Cdc25c, procaspase-3, -8, and -9, Mcl-1, Bcl-xL, STAT3, p-AKT, p-ERK | [87,88,89,90] |
| LoVo | p21, NAG-1 | [88] | |
| DLD-1 | PARP cleavage | Mcl-1, p-AKT, p-ERK, Bcl-xL, Mcl-1, STAT3 | [87,90] |
| SW480 | Cdc2, cyclin B1 | [91] | |
| HT-29 | Bax, PARP cleavage, caspase-3 and -8 | Cdc2, Bcl-2, m-TOR/PI3K/AKT, Bcl-xL, Mcl-1, STAT3, caspase-3 and -8, cyclin D1 | [90,91,92,93] |
| Caco-2 | Cdc2 | [91] | |
| COLO320 | PARP cleavage | Bcl-xL, Mcl-1, STAT3 | [90] |
| Esophageal | |||
| KYSE-510 | p21, PIG3, p63, p73, caspase-3 and -9, Bax | cyclin B1, Bcl-2 | [94] |
| Eca-109 and KYSE-30 | PARP cleavage, caspase-8 | IL-6, VEGF | [95] |
| Gastric | |||
| HGC-27 and SGC-7901 | Bax, Bcl-2, caspase-3 | MMP | [96] |
| Glioblastoma | |||
| U-1242MG | PARP cleavage | MAPK, AKT, mTOR, Bcl-xL | [97] |
| T98G and U-87MG | p-p38 MAPK, c-Jun1, caspase-3, -8, and -9, Bax, tBid, Smac (cytosol), SBDP, CAD (nuclear) | ROS, MMP, Bcl-2, Cyt c (mitochondrial), Smac (mitochondrial), calpastatin, ICAD | [98] |
| Head and Neck | |||
| SCC-25 | TRAIL, TRAIL-R1, and -R2, Fas, TNF-α, TNF-R1 and -R2, Bax, caspase-3 | Bcl-2 | [99] |
| Melanoma | |||
| A375 and C8161 | Cyt c release, Bax, Apaf-1, caspase-3, -9, and PARP cleavage | Bcl-2, Cyt c (mitochondrial), p-ERK1/2, p-AKT, p-mTOR | [50,100,101] |
| Leukemia | |||
| THP-1 | caspase-3 activity, p-p38, p-ERK, PKCδ activity, p-ATM | caspase-9 activity, p-H2AX | [102,103] |
| U937 | caspase-3, -7, -9, and PARP cleavage, p-JNK, Bcl-2 cleavage | hTERT, c-Myc, Mcl-1, p-AKT, AKT, p-Bad, p-mTOR, p-GSK3β, JNK, Mcl-1, Bcl-2 | [104,105] |
| HL60 | p-Cdc2, p-p38, caspase-3, -8, and PARP cleavage | PI3Kp85, p-AKT, p-GSK3β, p-JAK2, p-Src, p-STAT3 | [106,107] |
| TF-1 | LMWPTP | CDK6, p-Src, p-JAK2, p-SHP2, p-STAT3 and 5, p-p70S6K | [106] |
| Liver | |||
| Huh-7 | caspase-3, -8, and -9 cleavage, PARP, Bax/Bcl-2 ratio | [108,109] | |
| HepG2 | caspase-3, -7, -8, -9, and -10, Bid, p21, p16, PARP cleavage, Bax, DR5, ROS, TNF-α, IFN-γ | Bcl-2, PI3K/AKT/mTOR, p-LRP6, Skp2 | [48,110,111,112,113,114] |
| Hep3B | DR5, ROS, caspase activation | [115] | |
| SK-HEP-1 | ROS, caspase 3, PARP | MMP, Bcl-2 | [116] |
| BEL-7402 and BEL-7402 xenograft | ROS, caspase 3, PARP | MMP, Bcl-2, Nrf2 | [116,117] |
| Lung | |||
| A549 | p21, Cyt c release, Bax, p53, p-p53, Wee1, Chk2, Bid, GRP78, caspase-3, -9, and PARP cleavage, GADD153, AIF, MAPK, DR4, DR5 | XIAP, Bcl-2, MMP, cyclin B, Cdc25c, procaspase-8, Bcl-xL, NF-κB, ERK, AKT, Cyt c (mitochondrial) | [81,100,118,119,120] |
| H460 | p21, Bax, FasL, p53, AIF, Cyt c, caspase-3, GRP78, GADD153 | XIAP, Bcl-2, Bid, procaspase-8 | [118,121,122] |
| H1299 | MAPK, DR4, DR5, Bax, Bad | Bcl-xL, Bcl-2, NF-κB, ERK, AKT | [120] |
| Diffuse large B-cell lymphoma | |||
| U2932 and OCI-LY10 | caspase family, PARP cleavage | Bcl-xL, PI3K/mTOR, p-GS3K-β, MCL-X, p38, p-p65, p-AKT | [123] |
| Mesothelioma | |||
| MM-B1, H-Meso-1 and MM-F1 | Bax/Bcl-2 ratio, p53, caspase-8, -9, and PARP-1 cleavage | p-ERK1/2, p-JNK, p-p38 MAPK, p-AKT, c-Jun, p-c-Jun, NF-κB nuclear translocation | [124] |
| Multiple myeloma | |||
| U266 and RPMI 8226 | PARP cleavage | p-STAT3, p-ERK, p-AKT, NF-κB, Mcl-1, Bcl-2, Bcl-xL, XIAP, survivin | [125] |
| Neuroblastoma | |||
| SK-N-DZ, SK-N-BE2, SK-N-DZ and SK-N-BE2 xenograft | caspase-3, -8, and PARP cleavage, Bax, Bid, tBid, calpain, ICAD fragment, p21, Noxa, PUMA, p53, ICAD, SBDP | N-Myc, E-cadherin, Notch-1, hTERT, PCNA, Smac, survivin, SBDP, Bcl-2, Mcl-1 | [126,127,128,129] |
| NUB-7 | PARP cleavage, p53 (NE), p21, Bax, p-ERK | [130] | |
| IMR-32 | Bax, Noxa, PUMA, p53, caspase-3, ICAD | Bcl-2, Mcl-1 | [128] |
| Oral | |||
| SCC-25 | TRAIL, TRAIL-R1 and -R2, Fas, TNF-α, TNF-R1 and -R2, Bax, caspase-3 | cyclin D1 and E, CDK1 | [99] |
| Osteosarcoma | |||
| U-2 OS | Bax, PARP cleavage, p53, AIF | procaspase-3, -8, and -9, GADD153 (NE) | [131] |
| Ovarian | |||
| SKOV-3 | caspase-3 and -9, Bax, Bcl-2, COX-2, ROS | [132,133,134] | |
| A2780 and OVCAR-3 | ROS, MDA, caspase-3 and -9 | [133] | |
| Pancreatic | |||
| BxPC-3 | Ac-p53, p21, PUMA, Cyt c release, caspase-3 cleavage | Bcl-xL/p53 interaction, Bcl-xL/PUMA interaction, cyclin B1, Bcl-2, XIAP, p-GSK3β, NF-κB/p65 (NE) | [135,136,137] |
| MIA PaCa-2 | Ac-p53, p21, PUMA, Cyt c release, PARP cleavage | Bcl-xL/p53 interaction, Bcl-xL/PUMA interaction | [135,138] |
| PANC-1 | Cyt c release, caspase-3 cleavage | cyclin B1, XIAP, p-GSK3β, NF-κB/p65 (NE) | [136] |
| PEL | |||
| BC3, BCBL1, and B | p53 | STAT3, ROS | [139] |
| Prostate | |||
| 22Rv1 and 22Rv1 xenograft | p53, p-p53, p21, p14, Cyt c release, Bax, Apaf-1, caspase-3, -8, -9, and PARP cleavage | MDM2, MMP, Bcl-2, Bcl-xL, p-IKKα, NF-ĸB/p65, PCNA, HDAC1 and 3, Bcl-2 | [140,141,142] |
| PC-3 and PC-3 xenograft | caspase-3, -9, and PARP cleavage, Bax, Bad, Ku70, Cyt c release, p27, p21 | XIAP, cIAP-1, -2, Bcl-2, Bcl-xL, survivin, HDAC1, procaspase-3, -7, and -9, cyclin D1, p-IKKα, NF-ĸB/p65, PCNA, ER-β, PSMA5, PLK-1, HDAC1, and 3, Bcl-2 | [141,142,143,144,145,146,147] |
| LNCaP | p21, p27, Bax, PARP cleavage, Cyt c release | cyclin D1, D2, and E, CDK2, 4, and 6, Bcl-2, procaspase-3, -8, and -9, NF-κB/p65, PLK-1 | [51,145,147] |
| DU145 | caspase-3, -9, and PARP cleavage, DR5, Cyt c release | XIAP, cIAP-1 and -2, survivin, procaspase-3, -7, and -9 | [143,145,148] |
| Renal | |||
| ACHN, 786-O, and Caki-1 | p53, Bax, caspase-3 and -9 | [149] | |
| Thyroid | |||
| FRO | c-Myc, Bid, Fas, p-p53, caspase-3 and PARP cleavage | Bcl-2, p27, p21 | [150] |
| Cancer/Cell Lines | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|
| Mesothelioma | |||
| MSTO-211H and H2452 | ROS, γ-H2AX, p-ATM, p-ATR, p-CHK1, p-CHK2, Bax, caspase-3 and PARP cleavage, p-MLKL, p-RIP3, Bax/Bcl-2 ratio | MMP, ATP, Bcl-2 | [193] |
| Pancreatic | |||
| AsPC-1 | p-ATM, γ-H2AX, p-p53, Bim, Bid, Bax, PARP cleavage, caspasae-3, -8, and -9, Cyt c, AIF1, p62, LC3B, p-MLKL, p-RIP | Bcl-2 | [194] |
| Cancer/Cell Lines | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|
| Lung | |||
| A549 | ROS, COX-2, p53, MDA, Bax, caspase-3 and -8, Cyt c | GPX4, FTH1, SOD, Bcl-2 | [207] |
| Multiple Myeloma | |||
| HEK293 | caspase-3 and -9, p38, JNK, LC3-II, Beclin-1, ROS | AKT, MMP, STAT1, COX-2, iNOS | [176] |
| NCI-H929 | LC3-II, Beclin-1, ROS | MMP | [208] |
| Neuroblastoma | |||
| SH-SY5Y | GPX4 | MMP | [206] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.Y.; Sung, B.; Kim, N.D. Role of Induced Programmed Cell Death in the Chemopreventive Potential of Apigenin. Int. J. Mol. Sci. 2022, 23, 3757. https://doi.org/10.3390/ijms23073757
Jang JY, Sung B, Kim ND. Role of Induced Programmed Cell Death in the Chemopreventive Potential of Apigenin. International Journal of Molecular Sciences. 2022; 23(7):3757. https://doi.org/10.3390/ijms23073757
Chicago/Turabian StyleJang, Jung Yoon, Bokyung Sung, and Nam Deuk Kim. 2022. "Role of Induced Programmed Cell Death in the Chemopreventive Potential of Apigenin" International Journal of Molecular Sciences 23, no. 7: 3757. https://doi.org/10.3390/ijms23073757
APA StyleJang, J. Y., Sung, B., & Kim, N. D. (2022). Role of Induced Programmed Cell Death in the Chemopreventive Potential of Apigenin. International Journal of Molecular Sciences, 23(7), 3757. https://doi.org/10.3390/ijms23073757







