Design and Nonadiabatic Photoisomerization Dynamics Study of a Three-Stroke Light-Driven Molecular Rotary Motor
Abstract
:1. Introduction
2. Computational Details
2.1. Density Functional Methods
2.2. Semiempirical Methods
3. Results and Discussion
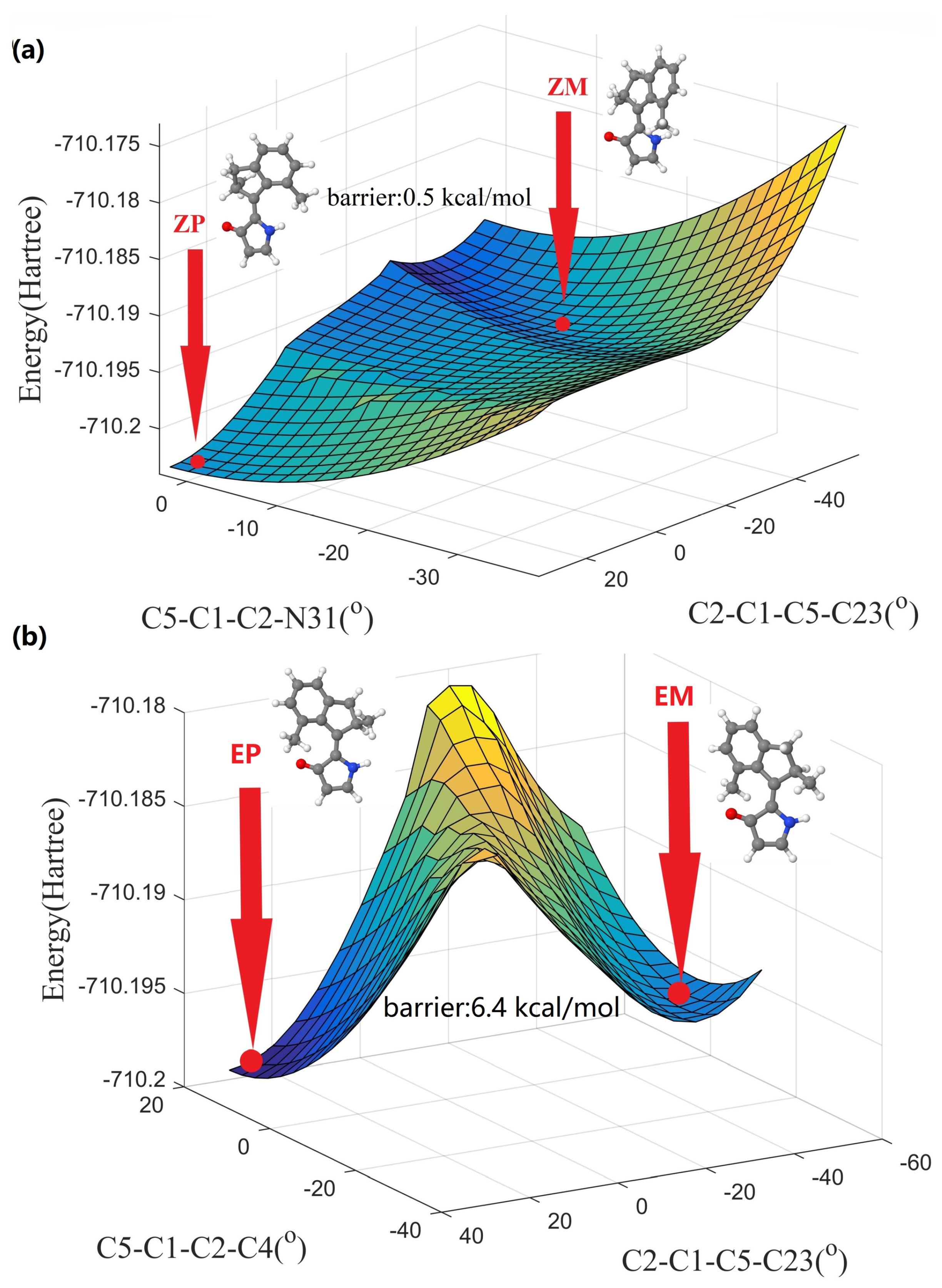
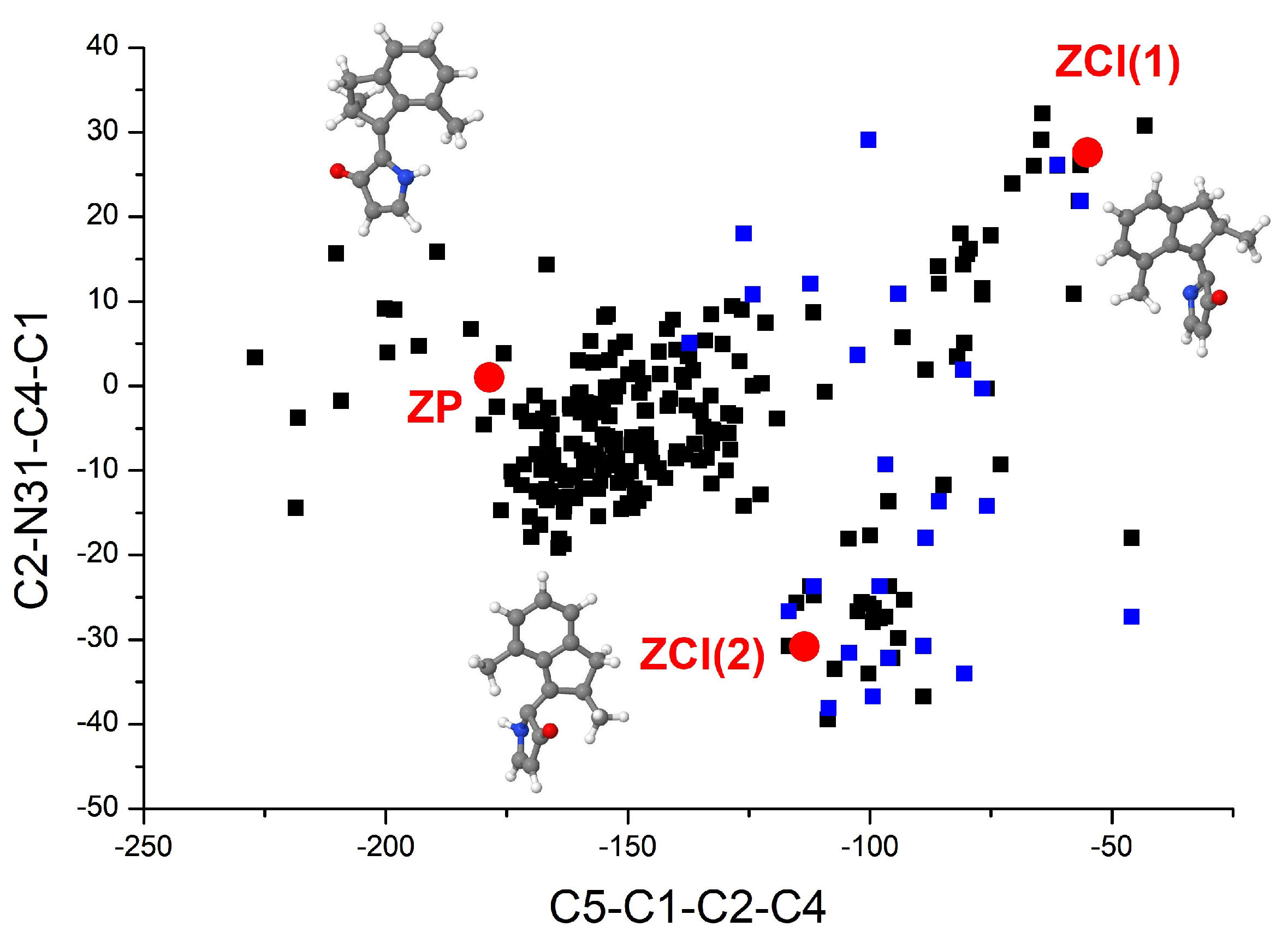
3.1. Equilibrium Structures
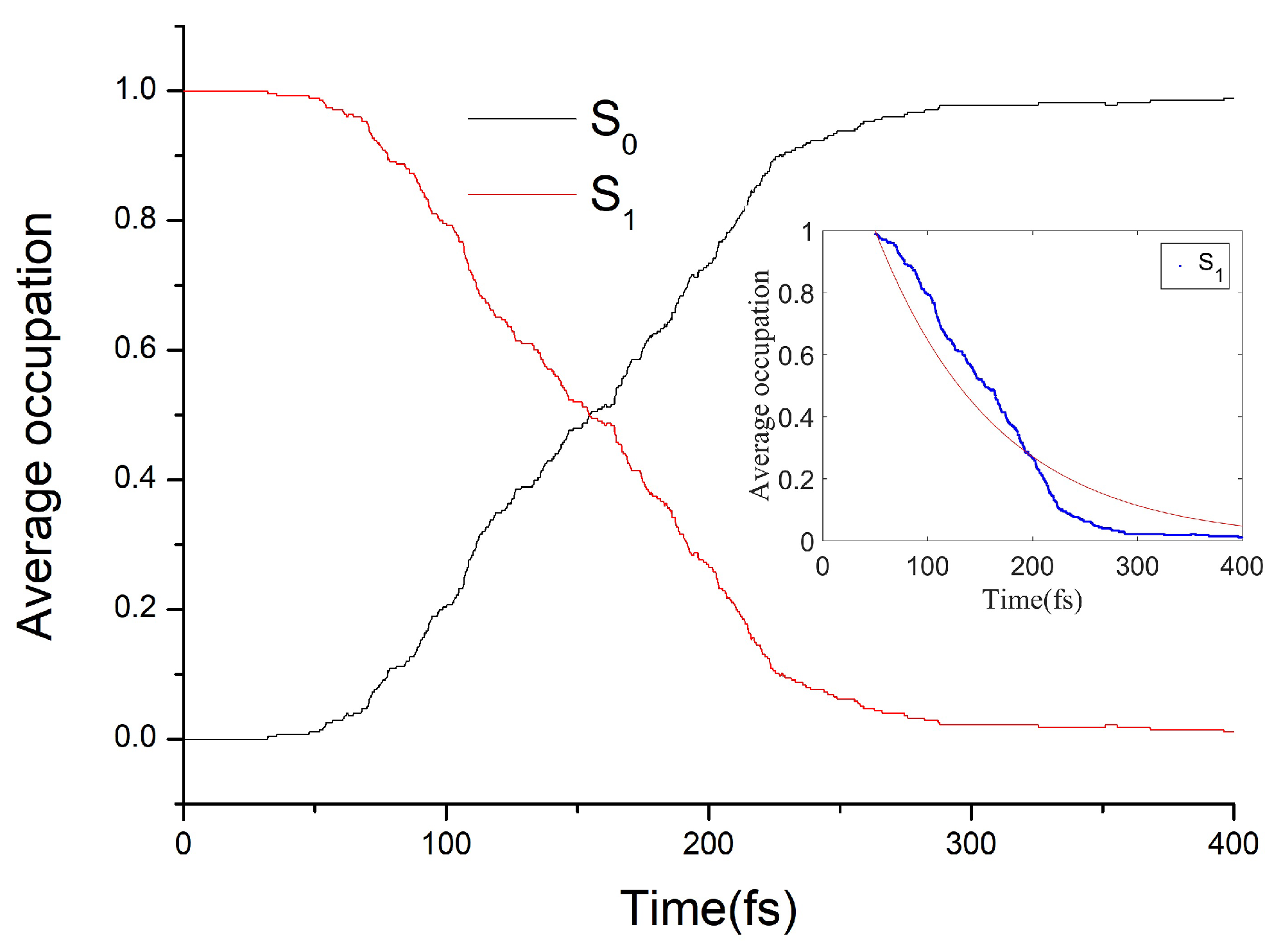
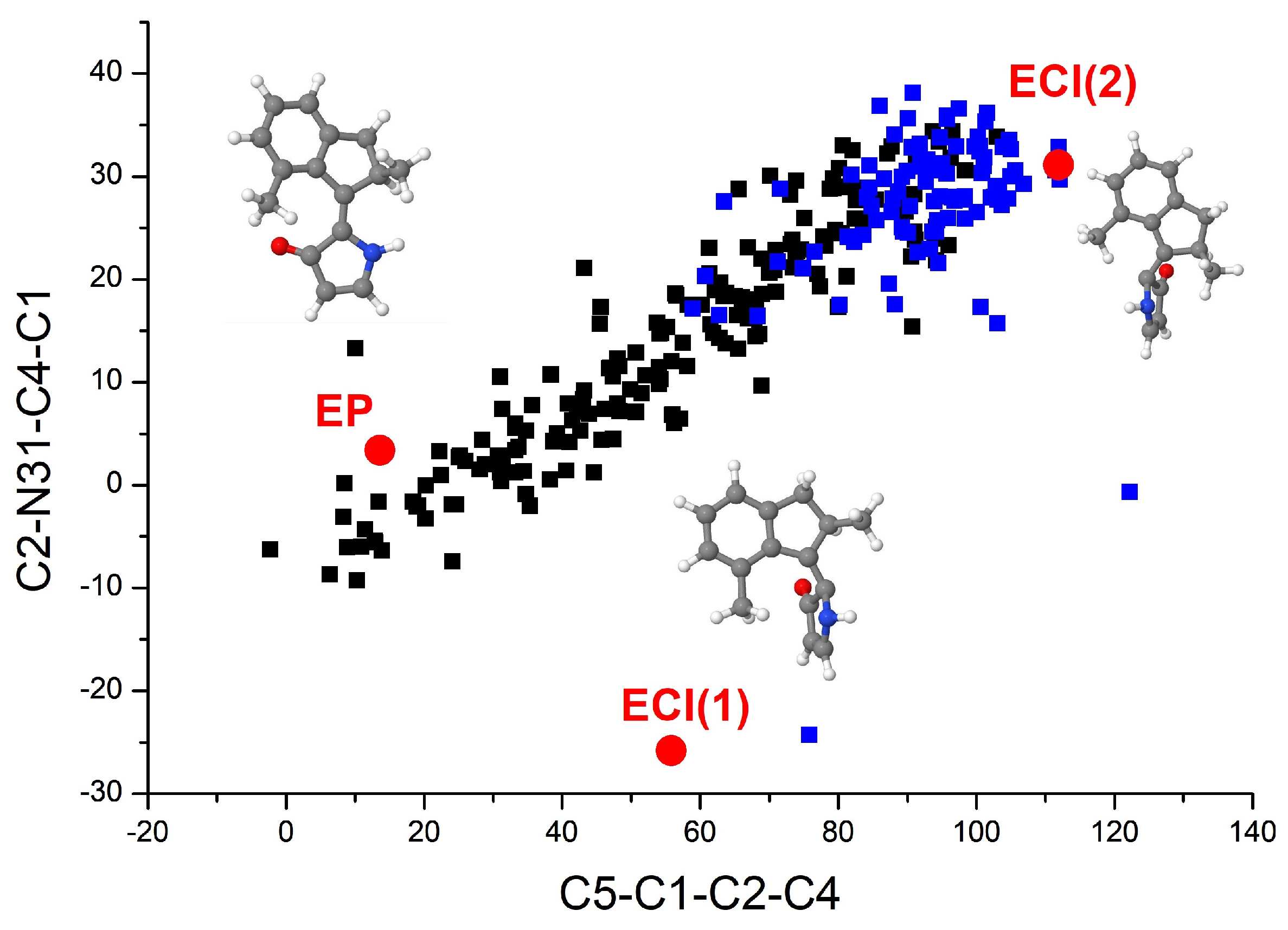
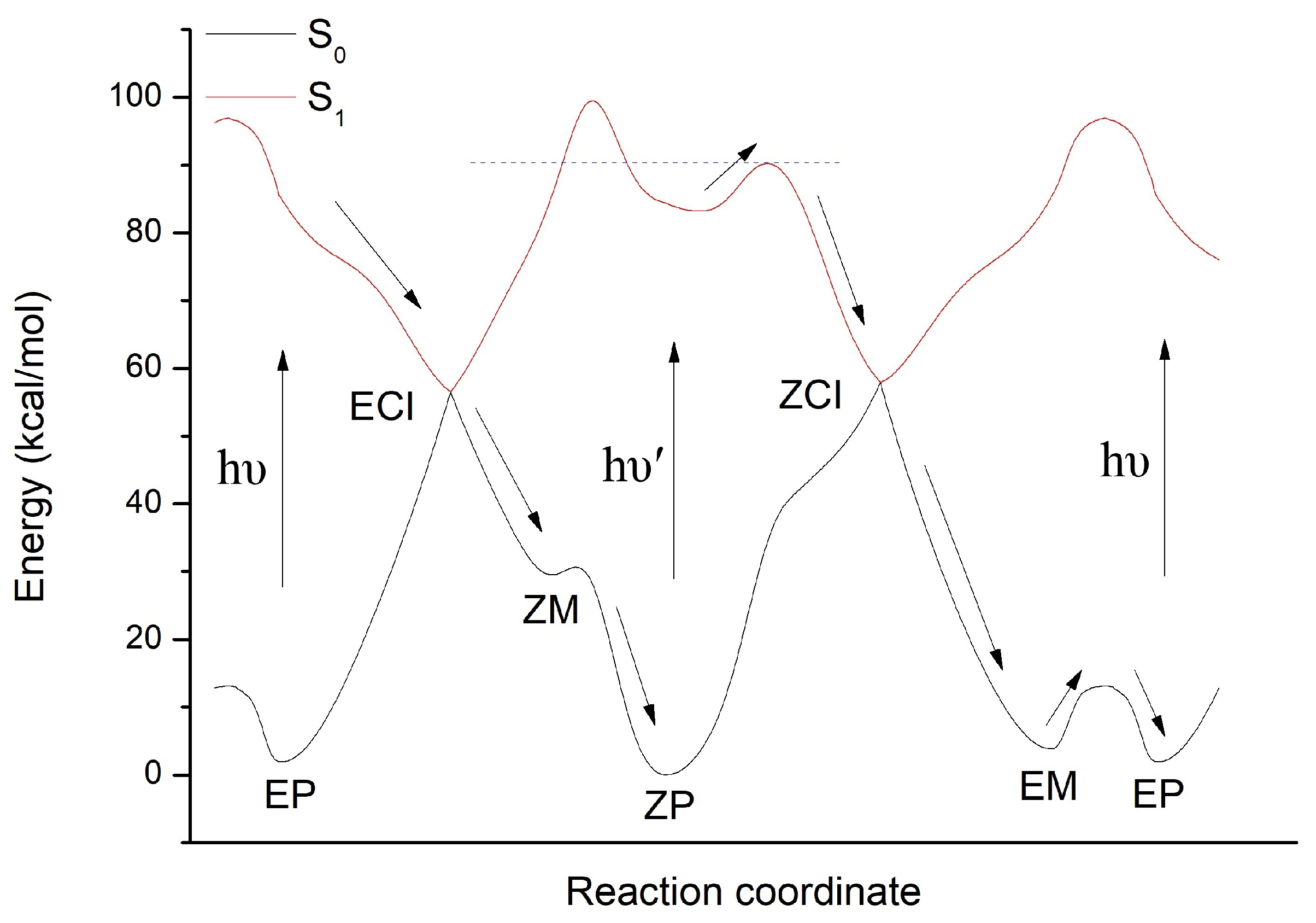
3.2. The Nonadiabatic Dynamics of EP→ZP Photoisomerization
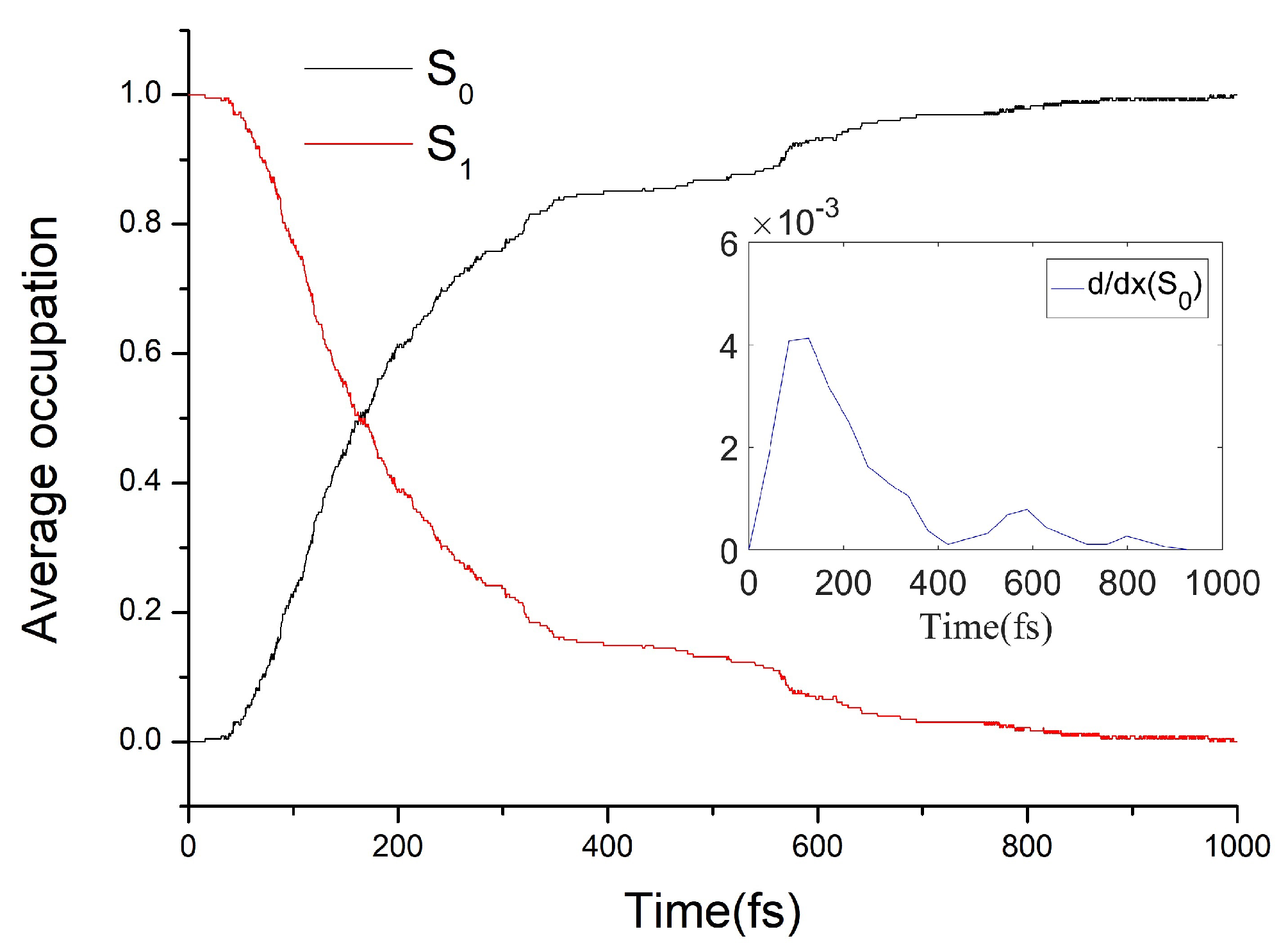
3.3. The Nonadiabatic Dynamics of ZP→EM Photoisomerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kassem, S.; Leeuwen, T.V.; Lubbe, A.S.; Wilson, M.R.; Feringa, B.L.; Leigh, D.A. Artificial Molecular Motors. Chem. Soc. Rev. 2017, 46, 2592–2621. [Google Scholar] [CrossRef] [Green Version]
- Kottas, G.S.; Clarke, L.I.; Horinek, D.; Michl, J. Artificial Molecular Rotors. Chem. Rev. 2005, 105, 1281–1376. [Google Scholar] [CrossRef]
- Feringa, B.L. In Control of Motion: From Molecular Switches to Molecular Motors. Acc. Chem. Res. 2001, 34, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Erbas-Cakmak, S.; Leigh, D.A.; Mcternan, C.T.; Nussbaumer, A.L. Artificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef] [Green Version]
- Baroncini, M.; Silvi, S.; Credi, A. Photo- and Redox-Driven Artificial Molecular Motors. Chem. Rev. 2019, 120, 200–268. [Google Scholar] [CrossRef] [Green Version]
- Groppi, J.; Baroncini, M.; Venturi, M.; Silvi, S.; Credi, A. Design of photo-activated molecular machines: Highlights from the past ten years. Chem. Commun. 2019, 55, 12595–12602. [Google Scholar] [CrossRef]
- Kelly, T.R.; Cai, X.; Damkaci, F.; Panicker, S.B.; Tu, B.; Bushell, S.M.; Cornella, I.; Piggott, M.J.; Salives, R.; Cavero, M.; et al. Progress toward a Rationally Designed, Chemically Powered Rotary Molecular Motor. J. Am. Chem. Soc. 2007, 129, 376–386. [Google Scholar] [CrossRef]
- Kelly, T.R.; De, S.H.; Silva, R.A. Unidirectional Rotary Motion in a Molecular System. Nature 1997, 401, 150–152. [Google Scholar] [CrossRef]
- Leeuwen, T.V.; Danowski, W.; Pizzolato, S.F.; Štacko, P.; Wezenberg, S.J.; Feringa, B.L. Braking of a Light-Driven Molecular Rotary Motor by Chemical Stimuli. Chem. Eur. J. 2018, 24, 81–84. [Google Scholar] [CrossRef]
- Seldenthuis, J.S.; Prins, F.; Thijssen, J.M.; vander Zant, H.S.J. An All-electric Single-molecule Motor. ACS Nano 2010, 4, 6681–6686. [Google Scholar] [CrossRef] [Green Version]
- Neumann, J.; Gottschalk, K.E.; Astumian, R.D. Driving and Controlling Molecular Surface Rotors with a Terahertz Electric Field. ACS Nano 2012, 6, 5242–5248. [Google Scholar] [CrossRef] [PubMed]
- Kudernac, T.; Ruangsupapichat, N.; Parschau, M.; Macia, B.; Katsonis, N.; Harutyunyan, S.R.; Ernst, K.-H.; Feringa, B.L. Electrically Driven Directional Motion of a Four-wheeled Molecule on a Metal Surface. Nature 2011, 479, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Feringa, B.L. The Art of Building Small: From Molecular Switches to Molecular Motors. J. Org. Chem. 2007, 72, 6635–6652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koumura, N.; Zijlstra, R.W.J.; van Delden, R.A.; Harada, N.; Feringa, B.L. Light-driven Monodirectional Molecular Rotor. Nature 1999, 401, 152–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greb, L.; Lehn, J.M. Light-Driven Molecular Motors: Imines as Four-Step or Two-Step Unidirectional Rotors. J. Am. Chem. Soc. 2014, 136, 13114–13117. [Google Scholar] [CrossRef] [PubMed]
- Koumura, N.; Geertsema, E.M.; Van Gelder, M.B.; Meetsma, A.; Feringa, B.L. Second Generation Light-Driven Molecular Motors. Unidirectional Rotation Controlled by a Single Stereogenic Center with Near-Perfect Photoequilibria and Acceleration of the Speed of Rotation by Structural Modification. J. Am. Chem. Soc. 2002, 124, 5037–5051. [Google Scholar] [CrossRef]
- Pollard, M.M.; Meetsma, A.; Feringa, B.L. A redesign of light-driven rotary molecular motors. Org. Biomol. Chem. 2008, 6, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Iriepa, C.; Marazzi, M.; Zapata, F.; Valentini, A.; Sampedro, D.; Frutos, L.M. Chiral Hydrogen Bond Environment Providing Unidirectional Rotation in Photoactive Molecular Motors. J. Phys. Chem. Lett. 2013, 4, 1389–1396. [Google Scholar] [CrossRef]
- Filatov, M.; Paolino, M.; Min, S.K.; Choi, C.H. Design and photoisomerization dynamics of a new family of synthetic 2-stroke light driven molecular rotary motors. Chem. Commun. 2019, 55, 5247–5250. [Google Scholar] [CrossRef]
- Gerwien, A.; Mayer, P.; Dube, H. Photon-Only Molecular Motor with Reverse Temperature-Dependent Efficiency. J. Am. Chem. Soc. 2018, 140, 16442–16445. [Google Scholar] [CrossRef]
- Wang, J.; Durbeej, B. Molecular motors with high quantum efficiency and visible-light responsiveness: Meeting two challenges in one design. Comput. Theor. Chem. 2019, 1148, 27–32. [Google Scholar] [CrossRef]
- Majumdar, A.; Jansen, T.L.C. Quantum-Classical Simulation of Molecular Motors Driven Only by Light. J. Phys. Chem. Lett. 2021, 12, 5512–5518. [Google Scholar] [CrossRef] [PubMed]
- Boursalian, G.; Nijboer, E.; Dorel, R.; Pfeifer, L.; Markovitch, O.; Blokhuis, A.; Feringa, B.L. All-Photochemical Rotation of Molecular Motors with a Phosphorus Stereoelement. J. Am. Chem. Soc. 2020, 142, 16868–16876. [Google Scholar] [CrossRef] [PubMed]
- Pooler, D.R.S.; Lubbe, A.S.; Crespi, S.; Feringa, B.L. Designing light-driven rotary molecular motors. Chem. Sci. 2021, 12, 14964–14986. [Google Scholar] [CrossRef] [PubMed]
- Tully, J.C. Molecular Dynamics with Electronic Transitions. J. Chem. Phys. 1990, 93, 1061–1071. [Google Scholar] [CrossRef]
- Schiffer, S.H.; Tully, J.C. Proton Transfer in Solution: Molecular Dynamics with Quantum Transitions. J. Chem. Phys. 1994, 101, 4657–4667. [Google Scholar] [CrossRef] [Green Version]
- Roke, D.; Sen, M.; Danowski, W.; Wezenberg, S.J.; Feringa, B.L. Visible-Light-Driven Tunable Molecular Motors Based on Oxindole. J. Am. Chem. Soc. 2019, 141, 7622–7627. [Google Scholar] [CrossRef]
- Pooler, D.R.S.; Pierron, R.; Crespi, S.; Costil, R.; Pfeifer, L.; Leonard, J.; Olivucci, M.; Feringa, B.L. Effect of charge-transfer enhancement on the efficiency and rotary mechanism of an oxindole-based molecular motor. Chem. Sci. 2021, 12, 7486–7497. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Thiel, W. MNDO Program, Version 6.1; Max-Planck-Institut fur Kohlenforschung: Mulheim, Germany, 2007. [Google Scholar]
- Weber, W.; Thiel, W. Orthogonalization Corrections for Semiempirical Methods. Theor. Chem. Acc. 2000, 103, 495–506. [Google Scholar] [CrossRef]
- Otte, N.; Scholten, M.; Thiel, W. Looking at Self-Consistent-Charge Density Functional Tight Binding from a Semiempirical Perspective. J. Phys. Chem. A 2007, 111, 5751–5755. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, J.; Lan, Z. Photoinduced Nonadiabatic Decay and Dissociation Dynamics of Dimethylnitramine. J. Phys. Chem. A 2013, 117, 4785–4793. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Cui, X.; Hu, D.; Jiang, C.; Zhao, D.; Lan, Z.; Li, F. “Watching” the Dark State in Ultrafast Nonadiabatic Photoisomerization Process of a Light-Driven Molecular Rotary Motor. J. Phys. Chem. A 2017, 121, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, A.; Gamez, J.A.; Thiel, W.; Filatov, M. Computational Design of a Family of Light-Driven Rotary Molecular Motors with Improved Quantum Efficiency. J. Phys. Chem. Lett. 2016, 7, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, M.; Gao, Y.J.; Zhang, Y.; Xia, S.H.; Cui, G. Electronic structure calculations and nonadiabatic dynamics simulations of excited-state relaxation of Pigment Yellow 101. Phys. Chem. Chem. Phys. 2018, 20, 6524–6532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Sun, X.W.; Zhang, T.S.; Liu, X.Y.; Cui, G. Nonadiabatic Dynamics Simulations on Early-Time Photochemistry of Spirobenzopyran. J. Phys. Chem. A 2020, 124, 2547–2559. [Google Scholar] [CrossRef]
- Gao, Y.J.; Chang, X.P.; Liu, X.Y.; Li, Q.S.; Cui, G. Excited-State Decay Paths in Tetraphenylethene Derivatives. J. Phys. Chem. A 2017, 121, 2572–2579. [Google Scholar] [CrossRef]
- Xia, S.H.; Che, M.; Liu, Y.; Zhang, Y.; Cui, G. Photochemical mechanism of 1,5-benzodiazepin-2-one: Electronic structure calculations and nonadiabatic surface-hopping dynamics simulations. Phys. Chem. Chem. Phys. 2019, 21, 10086–10094. [Google Scholar] [CrossRef]
- Weingart, O.; Lan, Z.; Koslowski, A.; Thiel, W. Chiral Pathways and Periodic Decay in cis-Azobenzene Photodynamics. J. Phys. Chem. Lett. 2011, 2, 1506–1509. [Google Scholar] [CrossRef]
- Lan, Z.; Lu, Y.; Weingart, O.; Thiel, W. Nonadiabatic Decay Dynamics of a Benzylidene Malononitrile. J. Phys. Chem. A 2012, 116, 1510–1518. [Google Scholar] [CrossRef]
- Wang, Y.T.; Liu, X.Y.; Cui, G.; Fang, W.H.; Thiel, W. Photoisomerization of Arylazopyrazole Photoswitches: Stereospecific Excited-State Relaxation. Angew. Chem. Int. Ed. 2016, 55, 14009–14013. [Google Scholar] [CrossRef]
- Pang, X.; Jiang, C.; Qi, Y.; Yuan, L.; Hu, D.; Zhang, X.; Zhao, D.; Wang, D.; Lan, Z.; Li, F. Ultrafast unidirectional chiral rotation in the Z-E photoisomerization of two azoheteroarene photoswitches. Phys. Chem. Chem. Phys. 2018, 20, 25910–25917. [Google Scholar] [CrossRef]
- Keal, T.W.; Wanki, M.; Thiel, W. Assessment of Semiempirical Methods for the Photoisomerisation of a Protonated Schiff Base. Theor. Chem. Acc. 2009, 123, 145–156. [Google Scholar] [CrossRef]
- Keal, T.W.; Koslowski, A.; Thiel, W. Comparison of Algorithms for Conical Intersection Optimization Using Semiempirical Methods. Theor. Chem. Acc. 2007, 118, 837–844. [Google Scholar] [CrossRef]
- Barbatti, M.; Granucci, G.; Persico, M.; Ruckenbauer, M.; Vazdar, M.; Eckert-Maksic, M.; Lischka, H.J. The On-the-fly Surface-hopping Program System NEWTON-X: Application to Ab Initio Simulation of the Nonadiabatic Photodynamics of Benchmark Systems. J. Photochem. Photobiol. A 2007, 190, 228–240. [Google Scholar] [CrossRef]
- Fabiano, E.; Groenhof, G.; Thiel, W. Approximate Switching Algorithms for Trajectory Surface Hopping. Chem. Phys. 2008, 351, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Fabiano, E.; Keal, T.W.; Thiel, W. Implementation of surface hopping molecular dynamics using semiempirical methods. Chem. Phys. 2008, 349, 334–347. [Google Scholar] [CrossRef]
- Granucci, G.; Persico, M.; Zoccante, A. Including quantum decoherence in surface hopping. J. Chem. Phys. 2010, 133, 134111. [Google Scholar] [CrossRef] [PubMed]
- Wigner, E. On the Quantum Correction for Thermodynamic Equilibrium. Phys. Rev. 1932, 40, 749–759. [Google Scholar] [CrossRef]
- Barbatti, M.; Sen, K. Effects of Different Initial Condition Samplings on Photodynamics and Specturm of Pyrrol. Int. J. Quantum Chem. 2016, 116, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Zobel, J.P.; Nogueira, J.J.; González, L. Finite-temperature Winger phase-space sampling and temperature effects on the excited-state dynamics of 2-nitronaphthalene. Phys. Chem. Chem. Phys. 2019, 21, 13906–13915. [Google Scholar] [CrossRef] [Green Version]
- Karnik, A.V.; Hasan, M. Stereochemistry: A Three-Dimensional Insight; Elements of Chirality and Chiral Stereoisomerism; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 3. [Google Scholar]
- Kazaryan, A.; Lan, Z.; Schafer, L.V.; Thiel, W.; Filatov, M. Surface Hopping Excited-State Dynamics Study of the Photoisomerization of a Light-Driven Fluorene Molecular Rotary Motor. J. Chem. Theory Comput. 2011, 7, 2189–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filatov, M.; Paolino, M.; Min, S.K.; Kim, K.S. Fulgides as Light-Driven Molecular Rotary Motors: Computational Design of a Prototype Compound. J. Phys. Chem. Lett. 2018, 9, 4995–5001. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Yang, S.; Zhao, D.; Jiang, C.; Lan, Z.; Li, F. Design and Nonadiabatic Photoisomerization Dynamics Study of a Three-Stroke Light-Driven Molecular Rotary Motor. Int. J. Mol. Sci. 2022, 23, 3908. https://doi.org/10.3390/ijms23073908
Ma J, Yang S, Zhao D, Jiang C, Lan Z, Li F. Design and Nonadiabatic Photoisomerization Dynamics Study of a Three-Stroke Light-Driven Molecular Rotary Motor. International Journal of Molecular Sciences. 2022; 23(7):3908. https://doi.org/10.3390/ijms23073908
Chicago/Turabian StyleMa, Jianzheng, Sujie Yang, Di Zhao, Chenwei Jiang, Zhenggang Lan, and Fuli Li. 2022. "Design and Nonadiabatic Photoisomerization Dynamics Study of a Three-Stroke Light-Driven Molecular Rotary Motor" International Journal of Molecular Sciences 23, no. 7: 3908. https://doi.org/10.3390/ijms23073908





