Store-Operated Ca2+ Entry Contributes to Piezo1-Induced Ca2+ Increase in Human Endometrial Stem Cells
Abstract
1. Introduction
2. Results
2.1. Expression of Mechanosensitive Piezo1 Channels in Human Endometrial Mesenchymal Stem Cells (eMSCs)
2.2. Piezo1 Channels as Ca2+ Entry Pathway in eMSCs
2.3. Expression of Orai1, STIM1 and STIM2 in eMSC Cells
2.4. Contribution of SOCE in Piezo1-Induced Ca2+ Influx
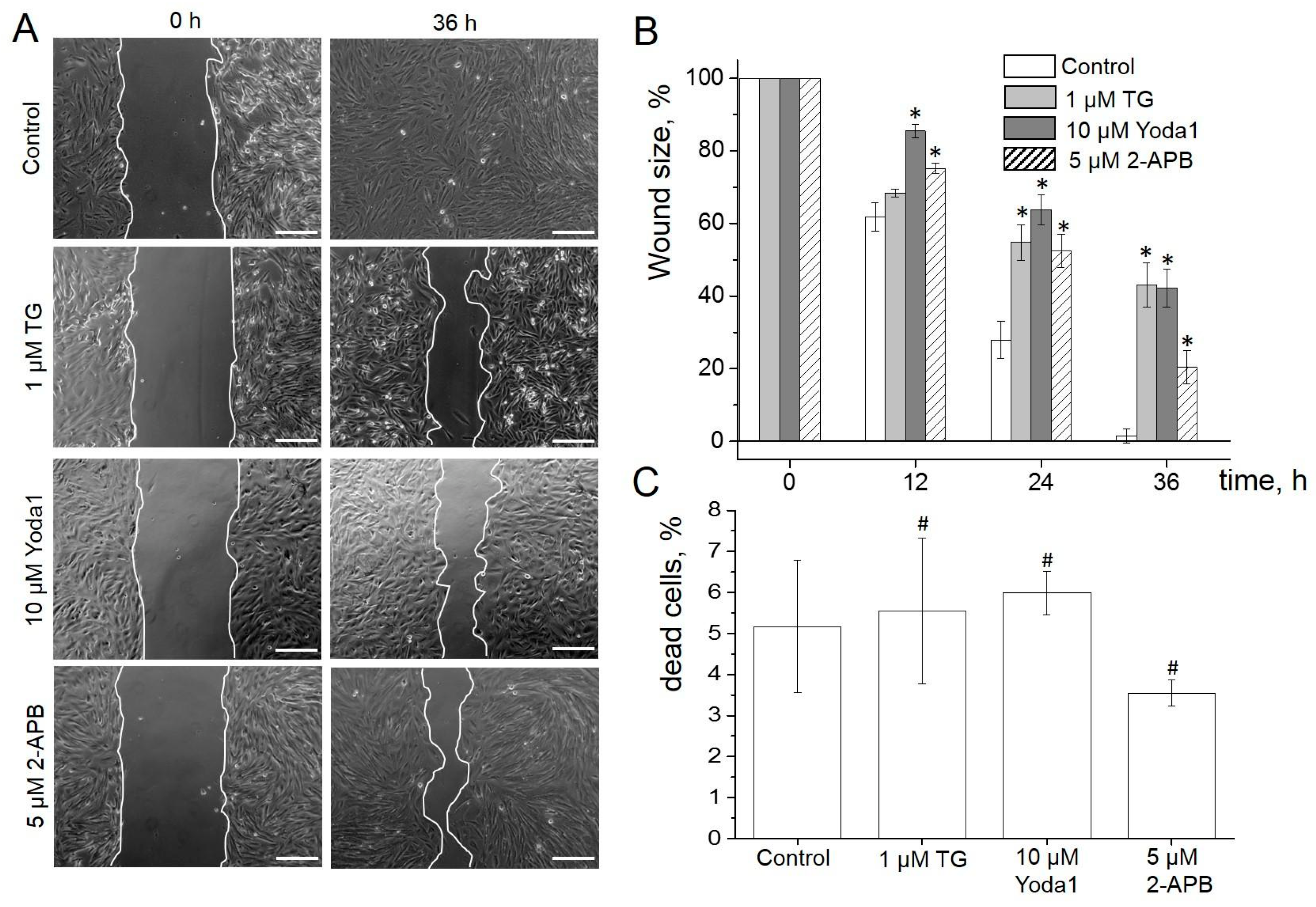
2.5. Role of SOCE and Piezo1 in eMSC Migration and Proliferation
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Reagents
4.2. Total RNA Extraction and Reverse Transcriptase (RT)-PCR
4.3. Immunofluorescence
4.4. Electrophysiology
4.5. Ca2+ Imaging
4.6. Quantification of Cell Migration (Wound Healing Assay)
4.7. FACS Analysis
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabapathy, V.; Ravi, S.; Srivastava, V.; Kumar, S. Long-term cultured human term placenta-derived mesenchymal stem cells of maternal origin displays plasticity. Stem Cells Int. 2012, 2012, 174–328. [Google Scholar] [CrossRef] [PubMed]
- Jazedje, T.; Perin, P.M.; Czeresnia, C.E.; Maluf, M.; Halpern, S.; Secco, M.; Bueno, D.F.; Vieira, N.M.; Zucconi, E.; Zatz, M. Human fallopian tube: A new source of multipotent adult mesenchymal stem cells discarded in surgical procedures. J. Transl. Med. 2009, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; Kim, H.; Hwang, K.J.; Kwon, H.C.; Kim, S.K. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007, 40, 75–90. [Google Scholar] [CrossRef] [PubMed]
- In ‘t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; Noort, W.A.; Claas, F.H.J.; Willemze, R.; Fibbe, W.E.; Kanhai, H.H. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003, 102, 1548–1549. [Google Scholar] [CrossRef] [PubMed]
- Warrior, S.; Haridas, N.; Bhonde, R. Inherent propensity of amnion-derived mesenchymal stem cells towards endothelial lineage: Vascularisation from an avascular tissue. Placenta 2012, 33, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Musina, R.A.; Belyavski, A.V.; Tarusova, O.V.; Solovyova, E.V.; Sukhikh, G.T. Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull. Exp. Biol. Med. 2008, 145, 539–543. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Zillwood, R.M.; Nguyen, H.P.T.; Wu, D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol. Reprod. 2009, 80, 1136–1145. [Google Scholar] [CrossRef]
- Schwab, K.E.; Hutchinson, P.; Gargett, C.E. Identification of surface markers for prospective isolation of human endometrial stromal colony forming cells. Hum. Reprod. 2008, 23, 934–943. [Google Scholar] [CrossRef]
- Ochoa-Bernal, M.A.; Fazleabas, A.T. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int. J. Mol. Sci. 2020, 21, 1973. [Google Scholar] [CrossRef]
- Figueira, P.G.; Abrão, M.S.; Krikun, G.; Taylor, H.S. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 2011, 1221, 10–17. [Google Scholar] [CrossRef]
- Zemelko, V.; Grinchuk, T.; Domnina, A.; Artsybasheva, I.; Zenin, V.; Kirsanov, A.; Bichevaia, N.; Korsak, V.; Nikolsky, N. Multipotent mesenchymal stem cells of desquamated endometrium: Isolation, characterization and use as feeder layer for maintenance of human embryonic stem cell lines. Cell Tiss. Biol. 2012, 6, 1–11. [Google Scholar] [CrossRef]
- Meng, X.; Ichim, T.E.; Zhong, J.; Rogers, A.; Yin, Z.; Jackson, J.; Wang, H.; Ge, W.; Bogin, V.; Chan, K.W.; et al. Endometrial regenerative cells: A novel stem cell population. J. Transl. Med. 2007, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D.T.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ahmad, M.; Perrimon, N. Mechanosensitive channels and their functions in stem cell differentiation. Exp. Cell Res. 2019, 374, 259–265. [Google Scholar] [CrossRef]
- Imura, T.; Otsuka, T.; Kawahara, Y.; Yuge, L. “Microgravity” as a unique and useful stem cell culture environment for cell-based therapy. Regen. Ther. 2019, 12, 2–5. [Google Scholar] [CrossRef]
- Danielyan, L.; Schwab, M.; Siegel, G.; Brawek, B.; Garaschuk, O.; Asavapanumas, N.; Buadze, M.; Lourhmati, A.; Wendel, H.P.; Avci-Adali, M.; et al. Cell motility and migration as determinants of stem cell efficacy. EBioMedicine 2020, 60, 102989. [Google Scholar] [CrossRef]
- Gillespie, P.G.; Walker, R.G. Molecular basis of mechanosensory transduction. Nature 2001, 413, 194–202. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Murthy, S.; Dubin, A.; Patapoutian, A. Piezos thrive under pressure: Mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–778. [Google Scholar] [CrossRef]
- Fang, X.Z.; Zhou, T.; Xu, J.Q.; Wang, Y.X.; Sun, M.M.; He, Y.J.; Pan, S.W.; Xiong, W.; Peng, Z.K.; Gao, X.H.; et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021, 11, 13. [Google Scholar] [CrossRef]
- Kusama, K.; Yoshie, M.; Tamura, K.; Imakawa, K.; Isaka, K.; Tachikawa, E. Regulatory action of calcium ion on cyclic AMP-enhanced expression of implantation—Related factors in human endometrial cells. PLoS ONE 2015, 10, e0132017. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; An, B.S.; Yang, H.; Jeung, E.B. Regulation and molecular mechanisms of calcium transport genes: Do they play a role in calcium transport in the uterine endometrium? J. Physiol. Pharmacol 2011, 62, 499–504. [Google Scholar] [PubMed]
- Hao, J.; Bao, X.; Jin, B.; Wang, X.; Mao, Z.; Li, X.; Wei, L.; Shen, D.; Wang, J.L. Ca2 channel subunit alpha 1D promotes proliferation and migration of endometrial cancer cells mediated by 17 beta-estradiol via the G protein-coupled estrogen receptor. FASEB J. 2015, 29, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.D.; Hayes, S.H.; Silvia, W.J. Cellular mechanisms by which oxytocin mediates uterine prostaglandin F2 alpha synthesis in bovine endometrium: Role of calcium. Domest. Anim. Endocrinol. 1998, 15, 477–487. [Google Scholar] [CrossRef]
- Sakoff, J.A.; Murdoch, R.N. Alterations in uterine calcium ions during induction of the decidual cell reaction in pseudopregnant mice. J. Reprod. Fertil. 1994, 101, 97–102. [Google Scholar] [CrossRef]
- Schmidt, S.; Schneider, S.; Yang, W.; Liu, G.; Schmidt, E.M.; Schmid, E.; Mia, S.; Brucker, S.; Stournaras, C.; Wallwiener, D.; et al. TGFbeta1 and SGK1-sensitive store-operated Ca2+ entry and ORAI1 expression in endometrial Ishikawa cells. Mol. Hum. Reprod. 2014, 20, 139–147. [Google Scholar] [CrossRef]
- Ruan, Y.C.; Chen, H.; Chan, H.C. Ion channels in the endometrium: Regulation of endometrial receptivity and embryo implantation. Hum. Reprod. Update 2014, 20, 517–529. [Google Scholar] [CrossRef]
- De Clercq, K.; Held, K.; van Bree, R.; Meuleman, C.; Peeraer, K.; Tomassetti, C.; Voets, T.; D’Hooghe, T.; Vriens, J. Functional expression of transient receptor potential channels in human endometrial stromal cells during the luteal phase of the menstrual cycle. Hum. Reprod. 2015, 30, 1421–1436. [Google Scholar] [CrossRef]
- Sugimoto, A.; Miyazaki, A.; Kawarabayashi, K.; Shono, M.; Akazawa, Y.; Hasegawa, T.; Ueda-Yamaguchi, K.; Kitamura, T.; Yoshizaki, K.; Fukumoto, S.; et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci. Rep. 2017, 18, 17696. [Google Scholar] [CrossRef]
- Mousawi, F.; Peng, H.; Li, J.; Ponnambalam, S.; Roger, S.; Zhao, H.; Yang, X.; Jiang, L.H. Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells 2020, 38, 410–421. [Google Scholar] [CrossRef]
- Chubinskiy-Nadezhdin, V.I.; Vasileva, V.Y.; Pugovkina, N.A.; Vassilieva, I.O.; Morachevskaya, E.A.; Nikolsky, N.N.; Negulyaev, Y.A. Local calcium signalling is mediated by mechanosensitive ion channels in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Park, H.J.; Kim, J.G.; Lee, I.H.; Cho, H.; Park, C.; Sung, T.S.; Koh, S.D.; Park, S.W.; Bae, Y.M. The Piezo2 ion channel is mechanically activated by low-threshold positive pressure. Sci. Rep. 2019, 9, 6446. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical activation of the mechanotransduction channel Piezo1. Elife 2015, 22, e07369. [Google Scholar] [CrossRef] [PubMed]
- Mendez, F.; Penner, R. Near-visible ultraviolet light induces a novel ubiquitous calcium-permeable cation current in mammalian cell lines. J. Physiol. 1998, 507, 365–377. [Google Scholar] [CrossRef]
- Patterson, R.; Rossum, D.; Gill, D. Store-Operated Ca2+ Entry: Evidence for a Secretion-like Coupling Model. Cell 1999, 98, 487–499. [Google Scholar] [CrossRef][Green Version]
- Cai, X. Molecular evolution and structural analysis of the Ca2 release activated Ca2 channel subunit, Orai. J. Mol. Biol. 2007, 368, 1284–1291. [Google Scholar] [CrossRef]
- Huang, B.; Qian, J.; Ma, J.; Huang, Z.; Shen, Y.; Chen, X.; Sun, A.; Ge, J.; Chen, H. Myocardial transfection of hypoxia-inducible factor-1α and co-transplantation of mesenchymal stem cells enhance cardiac repair in rats with experimental myocardial infarction. Stem Cell Res. Ther. 2014, 5, 22. [Google Scholar] [CrossRef]
- Folestad, E.; Kunath, A.; Wagsäter, D. PDGF-C and PDGF-D signaling in vascular diseases and animal models. Mol. Asp. Med. 2018, 62, 1–11. [Google Scholar] [CrossRef]
- Zhang, S.J.; Song, X.Y.; He, M.; Yu, S.B. Effect of TGF-β1/SDF-1/CXCR4 signal on BM-MSCs homing in rat heart of ischemia/perfusion injury. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 899–905. [Google Scholar] [PubMed]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef]
- Selvasandran, K.; Makhoul, G.; Jaiswal, P.K.; Jurakhan, R.; Li, L.; Ridwan, K.; Cecere, R.A. Tumor necrosis factor-α and hypoxia-induced secretome therapy for myocardial repair. Ann. Thorac. Surg. 2017, 105, 715–723. [Google Scholar] [CrossRef]
- Oh, E.J.; Lee, H.W.; Kalimuthu, S.; Kim, T.J.; Kim, H.M.; Baek, S.; Zhu, L.; Oh, J.M.; Son, S.H.; Chung, H.Y.; et al. In vivo migration of mesenchymal stem cells to burn injury sites and their therapeutic effects in a living mouse model. J. Control. Release 2018, 279, 79–88. [Google Scholar] [CrossRef]
- Kawai, T.; Katagiri, W.; Osugi, M.; Sugimura, Y.; Hibi, H.; Ueda, M. Secretomes from bone marrow–derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 2015, 17, 369–381. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, S.G.; Lee, S.W.; Oh, B.J.; Kim, J.H.; Kim, J.A.; Lee, G.; Jang, J.D.; Joe, Y.A. A Subset of paracrine factors as efficient biomarkers for predicting vascular regenerative efficacy of mesenchymal stromal/stem cells. Stem Cells 2018, 37, 77–88. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- De Donatis, A.; Ranaldi, F.; Cirri, P. Reciprocal control of cell proliferation and migration. Cell Commun. Signal. 2010, 8, 20. [Google Scholar] [CrossRef]
- Peng, H.; Hao, Y.; Mousawi, F.; Roger, S.; Li, J.; Joan, A.; Sim, J.A.; Ponnambalam, S.; Yang, X.; Jiang, L.H. Purinergic and Store-Operated Ca21 Signaling Mechanisms in Mesenchymal Stem Cells and Their Roles in ATP-Induced Stimulation of Cell Migration. Stem Cells 2016, 34, 2102–2114. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Tong, X. Cross-Talk between Mechanosensitive Ion Channels and Calcium Regulatory Proteins in Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 8782. [Google Scholar] [CrossRef]
- Zhang, T.; Chi, S.; Jiang, F.; Zhao, Q.; Xiao, B. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat. Commun. 2017, 8, 1797. [Google Scholar] [CrossRef]
- Wu, L.; Huang, X.; Kuang, Y.; Xing, Z.; Deng, X.; Luo, Z. Thapsigargin induces apoptosis in adrenocortical carcinoma by activating endoplasmic reticulum stress and the JNK signaling pathway: An in vitro and in vivo study. Drug Des. Devel. Ther. 2019, 13, 2787–2798. [Google Scholar] [CrossRef]
- Ma, Z.; Fan, C.; Yang, Y.; Di, S.; Hu, W.; Li, T.; Zhu, Y.; Han, J.; Xin, Z.; Wu, G.; et al. Thapsigargin sensitizes human esophageal cancer to TRAIL-induced apoptosis via AMPK activation. Sci. Rep. 2016, 6, 35196. [Google Scholar] [CrossRef]
- Jaskulska, A.; Janecka, A.E.; Gach-Janczak, K. Thapsigargin—From Traditional Medicine to Anticancer Drug. Int. J. Mol. Sci. 2021, 22, 4. [Google Scholar] [CrossRef]
- Chubinskiy-Nadezhdin, V.I.; Vasileva, V.Y.; Vassilieva, I.O.; Sudarikova, A.V.; Morachevskaya, E.A.; Negulyaev, Y.A. Agonist-induced Piezo1 activation suppresses migration of transformed fibroblasts. Biochem. Biophys. Res. Commun. 2019, 514, 173–179. [Google Scholar] [CrossRef]
- Holt, J.R.; Zeng, W.Z.; Evans, E.L.; Woo, S.-H.; Ma, S.; Abuwarda, H.; Loud, M.; Patapoutian, A.; Pathak, M.M. Spatiotemporal dynamics of PIEZO1 localization controls keratinocyte migration during wound healing. eLife 2021, 10, e65415. [Google Scholar] [CrossRef]
- Srivastava, N.; Traynor, D.; Piel, M.; Kabla, A.J.; Kay, R.R. Pressure sensing through Piezo channels controls whether cells migrate with blebs or pseudopods. Proc. Natl. Acad. Sci. USA 2020, 117, 2506–2512. [Google Scholar] [CrossRef]
- Yang, X.N.; Lu, Y.P.; Liu, J.J.; Huang, J.K.; Liu, Y.P.; Xiao, C.X.; Jazag, A.; Ren, J.L.; Guleng, B. Piezo1 is as a novel trefoil factor family 1 binding protein that promotes gastric cancer cell mobility in vitro. Dig. Dis. Sci. 2014, 59, 1428–1435. [Google Scholar] [CrossRef]
- Li, C.; Rezania, S.; Kammerer, S.; Sokolowski, A.; Devaney, T.; Gorischek, A.; Jahn, S.; Hackl, H.; Groschner, K.; Windpassinger, C.; et al. Piezo1 forms mechanosensitive ion channels in the human MCF-7 breast cancer cell line. Sci. Rep. 2015, 10, 8364. [Google Scholar] [CrossRef]
- Hung, W.C.; Yang, J.R.; Yankaskas, C.L.; Wong, B.S.; Wu, P.H.; Pardo-Pastor, C.; Serra, S.A.; Chiang, M.J.; Gu, Z.; Wirtz, D.; et al. Confinement Sensing and Signal Optimization via Piezo1/PKA and Myosin II Pathways. Cell Rep. 2016, 15, 1430–1441. [Google Scholar] [CrossRef]
- McHugh, B.J.; Murdoch, A.; Haslett, C.; Sethi, T. Loss of the integrin-activating transmembrane protein Fam38A (Piezo1) promotes a switch to a reduced integrin-dependent mode of cell migration. PLoS ONE 2012, 7, e40346. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, Z.; Zhang, X.; Niu, K.; Wang, Y.; Zheng, J.; Li, H.; Liu, Y. Loss of stretch-activated channels, PIEZOs, accelerates non-small cell lung cancer progression and cell migration. Biosci. Rep. 2019, 39, BSR20181679. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Vasileva, V.; Morachevskaya, E.; Sudarikova, A.; Negulyaev, Y.; Chubinskiy-Nadezhdin, V. Selective Chemical Activation of Piezo1 in Leukemia Cell Membrane: Single Channel Analysis. Int. J. Mol. Sci. 2021, 22, 7839. [Google Scholar] [CrossRef]
- Semenova, S.; Shatrova, A.; Vassilieva, I.; Shamatova, M.; Pugovkina, N.; Negulyaev, Y. Adenosine-5′-triphosphate suppresses proliferation and migration capacity of human endometrial stem cells. J. Cell Mol. Med. 2020, 24, 4580–4588. [Google Scholar] [CrossRef]





| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| hPIEZO1 | 3′-CCAGAACAGGTATCGGAAG-5′ | 5′-TGCTGTACCAGTACCTGCTG-3′ |
| hORAI1 | 5′-GACCTCGGCTCTGCTCTC-3′ | 5′-GGTGGGTACGTGGTCAG-3′ |
| hORAI2 | 5′-GCGGAAGCTCTACCTGAG-3′ | 5′-CCACATTGGGCAGGATGC-3′ |
| hORAI3 | 5′-CACGTCTGCCTTGCTCTC-3′ | 5′-ATGTTGCTCACAGCTTCAATG-3′ |
| hSTIM1 | 5′-GGCAGTCCGTAACATCCAC-3′ | 5′-TTGTATAC TTCTGATGACTTCC-3′ |
| hSTIM2 | 5′-ATGGTGGAATTGAAGTAGAGG-3′ | 5′-TTCCTTTGACATTGTTGTCTC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chubinskiy-Nadezhdin, V.; Semenova, S.; Vasileva, V.; Shatrova, A.; Pugovkina, N.; Negulyaev, Y. Store-Operated Ca2+ Entry Contributes to Piezo1-Induced Ca2+ Increase in Human Endometrial Stem Cells. Int. J. Mol. Sci. 2022, 23, 3763. https://doi.org/10.3390/ijms23073763
Chubinskiy-Nadezhdin V, Semenova S, Vasileva V, Shatrova A, Pugovkina N, Negulyaev Y. Store-Operated Ca2+ Entry Contributes to Piezo1-Induced Ca2+ Increase in Human Endometrial Stem Cells. International Journal of Molecular Sciences. 2022; 23(7):3763. https://doi.org/10.3390/ijms23073763
Chicago/Turabian StyleChubinskiy-Nadezhdin, Vladislav, Svetlana Semenova, Valeria Vasileva, Alla Shatrova, Natalia Pugovkina, and Yuri Negulyaev. 2022. "Store-Operated Ca2+ Entry Contributes to Piezo1-Induced Ca2+ Increase in Human Endometrial Stem Cells" International Journal of Molecular Sciences 23, no. 7: 3763. https://doi.org/10.3390/ijms23073763
APA StyleChubinskiy-Nadezhdin, V., Semenova, S., Vasileva, V., Shatrova, A., Pugovkina, N., & Negulyaev, Y. (2022). Store-Operated Ca2+ Entry Contributes to Piezo1-Induced Ca2+ Increase in Human Endometrial Stem Cells. International Journal of Molecular Sciences, 23(7), 3763. https://doi.org/10.3390/ijms23073763






