Transdermal Delivery of Indirubin-Loaded Microemulsion Gel: Preparation, Characterization and Anti-Psoriatic Activity
Abstract
:1. Introduction
2. Results
2.1. Preparation of IR-ME and IR-ME Gel
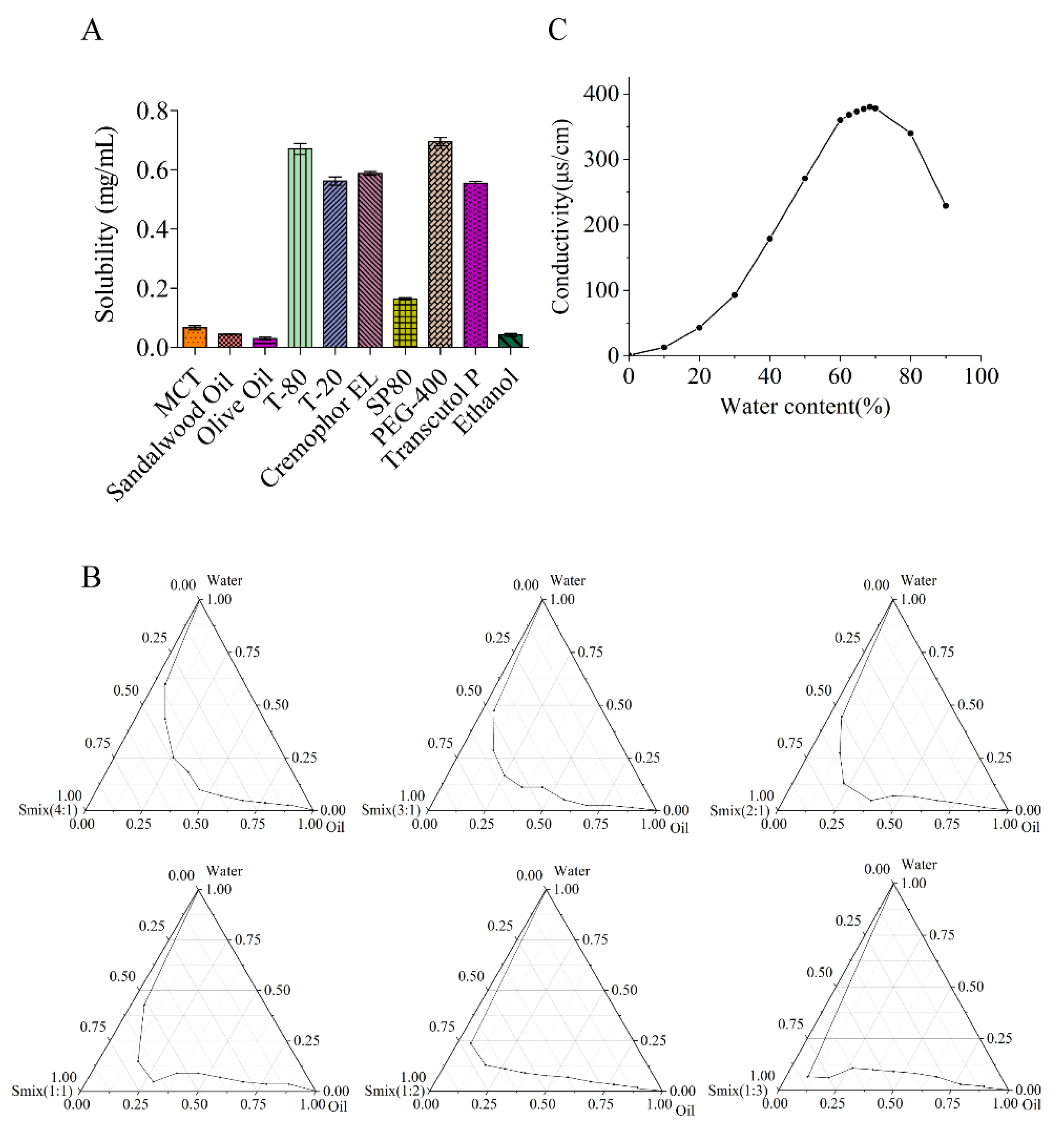
2.1.1. Solubility Study and Selection of Adjuvants for ME System
2.1.2. Determination of Efficient ME Region
2.1.3. Determination of Water Content of ME
2.1.4. Preparation of IR-ME Gel
2.2. Characterization of IR-ME and IR-ME Gel
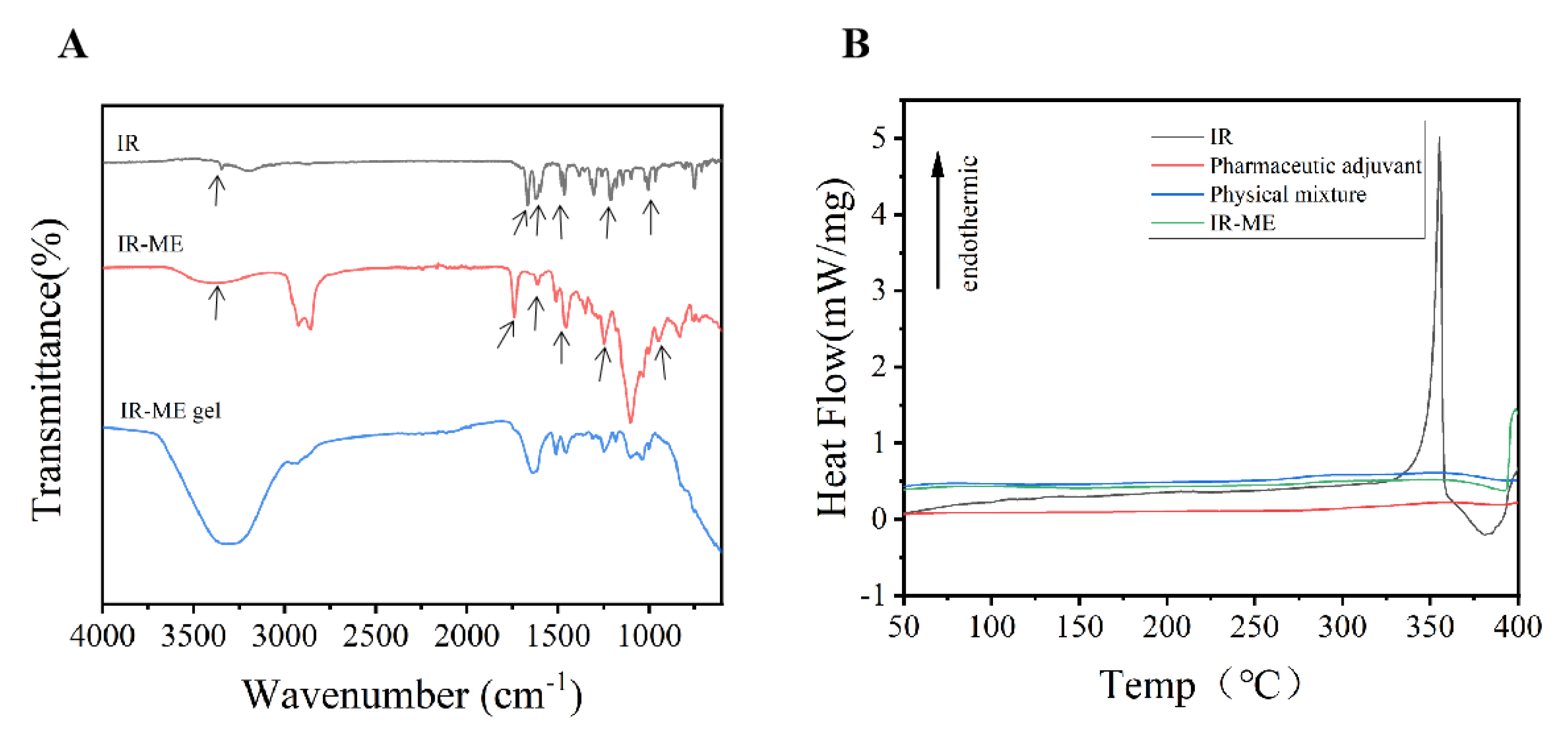
2.2.1. Fourier-Transform Infrared (FT-IR) Analysis
2.2.2. Differential Scanning Calorimetry (DSC) Analysis
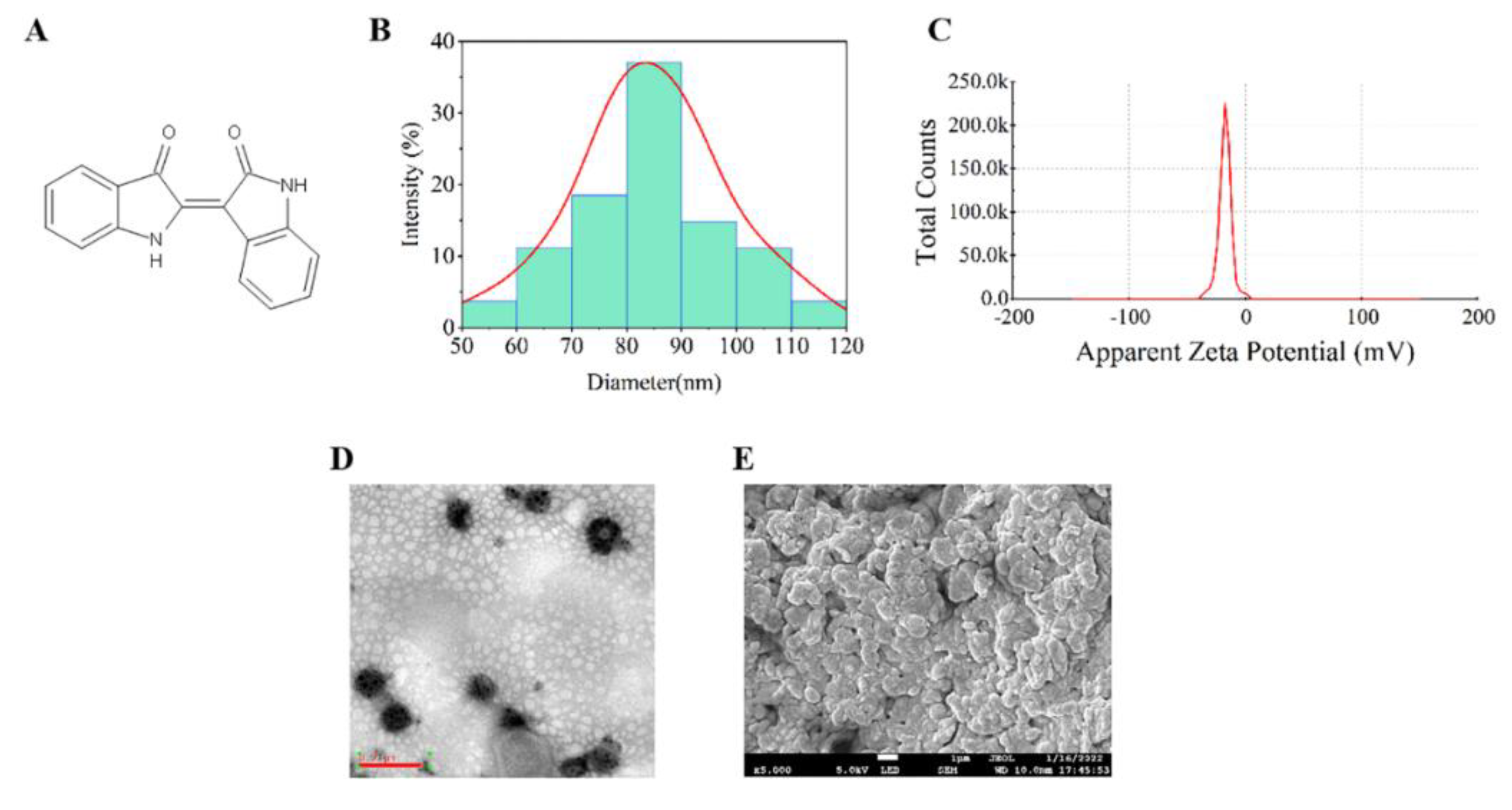
2.2.3. TEM and SEM Analysis
2.3. In Vitro Skin Permeation and Deposition Study
2.4. In Vivo Studies
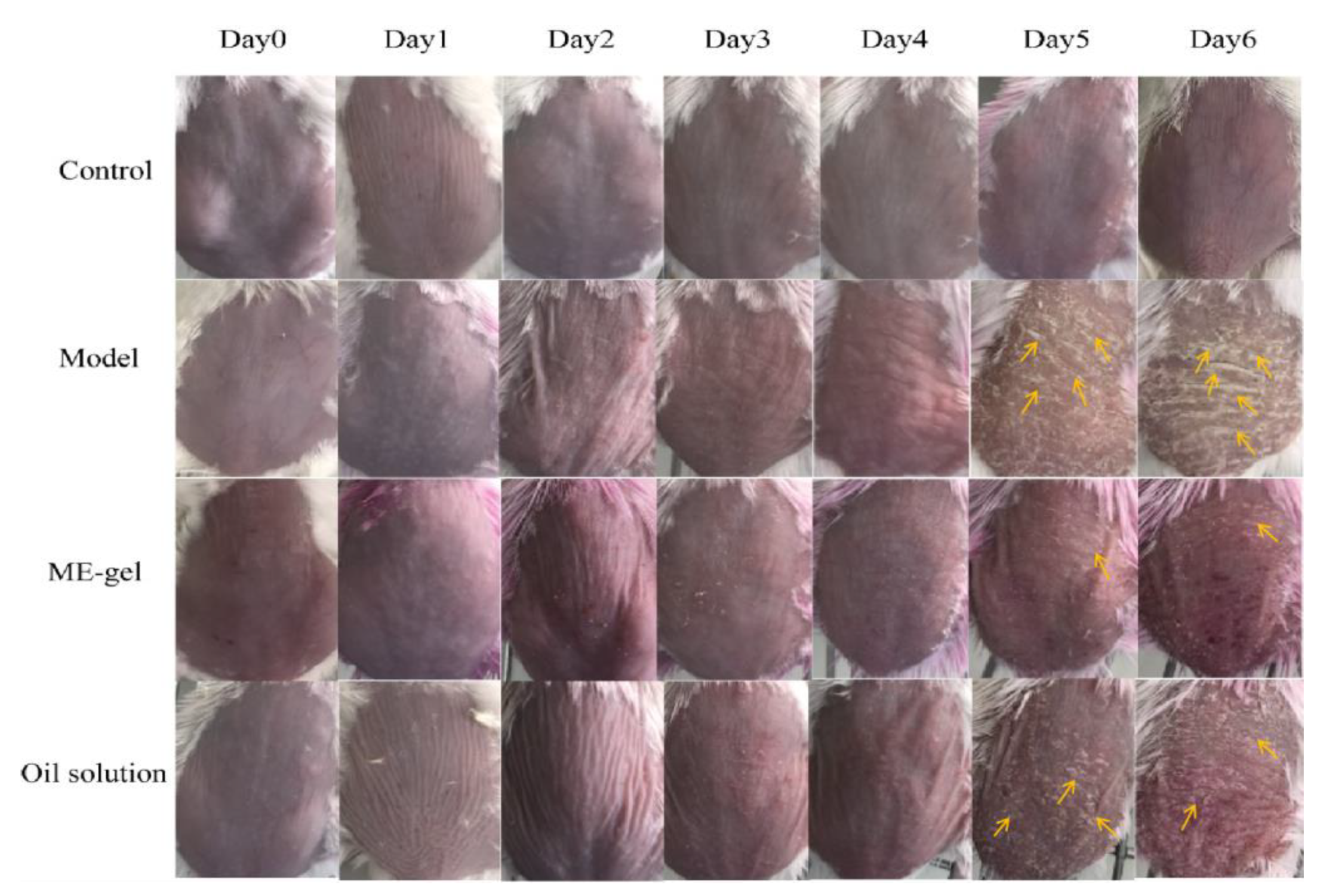
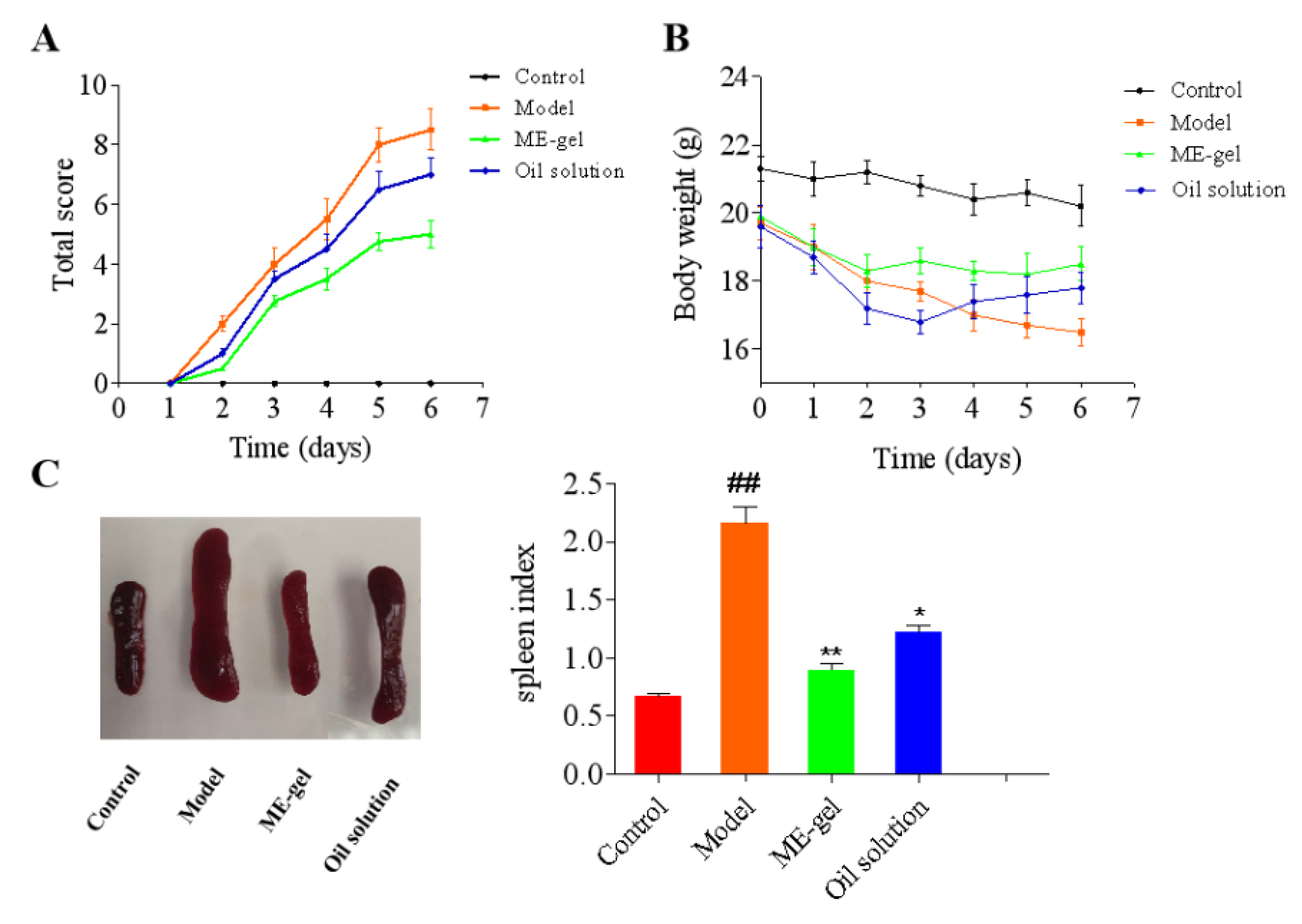
2.4.1. Macroscopic Observation of Dorsal Tissue
2.4.2. Body Weight and Spleen Index of Mice
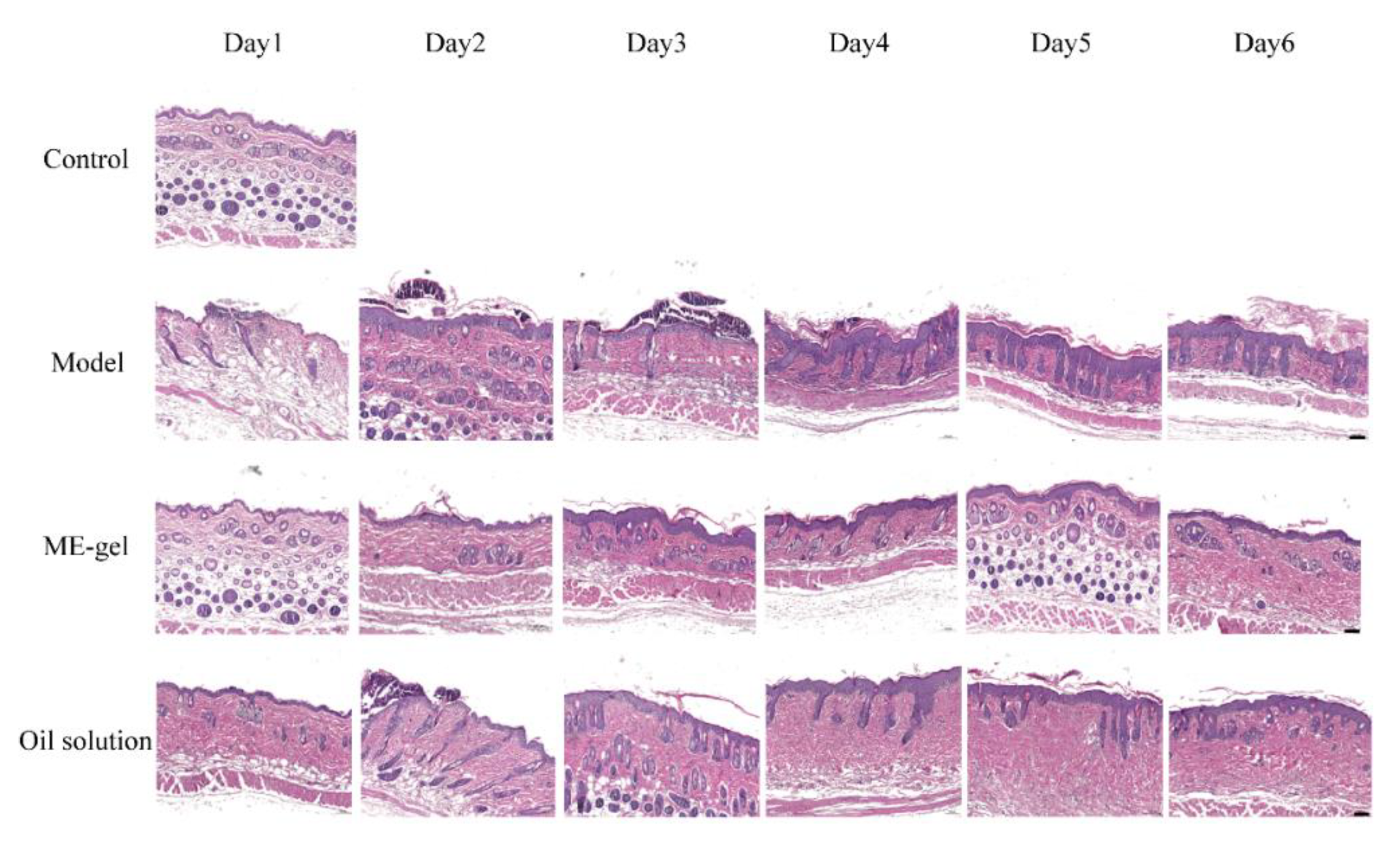
2.4.3. Skin Histopathology
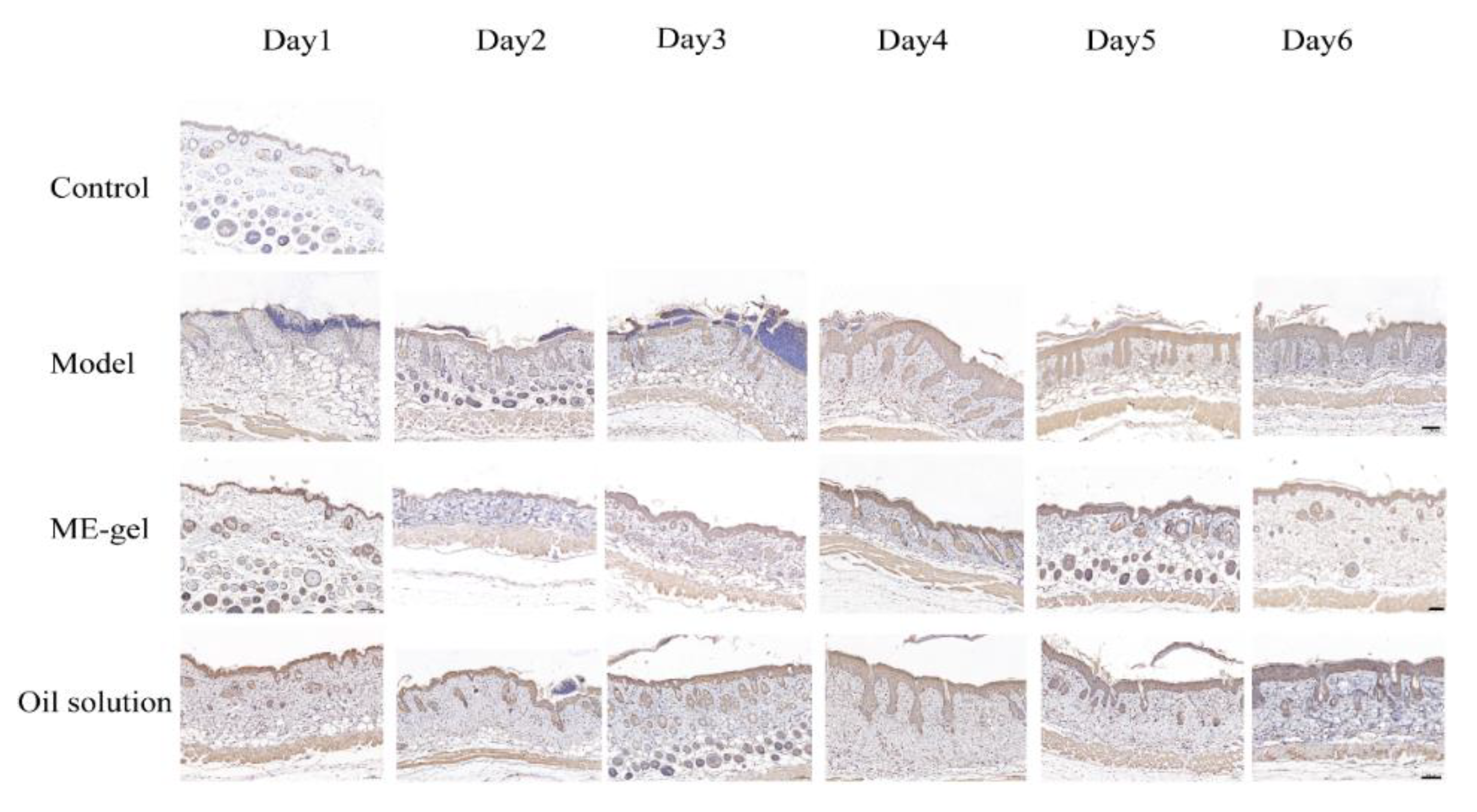
2.4.4. Immunohistochemical
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. IR Detection Method
4.3. Preparation of IR-ME and IR-ME Gel
4.4. Characterization of IR-ME and IR-ME Gel
4.5. In Vitro Transdermal Test
4.5.1. Preparation of Excised Skin
4.5.2. In Vitro Transdermal Test
4.6. In Vivo Studies
4.6.1. Experimental Design
4.6.2. Assessment of Psoriasis-like Symptoms
4.6.3. Histopathology
4.6.4. Immunohistochemistry
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bieber, T. AhR Modulating Agent for the Topical Therapy of Plaque Psoriasis. N. Engl. J. Med. 2021, 385, 2295–2296. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.-R.; Tampa, M.; Caruntu, C.; Sarbu, M.-I.; Mitran, C.-I.; Mitran, M.-I.; Matei, C.; Constantin, C.; Neagu, M. Advances in Understanding the Immunological Pathways in Psoriasis. Int. J. Mol. Sci. 2019, 20, 739. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Prinz, I.; Sandrock, I.; Mrowietz, U. Interleukin-17 cytokines: Effectors and targets in psoriasis-A breakthrough in understanding and treatment. J. Exp. Med. 2020, 217, e20191397. [Google Scholar] [CrossRef] [PubMed]
- Benezeder, T.; Wolf, P. Resolution of plaque-type psoriasis: What is left behind (and reinitiates the disease). Semin. Immunopathol. 2019, 41, 633–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, J.; Elman, S.; Perez-Chada, L.; Mita, C.; Merola, J.; LeBoeuf, N. Treatment of Immune Checkpoint Inhibitor-Mediated Psoriasis: A Systematic Review. J. Am. Acad. Dermatol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Rapalli, V.; Waghule, T.; Gorantla, S.; Dubey, S.; Saha, R.; Singhvi, G. Psoriasis: Pathological mechanisms, current pharmacological therapies, and emerging drug delivery systems. Drug Discov. Today 2020, 25, 2212–2226. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, C.; Alalaiwe, A.; Yang, S.; Fang, J. Apoptotic or Antiproliferative Activity of Natural Products against Keratinocytes for the Treatment of Psoriasis. Int. J. Mol. Sci. 2019, 20, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonesi, M.; Loizzo, M.; Provenzano, E.; Menichini, F.; Tundis, R. Anti-Psoriasis Agents from Natural Plant Sources. Curr. Med. Chem. 2016, 23, 1250–1267. [Google Scholar] [CrossRef] [PubMed]
- Gamret, A.; Price, A.; Fertig, R.; Lev-Tov, H.; Nichols, A. Complementary and Alternative Medicine Therapies for Psoriasis: A Systematic Review. JAMA Dermatol. 2018, 154, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, G.; Magiatis, P.; Velegraki, A.; Bassukas, I.D. A traditional Chinese remedy points to a natural skin habitat: Indirubin (indigo naturalis) for psoriasis and the Malassezia metabolome. Br. J. Dermatol. 2018, 179, 800. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-J.; Di, T.-T.; Wang, Y.; Wang, M.-X.; Meng, Y.-J.; Lin, Y.; Xu, X.-L.; Li, P.; Zhao, J.-X. Indirubin ameliorates imiquimod-induced psoriasis-like skin lesions in mice by inhibiting inflammatory responses mediated by IL-17A-producing γδ T cells. Mol. Immunol. 2018, 101, 386–395. [Google Scholar] [CrossRef]
- Lin, Y.K.; See, L.C.; Huang, Y.H.; Chi, C.C.; Hui, R.C. Comparison of indirubin concentrations in indigo naturalis ointment for psoriasis treatment: A randomized, double-blind, dosage-controlled trial. Br. J. Dermatol. 2018, 178, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Gaboriaud-Kolar, N.; Vougogiannopoulou, K.; Skaltsounis, A. Indirubin derivatives: A patent review (2010–present). Expert Opin. Ther. Pat. 2015, 25, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Akhter, S.; Beg, S. Nanomedicine Advances in Topical Infective and Non-Infective Skin Diseases Therapy. Recent Pat. Anti-Infect. Drug Discov. 2018, 13, 104. [Google Scholar] [CrossRef]
- Saleem, S.; Iqubal, M.; Garg, S.; Ali, J.; Baboota, S. Trends in nanotechnology-based delivery systems for dermal targeting of drugs: An enticing approach to offset psoriasis. Expert Opin. Drug Deliv. 2020, 17, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.; Hill, D.; Feldman, S. Current challenges and emerging drug delivery strategies for the treatment of psoriasis. Expert Opin. Drug Deliv. 2016, 13, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lin, H.; Wang, Y.; Wang, X.; Yao, J.; Fu, X.; Yu, X. Design, optimization and evaluation of a microemulsion-based hydrogel with high malleability for enhanced transdermal delivery of levamisole. Int. J. Pharm. 2021, 605, 120829. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shen, J.; Bi, J.; Tian, H.; Jin, Y.; Wang, Y.; Yang, X.; Yang, Z.; Kou, J. Preparation and evaluation of a self-nanoemulsifying drug delivery system loaded with Akebia saponin D-phospholipid complex. Int. J. Nanomed. 2016, 11, 4919–4929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salimi, A.; Sharif Makhmal Zadeh, B.; Hemati, A.; Akbari Birgani, S. Design and Evaluation of Self-Emulsifying Drug Delivery System (SEDDS) Of Carvedilol to Improve the Oral Absorption. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e16125. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Patel, N.; Shah, M.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Deore, G.; Chabukswar, A. Development of Microemulsion Based Nabumetone Transdermal Delivery for Treatment of Arthritis. Recent Pat. Drug Deliv. Formul. 2018, 12, 130–149. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduño-Ramirez, M.L.; Clares, B.; García, M.L.; Calpena, A.C. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Pajić, N.Z.B.; Todosijević, M.N.; Vuleta, G.M.; Cekic, N.; Dobričić, V.D.; Vučen, S.R.; Čalija, B.R.; Lukic, M.; Ilić, T.M.; Savic, S. Alkyl polyglucoside vs. ethoxylated surfactant-based microemulsions as vehicles for two poorly water-soluble drugs: Physicochemical characterization and in vivo skin performance. Acta Pharm. 2017, 67, 415–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szumała, P.; Macierzanka, A. Topical delivery of pharmaceutical and cosmetic macromolecules using microemulsion systems. Int. J. Pharm. 2022, 615, 121488. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent Update on Nanoemulgel as Topical Drug Delivery System. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Sun, J.; Liu, Y. Molecular Structures and Spectral Properties of Natural Indigo and Indirubin: Experimental and DFT Studies. Molecules 2019, 24, 3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jangdey, M.; Gupta, A.; Saraf, S. Fabrication, in-vitro characterization, and enhanced in-vivo evaluation of carbopol-based nanoemulsion gel of apigenin for UV-induced skin carcinoma. Drug Deliv. 2017, 24, 1026–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotta, S.; Khan, A.; Ansari, S.; Sharma, R.; Ali, J. Formulation of nanoemulsion: A comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015, 22, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hu, R.; Wang, B.; Gui, Y.; Cheng, G.; Gao, S.; Ye, L.; Tang, J. Self-microemulsifying drug delivery system for improving the bioavailability of huperzine A by lymphatic uptake. Acta Pharm. Sin. B 2017, 7, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Mao, X.; Zhang, J.; Sun, P.; Wang, H.; Zhang, Y.; Ma, Y.; Xu, S.; Lv, R.; Liu, X. Oral delivery of lycopene-loaded microemulsion for brain-targeting: Preparation, characterization, pharmacokinetic evaluation and tissue distribution. Drug Deliv. 2019, 26, 1191–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharib, R.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Drug-in-cyclodextrin-in-liposomes as a carrier system for volatile essential oil components: Application to anethole. Food Chem. 2017, 218, 365–371. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Q a | Jss b | Q (Skin) c |
|---|---|---|---|
| ME | 47.34 ± 3.59 | 1.96 ± 0.08 | 8.77 ± 1.26 |
| ME gel (1%) | 39.01 ± 3.21 | 1.65 ± 0.12 | 6.5 ± 0.6 |
| Oil solution | 22.21 ± 2.14 | 0.94 ± 0.09 | 4.18 ± 0.79 |
| Aqueous solution | 3.60 ± 0.11 | 0.05 ± 0.01 | 2.68 ± 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, E.; Li, H.; Li, X.; Wu, X.; Lei, K.; Diao, Y. Transdermal Delivery of Indirubin-Loaded Microemulsion Gel: Preparation, Characterization and Anti-Psoriatic Activity. Int. J. Mol. Sci. 2022, 23, 3798. https://doi.org/10.3390/ijms23073798
He E, Li H, Li X, Wu X, Lei K, Diao Y. Transdermal Delivery of Indirubin-Loaded Microemulsion Gel: Preparation, Characterization and Anti-Psoriatic Activity. International Journal of Molecular Sciences. 2022; 23(7):3798. https://doi.org/10.3390/ijms23073798
Chicago/Turabian StyleHe, Enxue, Hailing Li, Xiaokun Li, Xunxun Wu, Kun Lei, and Yong Diao. 2022. "Transdermal Delivery of Indirubin-Loaded Microemulsion Gel: Preparation, Characterization and Anti-Psoriatic Activity" International Journal of Molecular Sciences 23, no. 7: 3798. https://doi.org/10.3390/ijms23073798
APA StyleHe, E., Li, H., Li, X., Wu, X., Lei, K., & Diao, Y. (2022). Transdermal Delivery of Indirubin-Loaded Microemulsion Gel: Preparation, Characterization and Anti-Psoriatic Activity. International Journal of Molecular Sciences, 23(7), 3798. https://doi.org/10.3390/ijms23073798








