Use of Melatonin in Cancer Treatment: Where Are We?
Abstract
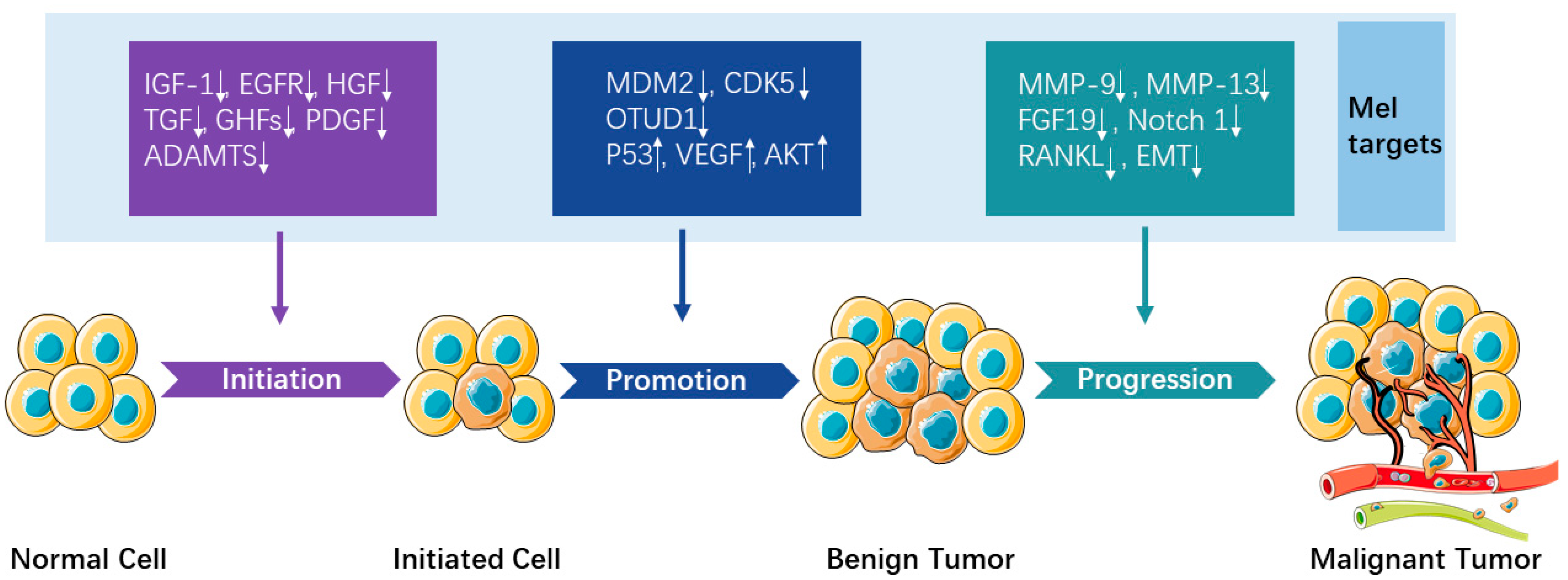
:1. Introduction
2. Basic Biology of Melatonin
2.1. Biosynthesis and Secretion of Melatonin
2.2. Biological Effects and Molecular Mechanisms of Melatonin
3. Anticancer Effect of Melatonin
3.1. Underlying Mechanisms/Signaling Pathways
3.2. Modulation of Immune Responses by Melatonin
3.3. Relationship between Melatonin, ROS Metabolism, and Tumor Progression
4. Clinical Trial Based on the Anticancer Effects of Melatonin
5. Melatonin in Combination with Other Anticancer Therapies
| Combination Therapy | Melatonin Effect | Mechanisms of Action | Reference |
|---|---|---|---|
| Mel + chemotherapy |
|
| [91] |
|
| [92] | |
|
| [93] | |
|
| [96] | |
|
| [97] | |
|
| [98] | |
| Mel + radiotherapy |
|
| [99] |
|
| [100] | |
| Mel + cancer vaccination |
|
| [103] |
|
| [104] | |
| Mel + immunotherapy |
|
| [107] |
|
| [110] |
6. Problems with Using Melatonin as an Anticancer Drug
6.1. Chemical Properties of Melatonin
6.2. The Measurement of Melatonin
6.3. The Safety Profile and Dosing of Melatonin
6.4. As a Single Anticancer Drug or Adjuvant Anticancer Drug?
7. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, the Pineal Gland Factor that Lightens Melanocytes1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Simopoulos, A.P.; Maldonado, M.D.; Flores, L.J.; Terron, M.P. Melatonin in Edible Plants (Phytomelatonin): Identification, Concentrations, Bioavailability and Proposed Functions. World Rev. Nutr. Diet. 2007, 97, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.D.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Reiter, R.J. Phytomelatonin: A review. J. Exp. Bot. 2009, 60, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Zheng, X.; Kong, J.; Manchester, L.C.; Hardeland, R.; Kim, S.J.; Xu, X.; Reiter, R.J. Fundamental Issues Related to the Origin of Melatonin and Melatonin Isomers during Evolution: Relation to Their Biological Functions. Int. J. Mol. Sci. 2014, 15, 15858–15890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.-X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. J. Cereb. Blood Flow Metab. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Karasek, M.; Winczyk, K. Melatonin in humans. J. Physiol. Pharmacol. J. Pol. Physiol. Soc. 2006, 57 (Suppl. 5), 19–39. [Google Scholar]
- Moroni, I.; Garcia-Bennett, A.; Chapman, J.; Grunstein, R.R.; Gordon, C.J.; Comas, M. Pharmacokinetics of exogenous melatonin in relation to formulation, and effects on sleep: A systematic review. Sleep Med. Rev. 2021, 57, 101431. [Google Scholar] [CrossRef] [PubMed]
- Härtter, S.; Ursing, C.; Morita, S.; Tybring, G.; Bahr, C.; Christensen, M.; Röjdmark, S.; Bertilsson, L. Orally given melatonin may serve as a probe drug for cytochrome P450 1A2 activity in vivo: A pilot study. Clin. Pharmacol. Ther. 2001, 70, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zetner, D.; Andersen, L.P.K.; Alder, R.; Jessen, M.L.; Tolstrup, A.; Rosenberg, J. Pharmacokinetics and Safety of Intravenous, Intravesical, Rectal, Transdermal, and Vaginal Melatonin in Healthy Female Volunteers: A Cross-Over Study. Pharmacology 2021, 106, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Skwarlo-Sonta, K.; Majewski, P.; Markowska, M.; Oblap, R.; Olszanska, B. Bidirectional communication between the pineal gland and the immune system. Can. J. Physiol. Pharmacol. 2003, 81, 342–349. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [Green Version]
- Talib, W.; Alsayed, A.; Abuawad, A.; Daoud, S.; Mahmod, A. Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 2021, 26, 2506. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8 (Suppl. 3), 34–42. [Google Scholar] [CrossRef]
- Becker-André, M.; Wiesenberg, I.; Schaeren-Wiemers, N.; André, E.; Missbach, M.; Saurat, J.H.; Carlberg, C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1994, 269, 28531–28534. [Google Scholar] [CrossRef]
- Ma, H.; Kang, J.; Fan, W.; He, H.; Huang, F. ROR: Nuclear Receptor for Melatonin or Not? Molecules 2021, 26, 2693. [Google Scholar] [CrossRef]
- Boutin, J.A.; Ferry, G. Is There Sufficient Evidence that the Melatonin Binding Site MT(3) Is Quinone Reductase 2? J. Pharmacol. Exp. Ther. 2019, 368, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Hunt, A.E.; Al-Ghoul, W.M.; Gillette, M.U.; Dubocovich, M.L. Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am. J. Physiol. Physiol. 2001, 280, C110–C118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin receptors, heterodimerization, signal transduction and binding sites: What’s new? Br. J. Pharmacol. 2008, 154, 1182–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 Melatonin Receptors in Mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Patel, K.K.; Dehari, D.; Agrawal, A.K.; Singh, S. Melatonin and its ubiquitous anticancer effects. Mol. Cell. Biochem. 2019, 462, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Lin, Y.; Chu, C.; Yang, Y.; Yang, S.; Liu, Y.; Hsiao, M.; Lee, W.; Chien, M. Melatonin-triggered post-transcriptional and post-translational modifications of ADAMTS1 coordinately retard tumorigenesis and metastasis of renal cell carcinoma. J. Pineal Res. 2020, 69, e12668. [Google Scholar] [CrossRef]
- Wang, P.; Ren, F.M.; Lin, Y.; Su, F.X.; Jia, W.H.; Su, X.F.; Tang, L.; Rene, Z. Night-shift work, sleep duration, daytime napping, and breast cancer risk. Sleep Med. 2015, 16, 462–468. [Google Scholar] [CrossRef]
- Song, J.; Ma, S.; Luo, J.; Liu, H.; Li, L.; Zhang, Z.; Chen, L.; Zhou, R. Downregulation of AKT and MDM2, Melatonin Induces Apoptosis in AGS and MGC803 Cells. Anat. Rec. 2019, 302, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tan, Z.; Li, H.; Lin, M.; Jiang, Y.; Liang, L.; Ma, Q.; Gou, J.; Ning, L.; Li, X.; et al. Melatonin reduces proliferation and promotes apoptosis of bladder cancer cells by suppressing O-GlcNAcylation of cyclin-dependent-like kinase 5. J. Pineal Res. 2021, 71, e12765. [Google Scholar] [CrossRef]
- Woo, S.M.; Seo, S.U.; Min, K.; Kwon, T.K. Melatonin induces apoptotic cell death through Bim stabilization by Sp1-mediated OTUD1 upregulation. J. Pineal Res. 2022, 72, e12781. [Google Scholar] [CrossRef] [PubMed]
- Santoro, R.; Marani, M.; Blandino, G.; Muti, P.; Strano, S. Melatonin triggers p53Ser phosphorylation and prevents DNA damage accumulation. Oncogene 2012, 31, 2931–2942. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Liu, H.; Song, J.; Wu, Q. Activity of Melatonin Against Gastric Cancer Growth in a Chick Embryo Tumor Xenograft Model. Cancer Manag. Res. 2021, 13, 8803–8808. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, L.; Chen, S.; Li, S.; Wang, B.; Zhang, C.; Chen, Y.; Yang, L.; Xin, H.; Chen, C.; et al. Melatonin protects against apoptosis of megakaryocytic cells via its receptors and the AKT/mitochondrial/caspase pathway. Aging 2020, 12, 13633–13646. [Google Scholar] [CrossRef]
- Gu, H.; Shen, Q.; Mei, D.; Yang, Y.; Wei, R.; Ni, M. Melatonin Inhibits TE-1 Esophageal Cancer Cells Metastasis by Suppressing the NF-κB Signaling Pathway and Decreasing MMP-9. Ann. Clin. Lab. Sci. 2020, 50, 65–72. [Google Scholar] [PubMed]
- Wang, L.; Su, Y.; Choi, W.S. Melatonin Suppresses Oral Squamous Cell Carcinomas Migration and Invasion through Blocking FGF19/FGFR 4 Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 9907. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Huang, C.-R.; Chang, C.-L.; Chiang, J.Y.; Luo, C.-W.; Chen, H.-H.; Yip, H.-K. Jagged2 progressively increased expression from Stage I to III of Bladder Cancer and Melatonin-mediated downregulation of Notch/Jagged2 suppresses the Bladder Tumorigenesis via inhibiting PI3K/AKT/mTOR/MMPs signaling. Int. J. Biol. Sci. 2020, 16, 2648–2662. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Yang, H.; Chang, K.; Zhang, B.; Xie, F.; Ye, J.; Chang, R.; Qiu, X.; Wang, Y.; Qu, Y.; et al. Melatonin alleviates progression of uterine endometrial cancer by suppressing estrogen/ubiquitin C/SDHB-mediated succinate accumulation. Cancer Lett. 2020, 476, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tai, H.; Tang, C.; Lin, L.; Lin, T.; Chang, A.; Chen, P.; Chen, Y.; Wang, P.; Lai, Y.; et al. Melatonin impedes prostate cancer metastasis by suppressing MMP-13 expression. J. Cell. Physiol. 2021, 236, 3979–3990. [Google Scholar] [CrossRef]
- Liu, P.-I.; Chang, A.-C.; Lai, J.-L.; Lin, T.-H.; Tsai, C.-H.; Chen, P.-C.; Jiang, Y.-J.; Lin, L.-W.; Huang, W.-C.; Yang, S.-F.; et al. Melatonin interrupts osteoclast functioning and suppresses tumor-secreted RANKL expression: Implications for bone metastases. Oncogene 2021, 40, 1503–1515. [Google Scholar] [CrossRef]
- Tian, Q.-X.; Zhang, Z.-H.; Ye, Q.-L.; Xu, S.; Hong, Q.; Xing, W.-Y.; Chen, L.; Yu, D.-X.; Xu, D.-X.; Xie, D.-D. Melatonin Inhibits Migration and Invasion in LPS-Stimulated and -Unstimulated Prostate Cancer Cells Through Blocking Multiple EMT-Relative Pathways. J. Inflamm. Res. 2021, 14, 2253–2265. [Google Scholar] [CrossRef]
- Tang, H.; Shi, X.; Zhu, P.; Guo, W.; Li, J.; Yan, B.; Zhang, S. Melatonin inhibits gallbladder cancer cell migration and invasion via ERK-mediated induction of epithelial-to-mesenchymal transition. Oncol. Lett. 2021, 22, 609. [Google Scholar] [CrossRef]
- Oh, B.S.; Im, E.; Lee, H.; Sim, D.Y.; Park, J.E.; Park, W.Y.; Park, Y.; Koo, J.; Pak, J.; Kim, D.H.; et al. Inhibition of TMPRSS4 mediated epithelial-mesenchymal transition is critically involved in antimetastatic effect of melatonin in colorectal cancers. Phytother. Res. 2021, 35, 4538–4546. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Chiou, P.-C.; Chen, P.-C.; Liu, P.-Y.; Huang, W.-C.; Chao, C.-C.; Tang, C.-H. Melatonin reduces lung cancer stemness through inhibiting of PLC, ERK, p38, β-catenin, and Twist pathways. Environ. Toxicol. 2019, 34, 203–209. [Google Scholar] [CrossRef]
- Shin, Y.Y.; Seo, Y.; Oh, S.; Ahn, J.; Song, M.; Kang, M.; Oh, J.; Lee, D.; Kim, Y.H.; Sung, E.; et al. Melatonin and verteporfin synergistically suppress the growth and stemness of head and neck squamous cell carcinoma through the regulation of mitochondrial dynamics. J. Pineal Res. 2022, 72, e12779. [Google Scholar] [CrossRef] [PubMed]
- Maroufi, N.F.; Amiri, M.; Dizaji, B.F.; Vahedian, V.; Akbarzadeh, M.; Roshanravan, N.; Haiaty, S.; Nouri, M.; Rashidi, M.-R. Inhibitory effect of melatonin on hypoxia-induced vasculogenic mimicry via suppressing epithelial-mesenchymal transition (EMT) in breast cancer stem cells. Eur. J. Pharmacol. 2020, 881, 173282. [Google Scholar] [CrossRef]
- Hao, J.; Fan, W.; Li, Y.; Tang, R.; Tian, C.; Yang, Q.; Zhu, T.; Diao, C.; Hu, S.; Chen, M.; et al. Melatonin synergizes BRAF-targeting agent vemurafenib in melanoma treatment by inhibiting iNOS/hTERT signaling and cancer-stem cell traits. J. Exp. Clin. Cancer Res. 2019, 38, 48. [Google Scholar] [CrossRef] [Green Version]
- Hunsaker, M.; Barba, G.; Kingsley, K.; Howard, K.M. Differential MicroRNA Expression of miR-21 and miR-155 within Oral Cancer Extracellular Vesicles in Response to Melatonin. Dent. J. 2019, 7, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doğanlar, O.; Doğanlar, Z.B.; Delen, E.; Doğan, A. The role of melatonin in angio-miR-associated inhibition of tumorigenesis and invasion in human glioblastoma tumour spheroids. Tissue Cell 2021, 73, 101617. [Google Scholar] [CrossRef]
- Oshiba, R.T.; Touson, E.; Elsherbini, Y.M.; Abdraboh, M.E. Melatonin: A regulator of the interplay between FoxO1, miR96, and miR215 signaling to diminish the growth, survival, and metastasis of murine adenocarcinoma. BioFactors 2021, 47, 740–753. [Google Scholar] [CrossRef]
- Ji, G.; Zhou, W.; Li, X.; Du, J.; Li, X.; Hao, H. Melatonin inhibits proliferation and viability and promotes apoptosis in colorectal cancer cells via upregulation of the microRNA-34a/449a cluster. Mol. Med. Rep. 2021, 23, 187. [Google Scholar] [CrossRef]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965–3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.; Yeh, C.; Lin, C.; Hsieh, Y.; Chuang, C.; Tang, C.; Lee, Y.; Yang, S. A novel melatonin-regulated lncRNA suppresses TPA-induced oral cancer cell motility through replenishing PRUNE2 expression. J. Pineal Res. 2021, 71, e12760. [Google Scholar] [CrossRef]
- Li, Y.; Zou, J.; Li, B.; Du, J. Anticancer effects of melatonin via regulating lncRNA JPX-Wnt/β-catenin signalling pathway in human osteosarcoma cells. J. Cell. Mol. Med. 2021, 25, 9543–9556. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Peng, F.; Zhu, L.; Li, X.; Ou, S.; Huang, Z.; Wu, S.; Peng, C.; Liu, P.; Kong, Y. Melatonin inhibits triple-negative breast cancer progression through the Lnc049808-FUNDC1 pathway. Cell Death Dis. 2021, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Potes, Y.; Mahyari, H.M.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Boosting immune system against cancer by melatonin: A mechanistic viewpoint. Life Sci. 2019, 238, 116960. [Google Scholar] [CrossRef]
- Moradkhani, F.; Moloudizargari, M.; Fallah, M.; Asghari, N.; Heidari Khoei, H.; Asghari, M.H. Immunoregulatory role of melatonin in cancer. J. Cell. Physiol. 2020, 235, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Pandi-Perumal, S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J. The role of melatonin in immuno-enhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87. [Google Scholar] [CrossRef]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Xu, L.; Wei, J.-E.; Xie, M.-R.; Wang, S.-E.; Zhou, R.-X. Role of CD4+CD25+ Regulatory T Cells in Melatonin-Mediated Inhibition of Murine Gastric Cancer Cell Growth In Vivo and In Vitro. Anat. Rec. 2011, 294, 781–788. [Google Scholar] [CrossRef]
- Vigoré, L.; Messina, G.; Brivio, F.; Fumagalli, L.; Rovelli, F.; Di Fede, G.; Lissoni, P. Psychoneuroendocrine modulation of regulatory T lymphocyte system: In vivo and in vitro effects of the pineal immunomodulating hormone melatonin. In Vivo 2010, 24, 787–789. [Google Scholar] [PubMed]
- Li, M.; Hao, B.; Zhang, M.; Reiter, R.J.; Lin, S.; Zheng, T.; Chen, X.; Ren, Y.; Yue, L.; Abay, B.; et al. Melatonin enhances radiofrequency-induced NK antitumor immunity, causing cancer metabolism reprogramming and inhibition of multiple pulmonary tumor development. Signal Transduct. Target. Ther. 2021, 6, 330. [Google Scholar] [CrossRef] [PubMed]
- Currier, N.L.; Miller, S.C. Echinacea purpurea and melatonin augment natural-killer cells in leukemic mice and prolong life span. J. Altern. Complementary Med. 2001, 7, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [Green Version]
- Muxel, S.M.; Pires-Lapa, M.A.; Monteiro, A.W.A.; Cecon, E.; Tamura, E.K.; Floeter-Winter, L.M.; Markus, R.P. NF-κB Drives the Synthesis of Melatonin in RAW 264.7 Macrophages by Inducing the Transcription of the Arylalkylamine-N-Acetyltransferase (AA-NAT) Gene. PLoS ONE 2012, 7, e52010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muxel, S.M.; Laranjeira-Silva, M.F.; Carvalho-Sousa, C.E.; Floeter-Winter, L.M.; Markus, R.P. The RelA/cRel nuclear factor-κ B (NF-κ B) dimer, crucial for inflammation resolution, mediates the transcription of the key enzyme in melatonin synthesis in RAW 264.7 macrophages. J. Pineal Res. 2016, 60, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, S.; Zeng, S.; Zhao, Y.; Zhu, C.; Deng, B.; Zhu, G.; Yin, Y.; Wang, W.; Hardeland, R.; et al. Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 2019, 66, e12547. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Shi, K.; Fu, M.; Chen, F. Melatonin indirectly decreases gastric cancer cell proliferation and invasion via effects on cancer-associated fibroblasts. Life Sci. 2021, 277, 119497. [Google Scholar] [CrossRef]
- Hanikoglu, A.; Kucuksayan, E.; Akduman, R.C.; Ozben, T. A Review on Melatonin’s Effects in Cancer: Potential Mechanisms. Anti-Cancer Agents Med. Chem. 2018, 18, 985–992. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-L.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Domínguez, N.; Ordóñez, R.; Fernández, A.; Blanco, C.M.; Baulies, A.; Garcia-Ruiz, C.; Fernández-Checa, J.C.; Mauriz, J.L.; González-Gallego, J. Melatonin-induced increase in sensitivity of human hepatocellular carcinoma cells to sorafenib is associated with reactive oxygen species production and mitophagy. J. Pineal Res. 2016, 61, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.E.; Coomber, B.L.; Bridle, B.W. Metabolic reprogramming in the tumour microenvironment: A hallmark shared by cancer cells and T lymphocytes. Immunology 2017, 152, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.-M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Martıín, M.; Macıías, M.; León, J.; Escames, G.; Khaldy, H.; Acuña-Castroviejo, D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell Biol. 2002, 34, 348–357. [Google Scholar] [CrossRef]
- Bizzarri, M.; Proietti, S.; Cucina, A.; Reiter, R.J. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: A review. Expert Opin. Ther. Targets 2013, 17, 1483–1496. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Chiu, K.-C.; Wang, T.-H.; Lan, W.-C.; Tsai, B.-H.; Wu, L.-J.; Hsia, S.-M.; Shieh, T.-M. Effects of melatonin to arecoline-induced reactive oxygen species production and DNA damage in oral squamous cell carcinoma. J. Formos. Med Assoc. 2021, 120, 668–678. [Google Scholar] [CrossRef]
- Chok, K.C.; Koh, R.Y.; Ng, M.G.; Ng, P.Y.; Chye, S.M. Melatonin Induces Autophagy via Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress Pathway in Colorectal Cancer Cells. Molecules 2021, 26, 5038. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Li, L.; Rui, C.; Liu, Y.; Liu, Z.; Tao, Z.; Cheng, H.; Liu, P. Induction of EnR stress by Melatonin enhances the cytotoxic effect of Lapatinib in HER2-positive breast cancer. Cancer Lett. 2021, 518, 82–93. [Google Scholar] [CrossRef]
- Innominato, P.F.; Lim, A.S.; Palesh, O.; Clemons, M.; Trudeau, M.; Eisen, A.; Wang, C.; Kiss, A.; Pritchard, K.I.; Bjarnason, G.A. The effect of melatonin on sleep and quality of life in patients with advanced breast cancer. Supportive Care Cancer Off. J. Multinatl. Assoc. Supportive Care Cancer 2016, 24, 1097–1105. [Google Scholar] [CrossRef]
- Lozano, A.; Marruecos, J.; Rubió, J.; Farré, N.; Gómez-Millán, J.; Morera, R.; Planas, I.; Lanzuela, M.; Vázquez-Masedo, M.G.; Cascallar, L.; et al. Randomized placebo-controlled phase II trial of high-dose melatonin mucoadhesive oral gel for the prevention and treatment of oral mucositis in patients with head and neck cancer undergoing radiation therapy concurrent with systemic treatment. Clin. Transl. Oncol. 2021, 23, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Elsabagh, H.H.; Moussa, E.; Mahmoud, S.A.; Elsaka, R.O.; Abdelrahman, H.; Hamed, H. Efficacy of Melatonin in prevention of radiation-induced oral mucositis: A randomized clinical trial. Oral Dis. 2020, 26, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Malugani, F.; Bukovec, R.; Bordin, V.; Perego, M.; Mengo, S.; Ardizzoia, A.; Tancini, G. Reduction of cisplatin-induced anemia by the pineal indole 5-methoxytryptamine in metastatic lung cancer patients. Neuro Endocrinol. Lett. 2003, 24, 83–85. [Google Scholar] [PubMed]
- Persson, C.; Glimelius, B.; Rönnelid, J.; Nygren, P. Impact of fish oil and melatonin on cachexia in patients with advanced gastrointestinal cancer: A randomized pilot study. Nutrition 2005, 21, 170–178. [Google Scholar] [CrossRef]
- Barni, S.; Lissoni, P.; Cazzaniga, M.; Ardizzoia, A.; Meregalli, S.; Fossati, V.; Fumagalli, L.; Brivio, F.; Tancini, G. A randomized study of low-dose subcutaneous interleukin-2 plus melatonin versus supportive care alone in metastatic colorectal cancer patients progressing under 5-fluorouracil and folates. Oncology 1995, 52, 243–245. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Meregalli, S.; Fossati, V.; Cazzaniga, M.E.; Esposti, D.; Tancini, G. Modulation of cancer endocrine therapy by melatonin: A phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. Br. J. Cancer 1995, 71, 854–856. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Giobbie-Hurder, A.; Gantman, K.; Savoie, J.; Scheib, R.; Parker, L.M.; Chen, W.Y. A randomized controlled trial of oral melatonin supplementation and breast cancer biomarkers. Cancer Causes Control 2012, 23, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Sookprasert, A.; Johns, N.P.; Phunmanee, A.; Pongthai, P.; Cheawchanwattana, A.; Johns, J.; Konsil, J.; Plaimee, P.; Porasuphatana, S.; Jitpimolmard, S. Melatonin in patients with cancer receiving chemotherapy: A randomized, double-blind, placebo-controlled trial. Anticancer Res. 2014, 34, 7327–7337. [Google Scholar]
- Seely, D.; Legacy, M.; Auer, R.C.; Fazekas, A.; Delic, E.; Anstee, C.; Angka, L.; Kennedy, M.A.; Tai, L.-H.; Zhang, T.; et al. Adjuvant melatonin for the prevention of recurrence and mortality following lung cancer resection (AMPLCaRe): A randomized placebo controlled clinical trial. eClinicalMedicine 2021, 33, 100763. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.M.; Han, Y.-S.; Yun, C.W.; Lee, S.H. Melatonin Promotes Apoptosis of Oxaliplatin-resistant Colorectal Cancer Cells Through Inhibition of Cellular Prion Protein. Anticancer Res. 2018, 38, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, A.; Sonohara, F.; Goel, A. Melatonin-mediated downregulation of thymidylate synthase as a novel mechanism for overcoming 5-fluorouracil associated chemoresistance in colorectal cancer cells. Carcinogenesis 2019, 40, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sang, X.; Wang, M.; Liu, Y.; Liu, J.; Wang, X.; Liu, P.; Cheng, H. Melatonin potentiates the cytotoxic effect of Neratinib in HER2+ breast cancer through promoting endocytosis and lysosomal degradation of HER2. Oncogene 2021, 40, 6273–6283. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Salehi, E.; Farhood, B.; Nashtaei, M.S.; Goradel, N.H.; Khanlarkhani, N.; Namjoo, Z.; Mortezaee, K. Adjuvant chemotherapy with melatonin for targeting human cancers: A review. J. Cell. Physiol. 2019, 234, 2356–2372. [Google Scholar] [CrossRef] [PubMed]
- Alonso-González, C.; González, A.; Menéndez-Menéndez, J.; Martínez-Campa, C.; Cos, S. Melatonin as a Radio-Sensitizer in Cancer. Biomedicines 2020, 8, 247. [Google Scholar] [CrossRef]
- Zhang, M.; Li, R.; Zhang, R.; Zhang, Y. Melatonin sensitizes esophageal cancer cells to 5-fluorouracil via promotion of apoptosis by regulating EZH2 expression. Oncol. Rep. 2021, 45, 22. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Goel, A. A combined treatment with melatonin and andrographis promotes autophagy and anti-cancer activity in colorectal cancer. Carcinogenesis 2022, bgac008. [Google Scholar] [CrossRef]
- Tran, Q.H.; Hoang, D.H.; Song, M.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Melatonin and doxorubicin synergistically enhance apoptosis via autophagy-dependent reduction of AMPKα1 transcription in human breast cancer cells. Exp. Mol. Med. 2021, 53, 1413–1422. [Google Scholar] [CrossRef]
- González-González, A.; Nieto, E.G.; Sánchez-Fernández, C.; Alonso-González, C.; Menéndez-Menéndez, J.; Gómez-Arozamena, J.; Cos, S.; Martínez-Campa, C. Melatonin Modulation of Radiation and Chemotherapeutics-induced Changes on Differentiation of Breast Fibroblasts. Int. J. Mol. Sci. 2019, 20, 3935. [Google Scholar] [CrossRef] [Green Version]
- Lissoni, P.; Meregalli, S.; Nosetto, L.; Barni, S.; Tancini, G.; Fossati, V.; Maestroni, G. Increased Survival Time in Brain Glioblastomas by a Radioneuroendocrine Strategy with Radiotherapy plus Melatonin Compared to Radiotherapy Alone. Oncology 1996, 53, 43–46. [Google Scholar] [CrossRef]
- Berk, L.; Berkey, B.; Rich, T.; Hrushesky, W.; Blask, D.; Gallagher, M.; Kudrimoti, M.; McGarry, R.C.; Suh, J.; Mehta, M. Randomized Phase II Trial of High-Dose Melatonin and Radiation Therapy for RPA Class 2 Patients With Brain Metastases (RTOG 0119). Int. J. Radiat. Oncol. 2007, 68, 852–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Baghban Rahimi, S.; Mohebbi, A.; Vakilzadeh, G.; Biglari, P.; Razeghi Jahromi, S.; Mohebi, S.R.; Shirian, S.; Gorji, A.; Ghaemi, A. Enhancement of therapeutic DNA vaccine potency by melatonin through inhibiting VEGF expression and induction of antitumor immunity mediated by CD8+ T cells. Arch. Virol. 2018, 163, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.C.R.; Porchia, B.F.M.M.; Pagni, R.L.; Souza, P.D.C.; Pegoraro, R.; Rodrigues, K.B.; Barros, T.B.; Aps, L.R.D.M.M.; De Araújo, E.F.; Calich, V.L.G.; et al. The Combined Use of Melatonin and an Indoleamine 2,3-Dioxygenase-1 Inhibitor Enhances Vaccine-Induced Protective Cellular Immunity to HPV16-Associated Tumors. Front. Immunol. 2018, 9, 1914. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, C.; Fu, Y.-X. Targeting innate sensing in the tumor microenvironment to improve immunotherapy. Cell. Mol. Immunol. 2020, 17, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Barni, S.; Cazzaniga, M.E.; Ardizzoia, A.; Rovelli, F.; Brivio, F.; Tancini, G. Efficacy of the Concomitant Administration of the Pineal Hormone Melatonin in Cancer Immunotherapy with Low-Dose IL-2 in Patients with Advanced Solid Tumors Who Had Progressed on IL-2 Alone. Oncology 1994, 51, 344–347. [Google Scholar] [CrossRef]
- Capelli, E.; Campo, I.; Panelli, S.; Damiani, G.; Barbone, M.G.S.; Lucchelli, A.; Cuccia, M. Evaluation of gene expression in human lymphocytes activated in the presence of melatonin. Int. Immunopharmacol. 2002, 2, 885–892. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Lee, K.-Y.; Wu, S.-M.; Kuo, D.-Y.; Shueng, P.-W.; Lin, C.-W. Melatonin Downregulates PD-L1 Expression and Modulates Tumor Immunity in KRAS-Mutant Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 5649. [Google Scholar] [CrossRef]
- Lissoni, P.M.G.; Borsotti, G.; Tosatto, A.; Frigerio, S.; Tassoni, S.; Fede, D.G. Modulation of Immune and Anti-Tumor Effects of Cancer Immunotherapy With Anti-Pd-1 Monoclonal Antibodies by the Pineal Hormone Melatonin: Preliminary Clinical Results. J. Immuno. Allerg 2020, 1, 1–6. [Google Scholar]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Pranil, T.; Moongngarm, A.; Loypimai, P. Influence of pH, temperature, and light on the stability of melatonin in aqueous solutions and fruit juices. Heliyon 2020, 6, e03648. [Google Scholar] [CrossRef] [PubMed]
- Daya, S.; Walker, R.; Glass, B.D.; Anoopkumar-Dukie, S. The effect of variations in pH and temperature on stability of melatonin in aqueous solution. J. Pineal Res. 2001, 31, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Zetner, D.; Rosenberg, J. Solubility and stability of melatonin in propylene glycol, glycofurol, and dimethyl sulfoxide. F1000Research 2020, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.; Yousefi, B.; Qujeq, D.; Marjani, A.; Asadi, J.; Wang, Z.; Mir, S.M. Melatonin and doxorubicin co-delivered via a functionalized graphene-dendrimeric system enhances apoptosis of osteosarcoma cells. Mater. Sci. Eng. C 2020, 119, 111554. [Google Scholar] [CrossRef]
- Sabzichi, M.; Samadi, N.; Mohammadian, J.; Hamishehkar, H.; Akbarzadeh, M.; Molavi, O. Sustained release of melatonin: A novel approach in elevating efficacy of tamoxifen in breast cancer treatment. Colloids Surf. B Biointerfaces 2016, 145, 64–71. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Ghassemi-Barghi, N. Melatonin Loading Chitosan-Tripolyphosphate Nanoparticles: Application in Attenuating Etoposide-Induced Genotoxicity in HepG2 Cells. Pharmacology 2018, 102, 74–80. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, W.; Li, Q.; Zhao, D.; Qu, J.; Yuan, Z.; Cheng, Z.; Zhu, X.; Zhuang, X.; Zhang, Z. 3D-printing magnesium–polycaprolactone loaded with melatonin inhibits the development of osteosarcoma by regulating cell-in-cell structures. J. Nanobiotechnology 2021, 19, 263. [Google Scholar] [CrossRef]
- Mirza-Aghazadeh-Attari, M.; Mihanfar, A.; Yousefi, B.; Majidinia, M. Nanotechnology-based advances in the efficient delivery of melatonin. Cancer Cell Int. 2022, 22, 43. [Google Scholar] [CrossRef]
- Kennaway, D.J. Measuring melatonin by immunoassay. J. Pineal Res. 2020, 69, e12657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Faassen, M.; Bischoff, R.; Kema, I.P. Relationship between plasma and salivary melatonin and cortisol investigated by LC-MS/MS. Clin. Chem. Lab. Med. 2017, 55, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzepka-Migut, B.; Paprocka, J. Melatonin-Measurement Methods and the Factors Modifying the Results. A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, B. Measurement of melatonin and 6-sulphatoxymelatonin. Methods Mol. Biol. 2013, 1065, 171–199. [Google Scholar] [PubMed]
- Kennaway, D.J. A critical review of melatonin assays: Past and present. J. Pineal Res. 2019, 67, e12572. [Google Scholar] [CrossRef] [Green Version]
- Foley, H.M.; Steel, A.E. Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 2018, 42, 65–81. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Du, K.; Xie, T.; Wang, B.; Chen, C.; Reiter, R.J.; Cen, B.; Yuan, Y. Pan-cancer analyses reveal genomics and clinical characteristics of the melatonergic regulators in cancer. J. Pineal Res. 2021, 71, e12758. [Google Scholar] [CrossRef]
- Bagheri, H.; Jafarpour, S.M.; Shekarchi, B.; Farhood, B. The radioprotective effects of melatonin and nanoselenium on DNA double-strand breaks in peripheral lymphocytes caused by I-131. Indian J. Nucl. Med. 2021, 36, 134–139. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Sundquist, J.; Sundquist, K.; Ji, J. Use of Melatonin Is Associated With Lower Risk of Colorectal Cancer in Older Adults. Clin. Transl. Gastroenterol. 2021, 12, e00396. [Google Scholar] [CrossRef]
- Wong, A.T.; Fensom, G.K.; Key, T.J.; Onland-Moret, N.C.; Tong, T.Y.; Travis, R.C. Urinary Melatonin in Relation to Breast Cancer Risk: Nested Case–Control Analysis in the DOM Study and Meta-analysis of Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2021, 30, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Artime, A.; Cernuda-Cernuda, R.; Cepas, V.; Gonzalez-Menendez, P.; Fernadez-Vega, S.; Quiros-Gonzalez, I.; Sainz, R.M.; Mayo, J.C.; Naveda, F.A. Melatonin-Induced Cytoskeleton Reorganization Leads to Inhibition of Melanoma Cancer Cell Proliferation. Int. J. Mol. Sci. 2020, 21, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Kwok, C.C.-H.; Chan, D.C.-W.; Ho, A.W.-Y.; Ho, C.-S.; Zhang, J.; Wing, Y.K.; Wang, F.; Tse, L.A. Disruption of sleep, sleep-wake activity rhythm, and nocturnal melatonin production in breast cancer patients undergoing adjuvant chemotherapy: Prospective cohort study. Sleep Med. 2019, 55, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Novik, A.V.; Protsenko, S.A.; Baldueva, I.A.; Berstein, L.M.; Anisimov, V.N.; Zhuk, I.N.; Semenova, A.I.; Latipova, D.K.; Tkachenko, E.V.; Semiglazova, T.Y. Melatonin and Metformin Failed to Modify the Effect of Dacarbazine in Melanoma. Oncologist 2021, 26, 364–e734. [Google Scholar] [CrossRef] [PubMed]
| Evidence Type | Study Type | Conclusion | Reference |
|---|---|---|---|
| Positive evidence |
|
| [27] |
|
| [81] | |
|
| [82,83] | |
|
| [84] | |
|
| [85] | |
|
| [86] | |
|
| [87] | |
| Negative evidence |
|
| [88] |
|
| [89] | |
|
| [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, C.; Choi, W.S. Use of Melatonin in Cancer Treatment: Where Are We? Int. J. Mol. Sci. 2022, 23, 3779. https://doi.org/10.3390/ijms23073779
Wang L, Wang C, Choi WS. Use of Melatonin in Cancer Treatment: Where Are We? International Journal of Molecular Sciences. 2022; 23(7):3779. https://doi.org/10.3390/ijms23073779
Chicago/Turabian StyleWang, Leilei, Chuan Wang, and Wing Shan Choi. 2022. "Use of Melatonin in Cancer Treatment: Where Are We?" International Journal of Molecular Sciences 23, no. 7: 3779. https://doi.org/10.3390/ijms23073779
APA StyleWang, L., Wang, C., & Choi, W. S. (2022). Use of Melatonin in Cancer Treatment: Where Are We? International Journal of Molecular Sciences, 23(7), 3779. https://doi.org/10.3390/ijms23073779






