Emerging Roles of Motile Epidermal Chloroplasts in Plant Immunity
Abstract
:1. Introduction
2. Epidermal Chloroplast Response Controls the Entry of Fungal Pathogens in A. thaliana
2.1. Intracellular Movements of Epidermal Chloroplasts in Response to Fungal Pathogens in A. thaliana
2.2. The Trigger of the Epidermal Chloroplast Response
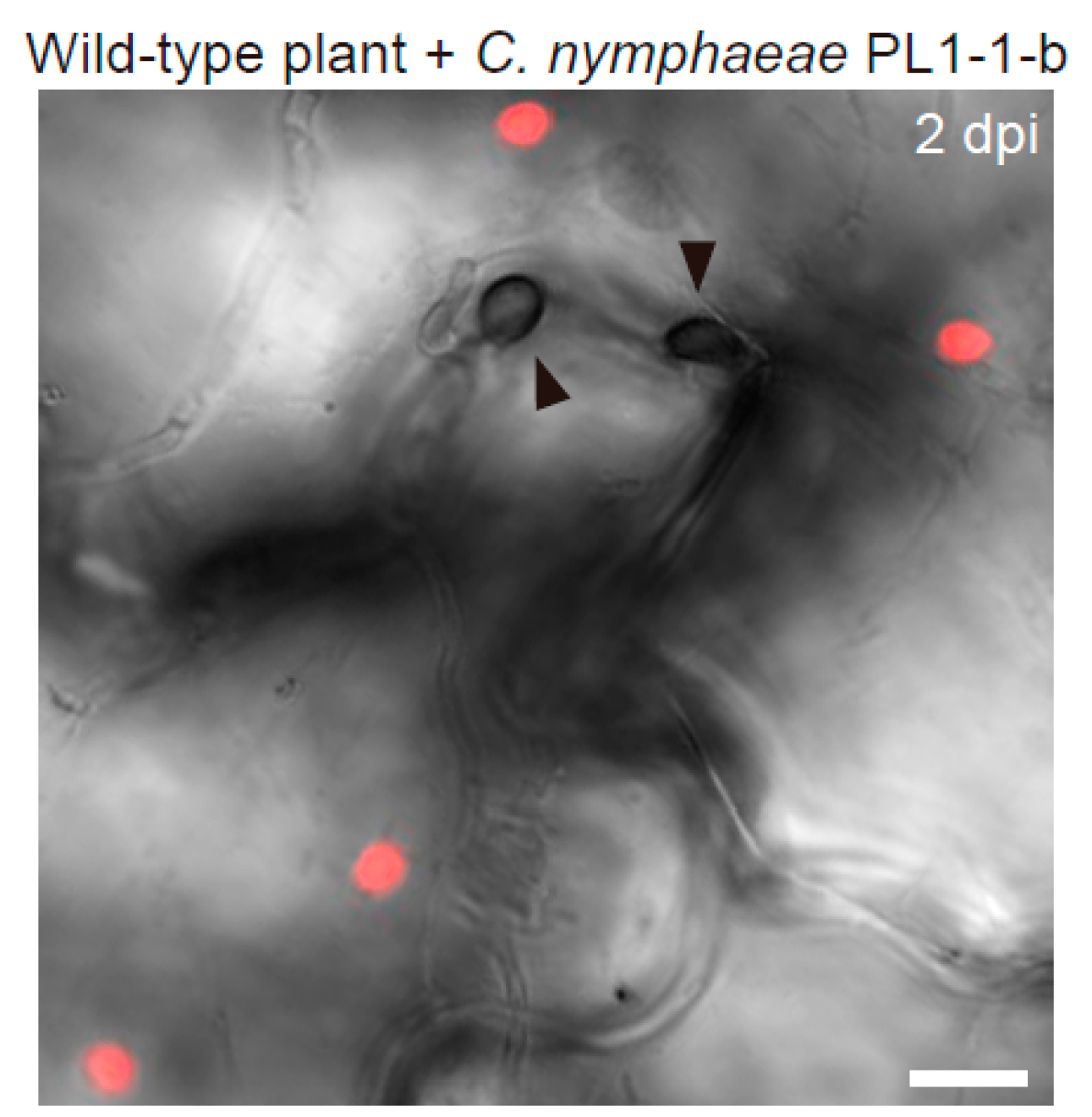
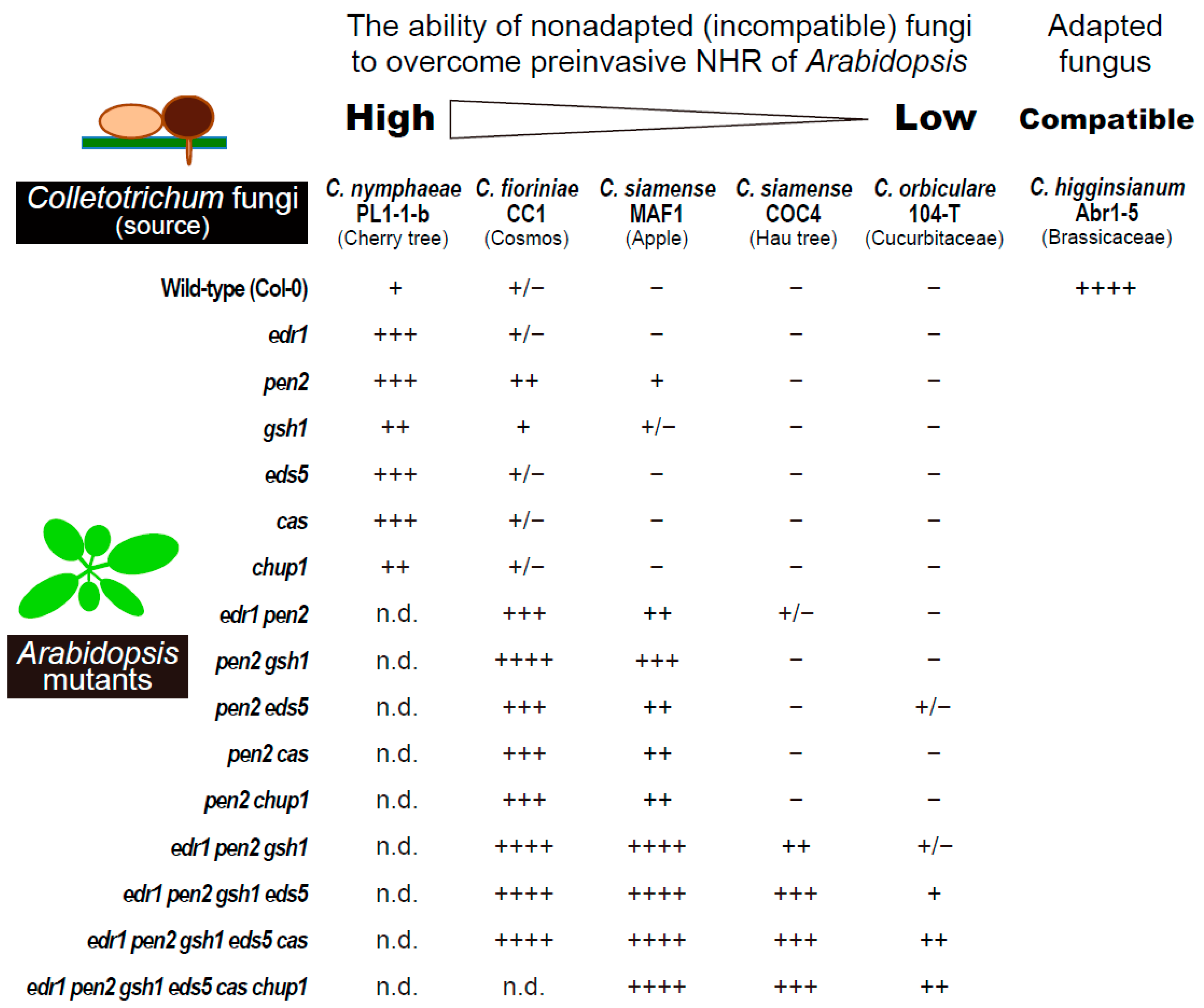
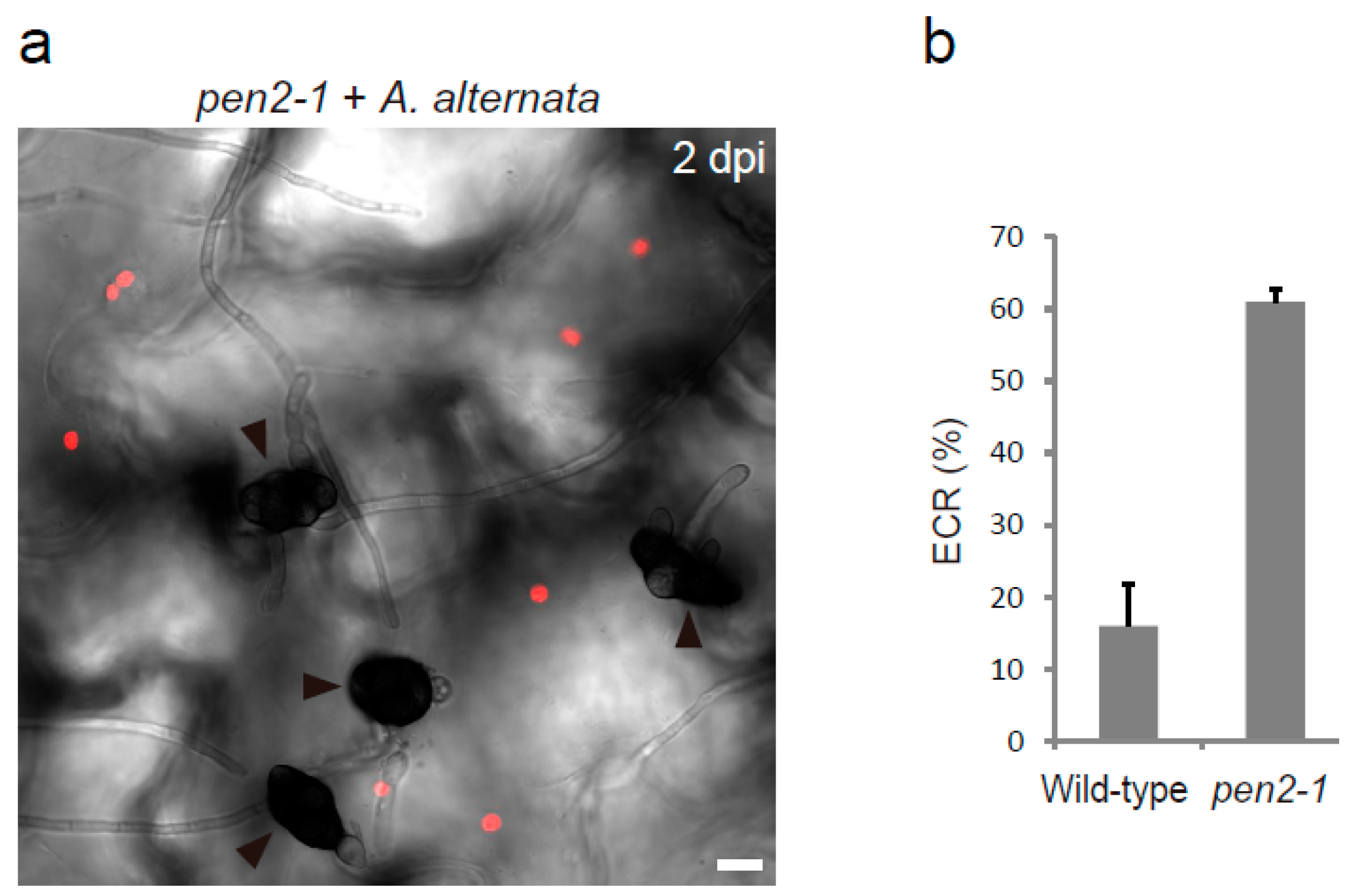
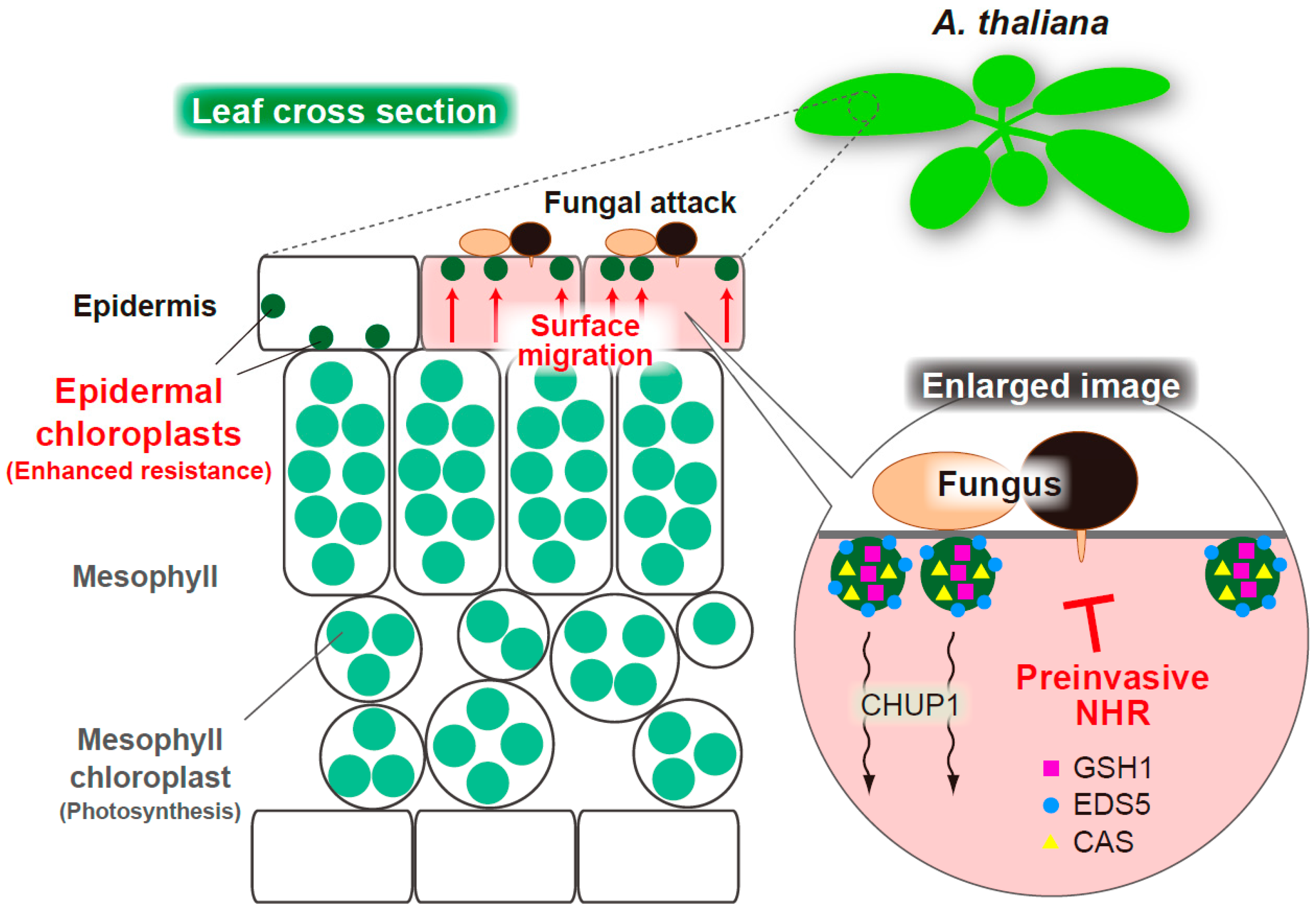
2.3. The Regulators of Epidermal Chloroplast Response and Preinvasive Nonhost Resistance of Arabidopsis
3. Epidermal Chloroplast-Localized Immune Components Contribute to Preinvasive Antifungal Nonhost Resistance of Arabidopsis
3.1. The Preferential Localization of Immune-Related Components to Motile Epidermal Chloroplasts in A. thaliana
3.2. Epidermal Chloroplast-Localized Immune Components and Preinvasive Defense in A. thaliana
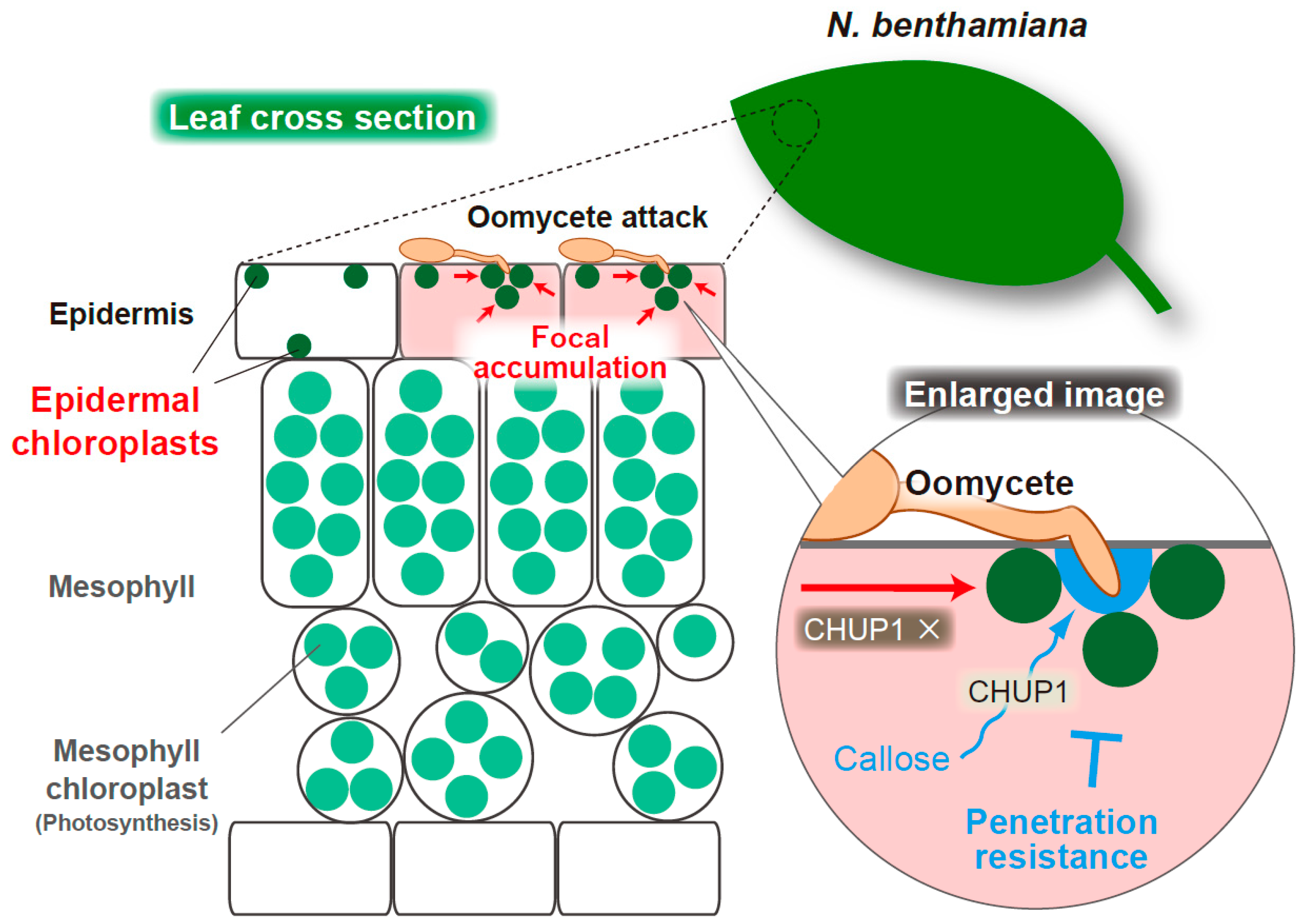
4. Epidermal Chloroplasts Accumulate at the Interface with Oomycete Pathogen and CHUP1 Is Required for Penetration Resistance in Nicotiana benthamiana
4.1. Focal Accumulation of Epidermal Chloroplasts at the Interface with Oomycete Pathogen in N. benthamiana
4.2. CHUP1-Dependent Callose Deposition at the Oomycete Penetration Site during the Epidermal Resistance of N. benthamiana
5. Dynamic Morphology of Epidermal Chloroplasts and Inter-Organelle Interactions in Plant Immunity
5.1. Stromule Formation, Enlargement, and Cavity Formation
5.2. Perinuclear Clustering of Epidermal Chloroplasts and Nuclear Movements
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kubo, Y.; Furusawa, I. Melanin biosynthesis: Prerequisite for successful invasion of the plant host by appressoria of Colle-totrichum and Pyricularia. In The Fungal Spore and Disease Initiation in Plants and Animals; Cole, G.T., Hoch, H.C., Eds.; Plenum Publishing: New York, NY, USA, 1991; pp. 205–217. [Google Scholar]
- Heath, M.C. Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant Biol. 2000, 3, 315–319. [Google Scholar] [CrossRef]
- Lee, H.-A.; Lee, H.-Y.; Seo, E.; Lee, J.; Kim, S.-B.; Oh, S.; Choi, E.; Choi, E.; Lee, S.E.; Choi, D. Current Understandings of Plant Nonhost Resistance. Mol. Plant-Microbe Interact. 2017, 30, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, N.C.; Thordal-Christensen, H.; Lipka, V.; Bau, S.; Kombrink, E.; Qiu, J.-L.; Hückelhoven, R.; Stein, M.; Freialdenhoven, A.; Somerville, S.C.; et al. SNARE-protein-mediated disease resistance at the plant cell wall. Nature 2003, 425, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.K.; Lipka, V.; Burton, R.; Panstruga, R.; Strizhov, N.; Schulze-Lefert, P.; Fincher, G.B. An Arabidopsis Callose Synthase, GSL5, Is Required for Wound and Papillary Callose Formation. Plant Cell 2003, 15, 2503–2513. [Google Scholar] [CrossRef] [Green Version]
- Lipka, V.; Dittgen, J.; Bednarek, P.; Bhat, R.; Wiermer, M.; Stein, M.; Landtag, J.; Brandt, W.; Rosahl, S.; Scheel, D.; et al. Pre- and Postinvasion Defenses Both Contribute to Nonhost Resistance in Arabidopsis. Science 2005, 310, 1180–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, M.; Dittgen, J.; Sánchez-Rodríguez, C.; Hou, B.-H.; Molina, A.; Schulze-Lefert, P.; Lipka, V.; Somerville, S. Arabidopsis PEN3/PDR8, an ATP Binding Cassette Transporter, Contributes to Nonhost Resistance to Inappropriate Pathogens That Enter by Direct Penetration. Plant Cell 2006, 18, 731–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, M.K.; Hagedorn, P.H.; de Torres-Zabala, M.; Grant, M.R.; Rung, J.H.; Collinge, D.B.; Lyngkjaer, M.F. Transcriptional regulation by an NAC (NAM-ATAF1,2-CUC2) transcription factor attenuates ABA signalling for efficient basal defence towards Blumeria graminis f. sp. hordei in Arabidopsis. Plant J. 2008, 56, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P.; Piślewska-Bednarek, M.; Svatoš, A.; Schneider, B.; Doubský, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A Glucosinolate Metabolism Pathway in Living Plant Cells Mediates Broad-Spectrum Antifungal Defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef]
- Pinosa, F.; Buhot, N.; Kwaaitaal, M.; Fahlberg, P.; Thordal-Christensen, H.; Ellerström, M.; Andersson, M.X. Arabidopsis Phospholipase Dδ Is Involved in Basal Defense and Nonhost Resistance to Powdery Mildew Fungi. Plant Physiol. 2013, 163, 896–906. [Google Scholar] [CrossRef] [Green Version]
- Campe, R.; Langenbach, C.; Leissing, F.; Popescu, G.; Popescu, S.C.; Goellner, K.; Beckers, G.J.M.; Conrath, U. ABC transporter PEN 3/ PDR 8/ ABCG 36 interacts with calmodulin that, like PEN 3, is required for Arabidopsis nonhost resistance. New Phytol. 2015, 209, 294–306. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Jürgens, G.; Thordal-Christensen, H. VPS9a Activates the Rab5 GTPase ARA7 to Confer Distinct Pre- and Postinvasive Plant Innate Immunity. Plant Cell 2017, 29, 1927–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiruma, K.; Onozawa-Komori, M.; Takahashi, F.; Asakura, M.; Bednarek, P.; Okuno, T.; Schulze-Lefert, P.; Takano, Y. Entry Mode–Dependent Function of an Indole Glucosinolate Pathway in Arabidopsis for Nonhost Resistance against Anthracnose Pathogens. Plant Cell 2010, 22, 2429–2443. [Google Scholar] [CrossRef] [Green Version]
- Hiruma, K.; Nishiuchi, T.; Kato, T.; Bednarek, P.; Okuno, T.; Schulze-Lefert, P.; Takano, Y. Arabidopsis ENHANCED DISEASE RESISTANCE 1 is required for pathogen-induced expression of plant defensins in nonhost resistance, and acts through interference of MYC2-mediated repressor function. Plant J. 2011, 67, 980–992. [Google Scholar] [CrossRef]
- Hiruma, K.; Fukunaga, S.; Bednarek, P.; Piślewska-Bednarek, M.; Watanabe, S.; Narusaka, Y.; Shirasu, K.; Takano, Y. Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. Proc. Natl. Acad. Sci. USA 2013, 110, 9589–9594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irieda, H.; Inoue, Y.; Mori, M.; Yamada, K.; Oshikawa, Y.; Saitoh, H.; Uemura, A.; Terauchi, R.; Kitakura, S.; Kosaka, A.; et al. Conserved fungal effector suppresses PAMP-triggered immunity by targeting plant immune kinases. Proc. Natl. Acad. Sci. USA 2018, 116, 496–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, K.; Houjyou, Y.; Komatsu, T.; Hori, H.; Kodaira, T.; Ishikawa, A. AGB1 and PMR5 Contribute to PEN2-Mediated Preinvasion Resistance to Magnaporthe oryzae in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2009, 22, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Nakao, M.; Nakamura, R.; Kita, K.; Inukai, R.; Ishikawa, A. Non-host resistance to penetration and hyphal growth of Magnaporthe oryzae in Arabidopsis. Sci. Rep. 2011, 1, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okawa, C.; Ishikawa, A. MPK6 Contributes to Non-Host Resistance to Magnaporthe oryzae in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013, 77, 1320–1322. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Shibuya, H.; Ishikawa, A. SOBIR1 contributes to non-host resistance to Magnaporthe oryzae in Arabidopsis. Biosci. Biotechnol. Biochem. 2016, 80, 1577–1579. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Shibuya, H.; Ishikawa, A. ERECTA contributes to non-host resistance to Magnaporthe oryzae in Arabidopsis. Biosci. Biotechnol. Biochem. 2016, 80, 1390–1392. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Murano, T.; Ishikawa, A. SOBIR1 and AGB1 independently contribute to nonhost resistance to Pyricularia oryzae (syn. Magnaporthe oryzae) in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2018, 82, 1922–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuPree, P.; Pwee, K.-H.; Gray, J.C. Expression of photosynthesis gene-promoter fusions in leaf epidermal cells of transgenic tobacco plants. Plant J. 1991, 1, 115–120. [Google Scholar] [CrossRef]
- Bowes, B.G.; Mauseth, J.D. Plant Structure—A Colour Guide, 2nd ed.; Manson Publishing, Ltd.: London, UK, 2008. [Google Scholar]
- Vaughan, K. Immunocytochemistry of Plant Cells; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Brunkard, J.O.; Runkel, A.M.; Zambryski, P.C. Chloroplasts extend stromules independently and in response to internal redox signals. Proc. Natl. Acad. Sci. USA 2015, 112, 10044–10049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullet, J.E. Chloroplast Development and Gene Expression. Annu. Rev. Plant Biol. 1988, 39, 475–502. [Google Scholar] [CrossRef]
- Kong, S.-G.; Wada, M. New Insights into Dynamic Actin-Based Chloroplast Photorelocation Movement. Mol. Plant 2011, 4, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Osteryoung, K.W.; Pyke, K.A. Division and Dynamic Morphology of Plastids. Annu. Rev. Plant Biol. 2014, 65, 443–472. [Google Scholar] [CrossRef]
- Pyke, K.; Leech, R.M. A Genetic Analysis of Chloroplast Division and Expansion in Arabidopsis thaliana. Plant Physiol. 1994, 104, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Robertson, E.J.; Rutherford, S.M.; Leech, R.M. Characterization of Chloroplast Division Using the Arabidopsis Mutant arc5. Plant Physiol. 1996, 112, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Vitha, S.; McAndrew, R.S.; Osteryoung, K.W. Ftsz Ring Formation at the Chloroplast Division Site in Plants. J. Cell Biol. 2001, 153, 111–120. [Google Scholar] [CrossRef]
- Joo, J.H.; Wang, S.; Chen, J.-G.; Jones, A.; Fedoroff, N.V. Different Signaling and Cell Death Roles of Heterotrimeric G Protein α and β Subunits in the Arabidopsis Oxidative Stress Response to Ozone. Plant Cell 2005, 17, 957–970. [Google Scholar] [CrossRef] [Green Version]
- Pyke, K.A. Plastid Biology; Cambridge University Press: New York, NY, USA, 2009; pp. 13–18. [Google Scholar]
- Barton, K.A.; Schattat, M.H.; Jakob, T.; Hause, G.; Wilhelm, C.; McKenna, J.F.; Máthé, C.; Runions, J.; Van Damme, D.; Mathur, J. Epidermal Pavement Cells of Arabidopsis Have Chloroplasts. Plant Physiol. 2016, 171, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.A.; Wozny, M.; Mathur, N.; Jaipargas, E.-A.; Mathur, J. Pavement cell chloroplast behaviour and interactions with other organelles in Arabidopsis thaliana. J. Cell Sci. 2017, 131, jcs202275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.X.; Phua, S.Y.; Crisp, P.; McQuinn, R.; Pogson, B.J. Learning the Languages of the Chloroplast: Retrograde Signaling and Beyond. Annu. Rev. Plant Biol. 2016, 67, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Feng, L.; Alyafei, M.A.M.; Jaleel, A.; Ren, M. Function of Chloroplasts in Plant Stress Responses. Int. J. Mol. Sci. 2021, 22, 13464. [Google Scholar] [CrossRef] [PubMed]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stael, S.; Kmiecik, P.; Willems, P.; Van Der Kelen, K.; Coll, N.S.; Teige, M.; Van Breusegem, F. Plant innate immunity—sunny side up? Trends Plant Sci. 2014, 20, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transciption. J. Exp. Bot. 2016, 68, 1303–1321. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Serrano, I.; Audran, C.; Rivas, S. Chloroplasts at work during plant innate immunity. J. Exp. Bot. 2016, 67, 3845–3854. [Google Scholar] [CrossRef]
- Sowden, R.G.; Watson, S.J.; Jarvis, P. The role of chloroplasts in plant pathology. Essays Biochem. 2017, 62, 21–39. [Google Scholar] [CrossRef]
- Kretschmer, M.; Damoo, D.; Djamei, A.; Kronstad, J. Chloroplasts and Plant Immunity: Where Are the Fungal Effectors? Pathogens 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Littlejohn, G.R.; Breen, S.; Smirnoff, N.; Grant, M. Chloroplast immunity illuminated. New Phytol. 2020, 229, 3088–3107. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, K.; Pan, H.; Liu, J. Chloroplast: The Emerging Battlefield in Plant–Microbe Interactions. Front. Plant Sci. 2021, 12, 63785. [Google Scholar] [CrossRef] [PubMed]
- Irieda, H.; Takano, Y. Epidermal chloroplasts are defense-related motile organelles equipped with plant immune components. Nat. Commun. 2021, 12, 2739. [Google Scholar] [CrossRef] [PubMed]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate Metabolites Required for an Arabidopsis Innate Immune Response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlaeppi, K.; Mansour, E.A.; Buchala, A.; Mauch, F. Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin. Plant J. 2010, 62, 840–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irieda, H. Preinvasive nonhost resistance of Arabidopsis against melanized appressorium-mediated entry of multiple nonadapted Colletotrichum fungi. Plant Signal. Behav. 2022, e2018218. [Google Scholar] [CrossRef] [PubMed]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Liu, Z.; Shen, H.; Wu, D. Damage-Associated Molecular Pattern-Triggered Immunity in Plants. Front. Plant Sci. 2019, 10, 646. [Google Scholar] [CrossRef]
- Chinchilla, D.; Zipfel, C.; Robatzek, S.; Kemmerling, B.; Nürnberger, T.; Jones, J.D.G.; Felix, G.; Boller, T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007, 448, 497–500. [Google Scholar] [CrossRef]
- Heese, A.; Hann, D.R.; Gimenez-Ibanez, S.; Jones, A.M.E.; He, K.; Li, J.; Schroeder, J.I.; Peck, S.C.; Rathjen, J.P. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA 2007, 104, 12217–12222. [Google Scholar] [CrossRef] [Green Version]
- Huffaker, A.; Ryan, C.A. Endogenous peptide defense signals in Arabidopsis differentially amplify signaling for the innate immune response. Proc. Natl. Acad. Sci. USA 2007, 104, 10732–10736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Wu, S.; Gao, X.; Zhang, Y.; Shan, L.; He, P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl. Acad. Sci. USA 2009, 107, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, W.; Xiang, T.; Liu, Z.; Laluk, K.; Ding, X.; Zou, Y.; Gao, M.; Zhang, X.; Chen, S.; et al. Receptor-like Cytoplasmic Kinases Integrate Signaling from Multiple Plant Immune Receptors and Are Targeted by a Pseudomonas syringae Effector. Cell Host Microbe 2010, 7, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Huffaker, A.; Bryan, A.C.; Tax, F.; Ryan, C.A. PEPR2 Is a Second Receptor for the Pep1 and Pep2 Peptides and Contributes to Defense Responses in Arabidopsis. Plant Cell 2010, 22, 508–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Fraiture, M.; Kolb, D.; Löffelhardt, B.; Desaki, Y.; Boutrot, F.F.G.; Tor, M.; Zipfel, C.; Gust, A.A.; Brunner, F. Arabidopsis receptor-like protein30 and receptor-like kinase suppressor of BIR1-1/EVERSHED mediate innate immunity to necrotrophic fungi. Plant Cell 2013, 25, 4227–4241. [Google Scholar] [CrossRef] [Green Version]
- Albert, I.; Böhm, H.; Albert, M.; Feiler, C.E.; Imkampe, J.; Wallmeroth, N.; Brancato, C.; Raaymakers, T.M.; Oome, S.; Zhang, H.; et al. An RLP23–SOBIR1–BAK1 complex mediates NLP-triggered immunity. Nat. Plants 2015, 1, 15140. [Google Scholar] [CrossRef]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Tang, D.; Wang, G.; Zhou, J.-M. Receptor Kinases in Plant-Pathogen Interactions: More Than Pattern Recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef] [Green Version]
- Saijo, Y.; Loo, E.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- Albert, I.; Hua, C.; Nürnberger, T.; Pruitt, R.N.; Zhang, L. Surface Sensor Systems in Plant Immunity. Plant Physiol. 2019, 182, 1582–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldo, M.; Valent, B. Filamentous plant pathogen effectors in action. Nat. Rev. Genet. 2013, 11, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Rovenich, H.; Boshoven, J.C.; Thomma, B.P. Filamentous pathogen effector functions: Of pathogens, hosts and microbiomes. Curr. Opin. Plant Biol. 2014, 20, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Garcia, E.; Valent, B. How eukaryotic filamentous pathogens evade plant recognition. Curr. Opin. Microbiol. 2015, 26, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; McLellan, H.; Boevink, P.C.; Birch, P.R.J. All roads lead to susceptibility: The many modes of action of fungal and oomycete intracellular effectors. Plant Commun. 2020, 1, 100050. [Google Scholar] [CrossRef]
- Figueroa, M.; Ortiz, D.; Henningsen, E.C. Tactics of host manipulation by intracellular effectors from plant pathogenic fungi. Curr. Opin. Plant Biol. 2021, 62, 102054. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, J.; Rincon-Rivera, L.J.; Takahara, H.; Neumann, U.; Van Themaat, E.V.L.; Van Der Does, H.C.; Hacquard, S.; Stüber, K.; Will, I.; Schmalenbach, W.; et al. Sequential Delivery of Host-Induced Virulence Effectors by Appressoria and Intracellular Hyphae of the Phytopathogen Colletotrichum higginsianum. PLoS Pathog. 2012, 8, e1002643. [Google Scholar] [CrossRef]
- Irieda, H.; Maeda, H.; Akiyama, K.; Hagiwara, A.; Saitoh, H.; Uemura, A.; Terauchi, R.; Takano, Y. Colletotrichum orbiculare Secretes Virulence Effectors to a Biotrophic Interface at the Primary Hyphal Neck via Exocytosis Coupled with SEC22-Mediated Traffic. Plant Cell 2014, 26, 2265–2281. [Google Scholar] [CrossRef] [Green Version]
- Wada, M. Chloroplast and nuclear photorelocation movements. Proc. Jpn. Acad. Ser. B 2016, 92, 387–411. [Google Scholar] [CrossRef] [Green Version]
- Higa, T.; Suetsugu, N.; Kong, S.-G.; Wada, M. Actin-dependent plastid movement is required for motive force generation in directional nuclear movement in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 4327–4331. [Google Scholar] [CrossRef] [Green Version]
- Suetsugu, N.; Higa, T.; Kong, S.-G.; Wada, M. PLASTID MOVEMENT IMPAIRED1 and PLASTID MOVEMENT IMPAIRED1-RELATED1 mediate photorelocation movements of both chloroplasts and nuclei. Plant Physiol. 2015, 169, 1155–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikawa, K.; Kasahara, M.; Kiyosue, T.; Kagawa, T.; Suetsugu, N.; Takahashi, F.; Kanegae, T.; Niwa, Y.; Kadota, A.; Wada, M. Chloroplast Unusual Positioning1 Is Essential for Proper Chloroplast Positioning. Plant Cell 2003, 15, 2805–2815. [Google Scholar] [CrossRef] [Green Version]
- Oikawa, K.; Yamasato, A.; Kong, S.-G.; Kasahara, M.; Nakai, M.; Takahashi, F.; Ogura, Y.; Kagawa, T.; Wada, M. Chloroplast Outer Envelope Protein CHUP1 Is Essential for Chloroplast Anchorage to the Plasma Membrane and Chloroplast Movement. Plant Physiol. 2008, 148, 829–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suetsugu, N.; Higa, T.; Gotoh, E.; Wada, M. Light-Induced Movements of Chloroplasts and Nuclei Are Regulated in Both Cp-Actin-Filament-Dependent and -Independent Manners in Arabidopsis thaliana. PLoS ONE 2016, 11, e0157429. [Google Scholar] [CrossRef] [Green Version]
- Suetsugu, N.; Kagawa, T.; Wada, M. An Auxilin-Like J-Domain Protein, JAC1, Regulates Phototropin-Mediated Chloroplast Movement in Arabidopsis. Plant Physiol. 2005, 139, 151–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarillo, J.A.; Gabrys, H.; Capel, J.; Alonso, J.; Ecker, J.; Cashmore, A.R. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 2001, 410, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Sakai, T.; Suetsugu, N.; Oikawa, K.; Ishiguro, S.; Kato, T.; Tabata, S.; Okada, K.; Wada, M. Arabidopsis NPL1: A Phototropin Homolog Controlling the Chloroplast High-Light Avoidance Response. Science 2001, 291, 2138–2141. [Google Scholar] [CrossRef]
- Sakai, T.; Kagawa, T.; Kasahara, M.; Swartz, T.E.; Christie, J.M.; Briggs, W.R.; Wada, M.; Okada, K. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 2001, 98, 6969–6974. [Google Scholar] [CrossRef] [Green Version]
- Nomura, H.; Komori, T.; Kobori, M.; Nakahira, Y.; Shiina, T. Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J. 2007, 53, 988–998. [Google Scholar] [CrossRef]
- Yamasaki, K.; Motomura, Y.; Yagi, Y.; Nomura, H.; Kikuchi, S.; Nakai, M.; Shiina, T. Chloroplast envelope localization of EDS5, an essential factor for salicylic acid biosynthesis in Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e23603. [Google Scholar] [CrossRef] [Green Version]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-C.; Engle, N.L.; Banday, Z.Z.; Cecchini, N.M.; Jung, H.W.; Tschaplinski, T.J.; Greenberg, J.T. ALD1 accumulation in Arabidopsis epidermal plastids confers local and non-autonomous disease resistance. J. Exp. Bot. 2021, 72, 2710–2726. [Google Scholar] [CrossRef] [PubMed]
- Parisy, V.; Poinssot, B.; Owsianowski, L.; Buchala, A.; Glazebrook, J.; Mauch, F. Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 2006, 49, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Yang, G.; Ma, M.; Liu, X.; Li, B.; Xie, J.; Fu, Y.; Chen, T.; Yu, Y.; Chen, W.; et al. An effector of a necrotrophic fungal pathogen targets the calcium-sensing receptor in chloroplasts to inhibit host resistance. Mol. Plant Pathol. 2020, 21, 686–701. [Google Scholar] [CrossRef] [Green Version]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Serrano, M.; Wang, B.; Aryal, B.; Garcion, C.; Abou-Mansour, E.; Heck, S.; Geisler, M.; Mauch, F.; Nawrath, C.; Métraux, J.-P. Export of Salicylic Acid from the Chloroplast Requires the Multidrug and Toxin Extrusion-Like Transporter EDS5. Plant Physiol. 2013, 162, 1815–1821. [Google Scholar] [CrossRef] [Green Version]
- Song, J.T.; Lu, H.; Greenberg, J. Divergent Roles in Arabidopsis thaliana Development and Defense of Two Homologous Genes, Aberrant Growth and Death2 and AGD2-LIKE Defense Response Protein1, Encoding Novel Aminotransferases. Plant Cell 2004, 16, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Song, J.T.; Lü, H.; McDowell, J.M.; Greenberg, J.T. A key role for ALD1 in activation of local and systemic defenses in Arabidopsis. Plant J. 2004, 40, 200–212. [Google Scholar] [CrossRef]
- Cecchini, N.; Jung, H.W.; Engle, N.L.; Tschaplinski, T.; Greenberg, J. ALD1 Regulates Basal Immune Components and Early Inducible Defense Responses in Arabidopsis. Mol. Plant-Microbe Interact. 2015, 28, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Caplan, J.L.; Kumar, A.S.; Park, E.; Padmanabhan, M.S.; Hoban, K.; Modla, S.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplast Stromules Function during Innate Immunity. Dev. Cell 2015, 34, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, Z.; Duggan, C.; Toufexi, A.; Pandey, P.; Liang, Y.; Segretin, M.E.; Yuen, L.H.; Gaboriau, D.C.A.; Leary, A.Y.; Tumtas, Y.; et al. Chloroplasts alter their morphology and accumulate at the pathogen interface during infection by Phytophthora infestans. Plant J. 2021, 107, 1771–1787. [Google Scholar] [CrossRef] [PubMed]
- Savage, Z.; Erickson, J.L.; Prautsch, J.; Balmez, A.I.; Tumtas, Y.; Yuen, E.L.H.; Stuttmann, J.; Fantino, E.; Duggan, C.; Molinari, C.; et al. Chloroplast movement and positioning protein CHUP1 is required for focal immunity against Phytophthora infestans. bioRxiv 2021. [Google Scholar] [CrossRef]
- Shibata, Y.; Kawakita, K.; Takemoto, D. SGT1 and HSP90 are essential for age-related non-host resistance of Nicotiana benthamiana against the oomycete pathogen Phytophthora infestans. Physiol. Mol. Plant Pathol. 2011, 75, 120–128. [Google Scholar] [CrossRef]
- Natesan, S.K.A.; Sullivan, J.A.; Gray, J.C. Stromules: A characteristic cell-specific feature of plastid morphology. J. Exp. Bot. 2005, 56, 787–797. [Google Scholar] [CrossRef]
- Kumar, A.S.; Park, E.; Nedo, A.; AlQarni, A.; Ren, L.; Hoban, K.; Modla, S.; McDonald, J.H.; Kambhamettu, C.; Dinesh-Kumar, S.P.; et al. Stromule extension along microtubules coordinated with actin-mediated anchoring guides perinuclear chloroplast movement during innate immunity. eLife 2018, 7, e23626. [Google Scholar] [CrossRef] [Green Version]
- Hanson, M.R.; Hines, K.M. Stromules: Probing Formation and Function. Plant Physiol. 2017, 176, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Caplan, J.L.; Mamillapalli, P.; Burch-Smith, T.M.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplastic Protein NRIP1 Mediates Innate Immune Receptor Recognition of a Viral Effector. Cell 2008, 132, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Tsushima, A.; Narusaka, M.; Gan, P.; Kumakura, N.; Hiroyama, R.; Kato, N.; Takahashi, S.; Takano, Y.; Narusaka, Y.; Shirasu, K. The Conserved Colletotrichum spp. Effector Candidate CEC3 Induces Nuclear Expansion and Cell Death in Plants. Front. Microbiol. 2021, 12, 682155. [Google Scholar] [CrossRef]
- De Souza, A.J.; Wang, J.-Z.; Dehesh, K. Retrograde Signals: Integrators of Interorganellar Communication and Orchestrators of Plant Development. Annu. Rev. Plant Biol. 2017, 68, 85–108. [Google Scholar] [CrossRef]
- Mielecki, J.; Gawroński, P.; Karpiński, S. Retrograde Signaling: Understanding the Communication between Organelles. Int. J. Mol. Sci. 2020, 21, 6173. [Google Scholar] [CrossRef] [PubMed]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N.; Park, E. Spatial chloroplast-to-nucleus signalling involving plastid–nuclear complexes and stromules. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190405. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Noshi, M.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. H2O2-triggered Retrograde Signaling from Chloroplasts to Nucleus Plays Specific Role in Response to Stress. J. Biol. Chem. 2012, 287, 11717–11729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiguzov, A.; Vainonen, J.P.; Wrzaczek, M.; Kangasjärvi, J. ROS-talk—how the apoplast, the chloroplast, and the nucleus get the message through. Front. Plant Sci. 2012, 3, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, P.; Julius, C.; Schmelzer, E.; Hahlbrock, K. Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defence gene activation in infected, cultured parsley cells. EMBO J. 1993, 12, 1735–1744. [Google Scholar] [CrossRef]
- Heath, M.C.; Nimichuk, Z.L.; Xu, H. Plant nuclear migrations as indicators of critical interactions between resistant or susceptible cowpea epidermal cells and invasion hyphae of the cowpea rust fungus. New Phytol. 1997, 135, 689–700. [Google Scholar] [CrossRef]
- Shan, X.C.; Goodwin, P.H. Reorganization of Filamentous Actin in Nicotiana benthamiana Leaf Epidermal Cells Inoculated with Colletotrichum destructivum and Colletotrichum graminicola. Bot. Gaz. 2005, 166, 31–39. [Google Scholar] [CrossRef]
- Opalski, K.S.; Schultheiss, H.; Kogel, K.; Hückelhoven, R. The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f.sp. hordei. Plant J. 2005, 41, 291–303. [Google Scholar] [CrossRef]
- Ding, X.; Jimenez-Gongora, T.; Krenz, B.; Lozano-Duran, R. Chloroplast clustering around the nucleus is a general response to pathogen perception in Nicotiana benthamiana. Mol. Plant Pathol. 2019, 20, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irieda, H. Emerging Roles of Motile Epidermal Chloroplasts in Plant Immunity. Int. J. Mol. Sci. 2022, 23, 4043. https://doi.org/10.3390/ijms23074043
Irieda H. Emerging Roles of Motile Epidermal Chloroplasts in Plant Immunity. International Journal of Molecular Sciences. 2022; 23(7):4043. https://doi.org/10.3390/ijms23074043
Chicago/Turabian StyleIrieda, Hiroki. 2022. "Emerging Roles of Motile Epidermal Chloroplasts in Plant Immunity" International Journal of Molecular Sciences 23, no. 7: 4043. https://doi.org/10.3390/ijms23074043
APA StyleIrieda, H. (2022). Emerging Roles of Motile Epidermal Chloroplasts in Plant Immunity. International Journal of Molecular Sciences, 23(7), 4043. https://doi.org/10.3390/ijms23074043





