Methylmercury Causes Neurodegeneration and Downregulation of Myelin Basic Protein in the Spinal Cord of Offspring Rats after Maternal Exposure
Abstract
:1. Introduction
2. Results
2.1. Offspring Details
2.2. MeHg Exposure during Pregnancy and Lactation Increased Total Mercury Levels in the Offspring Blood
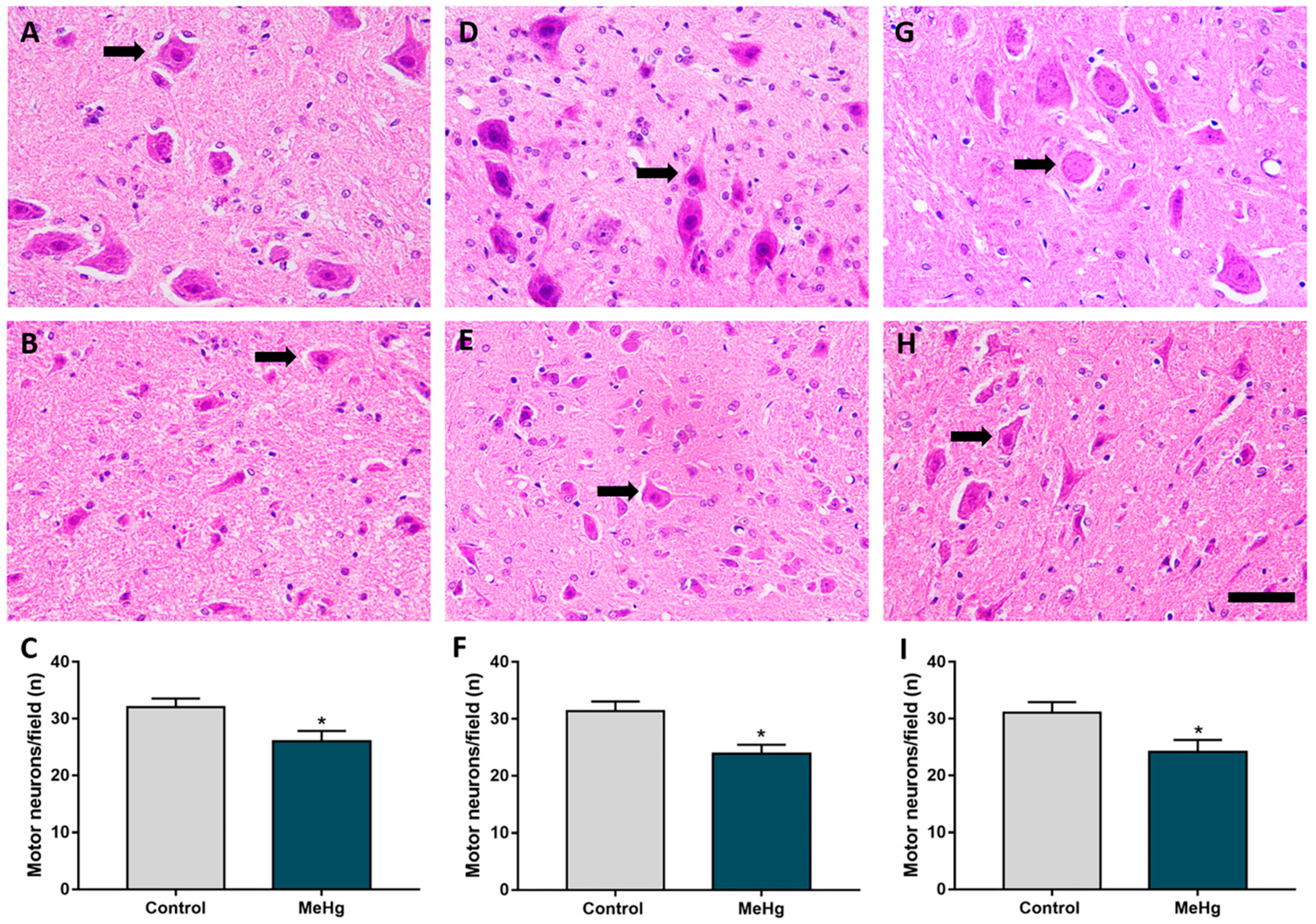
2.3. MeHg Exposure during Intrauterine and Perinatal Period Induces Neuron Degeneration in the Spinal Cord of Offspring Rats
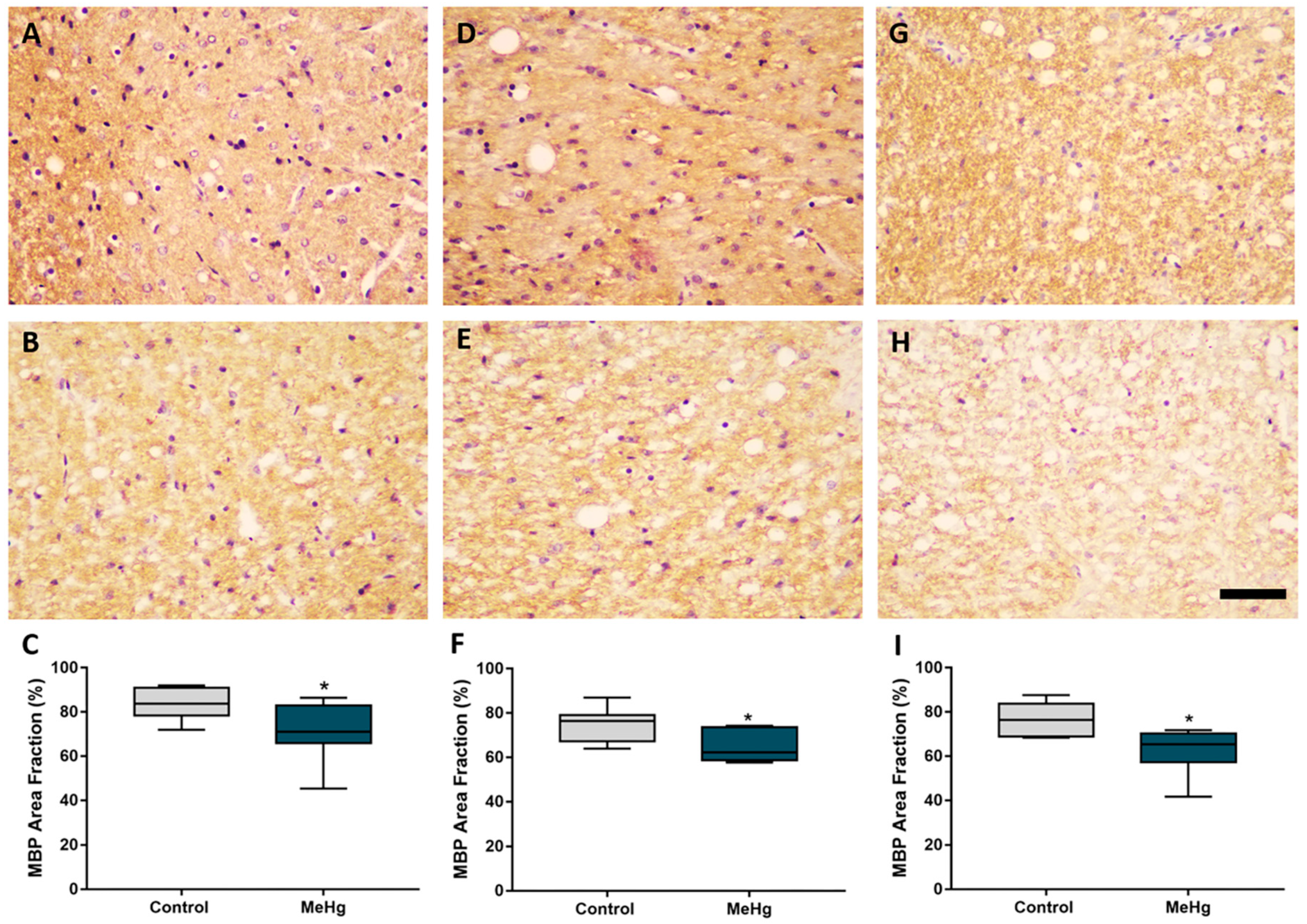
2.4. MeHg Exposure during Intrauterine and Lactational Periods Induces Downregulation of Myelin Basic Protein in Offspring Rats
3. Discussion
4. Materials and Methods
4.1. Ethical Aspects
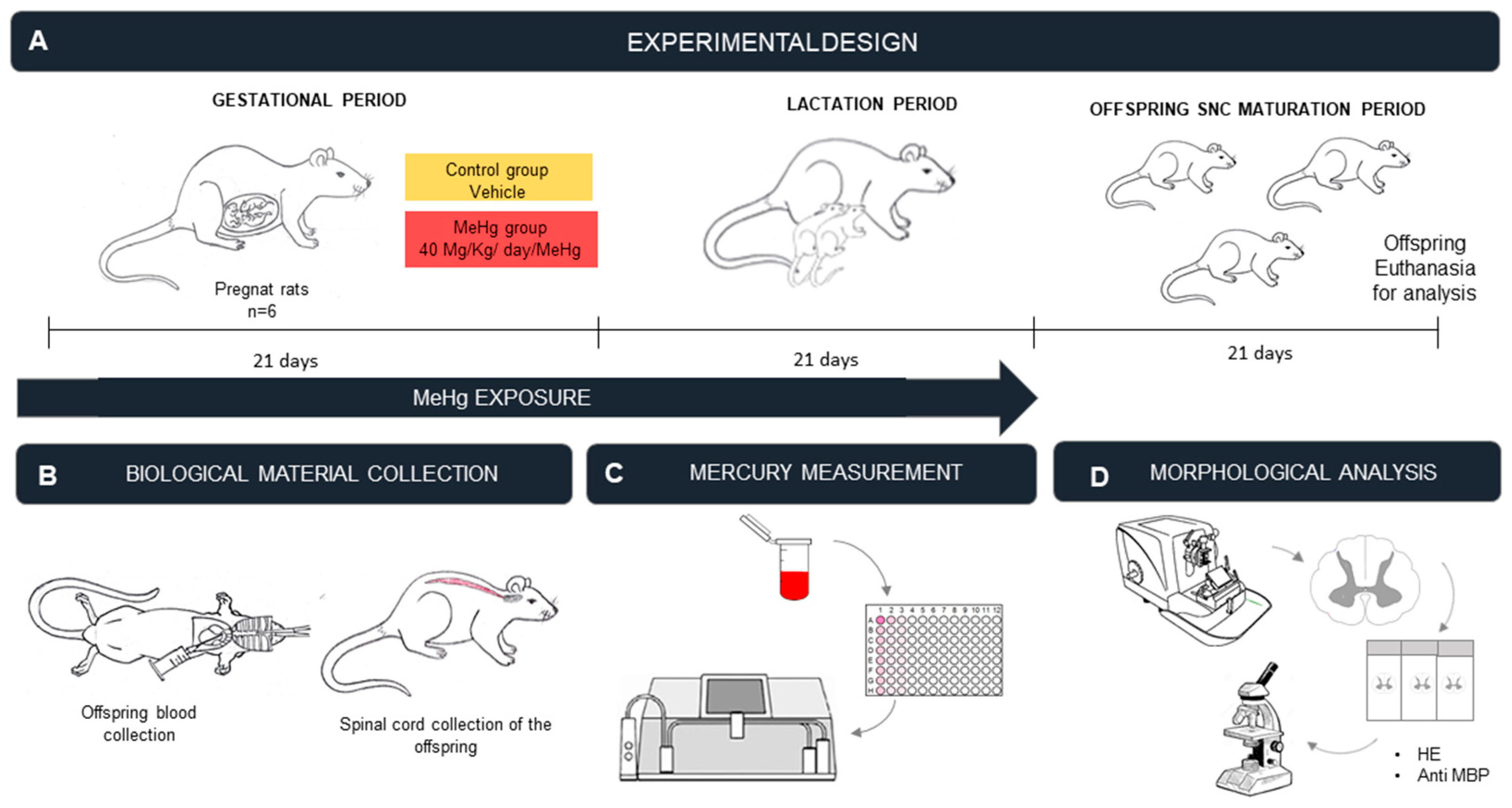
4.2. Experimental Animals
4.3. MeHg Exposure Protocol
4.4. Sample Collection and Perfusion
4.5. Measurement of THg Levels in the Blood
4.6. Histological Procedures
4.6.1. Counting of Motor Neurons
4.6.2. Assessment of Immunoreactivity of Myelin Basic Protein
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MeHg | methylmercury |
| MBP | myelin basic protein |
| Hg0 | elemental Mercury |
| HgCl2 | mercury chloride |
| CNS | central nervous system |
| SC | spinal cord |
| THg | total mercury |
| PB | phosphate buffer |
| HNO3 | nitric acid |
| HClO4 | perchloric acid |
| H2SO4 | sulfuric acid |
| PBS | phosphate buffer saline |
| DAB | 3,3′diaminobenzidine |
References
- UNEP United Nations Environment Program. Available online: https://www.unenvironment.org/resources/publication/global-mercury-assessment-2018 (accessed on 18 November 2021).
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Yuki Takeda, P.; Macchi, B.M.; do Nascimento, J.L.M.; Maia, C.S.F.; Lima, R.R.; Arrifano, G.P. Mercury: What can we learn from the Amazon? Environ. Int. 2021, 146, 106223. [Google Scholar] [CrossRef]
- Langeland, A.L.; Hardin, R.D.; Neitzel, R.L. Mercury levels in human hair and farmed fish near artisanal and small-scale gold mining communities in the Madre de Dios river basin, Peru. Int. J. Environ. Res. Public Health 2017, 14, 302. [Google Scholar] [CrossRef] [Green Version]
- Vega, C.M.; Orellana, J.D.Y.; Oliveira, M.W.; Hacon, S.S.; Basta, P.C. Human mercury exposure in Yanomami indigenous villages from the Brazilian Amazon. Int. J. Environ. Res. Public Health 2018, 15, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo-López, M.E.; Macêdo, G.L.; Pereira, S.I.; Arrifano, G.P.; Picanço-Diniz, D.L.; do Nascimento, J.L.; Herculano, A.M. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol. Res. 2009, 60, 212–220. [Google Scholar] [CrossRef]
- Rodríguez Martín-Doimeadios, R.C.; Berzas Nevado, J.J.; Guzmán Bernardo, F.J.; Jiménez Moreno, M.; Arrifano, G.P.; Herculano, A.M.; Do Nascimento, J.L.; Crespo-López, M.E. Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ. Sci. Pollut. Res. Int. 2014, 21, 7466–7479. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, F.; Majorani, C.; Domanico, F.; Alimonti, A. Mercury in Children: Current State on Exposure through Human Biomonitoring Studies. Int. J. Environ. Res. Public Health 2017, 14, 519. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.; Paxinos, G.; Kayalioglu, G. The Spinal Cord. A Christopher and Dana Reeve Foundation Text and Atlas, 1st ed.; Elsevier: Amsterdan, The Netherlands, 2009. [Google Scholar]
- Bican, O.; Minagar, A.; Pruitt, A.A. The spinal cord: A review of functional neuroanatomy. Neurol. Clin. 2013, 31, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Santana, L.N.D.S.; Bittencourt, L.O.; Nascimento, P.C.; Fernandes, R.M.; Teixeira, F.B.; Fernandes, L.M.P.; Freitas Silva, M.C.; Nogueira, L.S.; Amado, L.L.; Crespo-Lopez, M.E.; et al. Low doses of methylmercury exposure during adulthood in rats display oxidative stress, neurodegeneration in the motor cortex and lead to impairment of motor skills. J. Trace Elem. Med. Biol. 2019, 51, 19–27. [Google Scholar] [CrossRef]
- Ceccatelli, S.; Bose, R.; Edoff, K.; Onishchenko, N.; Spulber, S. Long-lasting neurotoxic effects of exposure to methylmercury during development. J. Intern. Med. 2013, 273, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Rand, M.D.; Conrad, K.; Marvin, E.; Harvey, K.; Henderson, D.; Tawil, R.; Sobolewski, M.; Cory-Slechta, D.A. Developmental exposure to methylmercury and resultant muscle mercury accumulation and adult motor deficits in mice. Neurotoxicology 2020, 81, 1–10. [Google Scholar] [CrossRef]
- Sakamoto, M.; Kakita, A.; Wakabayashi, K.; Takahashi, H.; Nakano, A.; Akagi, H. Evaluation of changes in methylmercury accumulation in the developing rat brain and its effects: A study with consecutive and moderate dose exposure throughout gestation and lactation periods. Brain Res. 2002, 949, 51–59. [Google Scholar] [CrossRef]
- Rock, K.D.; Patisaul, H.B. Environmental mechanisms of neurodevelopmental toxicity. Curr. Environ. Health Rep. 2018, 5, 145–157. [Google Scholar] [CrossRef]
- Singer, L.T.; Min, M.O.; Minnes, S.; Short, E.; Lewis, B.; Lang, A.; Wu, M. Prenatal and concurrent cocaine, alcohol, marijuana, and tobacco effects on adolescent cognition and attention. Drug Alcohol Depend. 2018, 191, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Mactier, H.; Hamilton, R. Prenatal opioid exposure—Increasing evidence of harm. Early Hum. Dev. 2020, 150, 105188. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Farzan, S.F.; Howe, C.G.; Chen, Y.; Gilbert-Diamond, D.; Korrick, S.; Jackson, B.P.; Weinstein, A.R.; Karagas, M.R. Prenatal and postnatal mercury exposure and blood pressure in childhood. Environ. Int. 2021, 146, 106201. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sacramento, L.; Arrifano, G.P.; Lopes-Araújo, A.; Augusto-Oliveira, M.; Albuquerque-Santos, R.; Takeda, P.Y.; Souza-Monteiro, J.R.; Macchi, B.M.; do Nascimento, J.L.M.; Lima, R.R.; et al. Human neurotoxicity of mercury in the Amazon: A scoping review with insights and critical considerations. Ecotoxicol. Environ. Saf. 2021, 15, 111686. [Google Scholar] [CrossRef]
- Freire, M.A.M.; Oliveira, R.B.; Picanço-Diniz, C.W.; Pereira, A., Jr. Differential effects of methylmercury intoxication in the rat’s barrel field as evidenced by NADPH diaphorase histochemistry. Neurotoxicology 2007, 28, 175–181. [Google Scholar] [CrossRef]
- Bittencourt, L.O.; Puty, B.; Charone, S.; Aragão, W.A.B.; Farias-Junior, P.M.; Silva, M.C.F.; Crespo-Lopez, M.E.; Leite, A.L.; Buzalaf, M.A.R.; Lima, R.R. Oxidative biochemistry disbalance and changes on proteomic profile in salivary glands of rats induced by chronic exposure to methylmercury. Oxid. Med. Cell Longev. 2017, 2017, 5653291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittencourt, L.O.; Dionizio, A.; Nascimento, P.C.; Puty, B.; Leão, L.K.R.; Luz, D.A.; Silva, M.C.F.; Amado, L.L.; Leite, A.; Buzalaf, M.R.; et al. Proteomic approach underlying the hippocampal neurodegeneration caused by low doses of methylmercury after long-term exposure in adult rats. Metallomics 2019, 11, 390–403. [Google Scholar] [CrossRef]
- Freire, M.A.M.; Santana, L.N.S.; Bittencourt, L.O.; Nascimento, P.C.; Fernandes, R.M.; Leão, L.K.R.; Fernandes, L.M.P.; Silva, M.C.F.; Amado, L.L.; Gomes-Leal, W.; et al. Methylmercury intoxication and cortical ischemia: Pre-clinical study of their comorbidity. Ecotoxicol. Environ. Saf. 2019, 15, 557–565. [Google Scholar] [CrossRef]
- Freire, M.A.M.; Lima, R.R.; Nascimento, P.C.; Gomes-Leal, W.; Pereira, A. Effects of methylmercury on the pattern of NADPH diaphorase expression and astrocytic activation in the rat. Ecotoxicol. Environ. Saf. 2020, 201, 110799. [Google Scholar] [CrossRef] [PubMed]
- Eiró, L.G.; Ferreira, M.K.M.; Bittencourt, L.O.; Aragão, W.A.B.; Souza, M.P.C.; Silva, M.C.F.; Dionizio, A.; Buzalaf, M.A.R.; Crespo-López, M.E.; Lima, R.R. Chronic methylmercury exposure causes spinal cord impairment: Proteomic modulation and oxidative stress. Food Chem. Toxicol. 2020, 146, 111772. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Lopes, G.; Aragão, W.A.B.; Bittencourt, L.O.; Puty, B.; Lopes, A.P.; Dos Santos, S.M.; Monteiro, M.C.; de Oliveira, E.H.C.; da Silva, M.C.F.; Lima, R.R. Imaging microstructural damage and alveolar bone loss in rats systemically exposed to methylmercury: First experimental evidence. Biol. Trace Elem. Res. 2021, 199, 3707–3717. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, P.C.; Aragão, W.A.B.; Bittencourt, L.O.; Silva, M.C.F.; Crespo-Lopez, M.E.; Lima, R.R. Salivary parameters alterations after early exposure to environmental methylmercury: A preclinical study in offspring rats. J. Trace Elem. Med. Biol. 2021, 68, 126820. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.A. Spinal cord functional anatomy. Continuum 2015, 21, 13–35, Erratum in Continuum 2015, 21, 590. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Bittencourt, L.O.; Nascimento, P.C.; Ferreira, R.O.; Aragão, W.A.B.; Silva, M.C.F.; Gomes-Leal, W.; Fernandes, M.S.; Dionizio, A.; Buzalaf, M.R.; et al. Spinal cord neurodegeneration after inorganic mercury long-term exposure in adult rats: Ultrastructural, proteomic and biochemical damages associated with reduced neuronal density. Ecotoxicol. Environ. Saf. 2020, 191, 110159. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.R.F.; Bittencourt, L.O.; Aragão, W.A.B.; Nascimento, P.C.; Leão, L.K.R.; Oliveira, A.C.A.; Crespo-López, M.E.; Lima, R.R. Long-term exposure to lead reduces antioxidant capacity and triggers motor neurons degeneration and demyelination in spinal cord of adult rats. Ecotoxicol. Environ. Saf. 2020, 194, 110358. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Kirkpatrick, M.; Benoit, J.; Everett, W.; Gibson, J.; Rist, M.; Fredette, N. The effects of methylmercury exposure on behavior and biomarkers of oxidative stress in adult mice. Neurotoxicology 2015, 50, 170–178. [Google Scholar] [CrossRef]
- Nascimento, P.C.; Ferreira, M.K.M.; Balbinot, K.M.; Alves-Júnior, S.M.; Viana Pinheiro, J.J.; Silveira, F.M.; Martins, M.D.; Crespo-Lopez, M.E.; Lima, R.R. Methylmercury-induced toxicopathologic findings in salivary glands of offspring rats after gestational and lactational exposure. Biol. Trace Elem. Res. 2021, 199, 2983–2991. [Google Scholar] [CrossRef]
- Kong, H.K.; Wong, M.H.; Chan, H.M.; Lo, S.C. Chronic exposure of adult rats to low doses of methylmercury induced a state of metabolic deficit in the somatosensory cortex. J. Proteome Res. 2013, 12, 5233–5245. [Google Scholar] [CrossRef] [PubMed]
- Chemelo, V.S.; Bittencourt, L.O.; Aragão, W.A.B.; Dos Santos, S.M.; Souza-Rodrigues, R.D.; Ribeiro, C.H.M.A.; Monteiro, M.C.; Lima, R.R. Long-term exposure to inorganic mercury leads to oxidative stress in peripheral blood of adult rats. Biol. Trace Elem. Res. 2021, 199, 2992–3000. [Google Scholar] [CrossRef]
- Ferrucci, M.; Lazzeri, G.; Flaibani, M.; Biagioni, F.; Cantini, F.; Madonna, M.; Bucci, D.; Limanaqi, F.; Soldani, P.; Fornai, F. In search for a gold-standard procedure to count motor neurons in the spinal cord. Histol. Histopathol. 2018, 33, 1021–1046. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.R.; Guimarães-Silva, J.; Souza-Rodrigues, R.D.; Costa, A.M.R.; Santos, C.D.; Picanco-Diniz, C.W.; Gomes-Leal, W. Diffuse axonal damage, myelin impairment, astrocytosis and inflammatory response following microinjections of NMDA into the rat striatum. Inflammation 2008, 31, 24–35. [Google Scholar] [CrossRef]
- Lima, R.R.; Santana, L.N.; Fernandes, R.M.; Nascimento, E.M.; Oliveira, A.C.; Fernandes, L.M.P.; Dos Santos, E.M.N.; Tavares, P.A.N.; Dos Santos, I.R.; Gimarães-Santos, A.; et al. Neurodegeneration and glial response after acute striatal stroke: Histological basis for neuroprotective studies. Oxid. Med. Cell. Longev. 2016, 2016, 3173564. [Google Scholar] [CrossRef] [PubMed]
- Boggs, J.M. Myelin basic protein: A multifunctional protein. Cell. Mol. Life Sci. 2006, 63, 1945–1961. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.C.; Duarte, R.B.; Sousa, T.B.; Santos, J.R.; Freire, M.A.M.; Costa, M.S.M.O. Expression of the immediate-early gene egr-1 and substance P in the spinal cord following locomotor training in adult rats. Brain Res. 2010, 1345, 125–136. [Google Scholar] [CrossRef]
- Fernandes, L.M.; Teixeira, F.B.; Alves-Junior, S.M.; Pinheiro Jde, J.; Maia, C.S.; Lima, R.R. Immunohistochemical changes and atrophy after chronic ethanol intoxication in rat salivary glands. Histol. Histopathol. 2015, 30, 1069–1078. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, D.C.B.; Bittencourt, L.O.; Baia-da-Silva, D.C.; Chemelo, V.S.; Eiró-Quirino, L.; Nascimento, P.C.; Silva, M.C.F.; Freire, M.A.M.; Gomes-Leal, W.; Crespo-Lopez, M.E.; et al. Methylmercury Causes Neurodegeneration and Downregulation of Myelin Basic Protein in the Spinal Cord of Offspring Rats after Maternal Exposure. Int. J. Mol. Sci. 2022, 23, 3777. https://doi.org/10.3390/ijms23073777
da Silva DCB, Bittencourt LO, Baia-da-Silva DC, Chemelo VS, Eiró-Quirino L, Nascimento PC, Silva MCF, Freire MAM, Gomes-Leal W, Crespo-Lopez ME, et al. Methylmercury Causes Neurodegeneration and Downregulation of Myelin Basic Protein in the Spinal Cord of Offspring Rats after Maternal Exposure. International Journal of Molecular Sciences. 2022; 23(7):3777. https://doi.org/10.3390/ijms23073777
Chicago/Turabian Styleda Silva, Diane Cleydes Baía, Leonardo Oliveira Bittencourt, Daiane Claydes Baia-da-Silva, Victoria Santos Chemelo, Luciana Eiró-Quirino, Priscila Cunha Nascimento, Márcia Cristina Freitas Silva, Marco Aurelio M. Freire, Walace Gomes-Leal, Maria Elena Crespo-Lopez, and et al. 2022. "Methylmercury Causes Neurodegeneration and Downregulation of Myelin Basic Protein in the Spinal Cord of Offspring Rats after Maternal Exposure" International Journal of Molecular Sciences 23, no. 7: 3777. https://doi.org/10.3390/ijms23073777
APA Styleda Silva, D. C. B., Bittencourt, L. O., Baia-da-Silva, D. C., Chemelo, V. S., Eiró-Quirino, L., Nascimento, P. C., Silva, M. C. F., Freire, M. A. M., Gomes-Leal, W., Crespo-Lopez, M. E., & Lima, R. R. (2022). Methylmercury Causes Neurodegeneration and Downregulation of Myelin Basic Protein in the Spinal Cord of Offspring Rats after Maternal Exposure. International Journal of Molecular Sciences, 23(7), 3777. https://doi.org/10.3390/ijms23073777







