IGFBP-6: At the Crossroads of Immunity, Tissue Repair and Fibrosis
Abstract
1. Introduction: The Insulin-Like Growth Factors-Binding Proteins
2. Evolution, Immunity, and Tissue Repair
3. IGFBP-6 Has an Important Role in the Immune Response
4. IGFBPs Are Involved in the Regulation of Connective Tissues and of Fibrosis
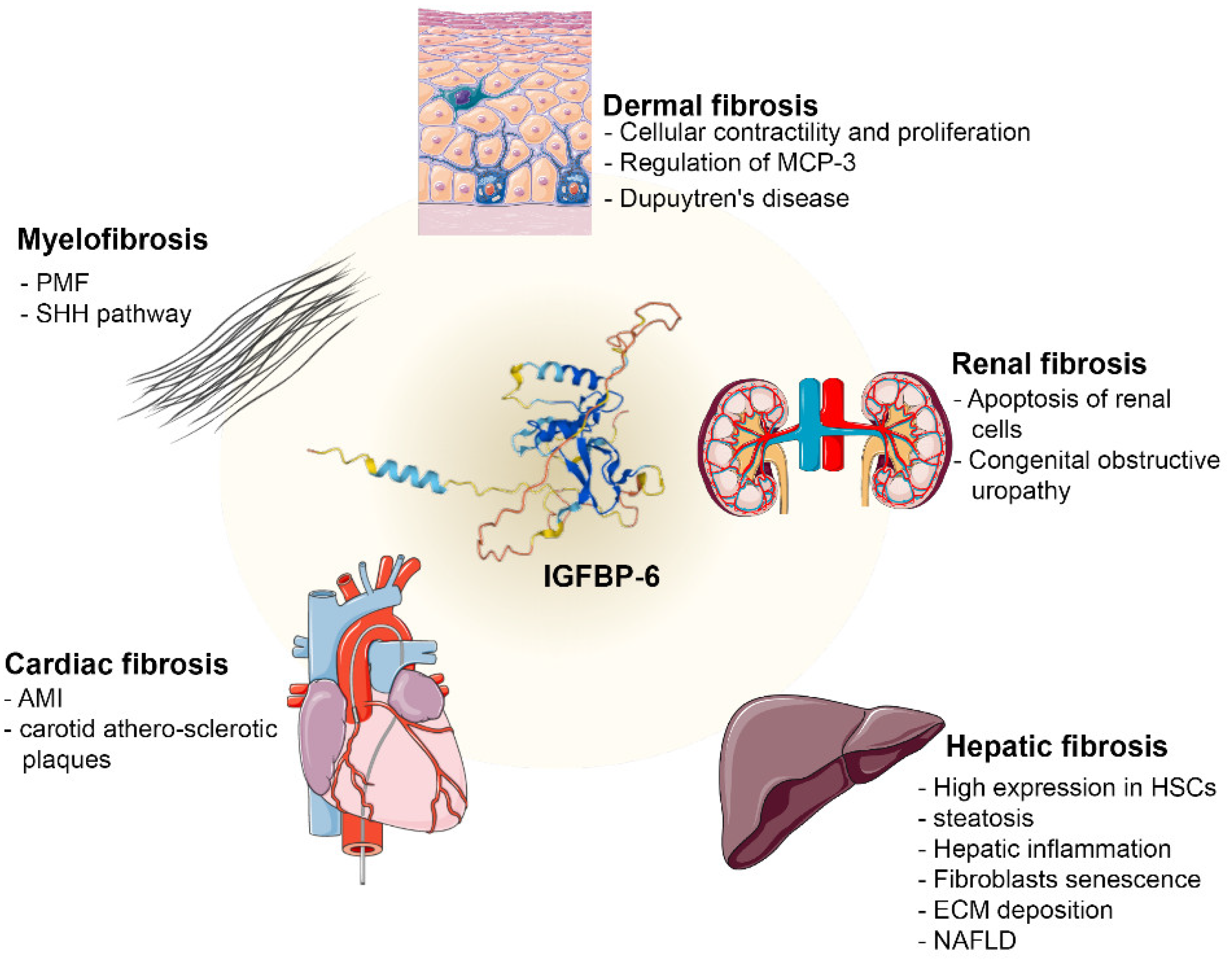
4.1. IGFBP-6 Regulates Several Fibrosis Mechanisms
4.1.1. Dermal Fibrosis
4.1.2. Renal Fibrosis
4.1.3. Hepatic Fibrosis
4.1.4. Cardiac Fibrosis
4.1.5. Myelofibrosis
5. IGFBP-6 Controls Fibroblasts and TME during Cancer Progression
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rajaram, S.; Baylink, D.J.; Mohan, S. Insulin-Like Growth Factor-Binding Proteins in Serum and Other Biological Fluids: Regulation and Functions. Endocr. Rev. 1997, 18, 801–831. [Google Scholar] [CrossRef]
- Wang, S.; Chi, K.; Wu, D.; Hong, Q. Insulin-Like Growth Factor Binding Proteins in Kidney Disease. Front. Pharmacol. 2021, 12, 807119. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A. Recent insights into the actions of IGFBP-6. J. Cell Commun. Signal. 2015, 9, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A. Current ideas on the biology of IGFBP-6: More than an IGF-II inhibitor? Growth Horm. IGF Res. 2016, 30–31, 81–86. [Google Scholar] [CrossRef]
- Bach, L.A. IGF-binding proteins. J. Mol. Endocrinol. 2018, 61, T11–T28. [Google Scholar] [CrossRef]
- Allen, J.E.; Wynn, T.A. Evolution of Th2 Immunity: A Rapid Repair Response to Tissue Destructive Pathogens. PLoS Pathog. 2011, 7, e1002003. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Allen, J. Mapping immune response profiles: The emerging scenario from helminth immunology. Eur. J. Immunol. 2007, 37, 3319–3326. [Google Scholar] [CrossRef]
- Gieseck, R.L., 3rd; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef]
- Atlas, H.P. Available online: https://www.proteinatlas.org/ENSG00000167779-IGFBP6/immune+cell (accessed on 24 January 2022).
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Martin, J.L.; Baxter, R. Oncogenic ras Causes Resistance to the Growth Inhibitor Insulin-like Growth Factor Binding Protein-3 (IGFBP-3) in Breast Cancer Cells. J. Biol. Chem. 1999, 274, 16407–16411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tsushima, T.; Miyakawa, M.; Isozaki, O.; Yamada, H.; Xu, Z.R.; Iwamoto, Y. Effect of Cytokines on Production of Insulin-like Growth Factor Binding Proteins (IGFBPs) from Human Fibroblasts in Culture. Endocr. J. 1999, 46, S63–S66. [Google Scholar] [CrossRef] [PubMed]
- Gabbitas, B.; Canalis, E. Growth factor regulation of insulin-like growth factor binding protein-6 expression in osteoblasts. J. Cell. Biochem. 1997, 66, 77–86. [Google Scholar] [CrossRef]
- Denys, H.; Jadidizadeh, A.; Amini Nik, S.; Van Dam, K.; Aerts, S.; Alman, B.A.; Cassiman, J.J.; Tejpar, S. Identification of IGFBP-6 as a significantly downregulated gene by beta-catenin in desmoid tumors. Oncogene 2004, 23, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Buechler, M.B.; Pradhan, R.N.; Krishnamurty, A.T.; Cox, C.; Calviello, A.K.; Wang, A.W.; Yang, Y.A.; Tam, L.; Caothien, R.; Roose-Girma, M.; et al. Cross-tissue organization of the fibroblast lineage. Nature 2021, 593, 575–579. [Google Scholar] [CrossRef]
- Liso, A.; Capitanio, N.; Gerli, R.; Conese, M. From fever to immunity: A new role for IGFBP-6? J. Cell Mol. Med. 2018, 22, 4588–4596. [Google Scholar] [CrossRef]
- Basu, S.; Srivastava, P.K. Fever-like temperature induces maturation of dendritic cells through induction of hsp90. Int. Immunol. 2003, 15, 1053–1061. [Google Scholar] [CrossRef]
- Liso, A.; Castellani, S.; Massenzio, F.; Trotta, R.; Pucciarini, A.; Bigerna, B.; De Luca, P.; Zoppoli, P.; Castiglione, F.; Palumbo, M.C.; et al. Human monocyte-derived dendritic cells exposed to hyperthermia show a distinct gene expression profile and selective upregulation of IGFBP6. Oncotarget 2017, 8, 60826–60840. [Google Scholar] [CrossRef]
- Alunno, A.; Bistoni, O.; Manetti, M.; Cafaro, G.; Valentini, V.; Bartoloni, E.; Gerli, R.; Liso, A. Insulin-Like Growth Factor Binding Protein 6 in Rheumatoid Arthritis: A Possible Novel Chemotactic Factor? Front. Immunol. 2017, 8, 554. [Google Scholar] [CrossRef]
- Conese, M.; Pace, L.; Pignataro, N.; Catucci, L.; Ambrosi, A.; Di Gioia, S.; Tartaglia, N.; Liso, A. Insulin-Like Growth Factor Binding Protein 6 Is Secreted in Extracellular Vesicles upon Hyperthermia and Oxidative Stress in Dendritic Cells but Not in Monocytes. Int. J. Mol. Sci. 2020, 21, 4428. [Google Scholar] [CrossRef]
- Xie, L.; Tsaprailis, G.; Chen, Q.M. Proteomic Identification of Insulin-like Growth Factor-binding Protein-6 Induced by Sublethal H2O2 Stress from Human Diploid Fibroblasts. Mol. Cell. Proteom. 2005, 4, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, L.; Li, Y.; Wang, X.; Zhou, J.; Liu, Y.; Fu, P.; Gallicchio, M.A.; Bach, L.; Duan, C. IGF binding protein-6 expression in vascular endothelial cells is induced by hypoxia and plays a negative role in tumor angiogenesis. Int. J. Cancer 2012, 130, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-J.; Park, J.; Shin, J.-H.; Chang, M.-S. Insulin-like growth factor binding protein-6 released from human mesenchymal stem cells confers neuronal protection through IGF-1R-mediated signaling. Int. J. Mol. Med. 2017, 40, 1860–1868. [Google Scholar] [CrossRef][Green Version]
- Reynolds, A.; Laurie, C.; Mosley, R.L.; Gendelman, H.E. Oxidative Stress and the Pathogenesis of Neurodegenerative Disorders. Int. Rev. Neurobiol. 2007, 82, 297–325. [Google Scholar] [CrossRef]
- Conese, M.; D’Oria, S.; Castellani, S.; Trotta, R.; Montemurro, P.; Liso, A. Insulin-like growth factor-6 (IGFBP-6) stimulates neutrophil oxidative burst, degranulation and chemotaxis. Inflamm. Res. 2018, 67, 107–109. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Lai, P.-L.; Chien, Y.; Lee, P.-H.; Lai, Y.-H.; Ma, H.-I.; Shiau, C.-Y.; Wang, K.-C. Improvement of Impaired Motor Functions by Human Dental Exfoliated Deciduous Teeth Stem Cell-Derived Factors in a Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3807. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Britschgi, M.; Herbert, C.; Takeda-Uchimura, Y.; Boxer, A.; Blennow, K.; Friedman, L.F.; Galasko, D.R.; Jutel, M.; Karydas, A.; et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat. Med. 2007, 13, 1359–1362. [Google Scholar] [CrossRef]
- Longhitano, L.; Forte, S.; Orlando, L.; Grasso, S.; Barbato, A.; Vicario, N.; Parenti, R.; Fontana, P.; Amorini, A.M.; Lazzarino, G.; et al. The Crosstalk between GPR81/IGFBP6 Promotes Breast Cancer Progression by Modulating Lactate Metabolism and Oxidative Stress. Antioxidants 2022, 11, 275. [Google Scholar] [CrossRef]
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science 2019, 366, eaax9198. [Google Scholar] [CrossRef]
- Kim, S.-D.; Kang, S.A.; Kim, Y.-W.; Yu, H.S.; Cho, K.-S.; Roh, H.-J. Screening and Functional Pathway Analysis of Pulmonary Genes Associated with Suppression of Allergic Airway Inflammation by Adipose Stem Cell-Derived Extracellular Vesicles. Stem Cells Int. 2020, 2020, 5684250. [Google Scholar] [CrossRef]
- Vaillancourt, V.T.; Bordeleau, M.; Laviolette, M.; Laprise, C. From expression pattern to genetic association in asthma and asthma-related phenotypes. BMC Res. Notes 2012, 5, 630. [Google Scholar] [CrossRef] [PubMed]
- Laprise, C.; Sladek, R.; Ponton, A.; Bernier, M.-C.; Hudson, T.J.; LaViolette, M. Functional classes of bronchial mucosa genes that are differentially expressed in asthma. BMC Genom. 2004, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.W.; Kim, I.T.; Shin, B.S.; Cheong, S.W.; Cho, U.H.; Huh, M.J.; Oh, G.S. TCDD-up-regulation of IGFBP-6 and IL-5R alpha subunit genes in vivo and in vitro. Mol. Cells 2001, 12, 372–379. [Google Scholar] [PubMed]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063. [Google Scholar] [CrossRef]
- Smeed, J.; Watkins, C.; Gossner, A.; Hopkins, J. Expression profiling reveals differences in immuno-inflammatory gene expression between the two disease forms of sheep paratuberculosis. Veter Immunol. Immunopathol. 2010, 135, 218–225. [Google Scholar] [CrossRef]
- Coussens, P.M.; Jeffers, A.; Colvin, C. Rapid and transient activation of gene expression in peripheral blood mononuclear cells from Johne’s disease positive cows exposed to Mycobacterium paratuberculosis in vitro. Microb. Pathog. 2004, 36, 93–108. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142. [Google Scholar] [CrossRef]
- Rosenbloom, J.; Macarak, E.; Piera-Velazquez, S.; Jimenez, S.A. Human Fibrotic Diseases: Current Challenges in Fibrosis Research. Methods Mol. Biol. 2017, 1627, 1–23. [Google Scholar]
- Ong, V.H.; Carulli, M.T.; Xu, S.; Khan, K.; Lindahl, G.; Abraham, D.J.; Denton, C.P. Cross-talk between MCP-3 and TGFbeta promotes fibroblast collagen biosynthesis. Exp. Cell Res. 2009, 315, 151–161. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Krtolica, A.; Beauséjour, C.M.; Parrinello, S.; Hodgson, J.G.; Chin, K.; Desprez, P.-Y.; Campisi, J. A Human-Like Senescence-Associated Secretory Phenotype Is Conserved in Mouse Cells Dependent on Physiological Oxygen. PLoS ONE 2010, 5, e9188. [Google Scholar] [CrossRef]
- Konermann, A.; Lossdörfer, S.; Jäger, A.; Chen, Y.; Götz, W. Autoregulation of insulin-like growth factor 2 and insulin-like growth factor-binding protein 6 in periodontal ligament cells in vitro. Ann. Anat. Anat. Anz. 2013, 195, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Takedachi, M.; Yamamoto, S.; Morimoto, C.; Ozasa, M.; Iwayama, T.; Lee, C.M.; Okura, H.; Matsuyama, A.; Kitamura, M.; et al. Trophic factors from adipose tissue-derived multi-lineage progenitor cells promote cytodifferentiation of periodontal ligament cells. Biochem. Biophys. Res. Commun. 2015, 464, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Lei, W.; Dai, L.; Yuan, Q.L.; Liu, L.; Zhou, H.; Zhang, J.; Zhang, Y.J. Whole Exome Sequencing Identified a Novel IGFBP6 Variant in a Disc Degeneration Pedigree. Genet. Test. Mol. Biomark. 2017, 21, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Mazzucchelli, G.; Lambert, C.; Comblain, F.; Depauw, E.; Henrotin, Y. Comparison of secretome from osteoblasts derived from sclerotic versus non-sclerotic subchondral bone in OA: A pilot study. PLoS ONE 2018, 13, e0194591. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.J.; Dave, A.; Charytonowicz, D.; Sebra, R.; Iatridis, J.C. Single-cell RNA-sequencing atlas of bovine caudal intervertebral discs: Discovery of heterogeneous cell populations with distinct roles in homeostasis. FASEB J. 2021, 35, e21919. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Smart Servier Medical Art. Available online: https://smart.servier.com/ (accessed on 23 February 2022).
- Ong, V.H.; Evans, L.A.; Shiwen, X.; Fisher, I.B.; Rajkumar, V.; Abraham, D.J.; Black, C.M.; Denton, C.P. Monocyte chemoattractant protein 3 as a mediator of fibrosis: Overexpression in systemic sclerosis and the type 1 tight-skin mouse. Arthritis Rheum. 2003, 48, 1979–1991. [Google Scholar] [CrossRef]
- Raykha, C.; Crawford, J.; Gan, B.S.; Fu, P.; Bach, L.A.; O’Gorman, D.B. IGF-II and IGFBP-6 regulate cellular contractility and proliferation in Dupuytren’s disease. Biochim. Biophys. Acta 2013, 1832, 1511–1519. [Google Scholar] [CrossRef]
- Christensson, A.; Ash, J.J.A.; DeLisle, R.K.R.K.; Gaspar, F.W.F.W.; Ostroff, R.; Grubb, A.; Lindström, V.; Bruun, L.; Williams, S.S.A. The Impact of the Glomerular Filtration Rate on the Human Plasma Proteome. Proteom. Clin. Appl. 2018, 12, e1700067. [Google Scholar] [CrossRef]
- Jarkovská, Z.; Rosická, M.; Kršek, M.; Sulkova, S.D.; Haluzik, M.; Justová, V.; Lacinová, Z.; Marek, J. Plasma ghrelin levels in patients with end-stage renal disease. Physiol. Res. 2005, 54, 403–408. [Google Scholar]
- Seseke, F.; Thelen, P.; Ringert, R.-H. Characterization of an Animal Model of Spontaneous Congenital Unilateral Obstructive Uropathy by cDNA Microarray Analysis. Eur. Urol. 2004, 45, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.L.; Fourman, L.T.; Zheng, I.; McClure, C.M.; Feldpausch, M.N.; Torriani, M.; Corey, E.K.; Chung, R.T.; Lee, H.; Kleiner, E.D.; et al. Relationship of IGF-1 and IGF-Binding Proteins to Disease Severity and Glycemia in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2021, 106, e520–e533. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castillo, M.; Rosique-Oramas, D.; Medina-Avila, Z.; Pérez-Hernández, J.L.; La Tijera, F.H.-D.; Santana-Vargas, D.; Montalvo-Jave, E.E.; Sanchez-Avila, F.; Torre, A.; Kershenobich, D.; et al. Differential production of insulin-like growth factor-binding proteins in liver fibrosis progression. Mol. Cell. Biochem. 2020, 469, 65–75. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, J.; Zhang, Y.; Chen, T.; Zhu, M.; Fang, C.; Mi, Y. Discovery of potential plasma protein biomarkers for acute myocardial infarction via proteomics. J. Thorac. Dis. 2019, 11, 3962–3972. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huan, W.; Wu, J.; Zou, S.; Qu, L. IGFBP6 Is Downregulated in Unstable Carotid Atherosclerotic Plaques According to an Integrated Bioinformatics Analysis and Experimental Verification. J. Atheroscler. Thromb. 2020, 27, 1068–1085. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Tibullo, D.; Vicario, N.; Giallongo, C.; La Spina, E.; Romano, A.; Lombardo, S.; Moretti, M.; Masia, F.; Coda, A.R.D.; et al. IGFBP-6/sonic hedgehog/TLR4 signalling axis drives bone marrow fibrotic transformation in primary myelofibrosis. Aging 2021, 13, 25055–25071. [Google Scholar] [CrossRef]
- Gentilini, A.; Feliers, D.; Pinzani, M.; Woodruff, K.; Abboud, S. Characterization and regulation of insulin-like growth factor binding proteins in human hepatic stellate cells. J. Cell Physiol. 1998, 174, 240–250. [Google Scholar] [CrossRef]
- Klahr, S. Urinary tract obstruction. Semin. Nephrol. 2001, 21, 133–145. [Google Scholar] [CrossRef]
- Stanley, T.L.; Fourman, L.T.; Feldpausch, M.N.; Purdy, J.; Zheng, I.; Pan, C.S.; Aepfelbacher, J.; Buckless, C.; Tsao, A.; Kellogg, A.; et al. Effects of tesamorelin on non-alcoholic fatty liver disease in HIV: A randomised, double-blind, multicentre trial. Lancet HIV 2019, 6, e821–e830. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Adverse Cardiac Remodelling after Acute Myocardial Infarction: Old and New Biomarkers. Dis. Markers 2020, 2020, 1215802. [Google Scholar] [CrossRef]
- Takenaka, K.; Shimoda, K.; Akashi, K. Recent advances in the diagnosis and management of primary myelofibrosis. Korean J. Intern. Med. 2018, 33, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, G.A.; Stella, S.; Pennisi, M.S.; Pirosa, C.; Fermo, E.; Fabris, S.; Cattaneo, D.; Iurlo, A. The Role of New Technologies in Myeloproliferative Neoplasms. Front. Oncol. 2019, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Latagliata, R.; Polverelli, N.; Tieghi, A.; Palumbo, G.A.M.; Breccia, M.; Sabattini, E.; Villari, L.; Riminucci, M.; Valli, R.; Catani, L.; et al. Comparison ofJAK2V617F-positive essential thrombocythaemia and early primary myelofibrosis: The impact of mutation burden and histology. Hematol. Oncol. 2018, 36, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hegde, S.; DeNardo, D.G. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol. Immunother. 2017, 66, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.-C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
- De Vincenzo, A.; Belli, S.; Franco, P.; Telesca, M.; Iaccarino, I.; Botti, G.; Carriero, M.V.; Ranson, M.; Stoppelli, M.P. Paracrine recruitment and activation of fibroblasts by c-Myc expressing breast epithelial cells through the IGFs/IGF-1R axis. Int. J. Cancer 2019, 145, 2827–2839. [Google Scholar] [CrossRef]
- De Jaeghere, E.A.; Denys, H.G.; De Wever, O. Fibroblasts Fuel Immune Escape in the Tumor Microenvironment. Trends Cancer 2019, 5, 704–723. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Brücher, B.L.; Jamall, I.S. Epistemology of the origin of cancer: A new paradigm. BMC Cancer 2014, 14, 331. [Google Scholar] [CrossRef]
- Yamauchi, M.; Barker, T.H.; Gibbons, D.L.; Kurie, J.M. The fibrotic tumor stroma. J. Clin. Investig. 2018, 128, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Huang, Q.; Shen, J.; Shi, J.; Shen, C.; Xu, P.; Chang, H.; Xia, X.; Xu, L.; Jianhong, S.; et al. IGFBP6 Regulates Cell Apoptosis and Migration in Glioma. Cell. Mol. Neurobiol. 2017, 37, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.; Xin, L.; Tang, X.; Guo, H. The clinical characteristics and prognostic value of IGFBP6 in glioma. Neurol. Res. 2022, 44, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hong, Q.; Geng, X.; Chi, K.; Cai, G.; Wu, D. Insulin-Like Growth Factor Binding Protein 5—A Probable Target of Kidney Renal Papillary Renal Cell Carcinoma. BioMed Res. Int. 2019, 2019, 3210324. [Google Scholar] [CrossRef]
- Katz, L.H.; Li, Y.; Chen, J.S.; Muñoz, N.M.; Majumdar, A.; Chen, J.; Mishra, L. Targeting TGF-beta signaling in cancer. Expert Opin. Ther. Targets 2013, 17, 743–760. [Google Scholar] [CrossRef]
- Weeks, B.H.; He, W.; Olson, K.L.; Wang, X.J. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res. 2001, 61, 7435–7443. [Google Scholar]
- Tu, M.; Liu, X.; Han, B.; Ge, Q.; Li, Z.; Lu, Z.; Wei, J.; Song, G.; Cai, B.; Lv, N.; et al. Vasohibin-2 promotes proliferation in human breast cancer cells via upregulation of fibroblast growth factor-2 and growth/differentiation factor-15 expression. Mol. Med. Rep. 2014, 10, 663–669. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Jiang, J.; Zhang, X.; Zhang, M.; Fu, Y. Comprehensive Analysis of IGFBPs as Biomarkers in Gastric Cancer. Front. Oncol. 2021, 11, 723131. [Google Scholar] [CrossRef]
- Hanna, A.; Shevde, L.A. Hedgehog signaling: Modulation of cancer properies and tumor mircroenvironment. Mol. Cancer 2016, 15, 24. [Google Scholar] [CrossRef]
- Wilkinson, S.E.; Furic, L.; Buchanan, G.; Larsson, O.; Pedersen, J.; Frydenberg, M.; Risbridger, G.P.; Taylor, R.A. Hedgehog signaling is active in human prostate cancer stroma and regulates proliferation and differentiation of adjacent epithelium. Prostate 2013, 73, 1810–1823. [Google Scholar] [CrossRef]
- Galimberti, F.; Busch, A.M.; Chinyengetere, F.; Ma, T.; Sekula, D.; Memoli, V.A.; Dragnev, K.H.; Liu, F.; Johnson, K.C.; Guo, Y.; et al. Response to inhibition of smoothened in diverse epithelial cancer cells that lack smoothened or patched 1 mutations. Int. J. Oncol. 2012, 41, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Xu, X.-F.; Xu, L.; Niu, P.-Q.; Wang, F.; Hu, G.-Y.; Wang, X.-P.; Guo, C.-Y. Cyclopamine Blocked the Growth of Colorectal Cancer SW116 Cells by Modulating Some Target Genes of Gli1 in vitro. Hepatogastroenterology 2011, 58, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-P.; Xu, X.-F.; Guo, C.; Liu, J.; Yang, W.-J.; Xia, Y.-J.; Xu, L.; Yu, Y.-C. Gli1 maintains cell survival by up-regulating IGFBP6 and Bcl-2 through promoter regions in parallel manner in pancreatic cancer cells. J. Carcinog. 2009, 8, 13. [Google Scholar] [CrossRef] [PubMed]

| Disease/Clinical Problem | Type of Sample | Number of Cases | Expression Level | Ref. | |
|---|---|---|---|---|---|
| IGFBP-6 | Dermal Fibrosis | ||||
| Systemic sclerosis | Skin biopsy samples | Skin biopsy samples from the interscapular region of 5 Type 1 tight-skin (Tsk1) compared to 5 wild-type littermate mice at between 3 days and 12 weeks of age | UP (2-fold) | [49] | |
| Cellular contractility and proliferation in Dupuytren’s Disease (DD) | Primary Fibroblasts (PF) from diseased palmar fascia | 3 affected patients compared to PF derived from the adjacent, phenotypically unaffected palmar fascia of the same patients | DOWN (p < 0.05) | [50] | |
| Renal Fibrosis | |||||
| Proteomic study in Chronic Kidney Disease (CKD) | Human plasma samples | 389 patients, 51% male (N = 201), with a median age of 64 years, and median body mass index of 25.3 | DOWN (rho = −0.81, p = 1.0 × 10−82) | [51] | |
| End Stage Renal Disease (ERSD) | Human blood samples | 16 patients with ESRD receiving hemodialysis (8 men and 8 women) compared to 19 control healthy subjects (10 men and 9 women). | UP (p < 0.05) | [52] | |
| Characterization of congenital obstructive uropathy | RNA from rats with congenital hydronephrosis | Total cellular RNA from obstructed (n = 16), contralateral (n = 10) compared to healthy control kidneys (n = 4) | UP (7.4 fold, p < 0.01) | [53] | |
| Hepatic Fibrosis | |||||
| Disease severity and glycemia in nonalcoholic fatty liver disease | Human liver biopsy | 61 patients with HIV-infection, ≥5% hepatic fat fraction | UP (Fibrosis stage p = 0.03; Steatosis grade p = 0.004; NAFLD activity score p = 0.003) | [54] | |
| Chronic hepatitis C (CHC) | Human blood samples | 128 CHC patients and 123 controls | DOWN (p ≤ 0.001) | [55] | |
| Cardiac Fibrosis | |||||
| Identification of new Acute myocardial infarction (AMI) biomarkers | Human plasma samples | 10 AMI patients compared to 5 controls with no myocardial infarction | DOWN (Ratio = 0.70) | [56] | |
| Atherosclerotic carotid plaques | Gene Expression Omnibus (GEO) data sets and the European Bioinformatics Institute (EBI) database; Atherosclerotic plaques from patients with high-grade carotid artery stenosis | GSE41571 (5 ruptured and 6 stable carotid plaques); E-MTAB-2055 (25 ruptured and 22 stable plaques); GSE118481 (10 clinical unstable and 6 stable plaques); 52 patients (28 with stable plaques and 24 with unstable plaques) | DOWN (p < 0.0001) | [57] | |
| Myelofibrosis | |||||
| Microenvironmental alteration of primary myelofibrosis | Healthy mesenchymal stem cells HS5 | - | UP (p < 0.05) | [58] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liso, A.; Venuto, S.; Coda, A.R.D.; Giallongo, C.; Palumbo, G.A.; Tibullo, D. IGFBP-6: At the Crossroads of Immunity, Tissue Repair and Fibrosis. Int. J. Mol. Sci. 2022, 23, 4358. https://doi.org/10.3390/ijms23084358
Liso A, Venuto S, Coda ARD, Giallongo C, Palumbo GA, Tibullo D. IGFBP-6: At the Crossroads of Immunity, Tissue Repair and Fibrosis. International Journal of Molecular Sciences. 2022; 23(8):4358. https://doi.org/10.3390/ijms23084358
Chicago/Turabian StyleLiso, Arcangelo, Santina Venuto, Anna Rita Daniela Coda, Cesarina Giallongo, Giuseppe Alberto Palumbo, and Daniele Tibullo. 2022. "IGFBP-6: At the Crossroads of Immunity, Tissue Repair and Fibrosis" International Journal of Molecular Sciences 23, no. 8: 4358. https://doi.org/10.3390/ijms23084358
APA StyleLiso, A., Venuto, S., Coda, A. R. D., Giallongo, C., Palumbo, G. A., & Tibullo, D. (2022). IGFBP-6: At the Crossroads of Immunity, Tissue Repair and Fibrosis. International Journal of Molecular Sciences, 23(8), 4358. https://doi.org/10.3390/ijms23084358









