Shining Damaged Hearts: Immunotherapy-Related Cardiotoxicity in the Spotlight of Nuclear Cardiology
Abstract
:1. Introduction
2. Molecular Imaging: Visualization of Metabolic Pathways and Body Function
2.1. Molecular Imaging Tracers and Target Structures
2.2. Planar Scintigraphy and Single Photon Emission Computed Tomography
2.3. Positron Emission Tomography
3. Imaging of Glucose Consumption
4. Imaging of Cardiac Remodeling and SSTR-Expression
5. Imaging of Myocardial Damage
6. Investigation of Myocardial Perfusion and Viability
7. Assessment of Ventricular Function and Chamber Morphology
8. Future Trends
8.1. New Molecular Targets
8.2. Technological Perspectives
8.3. Artificial Intelligence-Based Approaches and Dynamic PET
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2021, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Rassaf, T.; Totzeck, M.; Backs, J.; Bokemeyer, C.; Hallek, M.; Hilfiker-Kleiner, D.; Hochhaus, A.; Lüftner, D.; Müller, O.J.; Neudorf, U.; et al. Onco-Cardiology: Consensus Paper of the German Cardiac Society, the German Society for Pediatric Cardiology and Congenital Heart Defects and the German Society for Hematology and Medical Oncology. Clin. Res. Cardiol. 2020, 109, 1197–1222. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef]
- Campia, U.; Moslehi, J.J.; Amiri-Kordestani, L.; Barac, A.; Beckman, J.A.; Chism, D.D.; Cohen, P.; Groarke, J.D.; Herrmann, J.; Reilly, C.M.; et al. Cardio-Oncology: Vascular and Metabolic Perspectives: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e579–e602. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Michel, L.; Schadendorf, D.; Rassaf, T. Oncocardiology: New challenges, new opportunities. Herz 2020, 45, 619–625. [Google Scholar] [CrossRef]
- Lobenwein, D.; Kocher, F.; Dobner, S.; Gollmann-Tepeköylü, C.; Holfeld, J. Cardiotoxic mechanisms of cancer immunotherapy–A systematic review. Int. J. Cardiol. 2021, 323, 179–187. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Gronich, N.; Lavi, I.; Barnett-Griness, O.; Saliba, W.; Abernethy, D.R.; Rennert, G. Tyrosine kinase-targeting drugs-associated heart failure. Br. J. Cancer 2017, 116, 1366–1373. [Google Scholar] [CrossRef] [Green Version]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Totzeck, M.; Schuler, M.; Stuschke, M.; Heusch, G.; Rassaf, T. Cardio-oncology-strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 2019, 280, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Mincu, R.I.; Rassaf, T. Cardiovascular Adverse Events in Patients With Cancer Treated With Bevacizumab: A Meta-Analysis of More Than 20 000 Patients. J. Am. Heart Assoc. 2017, 6, e006278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mincu, R.I.; Mahabadi, A.A.; Michel, L.; Mrotzek, S.M.; Schadendorf, D.; Rassaf, T.; Totzeck, M. Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e198890. [Google Scholar] [CrossRef] [Green Version]
- Totzeck, M.; Lutgens, E.; Neilan, T.G. Are we underestimating the potential for cardiotoxicity related to immune checkpoint inhibitors? Eur. Heart J. 2021, 42, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, F.; Poto, R.; Aran, L.; Cuomo, A.; Galdiero, M.R.; Spadaro, G.; Abete, P.; Bonaduce, D.; Marone, G.; Tocchetti, C.G.; et al. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: Clinical Risk Factors. Curr. Oncol. Rep. 2021, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Nielsen, D.; Svane, I.M.; Iversen, K.; Rasmussen, P.V.; Madelaire, C.; Fosbøl, E.; Køber, L.; Gustafsson, F.; Andersson, C.; et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: A nationwide Danish study. Eur. Heart J. 2021, 42, 1621–1631. [Google Scholar] [CrossRef]
- Michel, L.; Totzeck, M.; Lehmann, L.; Finke, D. Emerging role of immune checkpoint inhibitors and their relevance for the cardiovascular system. Herz 2020, 45, 645–651. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Barrett, D.M.; Teachey, D.T.; Grupp, S.A. Toxicity management for patients receiving novel T-cell engaging therapies. Curr. Opin. Pediatr. 2014, 26, 43–49. [Google Scholar] [CrossRef]
- Bonifant, C.L.; Jackson, H.J.; Brentjens, R.J.; Curran, K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther.-Oncolytics 2016, 3, 16011. [Google Scholar] [CrossRef]
- Totzeck, M.; Michel, L.; Lin, Y.; Herrmann, J.; Rassaf, T. Cardiotoxicity from chimeric antigen receptor-T cell therapy for advanced malignancies. Eur. Heart J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.V.; Slomka, P.; Moody, J.B.; Germano, G.; Ficaro, E.P. Quantitative Clinical Nuclear Cardiology, Part 1: Established Applications. J. Nucl. Cardiol. 2020, 27, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Slomka, P.J.; Moody, J.B.; Miller, R.J.H.; Renaud, J.M.; Ficaro, E.P.; Garcia, E.V. Quantitative clinical nuclear cardiology, part 2: Evolving/emerging applications. J. Nucl. Med. 2021, 62, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ruth, T.J. The uses of radiotracers in the life sciences. Rep. Prog. Phys. 2008, 72, 016701. [Google Scholar] [CrossRef]
- Wadsak, W.; Mitterhauser, M. Basics and principles of radiopharmaceuticals for PET/CT. Eur. J. Radiol. 2010, 73, 461–469. [Google Scholar] [CrossRef]
- Bar-Shalom, R.; Valdivia, A.Y.; Blaufox, M.D. PET imaging in oncology. Semin. Nucl. Med. 2000, 30, 150–185. [Google Scholar] [CrossRef] [PubMed]
- Farwell, M.D.; Pryma, D.A.; Mankoff, D.A. PET/CT imaging in cancer: Current applications and future directions. Cancer 2014, 120, 3433–3445. [Google Scholar] [CrossRef]
- Siegel, B.A.; Dehdashti, F. Oncologic PET/CT: Current status and controversies. Eur. Radiol. 2005, 15 (Suppl. 4), D127–D132. [Google Scholar] [CrossRef]
- Brandon, D.; Alazraki, A.; Halkar, R.K.; Alazraki, N.P. The role of single-photon emission computed tomography and SPECT/computed tomography in oncologic imaging. Semin. Oncol. 2011, 38, 87–108. [Google Scholar] [CrossRef]
- Cai, J.; Li, F. Single-photon emission computed tomography tracers for predicting and monitoring cancer therapy. Curr. Pharm. Biotechnol. 2013, 14, 693–707. [Google Scholar] [CrossRef]
- Chowdhury, F.U.; Scarsbrook, A.F. The role of hybrid SPECT-CT in oncology: Current and emerging clinical applications. Clin. Radiol. 2008, 63, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Gerber, G.; Chodak, G.W. Assessment of value of routine bone scans in patients with newly diagnosed prostate cancer. Urology 1991, 37, 418–422. [Google Scholar] [CrossRef]
- Krasnow, A.Z.; Hellman, R.S.; Timins, M.E.; Collier, B.D.; Anderson, T.; Isitman, A.T. Diagnostic bone scanning in oncology. Semin. Nucl. Med. 1997, 27, 107–141. [Google Scholar] [CrossRef]
- Ryan, P.J.; Fogelman, I. The bone scan: Where are we now? Semin. Nucl. Med. 1995, 25, 76–91. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Strobel, K.; Kampen, W.U.; Kuwert, T.; van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; Beheshti, M.; et al. The EANM practice guidelines for bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boy, C.; Heusner, T.A.; Poeppel, T.D.; Redmann-Bischofs, A.; Unger, N.; Jentzen, W.; Brandau, W.; Mann, K.; Antoch, G.; Bockisch, A.; et al. 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1-sst5) expression in normal human tissue: Correlation of sst2 mRNA and SUVmax. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Deppen, S.A.; Liu, E.; Blume, J.D.; Clanton, J.; Shi, C.; Jones-Jackson, L.B.; Lakhani, V.; Baum, R.P.; Berlin, J.; Smith, G.T.; et al. Safety and Efficacy of 68Ga-DOTATATE PET/CT for Diagnosis, Staging, and Treatment Management of Neuroendocrine Tumors. J. Nucl. Med. 2016, 57, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Frilling, A.; Sotiropoulos, G.C.; Radtke, A.; Malago, M.; Bockisch, A.; Kuehl, H.; Li, J.; Broelsch, C.E. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann. Surg. 2010, 252, 850–856. [Google Scholar] [CrossRef]
- Herrmann, K.; Czernin, J.; Wolin, E.M.; Gupta, P.; Barrio, M.; Gutierrez, A.; Schiepers, C.; Mosessian, S.; Phelps, M.E.; Allen-Auerbach, M.S. Impact of 68Ga-DOTATATE PET/CT on the management of neuroendocrine tumors: The referring physician’s perspective. J. Nucl. Med. 2015, 56, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.S.; Lau, W.F.; Hicks, R.J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef] [Green Version]
- Sawicki, L.M.; Deuschl, C.; Beiderwellen, K.; Ruhlmann, V.; Poeppel, T.D.; Heusch, P.; Lahner, H.; Fuhrer, D.; Bockisch, A.; Herrmann, K.; et al. Evaluation of 68Ga-DOTATOC PET/MRI for whole-body staging of neuroendocrine tumours in comparison with 68Ga-DOTATOC PET/CT. Eur. Radiol. 2017, 27, 4091–4099. [Google Scholar] [CrossRef] [PubMed]
- Skoura, E.; Michopoulou, S.; Mohmaduvesh, M.; Panagiotidis, E.; Al Harbi, M.; Toumpanakis, C.; Almukhailed, O.; Kayani, I.; Syed, R.; Navalkissoor, S.; et al. The Impact of 68Ga-DOTATATE PET/CT Imaging on Management of Patients with Neuroendocrine Tumors: Experience from a National Referral Center in the United Kingdom. J. Nucl. Med. 2016, 57, 34–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fendler, W.P.; Schmidt, D.F.; Wenter, V.; Thierfelder, K.M.; Zach, C.; Stief, C.; Bartenstein, P.; Kirchner, T.; Gildehaus, F.J.; Gratzke, C.; et al. 68Ga-PSMA PET/CT Detects the Location and Extent of Primary Prostate Cancer. J. Nucl. Med. 2016, 57, 1720–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fendler, W.P.; Weber, M.; Iravani, A.; Hofman, M.S.; Calais, J.; Czernin, J.; Ilhan, H.; Saad, F.; Small, E.J.; Smith, M.R.; et al. Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 7448–7454. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of 68Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J.; Maurer, T.; Eiber, M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics 2018, 38, 200–217. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Maurer, T.; Eiber, M.; Schwaiger, M.; Gschwend, J.E. Current use of PSMA-PET in prostate cancer management. Nat. Rev. Urol 2016, 13, 226–235. [Google Scholar] [CrossRef]
- Weber, M.; Kurek, C.; Barbato, F.; Eiber, M.; Maurer, T.; Nader, M.; Hadaschik, B.; Grunwald, V.; Herrmann, K.; Wetter, A.; et al. PSMA-Ligand PET for Early Castration-Resistant Prostate Cancer: A Retrospective Single-Center Study. J. Nucl. Med. 2021, 62, 88–91. [Google Scholar] [CrossRef]
- Bodei, L.; Cremonesi, M.; Grana, C.; Rocca, P.; Bartolomei, M.; Chinol, M.; Paganelli, G. Receptor radionuclide therapy with 90Y-[DOTA]0-Tyr3-octreotide (90Y-DOTATOC) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Delpassand, E.S.; Samarghandi, A.; Zamanian, S.; Wolin, E.M.; Hamiditabar, M.; Espenan, G.D.; Erion, J.L.; O’Dorisio, T.M.; Kvols, L.K.; Simon, J.; et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: The first US phase 2 experience. Pancreas 2014, 43, 518–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frilling, A.; Weber, F.; Saner, F.; Bockisch, A.; Hofmann, M.; Mueller-Brand, J.; Broelsch, C.E. Treatment with 90Y- and 177Lu-DOTATOC in patients with metastatic neuroendocrine tumors. Surgery 2006, 140, 968–976; discussion 976–977. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Garaventa, A.; Bellagamba, O.; Lo Piccolo, M.S.; Milanaccio, C.; Lanino, E.; Bertolazzi, L.; Villavecchia, G.P.; Cabria, M.; Scopinaro, G.; Claudiani, F.; et al. 131I-metaiodobenzylguanidine (131I-MIBG) therapy for residual neuroblastoma: A mono-institutional experience with 43 patients. Br. J. Cancer 1999, 81, 1378–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langbein, T.; Weber, W.A.; Eiber, M. Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine. J. Nucl. Med. 2019, 60, 13S–19S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pryma, D.A.; Chin, B.B.; Noto, R.B.; Dillon, J.S.; Perkins, S.; Solnes, L.; Kostakoglu, L.; Serafini, A.N.; Pampaloni, M.H.; Jensen, J.; et al. Efficacy and Safety of High-Specific-Activity 131I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J. Nucl. Med. 2019, 60, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Fendler, W.P.; Rahbar, K.; Herrmann, K.; Kratochwil, C.; Eiber, M. 177Lu-PSMA Radioligand Therapy for Prostate Cancer. J. Nucl. Med. 2017, 58, 1196–1200. [Google Scholar] [CrossRef] [Green Version]
- Gafita, A.; Fendler, W.P.; Hui, W.; Sandhu, S.; Weber, M.; Esfandiari, R.; Calais, J.; Rauscher, I.; Rathke, H.; Tauber, R.; et al. Efficacy and Safety of 177Lu-labeled Prostate-specific Membrane Antigen Radionuclide Treatment in Patients with Diffuse Bone Marrow Involvement: A Multicenter Retrospective Study. Eur. Urol. 2020, 78, 148–154. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benesova, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Foldes, I.; Levay, A.; Stotz, G. Comparative scanning of thyroid nodules with technetium-99m pertechnetate and technetium-99m methoxyisobutylisonitrile. Eur. J. Nucl. Med. 1993, 20, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Avram, A.M.; Iakovou, I.; Kwak, J.; Lawson, S.A.; Lulaj, E.; Luster, M.; Piccardo, A.; Schmidt, M.; Tulchinsky, M.; et al. EANM practice guideline/SNMMI procedure standard for RAIU and thyroid scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Ianni, F.; Perotti, G.; Prete, A.; Paragliola, R.M.; Ricciato, M.P.; Carrozza, C.; Salvatori, M.; Pontecorvi, A.; Corsello, S.M. Thyroid scintigraphy: An old tool is still the gold standard for an effective diagnosis of autonomously functioning thyroid nodules. J. Endocrinol. Investig. 2013, 36, 233–236. [Google Scholar] [CrossRef]
- Meller, J.; Becker, W. The continuing importance of thyroid scintigraphy in the era of high-resolution ultrasound. Eur. J. Nucl. Med. Mol. Imaging 2002, 29 (Suppl. 2), S425–S438. [Google Scholar] [CrossRef]
- Sathekge, M.M.; Mageza, R.B.; Muthuphei, M.N.; Modiba, M.C.; Clauss, R.C. Evaluation of thyroid nodules with technetium-99m MIBI and technetium-99m pertechnetate. Head Neck 2001, 23, 305–310. [Google Scholar] [CrossRef]
- Chetelat, G.; Arbizu, J.; Barthel, H.; Garibotto, V.; Law, I.; Morbelli, S.; van de Giessen, E.; Agosta, F.; Barkhof, F.; Brooks, D.J.; et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020, 19, 951–962. [Google Scholar] [CrossRef]
- Johnson, K.A.; Minoshima, S.; Bohnen, N.I.; Donohoe, K.J.; Foster, N.L.; Herscovitch, P.; Karlawish, J.H.; Rowe, C.C.; Carrillo, M.C.; Hartley, D.M.; et al. Appropriate use criteria for amyloid PET: A report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013, 9, E1–E16. [Google Scholar] [CrossRef] [Green Version]
- Landau, S.M.; Thomas, B.A.; Thurfjell, L.; Schmidt, M.; Margolin, R.; Mintun, M.; Pontecorvo, M.; Baker, S.L.; Jagust, W.J.; Alzheimer’s Disease Neuroimaging, I. Amyloid PET imaging in Alzheimer’s disease: A comparison of three radiotracers. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1398–1407. [Google Scholar] [CrossRef]
- Rice, L.; Bisdas, S. The diagnostic value of FDG and amyloid PET in Alzheimer’s disease-A systematic review. Eur. J. Radiol. 2017, 94, 16–24. [Google Scholar] [CrossRef]
- Antonini, A.; Benti, R.; De Notaris, R.; Tesei, S.; Zecchinelli, A.; Sacilotto, G.; Meucci, N.; Canesi, M.; Mariani, C.; Pezzoli, G.; et al. 123I-Ioflupane/SPECT binding to striatal dopamine transporter (DAT) uptake in patients with Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Neurol. Sci. 2003, 24, 149–150. [Google Scholar] [CrossRef]
- Catafau, A.M.; Tolosa, E.; Da, T.C.U.P.S.S.G. Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain Parkinsonian syndromes. Mov. Disord. 2004, 19, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Gaig, C.; Marti, M.J.; Tolosa, E.; Valldeoriola, F.; Paredes, P.; Lomena, F.J.; Nakamae, F. 123I-Ioflupane SPECT in the diagnosis of suspected psychogenic Parkinsonism. Mov. Disord. 2006, 21, 1994–1998. [Google Scholar] [CrossRef]
- Vlaar, A.M.; de Nijs, T.; Kessels, A.G.; Vreeling, F.W.; Winogrodzka, A.; Mess, W.H.; Tromp, S.C.; van Kroonenburgh, M.J.; Weber, W.E. Diagnostic value of 123I-ioflupane and 123I-iodobenzamide SPECT scans in 248 patients with parkinsonian syndromes. Eur. Neurol. 2008, 59, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Bajc, M.; Neilly, B.; Miniati, M.; Mortensen, J.; Jonson, B. Methodology for ventilation/perfusion SPECT. Semin. Nucl. Med. 2010, 40, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Bajc, M.; Schumichen, C.; Gruning, T.; Lindqvist, A.; Le Roux, P.Y.; Alatri, A.; Bauer, R.W.; Dilic, M.; Neilly, B.; Verberne, H.J.; et al. EANM guideline for ventilation/perfusion single-photon emission computed tomography (SPECT) for diagnosis of pulmonary embolism and beyond. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2429–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roach, P.J.; Schembri, G.P.; Bailey, D.L. V/Q scanning using SPECT and SPECT/CT. J. Nucl. Med. 2013, 54, 1588–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunariu, N.; Gibbs, S.J.; Win, Z.; Gin-Sing, W.; Graham, A.; Gishen, P.; Al-Nahhas, A. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J. Nucl. Med. 2007, 48, 680–684. [Google Scholar] [CrossRef] [Green Version]
- Buck, A.K.; Nekolla, S.; Ziegler, S.; Beer, A.; Krause, B.J.; Herrmann, K.; Scheidhauer, K.; Wester, H.J.; Rummeny, E.J.; Schwaiger, M.; et al. Spect/Ct. J. Nucl. Med. 2008, 49, 1305–1319. [Google Scholar] [CrossRef] [Green Version]
- Conway, J. Lung imaging-two dimensional gamma scintigraphy, SPECT, CT and PET. Adv. Drug Deliv. Rev. 2012, 64, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Czernin, J.; Israel, O.; Herrmann, K.; Barrio, M.; Nathanson, D.; Allen-Auerbach, M. Clinical applications of PET/CT and SPECT/CT imaging. In Physics of PET and SPECT Imaging; CRC Press: Boca Raton, FL, USA, 2017; pp. 461–494. [Google Scholar]
- Banerjee, S.; Pillai, M.R.; Ramamoorthy, N. Evolution of Tc-99m in diagnostic radiopharmaceuticals. Semin. Nucl. Med. 2001, 31, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Bombardieri, E.; Giammarile, F.; Aktolun, C.; Baum, R.P.; Bischof Delaloye, A.; Maffioli, L.; Moncayo, R.; Mortelmans, L.; Pepe, G.; Reske, S.N.; et al. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Nensa, F.; Bamberg, F.; Rischpler, C.; Menezes, L.; Poeppel, T.D.; la Fougere, C.; Beitzke, D.; Rasul, S.; Loewe, C.; Nikolaou, K.; et al. Hybrid cardiac imaging using PET/MRI: A joint position statement by the European Society of Cardiovascular Radiology (ESCR) and the European Association of Nuclear Medicine (EANM). Eur. Radiol. 2018, 28, 4086–4101. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, J. Vascular toxic effects of cancer therapies. Nat. Rev. Cardiol. 2020, 17, 503–522. [Google Scholar] [CrossRef]
- Plana, J.C.; Thavendiranathan, P.; Bucciarelli-Ducci, C.; Lancellotti, P. Multi-Modality Imaging in the Assessment of Cardiovascular Toxicity in the Cancer Patient. JACC Cardiovasc. Imaging 2018, 11, 1173–1186. [Google Scholar] [CrossRef]
- Dou, K.F.; Yang, M.F.; Yang, Y.J.; Jain, D.; He, Z.X. Myocardial 18F-FDG uptake after exercise-induced myocardial ischemia in patients with coronary artery disease. J. Nucl. Med. 2008, 49, 1986–1991. [Google Scholar] [CrossRef] [Green Version]
- Choksey, A.; Timm, K.N. Cancer Therapy-Induced Cardiotoxicity-A Metabolic Perspective on Pathogenesis, Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 23, 441. [Google Scholar] [CrossRef]
- Biersmith, M.A.; Tong, M.S.; Guha, A.; Simonetti, O.P.; Addison, D. Multimodality Cardiac Imaging in the Era of Emerging Cancer Therapies. J. Am. Heart Assoc. 2020, 9, e013755. [Google Scholar] [CrossRef]
- Safi, M.; Ahmed, H.; Al-Azab, M.; Xia, Y.L.; Shan, X.; Al-Radhi, M.; Al-Danakh, A.; Shopit, A.; Liu, J. PD-1/PDL-1 Inhibitors and Cardiotoxicity; Molecular, Etiological and Management Outlines. J. Adv. Res. 2021, 29, 45–54. [Google Scholar] [CrossRef]
- Nensa, F.; Kloth, J.; Tezgah, E.; Poeppel, T.D.; Heusch, P.; Goebel, J.; Nassenstein, K.; Schlosser, T. Feasibility of FDG-PET in myocarditis: Comparison to CMR using integrated PET/MRI. J. Nucl. Cardiol. 2018, 25, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Rischpler, C.; Rassaf, T.; Umutlu, L.; Herrmann, K.; Schlosser, T.W.; Totzeck, M. Imaging the Inflammatory Response in Checkpoint Inhibition Myocarditis. J. Nucl. Med. 2022, 63, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Arponen, O.; Skytta, T. Immune checkpoint inhibitor-induced myocarditis not visible with cardiac magnetic resonance imaging but detected with PET-CT: A case report. Acta Oncol. 2020, 59, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Dudoignon, D.; Pattison, D.A.; Legallois, D.; Hicks, R.J.; Aide, N. The utility of pharmacological and radiological interventions to optimize diagnostic information from PET/CT. Cancer Imaging 2020, 20, 68. [Google Scholar] [CrossRef]
- Ley, K.; Roy, P. Blind Spot: 18F-FDG PET Fails to Reveal Atherosclerosis Aggravated by Cancer Immunotherapy. JACC CardioOncol. 2020, 2, 611–613. [Google Scholar] [CrossRef]
- Kessler, L.; Ferdinandus, J.; Hirmas, N.; Bauer, S.; Dirksen, U.; Zarrad, F.; Nader, M.; Chodyla, M.; Milosevic, A.; Umutlu, L.; et al. 68Ga-FAPI as a Diagnostic Tool in Sarcoma: Data from the 68Ga-FAPI PET Prospective Observational Trial. J. Nucl. Med. 2022, 63, 89–95. [Google Scholar] [CrossRef]
- Sollini, M.; Kirienko, M.; Gelardi, F.; Fiz, F.; Gozzi, N.; Chiti, A. State-of-the-art of FAPI-PET imaging: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4396–4414. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Fragoso Costa, P.; Kessler, L.; Weber, M.; Hirmas, N.; Kostbade, K.; Bauer, S.; Schuler, M.; Ahrens, M.; Schildhaus, H.U.; et al. Initial clinical experience with 90Y-FAPI-46 radioligand therapy for advanced stage solid tumors: A case series of nine patients. J. Nucl. Med. 2021, 63. [Google Scholar] [CrossRef]
- Totzeck, M.; Siebermair, J.; Rassaf, T.; Rischpler, C. Cardiac fibroblast activation detected by positron emission tomography/computed tomography as a possible sign of cardiotoxicity. Eur. Heart J. 2020, 41, 1060. [Google Scholar] [CrossRef]
- Finke, D.; Heckmann, M.B.; Herpel, E.; Katus, H.A.; Haberkorn, U.; Leuschner, F.; Lehmann, L.H. Early Detection of Checkpoint Inhibitor-Associated Myocarditis Using 68Ga-FAPI PET/CT. Front. Cardiovasc. Med. 2021, 8, 614997. [Google Scholar] [CrossRef]
- Niu, N.; Huo, L.; Zhang, S.; Liu, Y.; Li, X. Immune checkpoint inhibitor-associated cardiotoxicity detected by 68Ga-DOTATATE PET/CT and 68Ga-FAPI PET/CT. Eur. Heart J. Cardiovasc. Imaging 2021, 23, e123. [Google Scholar] [CrossRef]
- Pure, E.; Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018, 37, 4343–4357. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebermair, J.; Kohler, M.I.; Kupusovic, J.; Nekolla, S.G.; Kessler, L.; Ferdinandus, J.; Guberina, N.; Stuschke, M.; Grafe, H.; Siveke, J.T.; et al. Cardiac fibroblast activation detected by Ga-68 FAPI PET imaging as a potential novel biomarker of cardiac injury/remodeling. J. Nucl. Cardiol. 2021, 28, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular Imaging of Fibroblast Activity After Myocardial Infarction Using a 68Ga-Labeled Fibroblast Activation Protein Inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef]
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting cardiac fibrosis with engineered T cells. Nature 2019, 573, 430–433. [Google Scholar] [CrossRef]
- Lapa, C.; Reiter, T.; Li, X.; Werner, R.A.; Samnick, S.; Jahns, R.; Buck, A.K.; Ertl, G.; Bauer, W.R. Imaging of myocardial inflammation with somatostatin receptor based PET/CT-A comparison to cardiac MRI. Int. J. Cardiol. 2015, 194, 44–49. [Google Scholar] [CrossRef]
- Boughdad, S.; Obeid, M.; MEYER, M.; Peters, S.; Michielin, O.; Monney, P.; Jreige, M.; Kamani, C.; Schaefer, N.; Prior, J. 68Ga-DOTATOC PET/CT to detect immune checkpoint inhibitor-related myocarditis. J. Nucl. Med. 2021, 62, 48. [Google Scholar] [CrossRef]
- Jacobson, A.F.; Senior, R.; Cerqueira, M.D.; Wong, N.D.; Thomas, G.S.; Lopez, V.A.; Agostini, D.; Weiland, F.; Chandna, H.; Narula, J.; et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J. Am. Coll. Cardiol. 2010, 55, 2212–2221. [Google Scholar] [CrossRef] [Green Version]
- Verberne, H.J.; Brewster, L.M.; Somsen, G.A.; van Eck-Smit, B.L. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: A systematic review. Eur. Heart J. 2008, 29, 1147–1159. [Google Scholar] [CrossRef] [Green Version]
- de Geus-Oei, L.F.; Mavinkurve-Groothuis, A.M.; Bellersen, L.; Gotthardt, M.; Oyen, W.J.; Kapusta, L.; van Laarhoven, H.W. Scintigraphic techniques for early detection of cancer treatment-induced cardiotoxicity. J. Nucl. Med. 2011, 52, 560–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salm, L.P.; Bulten, B.F.; Van Laarhoven, H.W.M.; De Geus-Oei, L.F. Autonomic Imaging Cardiotoxicity with [123I]-MIBG: The Effects of Chemotherapy, Monoclonal Antibody Therapy, and Radiotherapy. In Autonomic Innervation of the Heart: Role of Molecular Imaging; Slart, R.H.J.A., Tio, R.A., Elsinga, P.H., Schwaiger, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 437–451. [Google Scholar]
- Panjrath, G.S.; Jain, D. Monitoring chemotherapy-induced cardiotoxicity: Role of cardiac nuclear imaging. J. Nucl. Cardiol. 2006, 13, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Dimitriu-Leen, A.C.; Scholte, A.J.; Jacobson, A.F. 123I-MIBG SPECT for Evaluation of Patients with Heart Failure. J. Nucl. Med. 2015, 56 (Suppl. 4), 25S–30S. [Google Scholar] [CrossRef] [Green Version]
- Burger, I.A.; Lohmann, C.; Messerli, M.; Bengs, S.; Becker, A.; Maredziak, M.; Treyer, V.; Haider, A.; Schwyzer, M.; Benz, D.C.; et al. Age- and sex-dependent changes in sympathetic activity of the left ventricular apex assessed by 18F-DOPA PET imaging. PLoS ONE 2018, 13, e0202302. [Google Scholar] [CrossRef]
- Werner, R.A.; Rischpler, C.; Onthank, D.; Lapa, C.; Robinson, S.; Samnick, S.; Javadi, M.; Schwaiger, M.; Nekolla, S.G.; Higuchi, T. Retention Kinetics of the 18F-Labeled Sympathetic Nerve PET Tracer LMI1195: Comparison with 11C-Hydroxyephedrine and 123I-MIBG. J. Nucl. Med. 2015, 56, 1429–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyafil, F.; Gimelli, A.; Slart, R.; Georgoulias, P.; Rischpler, C.; Lubberink, M.; Sciagra, R.; Bucerius, J.; Agostini, D.; Verberne, H.J.; et al. EANM procedural guidelines for myocardial perfusion scintigraphy using cardiac-centered gamma cameras. Eur. J. Hybrid. Imaging 2019, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Higgins, J.A.; Williams, G. Stress-induced abnormalities in myocardial perfusion imaging that are not related to perfusion but are of diagnostic and prognostic importance. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 584–595. [Google Scholar] [CrossRef]
- Elfigih, I.A.; Henein, M.Y. Non-invasive imaging in detecting myocardial viability: Myocardial function versus perfusion. Int. J. Cardiol. Heart Vasc. 2014, 5, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Verberne, H.J.; Acampa, W.; Anagnostopoulos, C.; Ballinger, J.; Bengel, F.; De Bondt, P.; Buechel, R.R.; Cuocolo, A.; van Eck-Smit, B.L.; Flotats, A.; et al. EANM procedural guidelines for radionuclide myocardial perfusion imaging with SPECT and SPECT/CT: 2015 revision. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1929–1940. [Google Scholar] [CrossRef] [Green Version]
- Lindner, O.; Bengel, F.; Burchert, W.; Dorr, R.; Hacker, M.; Schafer, W.; Schafers, M.A.; Schmidt, M.; Schwaiger, M.; Vom Dahl, J.; et al. [Myokard-Perfusions-SPECT. Myocardial perfusion SPECT-Update S1 guideline]. Nuklearmedizin 2017, 56, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Beller, G.A.; Glover, D.K.; Edwards, N.C.; Ruiz, M.; Simanis, J.P.; Watson, D.D. 99mTc-sestamibi uptake and retention during myocardial ischemia and reperfusion. Circulation 1993, 87, 2033–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piwnica-Worms, D.P.; Kronauge, J.F.; LeFurgey, A.; Backus, M.; Hockett, D.; Ingram, P.; Lieberman, M.; Holman, B.L.; Jones, A.G.; Davison, A. Mitochondrial localization and characterization of 99Tc-SESTAMIBI in heart cells by electron probe X-ray microanalysis and 99Tc-NMR spectroscopy. Magn. Reson. Imaging 1994, 12, 641–652. [Google Scholar] [CrossRef]
- Platts, E.A.; North, T.L.; Pickett, R.D.; Kelly, J.D. Mechanism of uptake of technetium-tetrofosmin. I: Uptake into isolated adult rat ventricular myocytes and subcellular localization. J. Nucl. Cardiol. 1995, 2, 317–326. [Google Scholar] [CrossRef]
- Flynn, B.; Wernovsky, G.; Summerville, D.A.; Castaneda, A.R.; Treves, S.T. Comparison of technetium-99m MIBI and thallium-201 chloride myocardial scintigraphy in infants. J. Nucl. Med. 1989, 30, 1176–1181. [Google Scholar]
- Sciagra, R.; Lubberink, M.; Hyafil, F.; Saraste, A.; Slart, R.; Agostini, D.; Nappi, C.; Georgoulias, P.; Bucerius, J.; Rischpler, C.; et al. EANM procedural guidelines for PET/CT quantitative myocardial perfusion imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1040–1069. [Google Scholar] [CrossRef] [PubMed]
- Inno, A.; Chiampan, A.; Lanzoni, L.; Verze, M.; Molon, G.; Gori, S. Immune Checkpoint Inhibitors and Atherosclerotic Vascular Events in Cancer Patients. Front. Cardiovasc. Med. 2021, 8, 652186. [Google Scholar] [CrossRef] [PubMed]
- Poels, K.; Neppelenbroek, S.I.M.; Kersten, M.J.; Antoni, M.L.; Lutgens, E.; Seijkens, T.T.P. Immune checkpoint inhibitor treatment and atherosclerotic cardiovascular disease: An emerging clinical problem. J. Immunother. Cancer 2021, 9, e002916. [Google Scholar] [CrossRef]
- Michel, L.; Helfrich, I.; Hendgen-Cotta, U.B.; Mincu, R.I.; Korste, S.; Mrotzek, S.M.; Spomer, A.; Odersky, A.; Rischpler, C.; Herrmann, K.; et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur. Heart J. 2021, 43, 316–329. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Russell, R.R. Cardio-oncology: The Nuclear Option. Curr. Cardiol. Rep. 2017, 19, 31. [Google Scholar] [CrossRef]
- Agrawal, N.; Khunger, A.; Vachhani, P.; Colvin, T.A.; Hattoum, A.; Spangenthal, E.; Curtis, A.B.; Dy, G.K.; Ernstoff, M.S.; Puzanov, I. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: Case Series and Review of the Literature. Case Rep. Oncol. 2019, 12, 260–276. [Google Scholar] [CrossRef]
- Chen, R.; Peng, L.; Qiu, Z.; Wang, Y.; Wei, F.; Zhou, M.; Zhu, F. Case Report: Cardiac Toxicity Associated With Immune Checkpoint Inhibitors. Front. Cardiovasc. Med. 2021, 8, 727445. [Google Scholar] [CrossRef] [PubMed]
- Norwood, T.G.; Lenneman, C.A.; Westbrook, B.C.; Litovsky, S.H.; McKee, S.B.; Conry, R.M. Evolution of Immune Checkpoint Blockade-Induced Myocarditis Over 2 Years. JACC Case Rep. 2020, 2, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Altena, R.; Perik, P.J.; van Veldhuisen, D.J.; de Vries, E.G.; Gietema, J.A. Cardiovascular toxicity caused by cancer treatment: Strategies for early detection. Lancet Oncol. 2009, 10, 391–399. [Google Scholar] [CrossRef]
- Laursen, A.H.; Elming, M.B.; Ripa, R.S.; Hasbak, P.; Kjaer, A.; Kober, L.; Marott, J.L.; Thune, J.J.; Hutchings, M. Rubidium-82 positron emission tomography for detection of acute doxorubicin-induced cardiac effects in lymphoma patients. J. Nucl. Cardiol. 2020, 27, 1698–1707. [Google Scholar] [CrossRef]
- Zyromska, A.; Malkowski, B.; Wisniewski, T.; Majewska, K.; Reszke, J.; Makarewicz, R. (15)O-H2O PET/CT as a tool for the quantitative assessment of early post-radiotherapy changes of heart perfusion in breast carcinoma patients. Br. J. Radiol. 2018, 91, 20170653. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Gimelli, A. Myocardial perfusion years after radiation therapy for left-sided breast cancer: Normal or abnormal? This is the question. J. Nucl. Cardiol. 2021, 28, 1933–1935. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Yan, R.; Wu, Z.; Li, J.; Yan, M.; Hao, X.; Liu, J.; Li, S. 13N-Ammonia PET/CT Detection of Myocardial Perfusion Abnormalities in Beagle Dogs After Local Heart Irradiation. J. Nucl. Med. 2017, 58, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Ziadi, M.C.; de Kemp, R.; Beanlands, R.S.B.; Small, G.R. Looking for trouble: Reduced myocardial flow reserve following anthracyclines. J. Nucl. Cardiol. 2020, 27, 1708–1713. [Google Scholar] [CrossRef] [Green Version]
- Driessen, R.S.; Raijmakers, P.G.; Stuijfzand, W.J.; Knaapen, P. Myocardial perfusion imaging with PET. Int. J. Cardiovasc. Imaging 2017, 33, 1021–1031. [Google Scholar] [CrossRef] [Green Version]
- Nakazato, R.; Berman, D.S.; Alexanderson, E.; Slomka, P. Myocardial perfusion imaging with PET. Imaging Med. 2013, 5, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Farrell, M.B.; Galt, J.R.; Georgoulias, P.; Malhotra, S.; Pagnanelli, R.; Rischpler, C.; Savir-Baruch, B. SNMMI Procedure Standard/EANM Guideline for Gated Equilibrium Radionuclide Angiography. J. Nucl. Med. Technol. 2020, 48, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Basu, S. Equilibrium radionuclide angiocardiography: Its usefulness in current practice and potential future applications. World J. Radiol. 2012, 4, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Suero-Abreu, G.A.; Kim, B. A Case of Acute Heart Failure due to Immune Checkpoint Blocker Nivolumab. Cardiol. Res. 2019, 10, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, A.; Parker, N.A.; Choucair, K.; Alderson, J.; Deutsch, J.M. A Case of Acute Heart Failure Following Immunotherapy for Metastatic Lung Cancer. Cureus 2020, 12, e8093. [Google Scholar] [CrossRef] [PubMed]
- Laubli, H.; Balmelli, C.; Bossard, M.; Pfister, O.; Glatz, K.; Zippelius, A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J. Immunother. Cancer 2015, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Bouwer, N.I.; Jager, A.; Liesting, C.; Kofflard, M.J.M.; Brugts, J.J.; Kitzen, J.; Boersma, E.; Levin, M.D. Cardiac monitoring in HER2-positive patients on trastuzumab treatment: A review and implications for clinical practice. Breast 2020, 52, 33–44. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Seidman, A.; Hudis, C.; Pierri, M.K.; Shak, S.; Paton, V.; Ashby, M.; Murphy, M.; Stewart, S.J.; Keefe, D. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002, 20, 1215–1221. [Google Scholar] [CrossRef]
- Hambye, A.S.; Everaert, H.; Maes, A.; Mesotten, L.; Vandevivere, J.; Mortelmans, L.; Franken, P.R. Nuclear cardiology, Part II: Scintigraphic evaluation of cardiac function. J. Nucl. Med. Technol. 1998, 26, 72–79; quiz 84, 86. [Google Scholar]
- Nichols, K.; Saouaf, R.; Ababneh, A.A.; Barst, R.J.; Rosenbaum, M.S.; Groch, M.W.; Shoyeb, A.H.; Bergmann, S.R. Validation of SPECT equilibrium radionuclide angiographic right ventricular parameters by cardiac magnetic resonance imaging. J. Nucl. Cardiol. 2002, 9, 153–160. [Google Scholar] [CrossRef]
- Foster, C.; Gal, R.A.; Port, S.C.; Schmidt, D.H. Left ventricular ejection fraction during incremental and steady state exercise. Med. Sci. Sports Exerc. 1995, 27, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Romero-Farina, G.; Aguade-Bruix, S. Equilibrium radionuclide angiography: Present and future. J. Nucl. Cardiol. 2021, 28, 1315–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachpekidis, C.; Sachpekidis, V.; Kopp-Schneider, A.; Arsos, G.; Moralidis, E. Equilibrium radionuclide angiography: Intra- and inter-observer repeatability and reproducibility in the assessment of cardiac systolic and diastolic function. J. Nucl. Cardiol. 2021, 28, 1304–1314. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Florea, V.G. Chapter 15-Alterations in Ventricular Structure: Role of Left Ventricular Remodeling. In Heart Failure: A Companion to Braunwald’s Heart Disease, 2nd ed.; Mann, D.L., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 232–253. [Google Scholar]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef] [PubMed]
- Broos, K.; Lecocq, Q.; Raes, G.; Devoogdt, N.; Keyaerts, M.; Breckpot, K. Noninvasive imaging of the PD-1:PD-L1 immune checkpoint: Embracing nuclear medicine for the benefit of personalized immunotherapy. Theranostics 2018, 8, 3559–3570. [Google Scholar] [CrossRef]
- van der Veen, E.L.; Giesen, D.; Pot-de Jong, L.; Jorritsma-Smit, A.; De Vries, E.G.E.; Lub-de Hooge, M.N. 89Zr-pembrolizumab biodistribution is influenced by PD-1-mediated uptake in lymphoid organs. J. Immunother. Cancer 2020, 8, e000938. [Google Scholar] [CrossRef]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schroder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- Leung, D.; Bonacorsi, S.; Smith, R.A.; Weber, W.; Hayes, W. Molecular Imaging and the PD-L1 Pathway: From Bench to Clinic. Front. Oncol. 2021, 11, 698425. [Google Scholar] [CrossRef]
- Sermer, D.; Brentjens, R. CAR T-cell therapy: Full speed ahead. Hematol. Oncol. 2019, 37 (Suppl. 1), 95–100. [Google Scholar] [CrossRef] [Green Version]
- Burns, E.A.; Gentille, C.; Trachtenberg, B.; Pingali, S.R.; Anand, K. Cardiotoxicity Associated with Anti-CD19 Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Recognition, Risk Factors, and Management. Diseases 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, B.; Kang, Y.; Smith, A.M.; Frey, N.V.; Carver, J.R.; Scherrer-Crosbie, M. Cardiovascular Effects of CAR T Cell Therapy: A Retrospective Study. JACC CardioOncol. 2020, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Sakemura, R.; Bansal, A.; Siegler, E.L.; Hefazi, M.; Yang, N.; Khadka, R.H.; Newsom, A.N.; Hansen, M.J.; Cox, M.J.; Manriquez Roman, C.; et al. Development of a Clinically Relevant Reporter for Chimeric Antigen Receptor T-cell Expansion, Trafficking, and Toxicity. Cancer Immunol. Res. 2021, 9, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Lang, C.; Lim, L.; Man, F.; Kurtys, E.; Ashmore-Harris, C.; Johnson, P.; Skourti, E.; de Rosales, R.T.M.; Fruhwirth, G.O. Spatiotemporal PET Imaging Reveals Differences in CAR-T Tumor Retention in Triple-Negative Breast Cancer Models. Mol. Ther. 2020, 28, 2271–2285. [Google Scholar] [CrossRef]
- Dittmann, M.; Gonzalez Carvalho, J.M.; Rahbar, K.; Schafers, M.; Claesener, M.; Riemann, B.; Seifert, R. Incremental diagnostic value of [18F]tetrafluoroborate PET-CT compared to [131I]iodine scintigraphy in recurrent differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2639–2646. [Google Scholar] [CrossRef] [Green Version]
- Arndt, C.; Feldmann, A.; Koristka, S.; Schafer, M.; Bergmann, R.; Mitwasi, N.; Berndt, N.; Bachmann, D.; Kegler, A.; Schmitz, M.; et al. A theranostic PSMA ligand for PET imaging and retargeting of T cells expressing the universal chimeric antigen receptor UniCAR. Oncoimmunology 2019, 8, 1659095. [Google Scholar] [CrossRef] [Green Version]
- Albert, S.; Arndt, C.; Koristka, S.; Berndt, N.; Bergmann, R.; Feldmann, A.; Schmitz, M.; Pietzsch, J.; Steinbach, J.; Bachmann, M. From mono- to bivalent: Improving theranostic properties of target modules for redirection of UniCAR T cells against EGFR-expressing tumor cells in vitro and in vivo. Oncotarget 2018, 9, 25597–25616. [Google Scholar] [CrossRef] [Green Version]
- Chae, S.Y.; Kwon, T.W.; Jin, S.; Kwon, S.U.; Sung, C.; Oh, S.J.; Lee, S.J.; Oh, J.S.; Han, Y.; Cho, Y.P.; et al. A phase 1, first-in-human study of 18F-GP1 positron emission tomography for imaging acute arterial thrombosis. EJNMMI Res. 2019, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Lohrke, J.; Siebeneicher, H.; Berger, M.; Reinhardt, M.; Berndt, M.; Mueller, A.; Zerna, M.; Koglin, N.; Oden, F.; Bauser, M.; et al. 18F-GP1, a Novel PET Tracer Designed for High-Sensitivity, Low-Background Detection of Thrombi. J. Nucl. Med. 2017, 58, 1094–1099. [Google Scholar] [CrossRef] [Green Version]
- Hoilund-Carlsen, P.F.; Sturek, M.; Alavi, A.; Gerke, O. Atherosclerosis imaging with 18F-sodium fluoride PET: State-of-the-art review. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1538–1551. [Google Scholar] [CrossRef] [Green Version]
- Irkle, A.; Vesey, A.T.; Lewis, D.Y.; Skepper, J.N.; Bird, J.L.; Dweck, M.R.; Joshi, F.R.; Gallagher, F.A.; Warburton, E.A.; Bennett, M.R.; et al. Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography. Nat. Commun. 2015, 6, 7495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chicheportiche, A.; Marciano, R.; Orevi, M. Comparison of NEMA characterizations for Discovery MI and Discovery MI-DR TOF PET/CT systems at different sites and with other commercial PET/CT systems. EJNMMI Phys. 2020, 7, 1–20. [Google Scholar] [CrossRef]
- Rausch, I.; Ruiz, A.; Valverde-Pascual, I.; Cal-González, J.; Beyer, T.; Carrio, I. Performance evaluation of the Vereos PET/CT system according to the NEMA NU2-2012 standard. J. Nucl. Med. 2019, 60, 561–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvadori, J.; Labour, J.; Odille, F.; Marie, P.-Y.; Badel, J.-N.; Imbert, L.; Sarrut, D. Monte Carlo simulation of digital photon counting PET. EJNMMI Phys. 2020, 7, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Maniawski, P.; Knopp, M.V. Performance evaluation of the next generation solid-state digital photon counting PET/CT system. EJNMMI Res. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Van Sluis, J.; De Jong, J.; Schaar, J.; Noordzij, W.; Van Snick, P.; Dierckx, R.; Borra, R.; Willemsen, A.; Boellaard, R. Performance characteristics of the digital biograph vision PET/CT system. J. Nucl. Med. 2019, 60, 1031–1036. [Google Scholar] [CrossRef]
- Gnesin, S.; Kieffer, C.; Zeimpekis, K.; Papazyan, J.P.; Guignard, R.; Prior, J.O.; Verdun, F.R.; Lima, T.V.M. Phantom-based image quality assessment of clinical 18F-FDG protocols in digital PET/CT and comparison to conventional PMT-based PET/CT. EJNMMI Phys. 2020, 7, 1. [Google Scholar] [CrossRef]
- Lopez-Mora, D.A.; Flotats, A.; Fuentes-Ocampo, F.; Camacho, V.; Fernandez, A.; Ruiz, A.; Duch, J.; Sizova, M.; Domenech, A.; Estorch, M.; et al. Comparison of image quality and lesion detection between digital and analog PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1383–1390. [Google Scholar] [CrossRef]
- Miwa, K.; Wagatsuma, K.; Nemoto, R.; Masubuchi, M.; Kamitaka, Y.; Yamao, T.; Hiratsuka, S.; Yamaguchi, M.; Yoshii, T.; Kobayashi, R.; et al. Detection of sub-centimeter lesions using digital TOF-PET/CT system combined with Bayesian penalized likelihood reconstruction algorithm. Ann. Nucl. Med. 2020, 34, 762–771. [Google Scholar] [CrossRef]
- Salvadori, J.; Odille, F.; Verger, A.; Olivier, P.; Karcher, G.; Marie, P.Y.; Imbert, L. Head-to-head comparison between digital and analog PET of human and phantom images when optimized for maximizing the signal-to-noise ratio from small lesions. EJNMMI Phys. 2020, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Surti, S.; Viswanath, V.; Daube-Witherspoom, M.E.; Conti, M.; Casey, M.E.; Karp, J.S. Benefit of improved performance with state-of-the art digital PET/CT for lesion detection in oncology. J. Nucl. Med. 2020, 61, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- van Sluis, J.; Boellaard, R.; Somasundaram, A.; van Snick, P.H.; Borra, R.J.H.; Dierckx, R.; Stormezand, G.N.; Glaudemans, A.; Noordzij, W. Image Quality and Semiquantitative Measurements on the Biograph Vision PET/CT System: Initial Experiences and Comparison with the Biograph mCT. J. Nucl. Med. 2020, 61, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Sonni, I.; Baratto, L.; Park, S.; Hatami, N.; Srinivas, S.; Davidzon, G.; Gambhir, S.S.; Iagaru, A. Initial experience with a SiPM-based PET/CT scanner: Influence of acquisition time on image quality. EJNMMI Phys. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- van Sluis, J.; Boellaard, R.; Dierckx, R.; Stormezand, G.N.; Glaudemans, A.; Noordzij, W. Image Quality and Activity Optimization in Oncologic 18F-FDG PET Using the Digital Biograph Vision PET/CT System. J. Nucl. Med. 2020, 61, 764–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.; Jentzen, W.; Hofferber, R.; Herrmann, K.; Fendler, W.P.; Rischpler, C.; Umutlu, L.; Conti, M.; Costa, P.F.; Sraieb, M.; et al. Evaluation of 18F-FDG PET/CT images acquired with a reduced scan time duration in lymphoma patients using the digital biograph vision. BMC Cancer 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Fragoso Costa, P.; Jentzen, W.; F, S.U.; Fendler, W.P.; Rischpler, C.; Herrmann, K.; Conti, M.; Kersting, D.; Weber, M. Reduction of emission time for [68Ga]Ga-PSMA PET/CT using the digital biograph vision: A Phantom study. Q. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef]
- Weber, M.; Jentzen, W.; Hofferber, R.; Herrmann, K.; Fendler, W.P.; Conti, M.; Wetter, A.; Kersting, D.; Rischpler, C.; Fragoso Costa, P. Evaluation of [68Ga]Ga-PSMA PET/CT images acquired with a reduced scan time duration in prostate cancer patients using the digital biograph vision. EJNMMI Res. 2021, 11, 21. [Google Scholar] [CrossRef]
- Lasnon, C.; Coudrais, N.; Houdu, B.; Nganoa, C.; Salomon, T.; Enilorac, B.; Aide, N. How fast can we scan patients with modern (digital) PET/CT systems? Eur. J. Radiol. 2020, 129, 109144. [Google Scholar] [CrossRef]
- Kersting, D.; Jentzen, W.; Fragoso Costa, P.; Sraieb, M.; Sandach, P.; Umutlu, L.; Conti, M.; Zarrad, F.; Rischpler, C.; Fendler, W.P.; et al. Silicon-photomultiplier-based PET/CT reduces the minimum detectable activity of iodine-124. Sci. Rep. 2021, 11, 17477. [Google Scholar] [CrossRef]
- Kersting, D.; Jentzen, W.; Sraieb, M.; Costa, P.F.; Conti, M.; Umutlu, L.; Antoch, G.; Nader, M.; Herrmann, K.; Fendler, W.P.; et al. Comparing lesion detection efficacy and image quality across different PET system generations to optimize the iodine-124 PET protocol for recurrent thyroid cancer. EJNMMI Phys. 2021, 8, 14. [Google Scholar] [CrossRef]
- Lopez-Mora, D.A.; Sizova, M.; Estorch, M.; Flotats, A.; Camacho, V.; Fernandez, A.; Abouzian, S.; Fuentes-Ocampo, F.; Garcia, J.I.P.; Ballesteros, A.I.C.; et al. Superior performance of 18F-fluorocholine digital PET/CT in the detection of parathyroid adenomas. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou-Strauss, A.; Pan, L.; Sachpekidis, C. Kinetic modeling and parametric imaging with dynamic PET for oncological applications: General considerations, current clinical applications, and future perspectives. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Alberts, I.; Hunermund, J.N.; Prenosil, G.; Mingels, C.; Bohn, K.P.; Viscione, M.; Sari, H.; Vollnberg, B.; Shi, K.; Afshar-Oromieh, A.; et al. Clinical performance of long axial field of view PET/CT: A head-to-head intra-individual comparison of the Biograph Vision Quadra with the Biograph Vision PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, Y.; Yu, H.; Chen, S.; Tan, H.; Qi, C.; Dong, Y.; Wang, Y.; Deng, Z.; Shi, H. Total-body 18F-FDG PET/CT scan in oncology patients: How fast could it be? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Hu, P.C.; Wu, R.Z.; Gu, Y.S.; Chen, S.G.; Yu, H.J.; Wang, X.Q.; Song, J.; Shi, H.C. The image quality, lesion detectability, and acquisition time of 18F-FDG total-body PET/CT in oncological patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2507–2515. [Google Scholar] [CrossRef]
- Schmidt, K.C.; Turkheimer, F.E. Kinetic modeling in positron emission tomography. Q. J. Nucl. Med. 2002, 46, 70–85. [Google Scholar]
- Ben Bouallegue, F.; Maimoun, L.; Kucharczak, F.; Le Fur, P.; Vauchot, F.; Hay, B.; Rondet, E.; Mariano-Goulart, D. Left ventricle function assessment using gated first-pass 18F-FDG PET: Validation against equilibrium radionuclide angiography. J. Nucl. Cardiol. 2021, 28, 594–603. [Google Scholar] [CrossRef]
- Shi, X.; Liu, S.; Lin, X.; Zhao, X.; Fang, L.; Ding, J.; Dang, Y.; Xing, H.; Han, C.; Dong, C.; et al. Characterization of myocardial oxidative metabolism and myocardial external efficiency in high-risk alcohol cardiotoxicity and alcoholic cardiomyopathy via dynamic 11C-Acetate positron emission tomography. J. Nucl. Cardiol. 2020, 29, 278–288. [Google Scholar] [CrossRef]
- Ouyang, D.; He, B.; Ghorbani, A.; Yuan, N.; Ebinger, J.; Langlotz, C.P.; Heidenreich, P.A.; Harrington, R.A.; Liang, D.H.; Ashley, E.A.; et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature 2020, 580, 252–256. [Google Scholar] [CrossRef]
- Demissei, B.G.; Fan, Y.; Qian, Y.; Cheng, H.G.; Smith, A.M.; Shimamoto, K.; Vedage, N.; Narayan, H.K.; Scherrer-Crosbie, M.; Davatzikos, C.; et al. Left ventricular segmental strain and the prediction of cancer therapy-related cardiac dysfunction. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 418–426. [Google Scholar] [CrossRef]
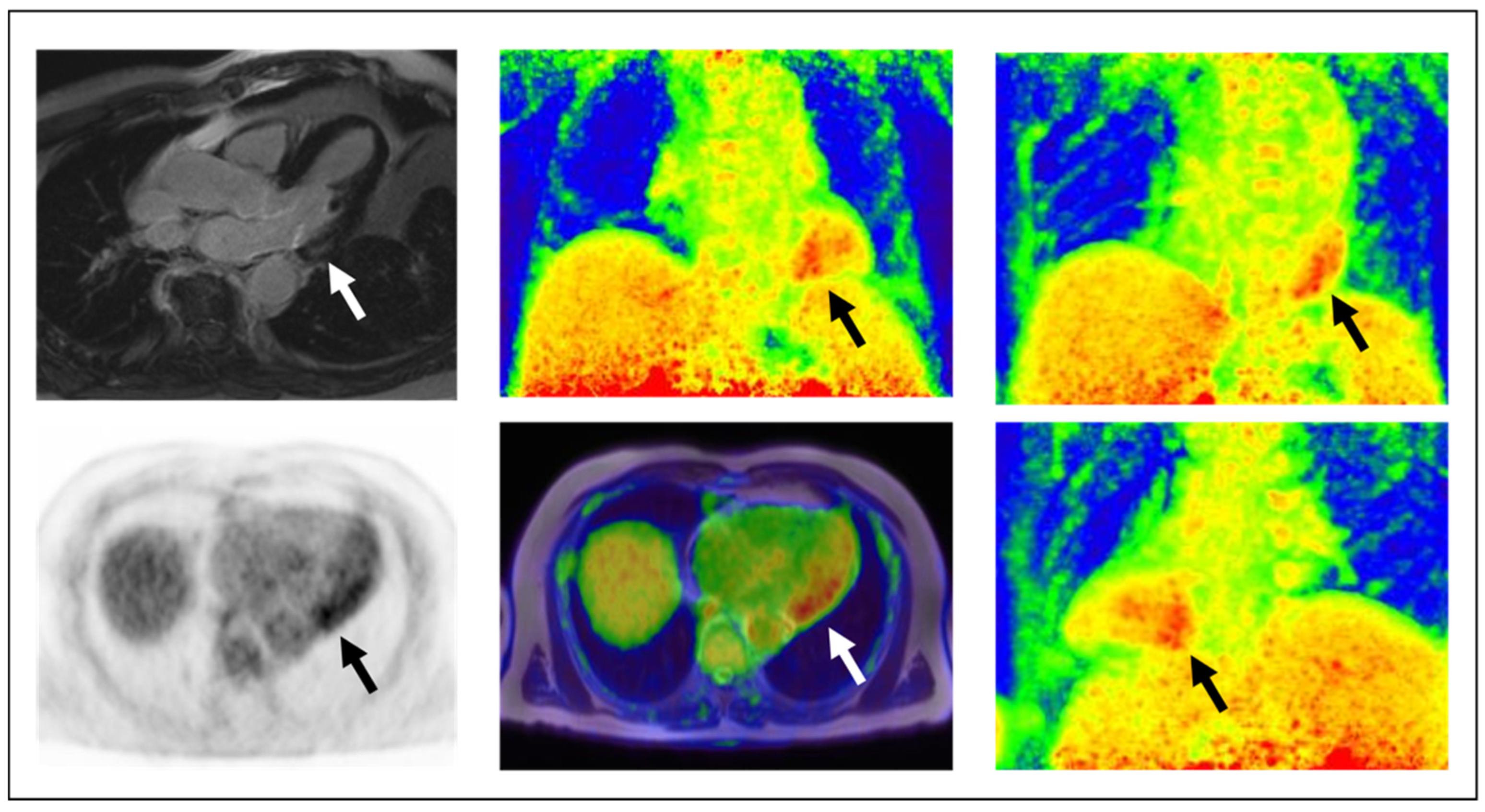

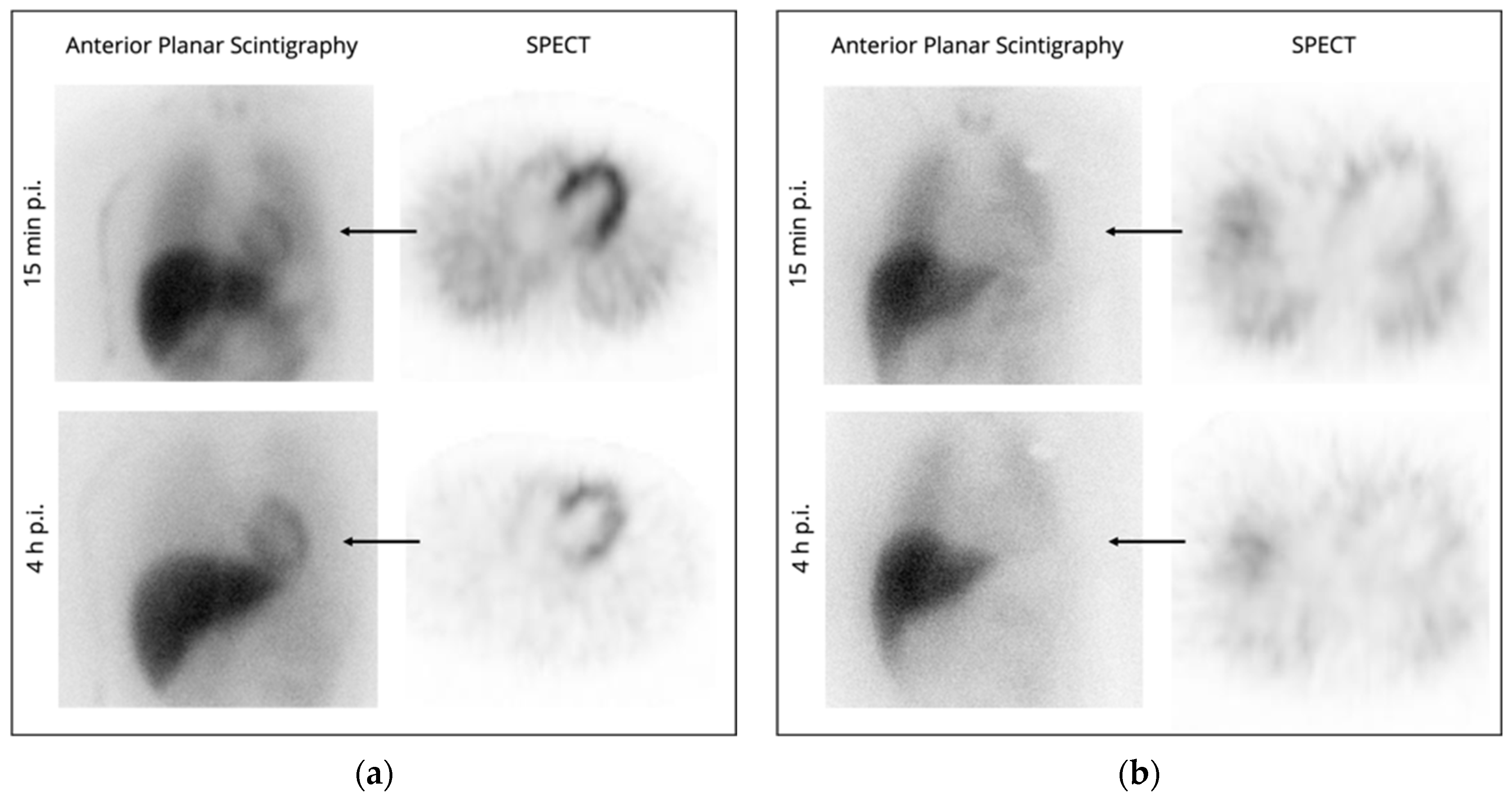
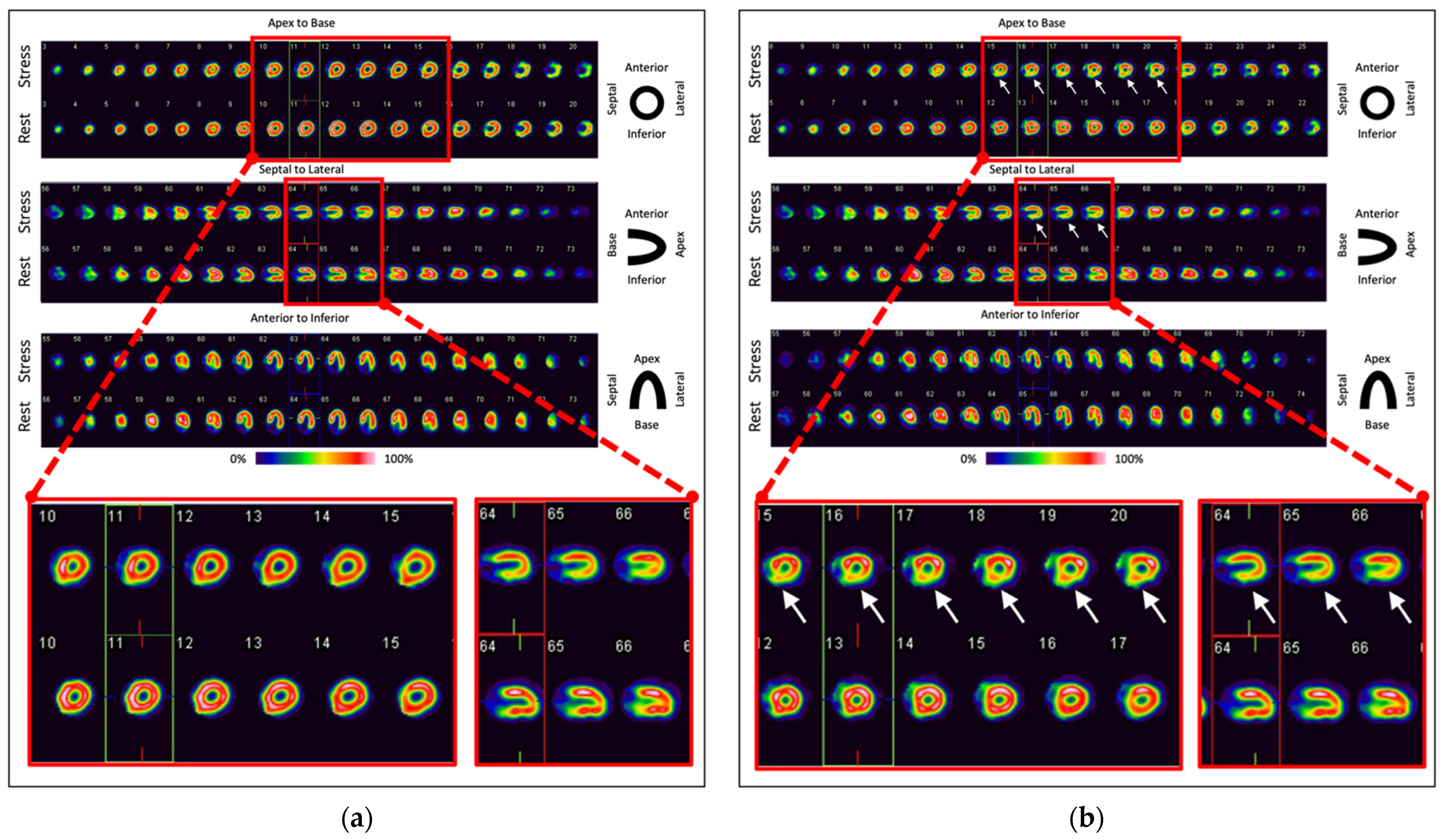
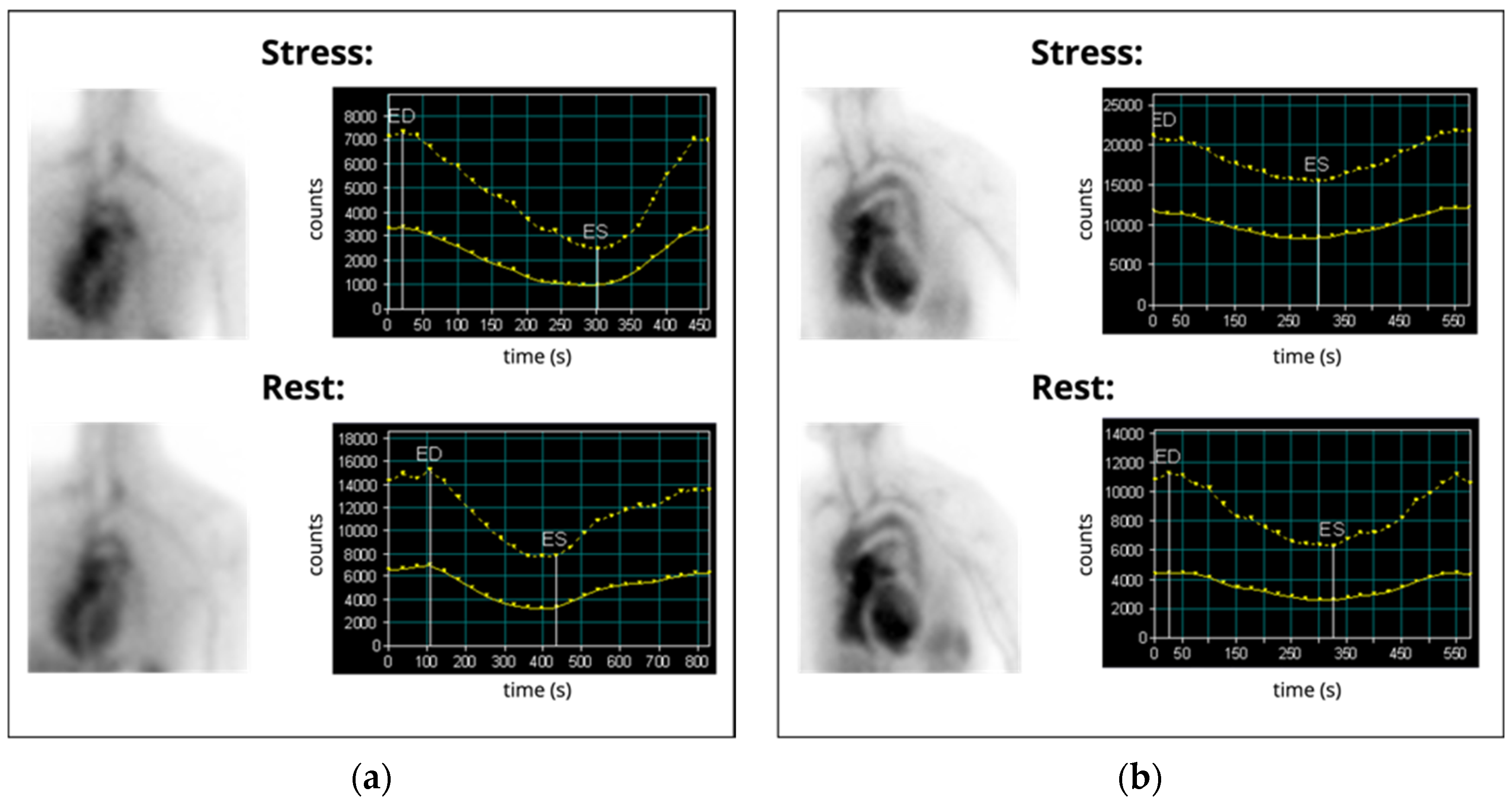
| Imaging Modality | Parameters | Advantages | Disadvantages | Publications |
|---|---|---|---|---|
| FDG PET | Glucose metabolism |
|
| Case reports |
| FAPI PET | Cardiac remodeling |
|
| Case reports |
| DOTATOC/ DOTATATE PET | SSTR *-Expression |
|
| Case reports |
| MIBG SPECT | Sympathetic innervation |
|
| - |
| Myocardial perfusion SPECT | LVEF † wall motion perfusion (stress/rest) |
|
| Case reports |
| ERNA | LVEF † wall motion |
|
| - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kersting, D.; Settelmeier, S.; Mavroeidi, I.-A.; Herrmann, K.; Seifert, R.; Rischpler, C. Shining Damaged Hearts: Immunotherapy-Related Cardiotoxicity in the Spotlight of Nuclear Cardiology. Int. J. Mol. Sci. 2022, 23, 3802. https://doi.org/10.3390/ijms23073802
Kersting D, Settelmeier S, Mavroeidi I-A, Herrmann K, Seifert R, Rischpler C. Shining Damaged Hearts: Immunotherapy-Related Cardiotoxicity in the Spotlight of Nuclear Cardiology. International Journal of Molecular Sciences. 2022; 23(7):3802. https://doi.org/10.3390/ijms23073802
Chicago/Turabian StyleKersting, David, Stephan Settelmeier, Ilektra-Antonia Mavroeidi, Ken Herrmann, Robert Seifert, and Christoph Rischpler. 2022. "Shining Damaged Hearts: Immunotherapy-Related Cardiotoxicity in the Spotlight of Nuclear Cardiology" International Journal of Molecular Sciences 23, no. 7: 3802. https://doi.org/10.3390/ijms23073802
APA StyleKersting, D., Settelmeier, S., Mavroeidi, I.-A., Herrmann, K., Seifert, R., & Rischpler, C. (2022). Shining Damaged Hearts: Immunotherapy-Related Cardiotoxicity in the Spotlight of Nuclear Cardiology. International Journal of Molecular Sciences, 23(7), 3802. https://doi.org/10.3390/ijms23073802






