Metformin Treatment Regulates the Expression of Molecules Involved in Adiponectin and Insulin Signaling Pathways in Endometria from Women with Obesity-Associated Insulin Resistance and PCOS
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Design
2.2. Materials and Reagents
2.3. Immunohistochemistry
2.4. Cell Culture and Treatments
2.5. Immunocytochemistry
2.6. Statistical Evaluation
3. Results
3.1. Effect of MTF on Molecules Associated with Insulin Action in Endometria from Women with IR, Obesity and PCOS
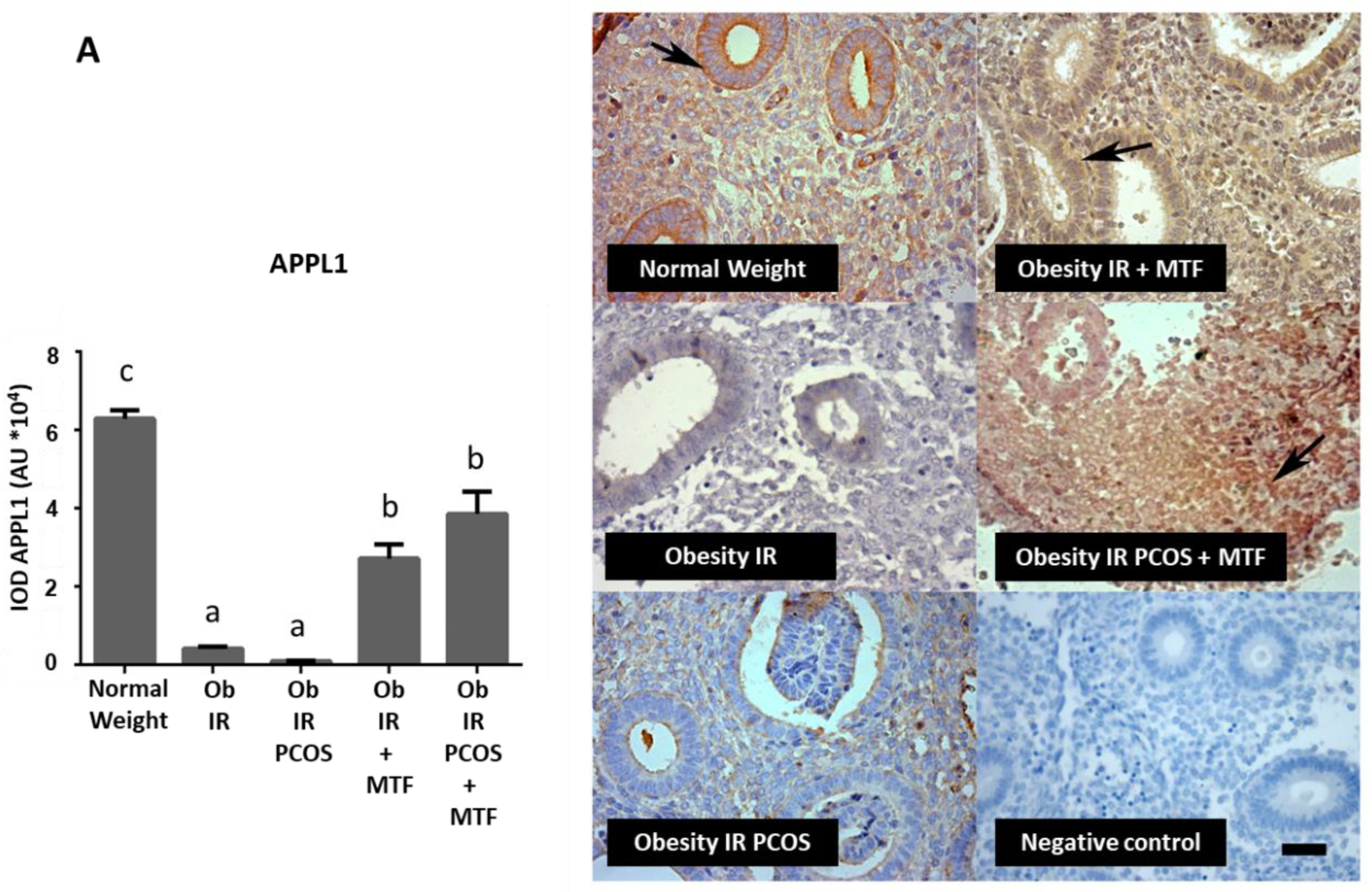
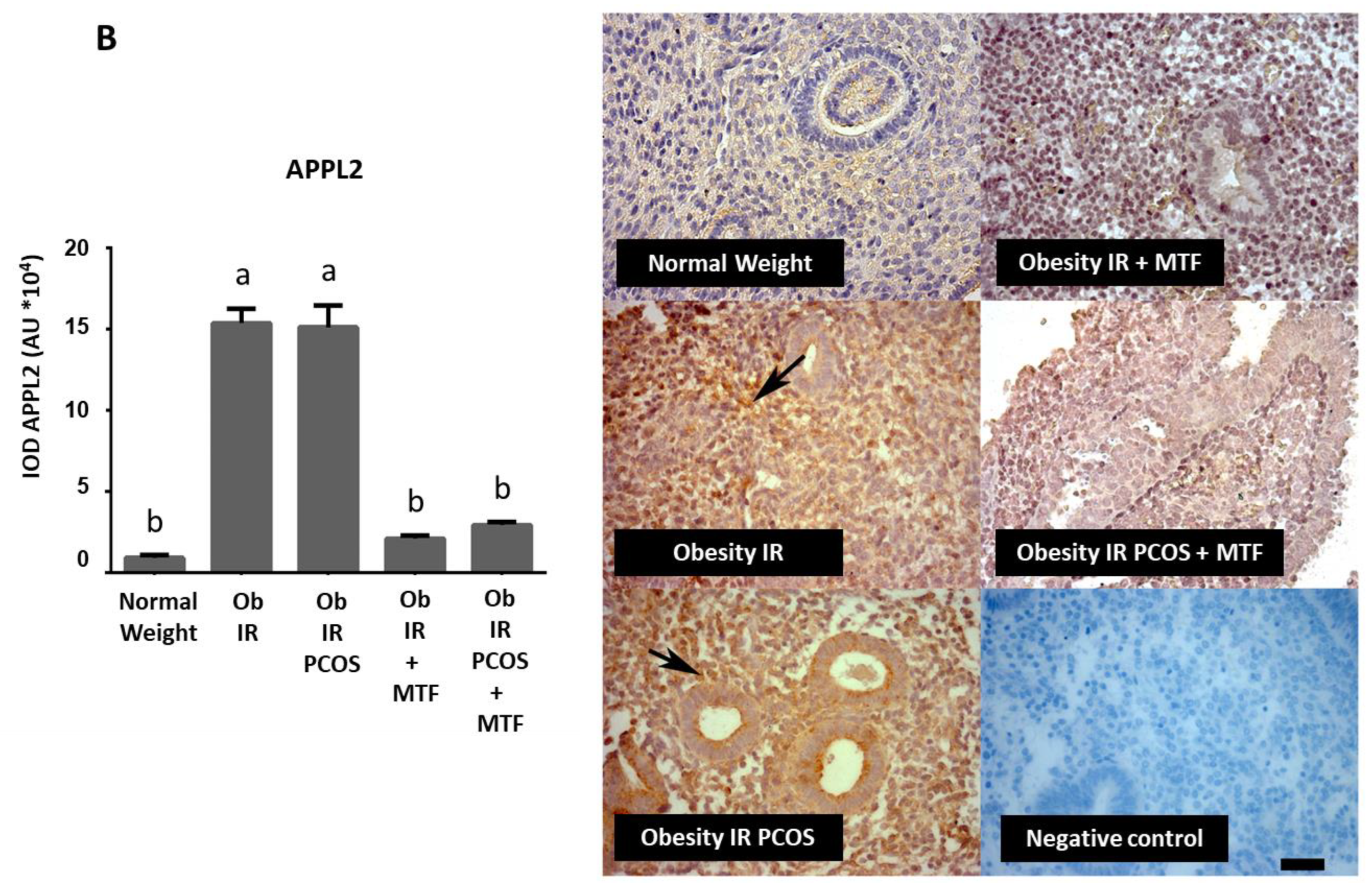
3.2. Effect of MTF on Molecules Associated with Adiponectin Signaling in Endometria from Women with IR, Obesity and PCOS
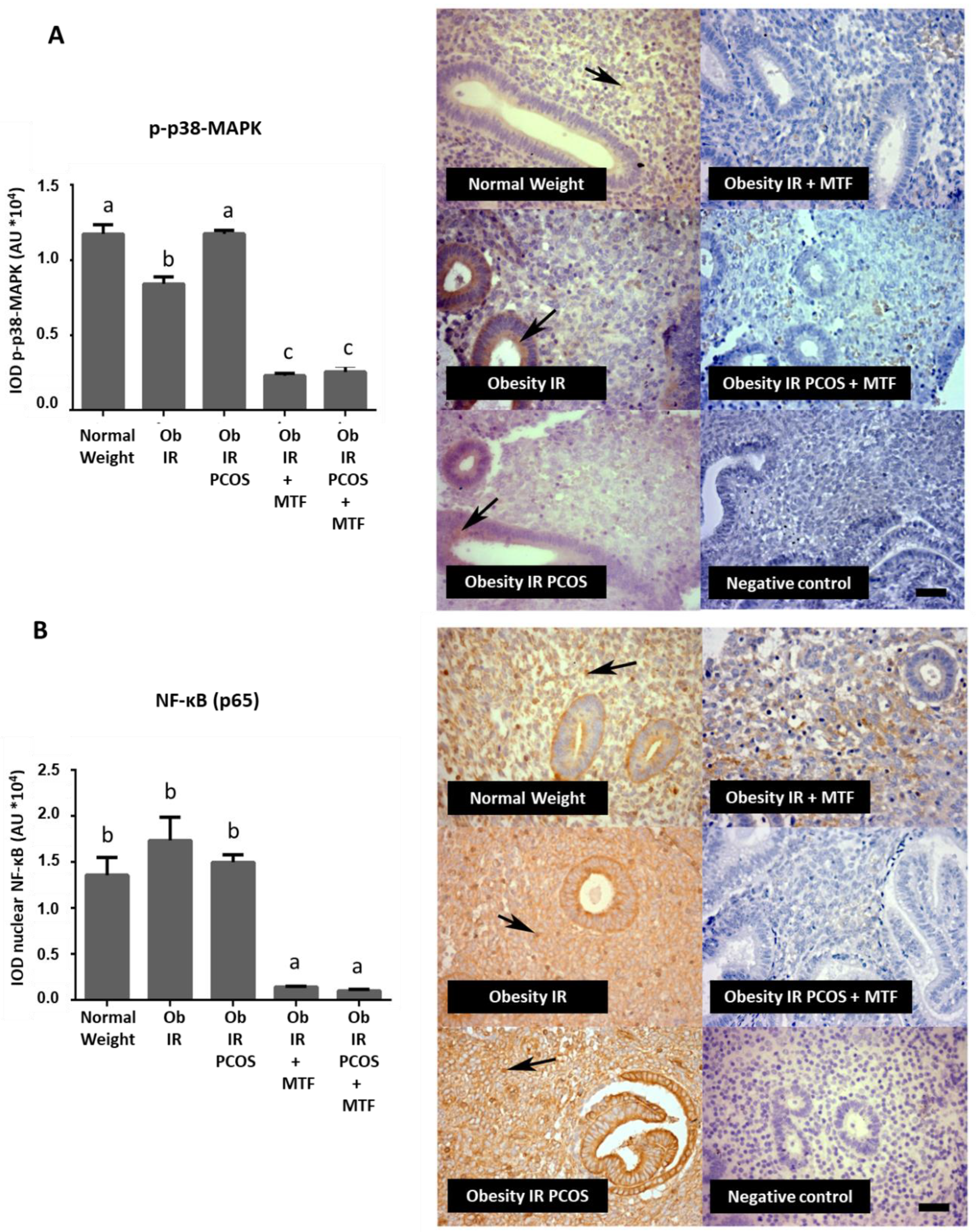
3.3. Effect of MTF on the Active Form of p38-MAPK and NF-κB Levels in Endometria from Women with Obesity-Associated IR and PCOS
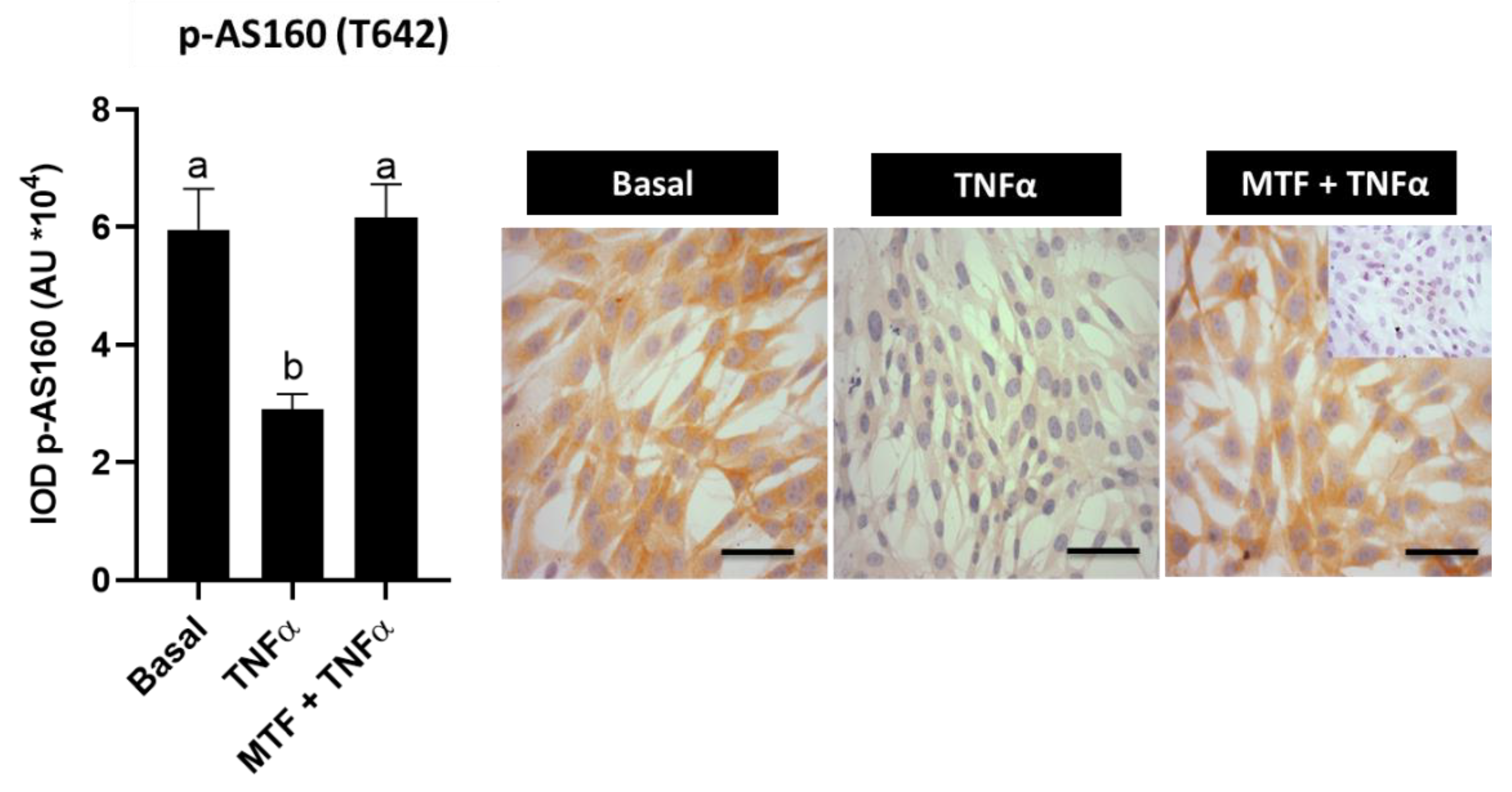
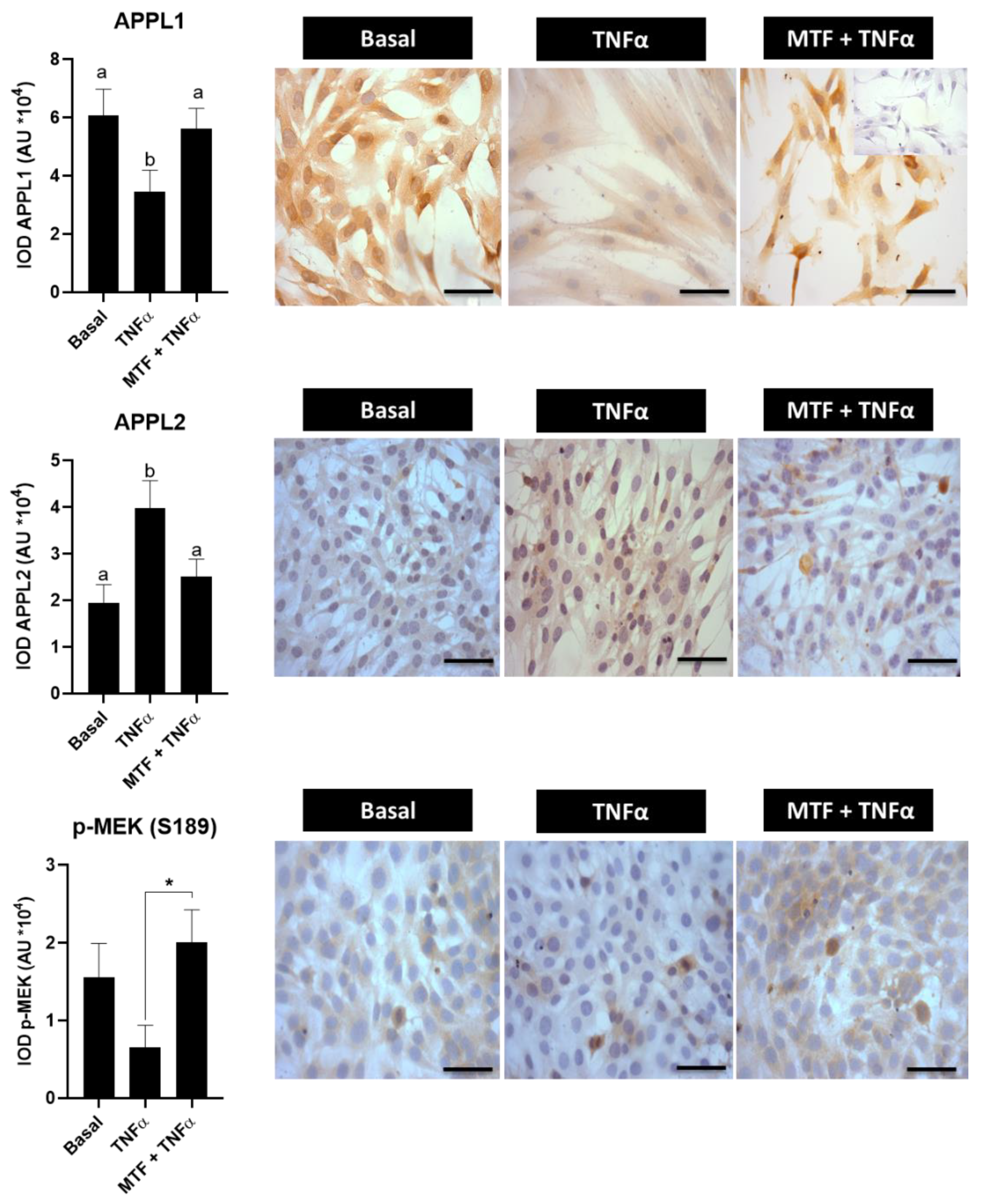
3.4. Metformin Re-Established Levels of Insulin and Adiponectin Signaling Molecules in Cultured Human Endometrial Cells
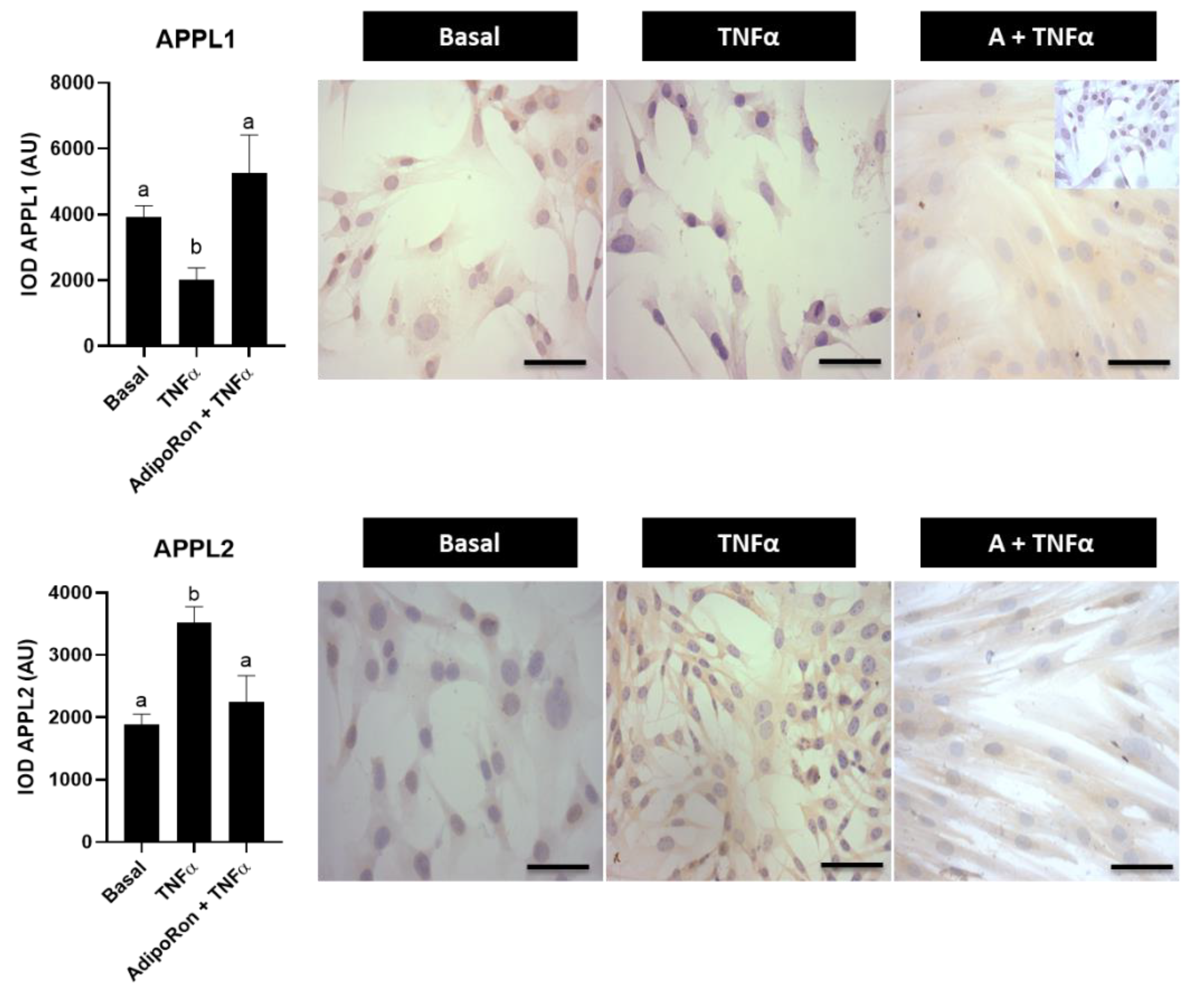
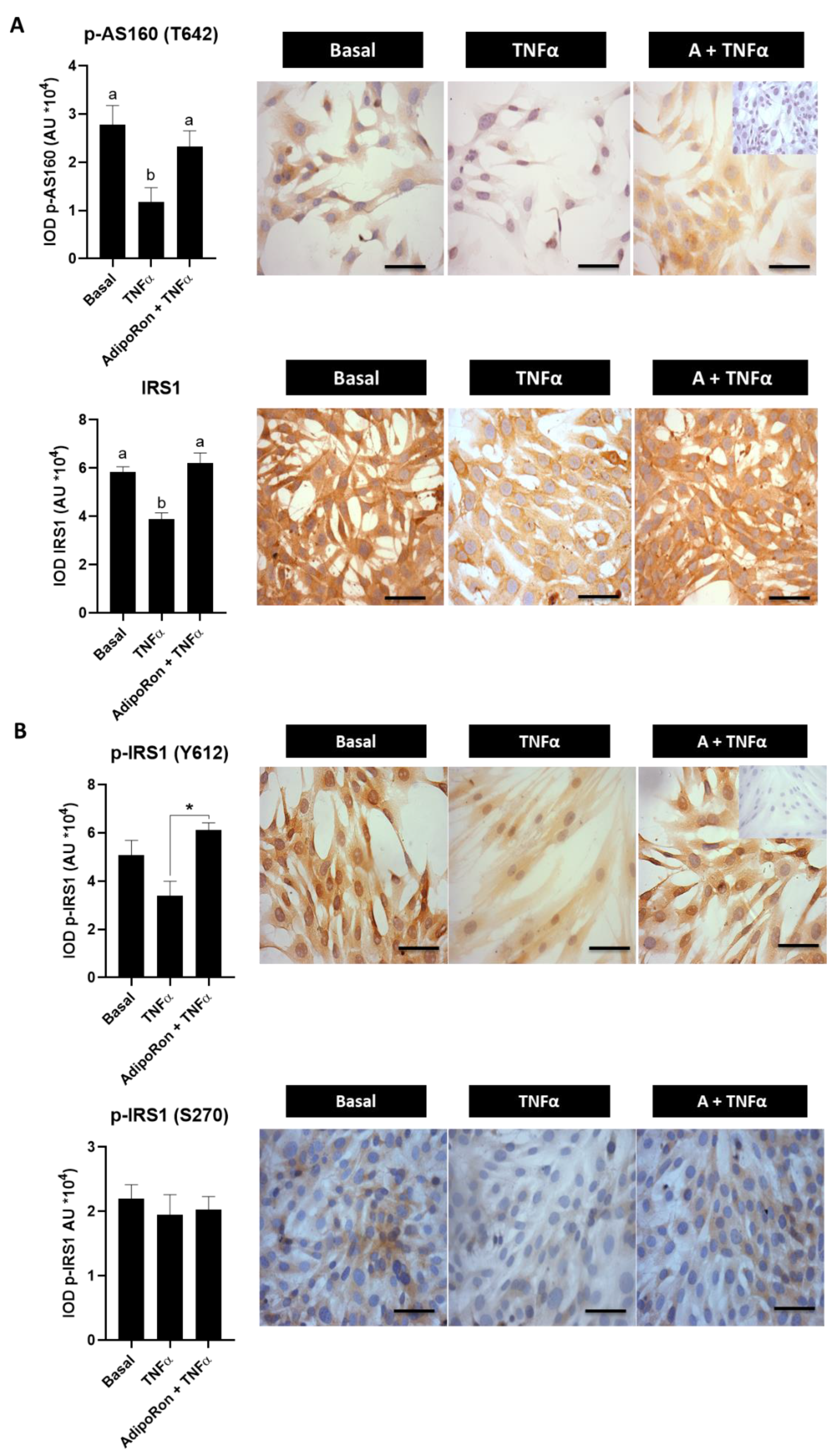
3.5. Effect of Adiponectin Agonist on Molecules Associated with Insulin Signaling in Human Endometrial Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diamanti-Kandarakis, E. Polycystic ovarian syndrome pathophysiology, molecular aspects and clinical implications. Expert Rev. Mol. Med. 2008, 10, e3. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Fornes, R.; Ormazabal, P.; Rosas, C.; Gabler, F.; Vantman, D.; Romero, C.; Vega, M. Changes in the expression of insulin signaling pathway molecules in endometria from PCOS women with or without insulin resistance. Mol. Med. 2010, 16, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Oróstica, L.; Rosas, C.; Plaza-Parrochia, F.; Astorga, I.; Gabler, F.; García, V.; Romero, C.; Vega, M. Altered Steroid Metabolism and Insulin Signaling in PCOS Endometria: Impact in Tissue Function. Curr. Pharm. Des. 2016, 22, 5614–5624. [Google Scholar] [CrossRef]
- Rosas, C.; Gabler, F.; Vantman, D.; Romero, C.; Vega, M. Levels of Rabs and WAVE family proteins associated with translocation of GLUT4 to the cell surface in endometria from hyperinsulinemic PCOS women. Hum. Reprod. 2010, 25, 2870–2877. [Google Scholar] [CrossRef]
- Oróstica, L.; Astorga, I.; Plaza-Parrochia, F.; Vera, C.; García, V.; Carvajal, R.; Gabler, F.; Romero, C.; Vega, M. Proinflammatory environment and role of TNF-α in endometrial function of obese women having polycystic ovarian syndrome. Int. J. Obes. 2016, 40, 1715–1722. [Google Scholar] [CrossRef]
- Messinis, I.E.; Messini, C.I. Obesity in PCOS and infertility. In Obesity: A Ticking Time Bomb for Reproductive Health, 1st ed.; Mahmood, T., Arulkumaran, S., Eds.; Elsevier Inc.: Waltham, MA, USA, 2013; pp. 99–116. [Google Scholar]
- García, V.; Oróstica, L.; Poblete, C.; Rosas, C.; Astorga, I.; Romero, C.; Vega, M. Endometria from obese PCOS women with hyperinsulinemia exhibit altered adiponectin signaling. Horm. Metab. Res. 2015, 47, 901–909. [Google Scholar] [CrossRef]
- Oróstica, L.; García, P.; Vera, C.; García, V.; Romero, C.; Vega, M. Effect of TNF-α on molecules related to the insulin action in endometrial cells exposed to hyperandrogenic and hyperinsulinic conditions characteristics of polycystic ovary syndrome. Reprod. Sci. 2018, 25, 1000–1009. [Google Scholar] [CrossRef]
- Guerre-Millo, M. Adiponectin: An update. Diabetes Metab. 2008, 34, 12–18. [Google Scholar] [CrossRef]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More than just another fat cell hormone? Diab. Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Deepa, S.; Dong, L. APPL1: Role in adiponectin signaling and beyond. American journal of physiology. Endocrinol. Metab. 2009, 296, E22–E36. [Google Scholar]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef]
- Adamczak, M.; Wiecek, A.; Funahashi, T.; Chudek, J.; Kokot, F.; Matsuzawa, Y. Decreased plasma adiponectin concentration in patients with essential hypertension. Am. J. Hypertens. 2003, 16, 72–75. [Google Scholar] [CrossRef]
- Carvajal, R.; Rosas, C.; Kohan, K.; Gabler, F.; Vantman, D.; Romero, C.; Vega, M. Metformin augments the levels of molecules that regulate the expression of the insulin-dependent glucose transporter GLUT4 in the endometria of hyperinsulinemic PCOS patients. Hum. Reprod. 2013, 28, 2235–2244. [Google Scholar] [CrossRef][Green Version]
- Liu, M.; Liu, F. Transcriptional and post-translational regulation of adiponectin. Biochem. J. 2009, 425, 41–52. [Google Scholar] [CrossRef]
- Saito, T.; Jones, C.C.; Huang, S.; Czech, M.P.; Pilch, P.F. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J. Biol. Chem 2007, 282, 32280–32287. [Google Scholar] [CrossRef]
- Wang, C.; Xin, X.; Ruihua Xiang, J.; Ramos, F.J.; Liu, M.; Lee, H.J.; Chen, H.; Mao, X.; Kikani, C.K.; Liu, F.; et al. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J. Biol. Chem. 2009, 284, 31608–31615. [Google Scholar] [CrossRef]
- Bluher, M.; Fasshauer, M.; Kralisch, S.; Schon, M.R.; Krohn, K.; Paschke, R. Regulation of adiponectin receptor R1 and R2 gene expression in adipocytes of C57BL/6 mice. Biochem. Biophys. Res. Commun. 2005, 329, 1127–1132. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, I.; Rasschaert, J.; Eizirik, D.L.; Cnop, M. Expression of adiponectina receptors in pancreatic beta cells. Biochem. Biophys. Res. Commun. 2003, 312, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.; Ferrannini, E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: A systematic review. Diabetologia 2006, 49, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Thong, F.S.L.; Bilan, P.J.; Amira Klip, A. The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes 2007, 56, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Gil-Campos, M.; Canete, R.R.; Gil, A. Adiponectin, the missing link in insulin resistance and obesity. Clin. Nutr. 2004, 23, 963–974. [Google Scholar] [CrossRef] [PubMed]
- McAuley, K.; Mann, J.; Chase, J.; Lotz, T.; Shaw, G. Point: HOMA—Satisfactory for the time being: HOMA: The best bet for the simple determination of insulin sensitivity, until something better comes along. Diabetes Care 2007, 30, 2411–2413. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Noyes, R.W.; Hertig, A.T.; Rock, J. Dating the endometrial biopsy. Fert. Steril. 1950, 1, 3–25. [Google Scholar] [CrossRef]
- Samalecos, A.; Reimann, K.; Wittmann, S.; Schulte, H.M.; Bronsens, J.J.; Bamberger, A.M.; Gellersen, B. Characterization of a novel telomerase-immortalized human endometrial stromal cell line, St-T1b. Reprod. Biol. Endocrinol. 2009, 7, 76. [Google Scholar] [CrossRef]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef]
- Oróstica, L.; Poblete, C.; Romero, C.; Vega, M. Pro-Inflammatory Markers Negatively Regulate IRS1 in Endometrial Cells and Endometrium from Women with Obesity and PCOS. Reprod. Sci. 2020, 27, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Kohan, K.; Carvajal, R.; Gabler, F.; Vantman, D.; Romero, C.; Vega, M. Role of the transcriptional factors FOXO1 and PPARG on gene expression of SLC2A4 in endometrial tissue from women with polycystic ovary syndrome. Reproduction 2010, 140, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Pfizenmainer, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhou, M.; Zhou, G.; Zhu, Q.; Li, W. Effect of metformin on adiponectin in PCOS: A meta-analysis and a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 61–67. [Google Scholar] [CrossRef]
- Zhou, L.; Deepa, S.S.; Etzler, J.C.; Ryu, J.; Mao, X.; Fang, Q.; Liu, D.D.; Torres, J.M.; Jia, W.; Lechleiter, J.D.; et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J. Biol. Chem. 2009, 284, 22426–22435. [Google Scholar] [CrossRef]
- Schmid, P.M.; Resch, M.; Schach, C.; Birner, C.; Riegger, G.A.; Luchner, A.; Endemann, D.H. Antidiabetic treatment restores adiponectin serum levels and APPL1 expression, but does not improve adiponectin-induced vasodilation and endothelial dysfunction in Zucker diabetic fatty rats. Cardiovasc. Diabetol. 2013, 18, 46. [Google Scholar] [CrossRef]
- Wang, B.; Jenkins, J.R.; Trayhurn, P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: Integrated response to TNF-alpha. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E731–E740. [Google Scholar] [CrossRef]
- Wang, B.; Trayhurn, P. Acute and prolonged effects of TNF-alpha on the expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture. Pflug. Arch. 2006, 452, 418–427. [Google Scholar] [CrossRef]
- Cabrera-Cruz, H.; Oróstica, L.; Plaza-Parrochia, F.; Torres-Pinto, I.; Romero, C.; Vega, M. The insulin-sensitizing mechanism of myo-inositol is associated with AMPK activation and GLUT-4 expression in human endometrial cells exposed to a PCOS environment. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E237–E248. [Google Scholar] [CrossRef]
- Villavicencio, A.; Bacallao, K.; Gabler, F.; Fuentes, A.; Albornoz, J.; Casals, A.; Vega, M. Deregulation of tissue homeostasis in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia. Gynecol. Oncol. 2007, 104, 290–295. [Google Scholar] [CrossRef]
- Villavicencio, A.; Goyeneche, A.; Telleria, C.; Bacallao, K.; Gabler, F.; Fuentes, A.; Vega, M. Involvement of Akt, Ras and cell cycle regulators in the potential development of endometrial hyperplasia in women with polycystic ovarian syndrome. Gynecol. Oncol. 2009, 115, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Parrochia, F.; Oróstica, L.; García, P.; Vera, C.; Romero, C.; Valladares, L.; Vega, M. Molecular mechanisms of androstenediol in the regulation of the proliferative process of human endometrial cells. Reprod. Sci. 2017, 24, 1079–1087. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Normal Weight | Obesity + IR | Obesity + IR + PCOS |
|---|---|---|---|
| Age (years) | 26.6 ± 5.6 | 26.9 ± 3.2 | 25.9 ± 2.4 |
| BMI (kg/m2) | 22.4 ± 2.1 | 34.2 ± 2.2 & | 32.6 ± 3.4 & |
| Estradiol (pmol/L) | 168.7 ± 77 | 159.5 ± 58 | 143.6 ± 40 |
| Testosterone (ng/dL) | 36.6 ± 10.7 | 31.8 ± 9.6 | 49.4 ± 9.2 * |
| Androstenedione (ng/mL) | 4.9 ± 1.3 | 4.5 ± 1.7 | 5.3 ± 1.4 * |
| SHBG (nmol/L) | 68.9 ± 0.8 | 52.0 ± 1.8 | 25.7 ± 11 * |
| FAI | 1.9 ± 0.6 | 2.1 ± 0.9 | 6.7 ± 1.9 * |
| HOMA-IR | 1.49 ± 1.1 | 4.1 ± 0.7 & | 4.5 ± 0.9 & |
| ISI Composite | 8.1 ± 4.7 | 2.4 ± 1.6 & | 2.09 ± 1.1 & |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oróstica, M.L.; Astorga, I.; Plaza-Parrochia, F.; Poblete, C.; Carvajal, R.; García, V.; Romero, C.; Vega, M. Metformin Treatment Regulates the Expression of Molecules Involved in Adiponectin and Insulin Signaling Pathways in Endometria from Women with Obesity-Associated Insulin Resistance and PCOS. Int. J. Mol. Sci. 2022, 23, 3922. https://doi.org/10.3390/ijms23073922
Oróstica ML, Astorga I, Plaza-Parrochia F, Poblete C, Carvajal R, García V, Romero C, Vega M. Metformin Treatment Regulates the Expression of Molecules Involved in Adiponectin and Insulin Signaling Pathways in Endometria from Women with Obesity-Associated Insulin Resistance and PCOS. International Journal of Molecular Sciences. 2022; 23(7):3922. https://doi.org/10.3390/ijms23073922
Chicago/Turabian StyleOróstica, Maria Lorena, Isis Astorga, Francisca Plaza-Parrochia, Cristian Poblete, Rodrigo Carvajal, Víctor García, Carmen Romero, and Margarita Vega. 2022. "Metformin Treatment Regulates the Expression of Molecules Involved in Adiponectin and Insulin Signaling Pathways in Endometria from Women with Obesity-Associated Insulin Resistance and PCOS" International Journal of Molecular Sciences 23, no. 7: 3922. https://doi.org/10.3390/ijms23073922
APA StyleOróstica, M. L., Astorga, I., Plaza-Parrochia, F., Poblete, C., Carvajal, R., García, V., Romero, C., & Vega, M. (2022). Metformin Treatment Regulates the Expression of Molecules Involved in Adiponectin and Insulin Signaling Pathways in Endometria from Women with Obesity-Associated Insulin Resistance and PCOS. International Journal of Molecular Sciences, 23(7), 3922. https://doi.org/10.3390/ijms23073922







