Lipoprotein(a): Cardiovascular Disease, Aortic Stenosis and New Therapeutic Option
Abstract
1. Introduction
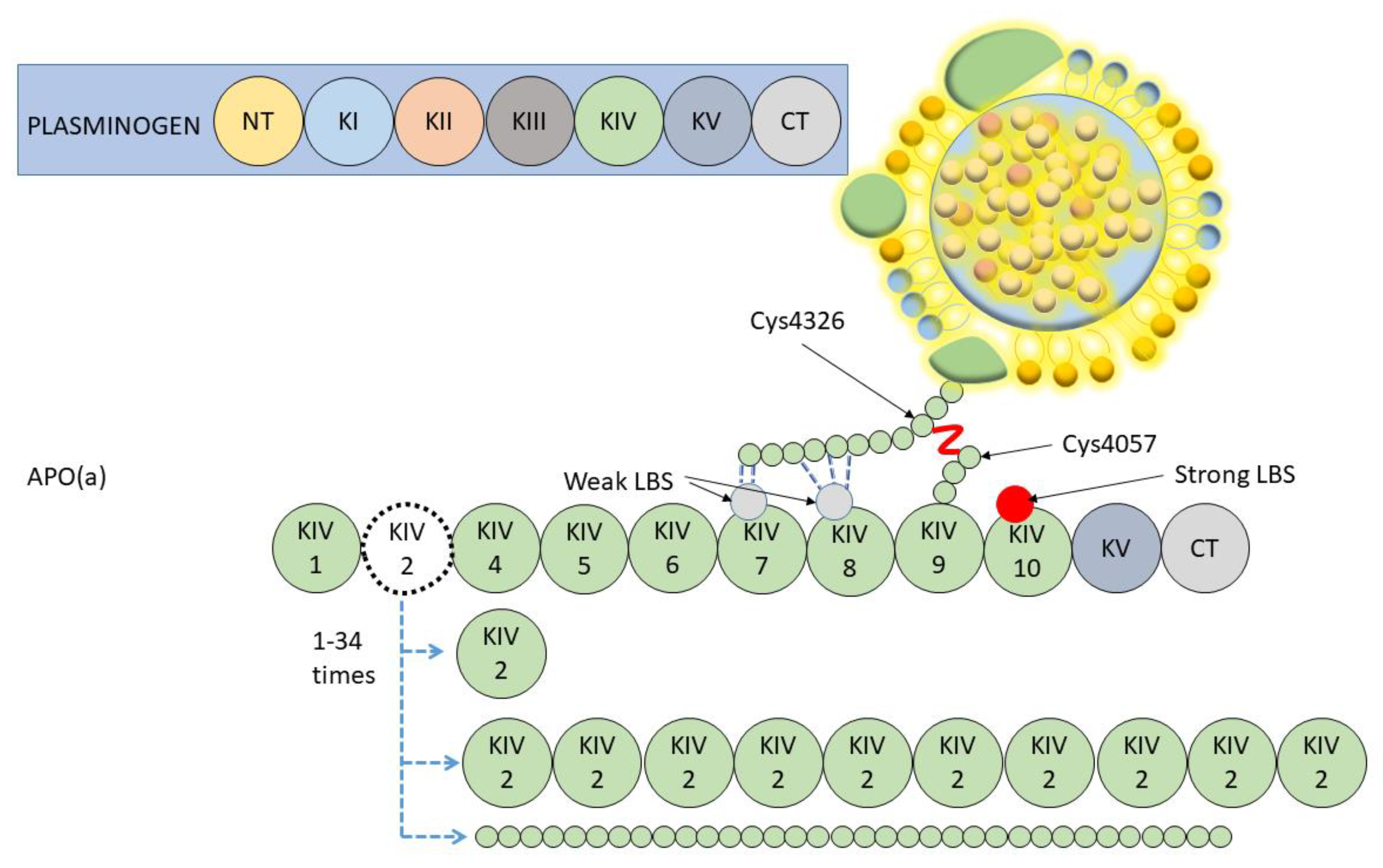
2. Lipoprotein(a) Molecule: Structure and Determinants of Plasma Levels
3. Lipoprotein(a) Molecule: Synthesis and Clearance
4. Lipoprotein(a) Measurements and Cut-Off
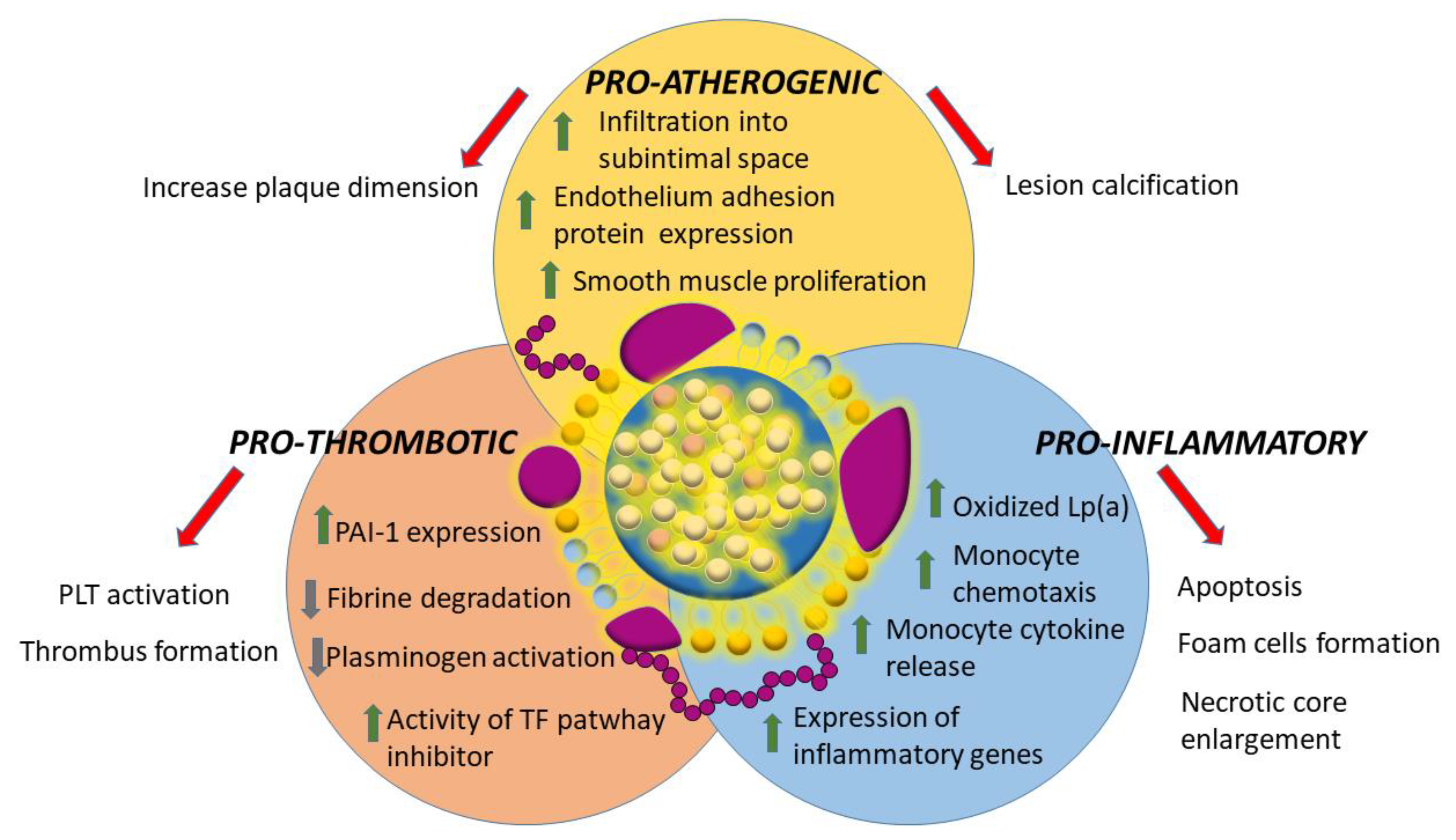
5. Lipoprotein(a) and Cardiovascular Disease: Evidences and Possible Mechanisms
6. Lipoprotein(a) and Aortic Valve Stenosis
7. Lipoprotein(a): Treatment
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Cardiovascular disease burden: Italian and global perspectives. Minerva Cardioangiol. 2021, 69, 231–240. [CrossRef]
- Xu, J.; Murphy, S.L.; Kockanek, K.D.; Arias, E. Mortality in the United States, 2018. NCHS Data Brief 2020, 355, 1–8. [Google Scholar]
- Patel, A.P.; Wang, M.; Pirruccello, J.P.; Ellinor, P.T.; Ng, K.; Kathiresan, S.; Khera, A.V. Lp(a) (Lipoprotein[a]) Concentrations and Incident Atherosclerotic Cardiovascular Disease New Insights from a Large National Biobank. Arter. Thromb. Vasc. Biol. 2021, 41, 465–474. [Google Scholar] [CrossRef]
- Nave, A.H.; Lange, K.S.; Leonards, C.O.; Siegerink, B.; Doehner, W.; Landmesser, U.; Steinhagen-Thiessen, E.; Endres, M.; Ebinger, M. Lipoprotein (a) as a risk factor for ischemic stroke: A meta-analysis. Atherosclerosis 2015, 242, 496–503. [Google Scholar] [CrossRef]
- Langsted, A.; Nordestgaard, B.G.; Kamstrup, P.R. Elevated Lipoprotein(a) and Risk of Ischemic Stroke. J. Am. Coll. Cardiol. 2019, 74, 54–66. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Extreme Lipoprotein(a) Levels and Improved Cardiovascular Risk Prediction. J. Am. Coll. Cardiol. 2013, 61, 1146–1156. [Google Scholar] [CrossRef]
- Larsson, S.C.; Gill, D.; Mason, A.M.; Jiang, T.; Bäck, M.; Butterworth, A.S.; Burgess, S. Lipoprotein(a) in Alzheimer, Atherosclerotic, Cerebrovascular, Thrombotic, and Valvular Disease. Circulation 2020, 141, 1826–1828. [Google Scholar] [CrossRef]
- The CARDIoGRAMplusC4D Consortium; Deloukas, P.; Kanoni, S.; Willenborg, C.; Farrall, M.; Assimes, T.L.; Thompson, J.R.; Ingelsson, E.; Saleheen, D.; Erdmann, J.; et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 2013, 45, 25–33. [Google Scholar] [CrossRef]
- Clarke, R.; Peden, J.F.; Hopewell, J.C.; Kyriakou, T.; Goel, A.; Heath, S.; Parish, S.; Barlera, S.; Franzosi, M.G.; Rust, S.; et al. Genetic Variants Associated with Lp(a) Lipoprotein Level and Coronary Disease. N. Engl. J. Med. 2009, 361, 2518–2528. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement from the American Heart Association. Arter. Thromb. Vasc. Biol. 2022, 42, E48–E60. [Google Scholar] [CrossRef]
- Cesaro, A.; Schiavo, A.; Moscarella, E.; Coletta, S.; Conte, M.; Gragnano, F.; Fimiani, F.; Monda, E.; Caiazza, M.; Limongelli, G.; et al. Lipoprotein(a): A genetic marker for cardiovascular disease and target for emerging therapies. J. Cardiovasc. Med. 2021, 22, 151–161. [Google Scholar] [CrossRef]
- Gentile, M.; Simeon, V.; Iannuzzo, G.; Mattiello, A.; di Taranto, M.D.; Panico, S.; Rubba, P. Lipoprotein (a) is an independent predictor of cardiovascular events in Mediterranean women (Progetto Atena). Eur. J. Prev. Cardiol. 2020, 27, 2248–2250. [Google Scholar] [CrossRef]
- Gragnano, F.; Fimiani, F.; Di Maio, M.; Cesaro, A.; Limongelli, G.; Cattano, D.; Calabrò, P. Impact of lipoprotein(a) levels on recurrent cardiovascular events in patients with premature coronary artery disease. Intern. Emerg. Med. 2019, 14, 621–625. [Google Scholar] [CrossRef]
- Kronenberg, F. Lipoprotein(a) in Prevention and Treatment of Atherosclerosis: Improving State-of-the-Art Management and Search for Novel Targets; von Eckardstein, A., Binder, C.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 201–232. [Google Scholar]
- Jawi, M.M.; Frohlich, J.; Chan, S.Y. Lipoprotein(a) the Insurgent: A New Insight into the Structure, Function, Metabolism, Pathogenicity, and Medications Affecting Lipoprotein(a) Molecule. J. Lipids 2020, 2020, 3491764. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.J.; Hazzard, W.R. Immunochemical quantification of human plasma Lp(a) lipoprotein. Lipids 1974, 9, 15–26. [Google Scholar] [CrossRef]
- Van Der Hoek, Y.Y.; Wittekoek, M.E.; Beisiegel, U.; Kastelein, J.J.P.; Koschinsky, M. The apolipoprotein(a) kringle IV repeats which differ from the major repeat kringle are present in variably-sized isoforms. Hum. Mol. Genet. 1993, 2, 361–366. [Google Scholar] [CrossRef]
- Leibundgut, G.; Scipione, C.; Yin, H.; Schneider, M.; Boffa, M.B.; Green, S.; Yang, X.; Dennis, E.; Witztum, J.L.; Koschinsky, M.L.; et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J. Lipid Res. 2013, 54, 2815–2830. [Google Scholar] [CrossRef]
- Shah, N.P.; Pajidipati, N.J.; McGarrah, R.W.; Navar, A.M.; Vemulapalli, S.; Blazing, M.A.; Shah, S.H.; Hernandez, A.F.; Patel, M.R. Lipoprotein (a): An Update on a Marker of Residual Risk and Associated Clinical Manifestations. Am. J. Cardiol. 2020, 126, 94–102. [Google Scholar] [CrossRef]
- White, A.L.; Lanford, R.E. Cell surface assembly of lipoprotein(a) in primary cultures of baboon hepatocytes. J. Biol. Chem. 1994, 269, 28716–28723. [Google Scholar] [CrossRef]
- Iannuzzo, G.; Tripaldella, M.; Mallardo, V.; Morgillo, M.; Vitelli, N.; Iannuzzi, A.; Aliberti, E.; Giallauria, F.; Tramontano, A.; Carluccio, R.; et al. Lipoprotein(a) Where Do We Stand? From the Physiopathology to Innovative Terapy. Biomedicines 2021, 9, 838. [Google Scholar] [CrossRef]
- Ober, C.; Nord, A.; Thompson, E.E.; Pan, L.; Tan, Z.; Cusanovich, D.; Sun, Y.; Nicolae, R.; Edelstein, C.; Schneider, D.H.; et al. Genome-wide association study of plasma lipoprotein(a) levels identifies multiple genes on chromosome 6q. J. Lipid Res. 2009, 50, 798–806. [Google Scholar] [CrossRef]
- Li, J.; Lange, L.A.; Sabourin, J.; Duan, Q.; Valdar, W.; Willis, M.S.; Li, Y.; Wilson, J.G.; Lange, E.M. Genome- and exome-wide association study of serum lipoprotein (a) in the Jackson Heart Study. J. Hum. Genet. 2015, 60, 755–761. [Google Scholar] [CrossRef]
- Mack, S.; Coassin, S.; Rueedi, R.; Yousri, N.A.; Seppälä, I.; Gieger, C.; Schönherr, S.; Forer, L.; Erhart, G.; Marques-Vidal, P.; et al. A genome-wide association meta-analysis on lipoprotein (a) concentrations adjusted for apolipoprotein (a) isoforms. J. Lipid Res. 2017, 58, 1834–1844. [Google Scholar] [CrossRef]
- Morgan, B.M.; Brown, A.N.; Deo, N.; Harrop, T.W.; Taiaroa, G.; Mace, P.D.; Wilbanks, S.M.; Merriman, T.R.; Williams, M.J.; McCormick, S.P. Nonsynonymous SNPs in LPA homologous to plasminogen deficiency mutants represent novel null apo(a) alleles. J. Lipid Res. 2020, 61, 432–444. [Google Scholar] [CrossRef]
- Tomlinson, J.E.; McLean, J.W.; Lawn, R.M. Rhesus Monkey Apolipoprotein(a). Sequence, evolution, and sites of synthesis. J. Biol. Chem. 1989, 264, 5957–5965. [Google Scholar] [CrossRef]
- Kraft, H.G.; Menzel, H.J.; Hoppichler, F.; Vogel, W.; Utermann, G. Changes of genetic apolipoprotein phenotypes caused by liver transplantation. Implications for apolipoprotein synthesis. J. Clin. Investig. 1989, 83, 137–142. [Google Scholar] [CrossRef]
- Cain, W.J.; Millar, J.S.; Himebauch, A.S.; Tietge, U.J.F.; Maugeais, C.; Usher, D.; Rader, D.J. Lipoprotein [a] is cleared from the plasma primarily by the liver in a process mediated by apolipoprotein [a]. J. Lipid Res. 2005, 46, 2681–2691. [Google Scholar] [CrossRef]
- Kronenberg, F.; Trenkwalder, E.; Lingenhel, A.; Friedrich, G.; Lhotta, K.; Schober, M.; Moes, N.; König, P.; Utermann, G.; Dieplinger, H. Renovascular arteriovenous differences in Lp[a] plasma concentrations suggest removal of Lp[a] from the renal circulation. J. Lipid Res. 1997, 38, 1755–1763. [Google Scholar] [CrossRef]
- Rader, D.J.; Cain, W.; Ikewaki, K.; Talley, G.; Zech, L.A.; Usher, D.; Brewer, H.B. The inverse association of plasma lipoprotein(a) concentrations with apolipoprotein(a) isoform size is not due to differences in Lp(a) catabolism but to differences in production rate. J. Clin. Investig. 1994, 93, 2758–2763. [Google Scholar] [CrossRef]
- Chennamsetty, I.; Kostner, K.M.; Claudel, T.; Vinod, M.; Frank, S.; Weiss, T.; Trauner, M.; Kostner, G.M. Nicotinic acid inhibits hepatic APOA gene expression: Studies in humans and in transgenic mice. J. Lipid Res. 2012, 53, 2405–2412. [Google Scholar] [CrossRef]
- Mooser, V.; Marcovina, S.M.; White, A.L.; Hobbs, H.H. Kringle-containing fragments of apolipoprotein(a) circulate in human plasma and are excreted into the urine. J. Clin. Investig. 1996, 98, 2414–2424. [Google Scholar] [CrossRef]
- Frischmann, M.; Kronenberg, F.; Trenkwalder, E.; Schaefer, J.; Schweer, H.; Dieplinger, B.; Koenig, P.; Ikewaki, K. In vivo turnover study demonstrates diminished clearance of lipoprotein(a) in hemodialysis patients. Kidney Int. 2007, 71, 1036–1043. [Google Scholar] [CrossRef]
- McCormick, S.P.; Schneider, W.J. Lipoprotein(a) catabolism: A case of multiple receptors. Pathology 2019, 51, 155–164. [Google Scholar] [CrossRef]
- Berman, A.N.; Blankstein, R. Current and future role of lipoprotein(a) in preventive cardiology. Curr. Opin. Cardiol. 2019, 34, 514–518. [Google Scholar] [CrossRef]
- Albers, J.J.; Adolphson, J.L.; Hazzard, W.R. Radioimmunoassay of human plasma Lp(a) lipoprotein. J. Lipid Res. 1977, 18, 331–338. [Google Scholar] [CrossRef]
- García-Gómez, C.; Garcí, C.; Gó, A.; Corbella, E. Inflammation, lipid metabolism and cardiovascular risk in rheumatoid arthritis: A qualitative relationship? World J. Orthop. 2014, 5, 304–311. [Google Scholar] [CrossRef]
- Pavanello, C.; Pirazzi, C.; Bjorkman, K.; Sandstedt, J.; Tarlarini, C.; Mosca, L.; Romeo, S.; Calabresi, L.; Mancina, R.M. Individuals with familial hypercholesterolemia and cardiovascular events have higher circulating Lp(a) levels. J. Clin. Lipidol. 2019, 13, 778–787.e6. [Google Scholar] [CrossRef]
- Marcovina, S.M.; Albers, J.J.; Gabel, B.; Koschinsky, M.L.; Gaur, V.P. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein (a). Clin. Chem. 1995, 41, 246–255. [Google Scholar] [CrossRef]
- Marcovina, S.M.; Clouet-Foraison, N.; Koschinsky, M.L.; Lowenthal, M.S.; Orquillas, A.; Boffa, M.B.; Hoofnagle, A.N.; Vaisar, T. Development of an LC-MS/MS Proposed Candidate Reference Method for the Standardization of Analytical Methods to Measure Lipoprotein(a). Clin. Chem. 2021, 67, 490–499. [Google Scholar] [CrossRef]
- Marcovina, S.M.; Albers, J.J.; Scanu, A.M.; Kennedy, H.; Giaculli, F.; Berg, K.; Couderc, R.; Dati, F.; Rifai, N.; Sakurabayashi, I.; et al. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein (a). Clin. Chem. 2000, 46, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Fazio, S.; Viney, N.J.; Xia, S.; Witztum, J.L.; Marcovina, S.M. Relationship of lipoprotein(a) molar concentrations and mass according to lipoprotein(a) thresholds and apolipoprotein(a) isoform size. J. Clin. Lipidol. 2018, 12, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F. Prediction of cardiovascular risk by Lp(a) concentrations or genetic variants within the LPA gene region. Clin. Res. Cardiol. Suppl. 2019, 14, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Langsted, A.; Mora, S.; Kolovou, G.; Baum, H.; Bruckert, E.; Watts, G.F.; Sypniewska, G.; Wiklund, O.; Borén, J.; et al. Fasting Is Not Routinely Required for Determination of a Lipid Profile: Clinical and Laboratory Implications Including Flagging at Desirable Concentration Cutpoints—A Joint Consensus Statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Clin. Chem. 2016, 62, 930–946. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration. Lipoprotein(a) Concentration and the Risk of Coronary Heart Disease, Stroke, and Nonvascular Mortality. JAMA J. Am. Med. Assoc. 2009, 302, 412–423. [Google Scholar] [CrossRef]
- Marcovina, S.M.; Viney, N.J.; Hughes, S.G.; Xia, S.; Witztum, J.L.; Tsimikas, S. Temporal variability in lipoprotein(a) levels in patients enrolled in the placebo arms of IONIS-APO(a)Rx and IONIS-APO(a)-LRx antisense oligonucleotide clinical trials. J. Clin. Lipidol. 2018, 12, 122–129.e2. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef]
- Guan, W.; Cao, J.; Steffen, B.T.; Post, W.S.; Stein, J.H.; Tattersall, M.C.; Kaufman, J.D.; McConnell, J.P.; Hoefner, D.M.; Warnick, R.; et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: The Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 996–1001. [Google Scholar] [CrossRef]
- Vasquez, N.; Joshi, P.H. Lp(a): Addressing a Target for Cardiovascular Disease Prevention. Curr. Cardiol. Rep. 2019, 21, 102. [Google Scholar] [CrossRef]
- Kostner, G.; Avogaro, P.; Cazzolato, G.; Marth, E.; Bittolo-Bon, G.; Qunici, G. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis 1981, 38, 51–61. [Google Scholar] [CrossRef]
- Kronenberg, F.; Utermann, G. Lipoprotein(a): Resurrected by genetics. J. Intern. Med. 2013, 273, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.P.; Jacobson, T.A.; Jones, P.H.; Koschinsky, M.L.; McNeal, C.J.; Nordestgaard, B.G.; Orringer, C.E. Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J. Clin. Lipidol. 2019, 13, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Kamstrup, P.R. Lipoprotein(a) and Cardiovascular Disease. Clin. Chem. 2021, 67, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, Z.; Zhang, H.; Ma, A.; Liao, Y.; Wang, D.; Zhao, B.; Zhu, Z.; Zhao, J.; Zhang, Z.; et al. Pentanucleotide TTTTA Repeat Polymorphism of Apolipoprotein(a) Gene and Plasma Lipoprotein(a) Are Associated with Ischemic and Hemorrhagic Stroke in Chinese: A multicenter case-control study in China. Stroke 2003, 34, 1617–1622. [Google Scholar] [CrossRef]
- Dieplinger, B.; Lingenhel, A.; Baumgartner, N.; Poelz, W.; Dieplinger, H.; Haltmayer, M.; Kronenberg, F.; Mueller, T. Increased Serum Lipoprotein(a) Concentrations and Low Molecular Weight Phenotypes of Apolipoprotein(a) Are Associated with Symptomatic Peripheral Arterial Disease. Clin. Chem. 2007, 53, 1298–1305. [Google Scholar] [CrossRef]
- Verwer, M.C.; Waissi, F.; Mekke, J.M.; Dekker, M.; Stroes, E.S.; de Borst, G.J.; Kroon, J.; Hazenberg, C.E.; de Kleijn, D.P. High lipoprotein(a) is associated with major adverse limb events after femoral artery endarterectomy. Atherosclerosis 2022, 349, 196–203. [Google Scholar] [CrossRef]
- Sakata, K.; Kumakura, H.; Funada, R.; Matsuo, Y.; Nakashima, K.; Iwasaki, T.; Ichikawa, S. Lipoprotein(a) is a Promising Residual Risk Factor for Long-Term Clinical Prognosis in Peripheral Arterial Disease. Ann. Vasc. Dis. 2022, 15, 186–192. [Google Scholar] [CrossRef]
- Norby, F.L.; Eryd, S.A.; Niemeijer, M.N.; Rose, L.M.; Smith, A.V.; Yin, X.; Agarwal, S.K.; Arking, D.E.; Chasman, D.L.; Chen, L.; et al. Association of Lipid-Related Genetic Variants with the Incidence of Atrial Fibrillation: The AFGen Consortium. PLoS ONE 2016, 11, e0151932. [Google Scholar] [CrossRef]
- Garg, P.K.; Guan, W.; Karger, A.B.; Steffen, B.T.; O’Neal, W.; Heckbert, S.R.; Michos, E.D.; Tsai, M.Y. Lp(a) (Lipoprotein [a]) and Risk for Incident Atrial Fibrillation: Multi-Ethnic Study of Atherosclerosis. Circ. Arrhythm. Electrophysiol. 2020, 13, e008401. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Guo, C.; Liu, K.; Xie, Y.; Cao, H.; Peng, W.; Sun, Y.; Liu, X.; Li, B.; Zhang, L. Association of Lipoprotein (a) variants with risk of cardiovascular disease: A Mendelian randomization study. Lipids Health Dis. 2021, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Yang, X.; Qiu, Q.; Gao, F.; Chen, W.; Hu, L.; Xu, Y.; Yi, Y.; Hu, H.; Jiang, L. Low lipoprotein(a) concentration is associated with atrial fibrillation: A large retrospective cohort study. Lipids Health Dis. 2022, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Shemirani, P.; Chong, M.; Narula, S.; Perrot, N.; Conen, D.; Roberts, J.D.; Thériault, S.; Bossé, Y.; Lanktree, M.B.; Pigeyre, M.; et al. Elevated Lipoprotein(a) and Risk of Atrial Fibrillation: An Observational and Mendelian Randomization Study. J. Am. Coll. Cardiol. 2022, 79, 1579–1590. [Google Scholar] [CrossRef]
- Papagrigorakis, E.; Iliopoulos, D.; Asimacopoulos, P.J.; Safi, H.J.; Weilbaecher, D.J.; Ghazzaly, K.G.; Nava, M.L.; Gaubatz, J.W.; Morrisett, J.D. Lipoprotein(a) in plasma, arterial wall, and thrombus from patients with aortic aneurysm. Clin. Genet. 1997, 52, 262–271. [Google Scholar] [CrossRef]
- Spence, J.D.; Koschinsky, M. Mechanisms of Lipoprotein(a) Pathogenicity. Arter. Thromb. Vasc. Biol. 2012, 32, 1550–1551. [Google Scholar] [CrossRef]
- Grainger, D.J.; Kirschenlohr, H.L.; Metcalfe, J.C.; Weissberg, P.L.; Wade, D.P.; Lawn, R.M. Proliferation of Human Smooth Muscle Cells Promoted by Lipoprotein(a). Science 1993, 260, 1655–1658. [Google Scholar] [CrossRef]
- Steinberg, D.; Witztum, J.L. History of discovery: Oxidized Low-Density Lipoprotein and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef]
- Scipione, C.A.; Sayegh, S.E.; Romagnuolo, R.; Tsimikas, S.; Marcovina, S.M.; Boffa, M.B.; Koschinsky, M.L. Mechanistic insights into Lp(a)-induced IL-8 expression: A role for oxidized phospholipid modification of apo(a). J. Lipid Res. 2015, 56, 2273–2285. [Google Scholar] [CrossRef]
- Lim, T.T.; Würtz, P.; Havulinna, A.S.; Palta, P.; Tukiainen, T.; Rehnström, K.; Esko, T.; Mägi, R.; Inouye, M.; Lappalainen, T.; et al. Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder Population. PLoS Genet. 2014, 10, e1004494. [Google Scholar] [CrossRef]
- Poon, M.; Zhang, X.; Dunsky, K.G.; Taubman, M.B.; Harpel, P.C. Apolipoprotein(a) Induces Monocyte Chemotactic Activity in Human Vascular Endothelial Cells. Circulation 1997, 96, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Thanassoulis, G.; Campbell, C.Y.; Owens, D.S.; Smith, J.G.; Smith, A.V.; Peloso, G.M.; Kerr, K.F.; Pechlivanis, S.; Budoff, M.J.; Harris, T.B.; et al. Genetic Associations with Valvular Calcification and Aortic Stenosis. N. Engl. J. Med. 2013, 368, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated Lipoprotein(a) and Risk of Aortic Valve Stenosis in the General Population. J. Am. Coll. Cardiol. 2014, 63, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Capoulade, R.; Chan, K.L.; Yeang, C.; Mathieu, P.; Bossé, Y.; Dumesnil, J.G.; Tam, J.W.; Teo, K.K.; Mahmut, A.; Yang, X.; et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015, 66, 1236–1246. [Google Scholar] [CrossRef]
- Kaiser, Y.; van der Toorn, J.E.; Singh, S.S.; Zheng, K.H.; Kavousi, M.; Sijbrands, E.J.G.; Stroes, E.S.G.; Vernooij, M.W.; de Rijke, Y.B.; Boekholdt, S.M.; et al. Lipoprotein(a) is associated with the onset but not the progression of aortic valve calcification. Eur. Heart J. 2022, 43, 3960–3967. [Google Scholar] [CrossRef]
- Kiechl, S.; Willeit, J.; Mayr, M.; Viehweider, B.; Oberhollenzer, M.; Kronenberg, F.; Wiedermann, C.J.; Oberthaler, S.; Xu, Q.; Witztum, J.L.; et al. Oxidized Phospholipids, Lipoprotein(a), Lipoprotein-Associated Phospholipase A2 Activity, and 10-Year Cardiovascular Outcomes: Prospective results from the bruneck study. Arter. Thromb. Vasc. Biol. 2007, 27, 1788–1795. [Google Scholar] [CrossRef]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef]
- Stulnig, T.M.; Morozzi, C.; Reindl-Schwaighofer, R.; Stefanutti, C. Looking at Lp(a) and Related Cardiovascular Risk: From Scientific Evidence and Clinical Practice. Curr. Atheroscler. Rep. 2019, 21, 37. [Google Scholar] [CrossRef]
- Peeters, F.E.C.M.; Meex, S.J.R.; Dweck, M.R.; Aikawa, E.; Crijns, H.J.G.M.; Schurgers, L.J.; Kietselaer, B.L.J.H. Calcific aortic valve stenosis: Hard disease in the heart. Eur. Heart J. 2017, 39, 2618–2624. [Google Scholar] [CrossRef]
- Schnitzler, J.G.; Ali, L.; Groenen, A.G.; Kaiser, Y.; Kroon, J. Lipoprotein(a) as Orchestrator of Calcific Aortic Valve Stenosis. Biomolecules 2019, 9, 760. [Google Scholar] [CrossRef]
- Rawadi, G.; Vayssière, B.; Dunn, F.; Baron, R.; Roman-Roman, S. BMP-2 Controls Alkaline Phosphatase Expression and Osteoblast Mineralization by a Wnt Autocrine Loop. J. Bone Miner. Res. 2003, 18, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Van Der Valk, F.M.; Bekkering, S.; Kroon, J.; Yeang, C.; Van den Bossche, J.; Van Buul, J.D.; Ravandi, A.; Nederveen, A.J.; Verberne, H.J.; Scipione, C.; et al. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation 2016, 134, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Bergmark, B.A.; O’Donoghue, M.L.; Murphy, S.A.; Kuder, J.F.; Ezhov, M.V.; Ceška, R.; Gouni-Berthold, I.; Jensen, H.K.; Tokgozoglu, S.L.; Mach, F.; et al. An Exploratory Analysis of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibition and Aortic Stenosis in the FOURIER Trial. JAMA Cardiol. 2020, 5, 709. [Google Scholar] [CrossRef] [PubMed]
- Hojo, Y.; Kumakura, H.; Kanai, H.; Iwasaki, T.; Ichikawa, S.; Kurabayashi, M. Lipoprotein(a) is a risk factor for aortic and mitral valvular stenosis in peripheral arterial disease. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Kaltoft, M.; Sigvardsen, P.E.; Afzal, S.; Langsted, A.; Fuchs, A.; Kühl, J.T.; Køber, L.; Kamstrup, P.R.; Kofoed, K.F.; Nordestgaard, B.G. Elevated lipoprotein(a) in mitral and aortic valve calcification and disease: The Copenhagen General Population Study. Atherosclerosis 2022, 349, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.K.; Guan, W.; Karger, A.B.; Steffen, B.T.; Budoff, M.; Tsai, M.Y. Lipoprotein (a) and risk for calcification of the coronary arteries, mitral valve, and thoracic aorta: The Multi-Ethnic Study of Atherosclerosis. J. Cardiovasc. Comput. Tomogr. 2020, 15, 154–160. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Stone, N.J.; Smith, S.C.; Orringer, C.E.; Rigotti, N.A.; Navar, A.M.; Khan, S.S.; Jones, D.W.; Goldberg, R.; Mora, S.; Blaha, M.; et al. Managing Atherosclerotic Cardiovascular Risk in Young Adults: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 819–836. [Google Scholar] [CrossRef]
- Lamina, C.; Kronenberg, F. For the Lp(a)-GWAS-Consortium Estimation of the Required Lipoprotein(a)-Lowering Therapeutic Effect Size for Reduction in Coronary Heart Disease Outcomes: A Mendelian Randomization Analysis. JAMA Cardiol. 2019, 4, 575–579. [Google Scholar] [CrossRef]
- Dyrbuś, K.; Gąsior, M.; Penson, P.E.; Banach, M. Extreme cardiovascular risk—Do we need a new risk category? Eur. Heart J. 2021, 43, 1784–1786. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Giugliano, R.P.; Sabatine, M.S.; Koren, M.J.; Langslet, G.; Bays, H.; Blom, D.; Eriksson, M.; Dent, R.; Wasserman, S.M.; et al. Reduction in Lipoprotein(a) With PCSK9 Monoclonal Antibody Evolocumab (AMG 145): A pooled analysis of more than 1300 patients in 4 phase II trials. J. Am. Coll. Cardiol. 2014, 63, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipoprotein (a): Impact by ethnicity and environmental and medical conditions. J. Lipid Res. 2016, 57, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Yahya, R.; Berk, K.; Verhoeven, A.; Bos, S.; van der Zee, L.; Touw, J.; Erhart, G.; Kronenberg, F.; Timman, R.; Sijbrands, E.; et al. Statin treatment increases lipoprotein(a) levels in subjects with low molecular weight apolipoprotein(a) phenotype. Atherosclerosis 2019, 289, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Borén, J.; Andreotti, F.; Watts, G.; Ginsberg, H.; Amarenco, P.; Catapano, A.L.; Descamps, O.S.; et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef]
- Sahebkar, A.; Reiner, Ž.; Simental-Mendía, L.E.; Ferretti, G.; Cicero, A.F. Effect of extended-release niacin on plasma lipoprotein(a) levels: A systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism 2016, 65, 1664–1678. [Google Scholar] [CrossRef]
- The AIM-HIGH Investigators Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [CrossRef]
- The HPS2-THRIVE Collaborative Group Effects of Extended-Release Niacin with Laropiprant in High-Risk Patients. N. Engl. J. Med. 2014, 371, 203–212. [CrossRef]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Pineda, A.L.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk insights from the FOURIER trial. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef]
- Bittner, V.A.; Szarek, M.; Aylward, P.E.; Bhatt, D.L.; Diaz, R.; Edelberg, J.M.; Fras, Z.; Goodman, S.G.; Halvorsen, S.; Hanotin, C.; et al. Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk After Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 75, 133–144. [Google Scholar] [CrossRef]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.S.; Cromwell, W.C.; Ali, S.; Chin, W.; Flaim, J.D.; Davidson, M. Mipomersen, an Apolipoprotein B Synthesis Inhibitor, Reduces Atherogenic Lipoproteins in Patients with Severe Hypercholesterolemia at High Cardiovascular Risk: A randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 2013, 62, 2178–2184. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, E.; Parhofer, K.G. Thematic review series: Lipoprotein (a): Coming of age at last Lipoprotein apheresis to treat elevated lipoprotein (a). J. Lipid Res. 2016, 57, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Stefanutti, C.; Pisciotta, L.; Favari, E.; Di Giacomo, S.; Vacondio, F.; Zenti, M.G.; Morozzi, C.; Berretti, D.; Mesce, D.; Vitale, M.; et al. Lipoprotein(a) concentration, genetic variants, apo(a) isoform size, and cellular cholesterol efflux in patients with elevated Lp(a) and coronary heart disease submitted or not to lipoprotein apheresis: An Italian case-control multicenter study on Lp(a). J. Clin. Lipidol. 2020, 14, 487–497.e1. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; de Ferranti, S.D.; Ito, M.K.; et al. Familial Hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients. J. Clin. Lipidol. 2011, 5, S1–S8. [Google Scholar] [CrossRef]
- Wang, A.; Richhariya, A.; Gandra, S.R.; Calimlim, B.; Kim, L.; Quek, R.G.W.; Nordyke, R.J.; Toth, P.P. Systematic Review of Low-Density Lipoprotein Cholesterol Apheresis for the Treatment of Familial Hypercholesterolemia. J. Am. Heart Assoc. 2016, 5, e003294. [Google Scholar] [CrossRef]
- Harada-Shiba, M.; Arai, H.; Oikawa, S.; Ohta, T.; Okada, T.; Okamura, T.; Nohara, A.; Bujo, H.; Yokote, K.; Wakatsuki, A.; et al. Guidelines for the Management of Familial Hypercholesterolemia. J. Atheroscler. Thromb. 2012, 19, 1043–1060. [Google Scholar] [CrossRef]
- Schettler, V.; The German Apheresis Working Group; Neumann, C.L.; Hulpke-Wette, M.; Hagenah, G.C.; Schulz, E.G.; Wieland, E. Current view: Indications for extracorporeal lipid apheresis treatment. Clin. Res. Cardiol. Suppl. 2012, 7, 15–19. [Google Scholar] [CrossRef]
- Macchi, C.; Sirtori, C.; Corsini, A.; Santos, R.; Watts, G.; Ruscica, M. A new dawn for managing dyslipidemias: The era of rna-based therapies. Pharmacol. Res. 2019, 150, 104413. [Google Scholar] [CrossRef]
- Viney, N.J.; van Capelleveen, J.C.; Geary, R.S.; Xia, S.; Tami, J.A.; Yu, R.Z.; Marcovina, S.M.; Hughes, S.G.; Graham, M.J.; Crooke, R.M.; et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): Two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016, 388, 2239–2253. [Google Scholar] [CrossRef]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I.; Tardif, J.-C.; Baum, S.J.; Steinhagen-Thiessen, E.; Shapiro, M.D.; Stroes, E.S.; Moriarty, P.M.; Nordestgaard, B.G.; et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. New Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Karwatowska-Prokopczuk, E.; Clouet-Foraison, N.; Xia, S.; Viney, N.J.; Witztum, J.L.; Marcovina, S.M.; Tsimikas, S. Prevalence and influence of LPA gene variants and isoform size on the Lp(a)-lowering effect of pelacarsen. Atherosclerosis 2021, 324, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Koren, M.J.; Moriarty, P.M.; Baum, S.J.; Neutel, J.; Hernandez-Illas, M.; Weintraub, H.S.; Florio, M.; Kassahun, H.; Melquist, S.; Varrieur, T.; et al. Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a). Nat. Med. 2022, 28, 96–103. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; López, J.A.G.; Knusel, B.; Gencer, B.; Wang, H.; Wu, Y.; Kassahun, H.; Sabatine, M.S. Study design and rationale for the Olpasiran trials of Cardiovascular Events And lipoproteiN(a) reduction-DOSE finding study (OCEAN(a)-DOSE). Am. Heart J. 2022, 251, 61–69. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K.; Balog, C.; Swerdlow, D.I.; Scrimgeour, A.C.; Rambaran, C.; Wilson, R.J.; Boyce, M.; Ray, K.K.; Cho, L.; et al. Single Ascending Dose Study of a Short Interfering RNA Targeting Lipoprotein(a) Production in Individuals with Elevated Plasma Lipoprotein(a) Levels. JAMA 2022, 327, 1679. [Google Scholar] [CrossRef]
| Non-Specific Therapies | ||||
| Name | %Lp(a) Reduction | Mechanism of Action | Administration | Experimental Phase |
| Statins | controversial | HMG-CoA reductase inhibitor | Oral | No |
| Niacin | 20 | Triglycerides production inhibitor | Oral | No |
| Alirocumab | 23.5 | Anti-PCSK9 antibodies | Subcutaneous (every 2 weeks) | No |
| Evolocumab | 29.5 | Anti-PCSK9 antibodies | Subcutaneous (every 2 weeks) | No |
| Inclisiran | 25.6 | siRNA inhibiting PCSK9 | Subcutaneous (every 6 months) | No |
| Mipomersen | 21–50 | Decreases apoB synthesis | Subcutaneous (weekly) | No |
| Specific Therapies (All Experimental) | ||||
| Name | %Lp(a) Reduction | Mechanism of Action | Administration | Experimental Phase |
| Pelacarsen | 80 | ASO inhibiting apo(a) | Subcutaneous (every 1 month) | Phase 3 ongoing (patients enrollment complete), result expected for mid 2025 |
| Olpasiran | 94–98 | siRNA inhibiting apo(a) | Subcutaneous (every 3 months) | Phase 2 finished (results not yet published), Phase 3 planned to start in December 2022 |
| SLN360 | 98 | siRNA inhibiting apo(a) | Subcutaneous (probably every 6 months) | Phase 1 finished, Phase 2 planned to start in December 2022 |
| LY3819469 | unknown | siRNA inhibiting apo(a) | Subcutaneous (timing unknown) | Phase 1 finished but results not yet presented, Phase 2 registered on clinicaltrial.gov |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maloberti, A.; Fabbri, S.; Colombo, V.; Gualini, E.; Monticelli, M.; Daus, F.; Busti, A.; Galasso, M.; De Censi, L.; Algeri, M.; et al. Lipoprotein(a): Cardiovascular Disease, Aortic Stenosis and New Therapeutic Option. Int. J. Mol. Sci. 2023, 24, 170. https://doi.org/10.3390/ijms24010170
Maloberti A, Fabbri S, Colombo V, Gualini E, Monticelli M, Daus F, Busti A, Galasso M, De Censi L, Algeri M, et al. Lipoprotein(a): Cardiovascular Disease, Aortic Stenosis and New Therapeutic Option. International Journal of Molecular Sciences. 2023; 24(1):170. https://doi.org/10.3390/ijms24010170
Chicago/Turabian StyleMaloberti, Alessandro, Saverio Fabbri, Valentina Colombo, Elena Gualini, Massimiliano Monticelli, Francesca Daus, Andrea Busti, Michele Galasso, Lorenzo De Censi, Michela Algeri, and et al. 2023. "Lipoprotein(a): Cardiovascular Disease, Aortic Stenosis and New Therapeutic Option" International Journal of Molecular Sciences 24, no. 1: 170. https://doi.org/10.3390/ijms24010170
APA StyleMaloberti, A., Fabbri, S., Colombo, V., Gualini, E., Monticelli, M., Daus, F., Busti, A., Galasso, M., De Censi, L., Algeri, M., Merlini, P. A., & Giannattasio, C. (2023). Lipoprotein(a): Cardiovascular Disease, Aortic Stenosis and New Therapeutic Option. International Journal of Molecular Sciences, 24(1), 170. https://doi.org/10.3390/ijms24010170






