Enhanced Bactericidal Effect of Calcinated Mg–Fe Layered Double Hydroxide Films Driven by the Fenton Reaction
Abstract
:1. Introduction
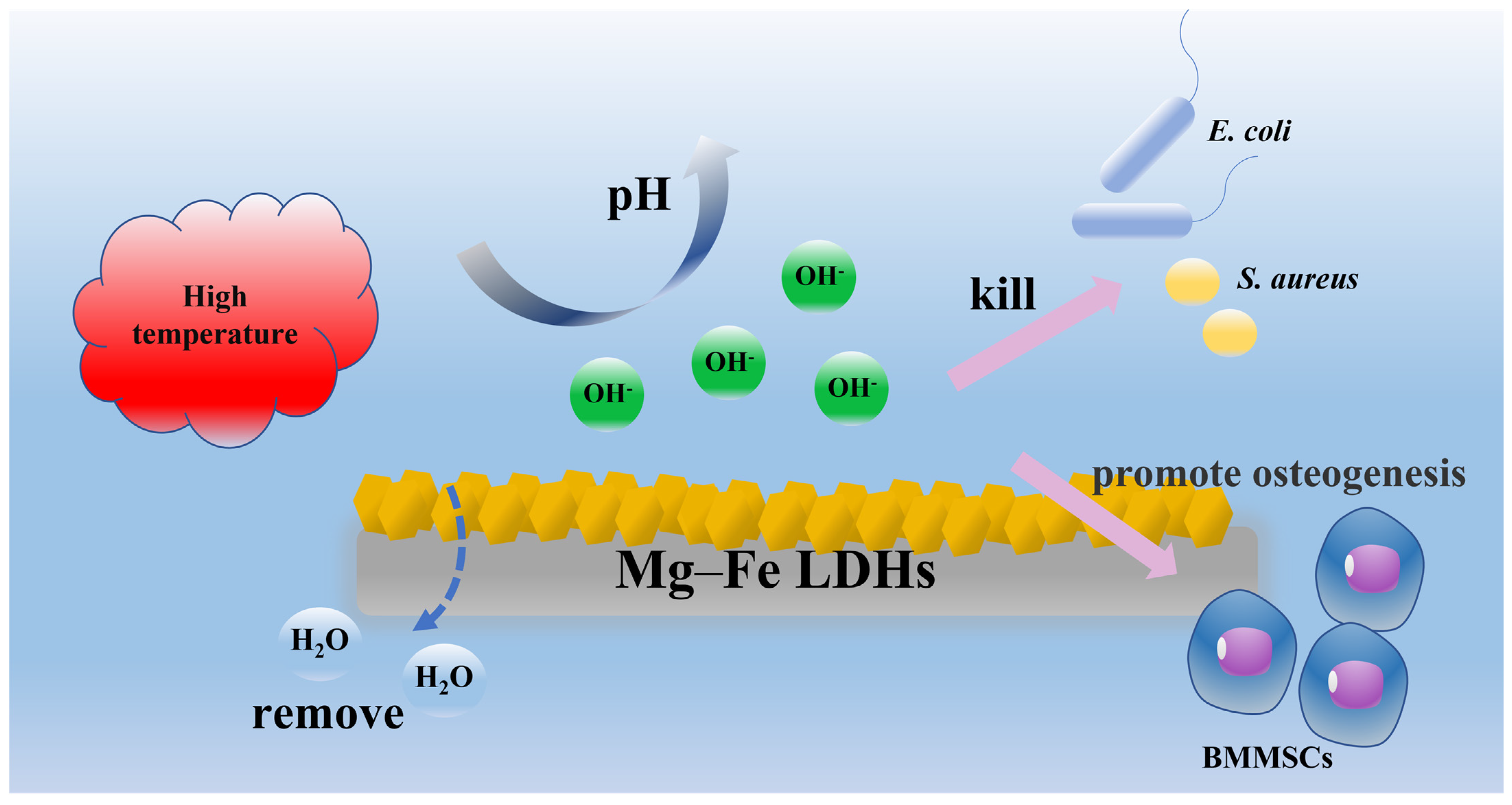
2. Results and Discussion
2.1. Sample Preparation and Characterization
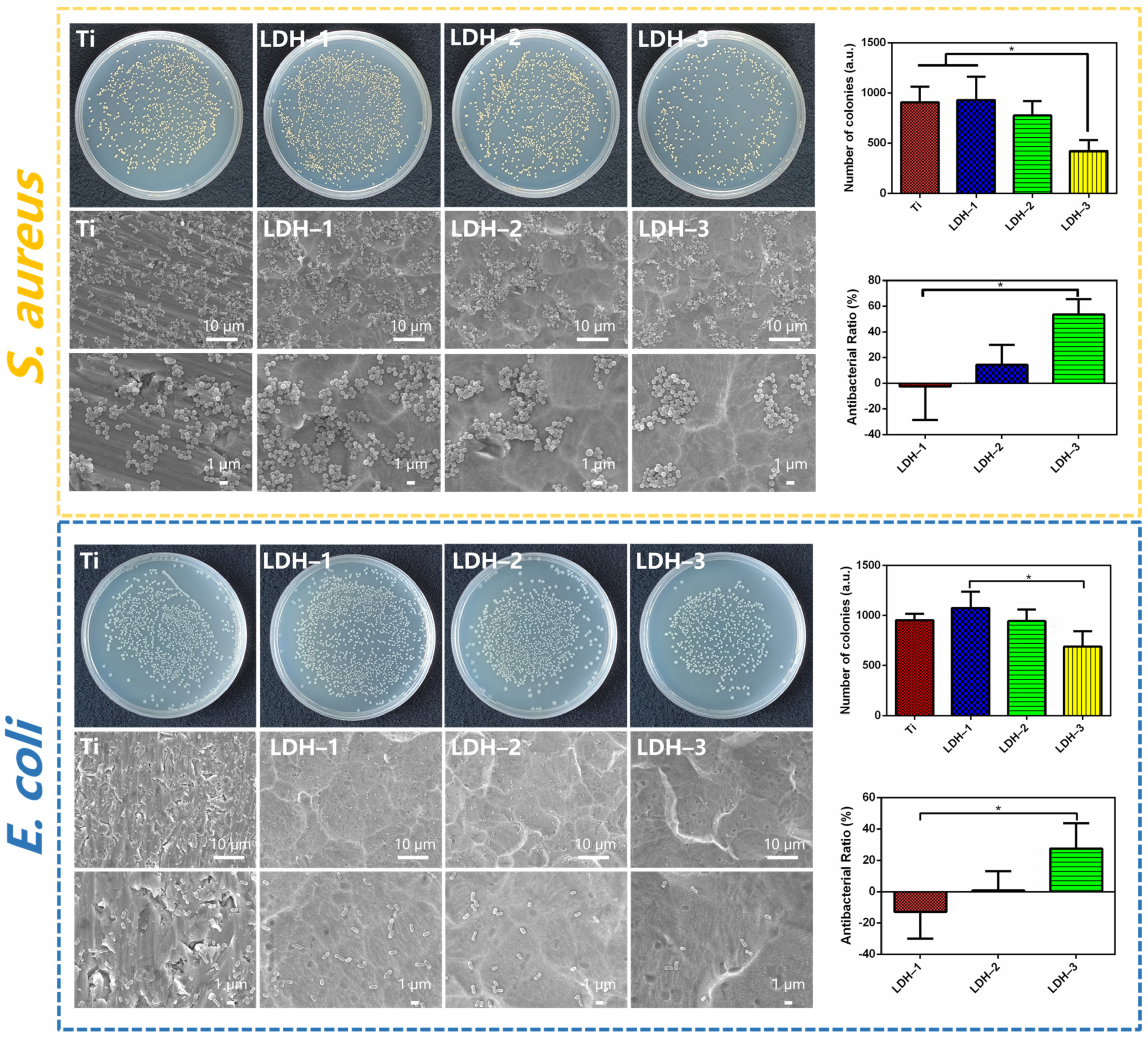
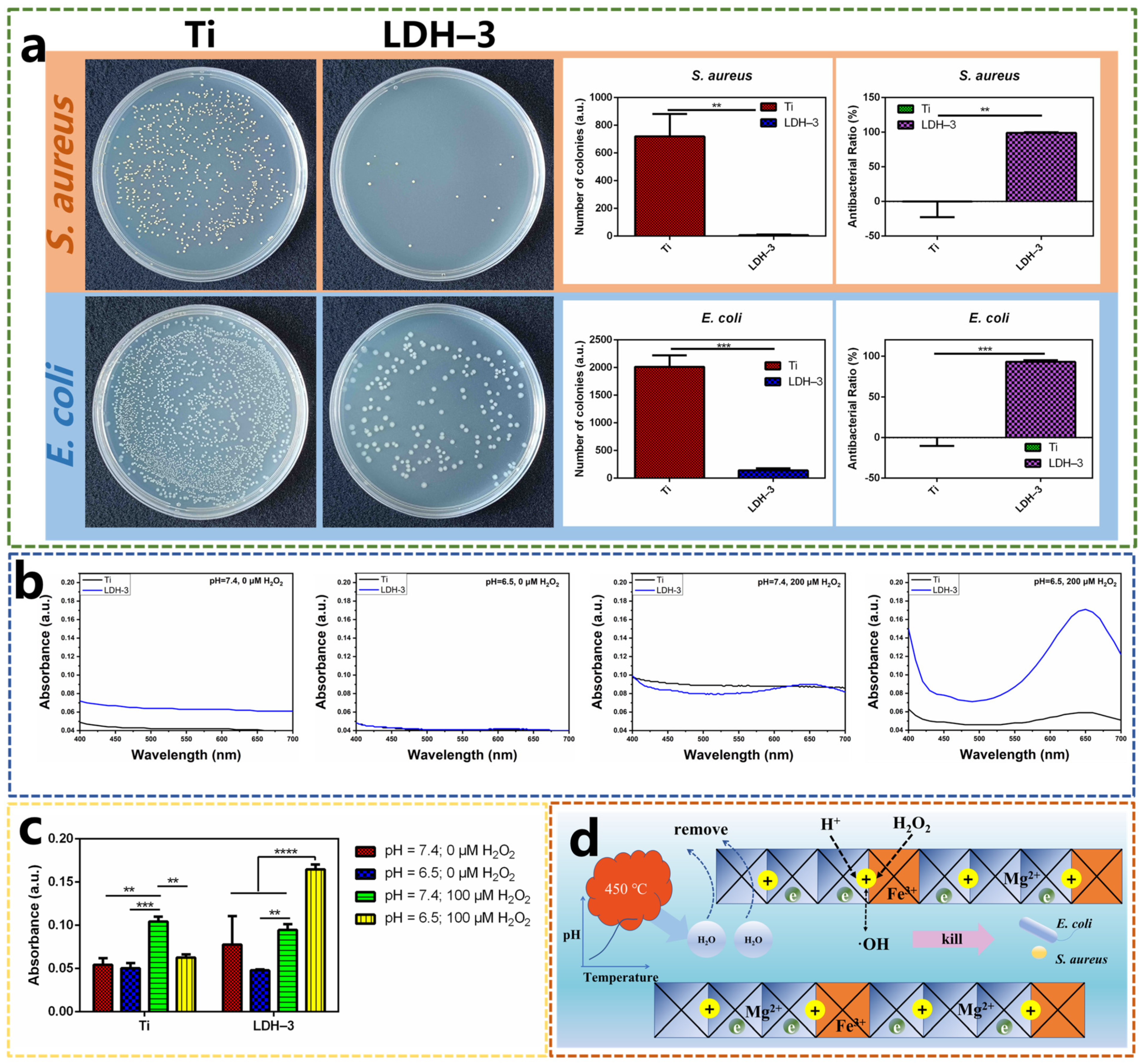
2.2. Antibacterial Performance of Samples
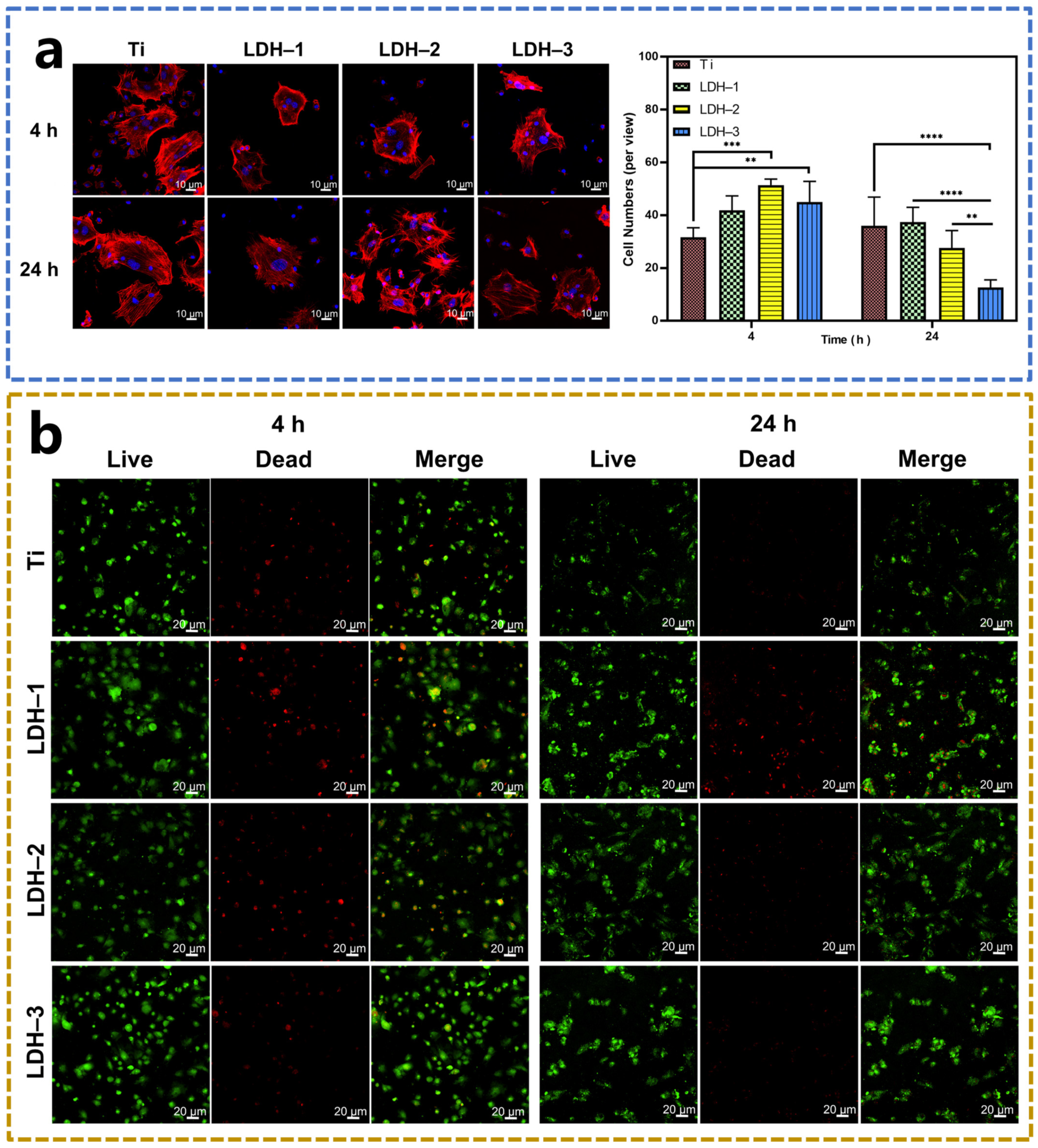
2.3. Biocompatibility of Samples
3. Materials and Methods
3.1. Sample Preparation
3.2. Surface Morphology and Crystalline Phase Characterization
3.3. pH Measurement
3.4. Electrochemical Performance
3.5. Hydroxy Radicals Detection
3.6. Bacteria Culture
3.7. Morphology of Bacteria
3.8. Bacteria Live/Dead Staining
3.9. Bacteria Culture under the Acidic and H2O2 Environment
3.10. Cell Initial Adhesion
3.11. Cell Live/Dead Staining
3.12. Cell Proliferation
3.13. Cell Proliferation under the H2O2 Environment
3.14. Gene Expression by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veletić, M.; Apu, E.H.; Simić, M.; Bergsland, J.; Balasingham, I.; Contag, C.H.; Ashammakhi, N. Implants with Sensing Capabilities. Chem. Rev. 2022, 122, 16329–16363. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Sarkar, N.; Banerjee, D. Natural medicine delivery from biomedical devices to treat bone disorders: A review. Acta Biomater. 2021, 126, 63–91. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Batra, U.; Kumar, K.; Ahuja, N.; Mahapatro, A. Progress in bioactive surface coatings on biodegradable Mg alloys: A critical review towards clinical translation. Bioact. Mater. 2023, 19, 717–757. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Fu, J.; Zhao, L.; Chu, P.K.; Huo, K. Biofunctional Elements Incorporated Nano/Microstructured Coatings on Titanium Implants with Enhanced Osteogenic and Antibacterial Performance. Adv. Healthc. Mater. 2020, 9, e2000681. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Ding, C.; Chen, Z.; Li, J. From molecules to macrostructures: Recent development of bioinspired hard tissue repair. Biomater. Sci. 2017, 5, 1435–1449. [Google Scholar] [CrossRef]
- Delanois, R.E.; Mistry, J.B.; Gwam, C.U.; Mohamed, N.S.; Choksi, U.S.; Mont, M.A. Current Epidemiology of Revision Total Knee Arthroplasty in the United States. J. Arthroplast. 2017, 32, 2663–2668. [Google Scholar] [CrossRef]
- Xing, F.; Li, S.; Yin, D.; Xie, J.; Rommens, P.M.; Xiang, Z.; Liu, M.; Ritz, U. Recent progress in Mg-based alloys as a novel bioabsorbable biomaterials for orthopedic applications. J. Magnes. Alloys 2022, 10, 1428–1456. [Google Scholar] [CrossRef]
- Balabiyev, A.; Podolnikova, N.P.; Kilbourne, J.A.; Baluch, D.P.; Lowry, D.; Zare, A.; Ros, R.; Flick, M.J.; Ugarova, T.P. Fibrin polymer on the surface of biomaterial implants drives the foreign body reaction. Biomaterials 2021, 277, 121087. [Google Scholar] [CrossRef]
- Chae, K.; Jang, W.Y.; Park, K.; Lee, J.; Kim, H.; Lee, K.; Lee, C.K.; Lee, Y.; Lee, S.H.; Seo, J. Antibacterial infection and immune-evasive coating for orthopedic implants. Sci. Adv. 2020, 6, eabb0025. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]
- van Hengel, I.A.J.; Gelderman, F.S.A.; Athanasiadis, S.; Minneboo, M.; Weinans, H.; Fluit, A.C.; van der Eerden, B.C.J.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Functionality-packed additively manufactured porous titanium implants. Mater. Today Bio 2020, 7, 100060. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Qian, Y.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. A surface-engineered polyetheretherketone biomaterial implant with direct and immunoregulatory antibacterial activity against methicillin-resistant Staphylococcus aureus. Biomaterials 2019, 208, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ren, L.; Zhang, S.; Wei, X.; Yang, K.; Dai, K. Antibacterial effect of a copper-containing titanium alloy against implant-associated infection induced by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2021, 119, 472–484. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef]
- Xu, Z.; Li, M.; Li, X.; Liu, X.; Ma, F.; Wu, S.; Yeung, K.W.; Han, Y.; Chu, P.K. Antibacterial Activity of Silver Doped Titanate Nanowires on Ti Implants. ACS Appl. Mater. Interfaces 2016, 8, 16584–16594. [Google Scholar] [CrossRef]
- Aggarwal, D.; Kumar, V.; Sharma, S. Drug-loaded biomaterials for orthopedic applications: A review. J. Control. Release 2022, 344, 113–133. [Google Scholar] [CrossRef]
- Xia, C.; Cai, D.; Tan, J.; Li, K.; Qiao, Y.; Liu, X. Synergistic Effects of N/Cu Dual Ions Implantation on Stimulating Antibacterial Ability and Angiogenic Activity of Titanium. ACS Biomater. Sci. Eng. 2018, 4, 3185–3193. [Google Scholar] [CrossRef]
- Liu, J.; Gefen, O.; Ronin, I.; Bar-Meir, M.; Balaban, N.Q. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 2020, 367, 200–204. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Z.; Wang, D.; Zhang, X.; Qian, S.; Liu, X. A facile and universal strategy to endow implant materials with antibacterial ability via alkalinity disturbing bacterial respiration. Biomater. Sci. 2020, 8, 1815–1829. [Google Scholar] [CrossRef]
- Wang, S.; Chen, B.; Ouyang, L.; Wang, D.; Tan, J.; Qiao, Y.; Ge, S.; Ruan, J.; Zhuang, A.; Liu, X.; et al. A Novel Stimuli-Responsive Injectable Antibacterial Hydrogel to Achieve Synergetic Photothermal/Gene-Targeted Therapy towards Uveal Melanoma. Adv. Sci. 2021, 8, e2004721. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, S.; Tan, J.; Xie, J.; Zhang, Y.; Chen, S.; Du, H.; Qian, S.; Qiao, Y.; Peng, F.; et al. Black Mn-containing layered double hydroxide coated magnesium alloy for osteosarcoma therapy, bacteria killing, and bone regeneration. Bioact. Mater. 2022, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xing, S.; Peng, F.; Zhang, X.; Tan, J.; Hao, X.; Qiao, Y.; Ge, N.; Liu, X. Microenvironment-responsive electrocution of tumor and bacteria by implants modified with degenerate semiconductor film. Bioact. Mater. 2023, 20, 472–488. [Google Scholar] [CrossRef] [PubMed]
- Albarqi, H.A.; Wong, L.H.; Schumann, C.; Sabei, F.Y.; Korzun, T.; Li, X.; Hansen, M.N.; Dhagat, P.; Moses, A.S.; Taratula, O.; et al. Biocompatible Nanoclusters with High Heating Efficiency for Systemically Delivered Magnetic Hyperthermia. ACS Nano 2019, 13, 6383–6395. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, C.; Wang, D.; Jiang, H.; Qiao, Y.; Zhang, D.; Zhang, X.; Xu, R.; Liu, C.; Su, J.; et al. Tailoring time-varying alkaline microenvironment on titanium for sequential anti-infection and osseointegration. Chem. Eng. J. 2022, 431, 133940. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, J.; Tan, J.; Zhang, D.; Wu, L.; Qiao, Y.; Wang, G.; Wu, J.; Yeung, K.W.K.; Liu, X. In-situ growth of vertical graphene on titanium by PECVD for rapid sterilization under near-infrared light. Carbon 2022, 192, 209–218. [Google Scholar] [CrossRef]
- Ameena Shirin, V.K.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Advanced drug delivery applications of layered double hydroxide. J. Control. Release 2021, 330, 398–426. [Google Scholar] [CrossRef]
- Hu, T.; Gu, Z.; Williams, G.R.; Strimaite, M.; Zha, J.; Zhou, Z.; Zhang, X.; Tan, C.; Liang, R. Layered double hydroxide-based nanomaterials for biomedical applications. Chem. Soc. Rev. 2022, 51, 6126–6176. [Google Scholar] [CrossRef]
- Wang, D.; Peng, F.; Li, J.; Qiao, Y.; Li, Q.; Liu, X. Butyrate-inserted Ni–Ti layered double hydroxide film for H2O2-mediated tumor and bacteria killing. Mater. Today 2017, 20, 238–257. [Google Scholar] [CrossRef]
- Yin, Y.; Jian, L.; Li, B.; Liang, C.; Han, X.; Zhao, X.; Wang, D. Mg-Fe layered double hydroxides modified titanium enhanced the adhesion of human gingival fibroblasts through regulation of local pH level. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112485. [Google Scholar] [CrossRef]
- Wang, D.; Ge, N.; Yang, T.; Peng, F.; Qiao, Y.; Li, Q.; Liu, X. NIR-Triggered Crystal Phase Transformation of NiTi-Layered Double Hydroxides Films for Localized Chemothermal Tumor Therapy. Adv. Sci. 2018, 5, 1700782. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg-Al-Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2016, 8, 35033–35044. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, D.; Cao, H.; Qiao, Y.; Zhu, H.; Liu, X. Effect of Local Alkaline Microenvironment on the Behaviors of Bacteria and Osteogenic Cells. ACS Appl. Mater. Interfaces 2018, 10, 42018–42029. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, D.; Qiu, J.; Peng, F.; Liu, X. Regulating the local pH level of titanium via Mg-Fe layered double hydroxides films for enhanced osteogenesis. Biomater. Sci. 2018, 6, 1227–1237. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Qiu, J.; Zhang, X.; Ge, N.; Liu, X. Corrosion Motivated ROS Generation Helps Endow Titanium with Broad-Spectrum Antibacterial Abilities. Adv. Mater. Interfaces 2019, 6, 1900514. [Google Scholar] [CrossRef]
- Lee, S.B.; Ko, E.H.; Park, J.Y.; Oh, J.M. Mixed Metal Oxide by Calcination of Layered Double Hydroxide: Parameters Affecting Specific Surface Area. Nanomaterials 2021, 11, 1153. [Google Scholar] [CrossRef]
- Merkl, P.; Aschtgen, M.S.; Henriques-Normark, B.; Sotiriou, G.A. Biofilm interfacial acidity evaluation by pH-Responsive luminescent nanoparticle films. Biosens. Bioelectron. 2021, 171, 112732. [Google Scholar] [CrossRef]
- Li, Y.; Xiu, W.; Yang, K.; Wen, Q.; Yuwen, L.; Luo, Z.; Liu, X.; Yang, D.; Xie, X.; Wang, L. A multifunctional Fenton nanoagent for microenvironment-selective anti-biofilm and anti-inflammatory therapy. Mater. Horiz. 2021, 8, 1264–1271. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Ma, S.; Guo, Q.; Zhang, W.; Cheng, L.; Ding, L.; Xu, Z.; Jiang, J.; Gao, L. Oral biofilm elimination by combining iron-based nanozymes and hydrogen peroxide-producing bacteria. Biomater. Sci. 2020, 8, 2447–2458. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Guo, F.; Shi, Y.; Li, L.; Xu, Z.; Yan, X.; Shi, W. Fe-doped g-C3N4 derived from biowaste material with Fe-N bonds for enhanced synergistic effect between photocatalysis and Fenton degradation activity in a broad pH range. J. Alloys Compd. 2022, 900, 163410. [Google Scholar] [CrossRef]
- Wang, P.; Shi, Y.; Zhang, S.; Huang, X.; Zhang, J.; Zhang, Y.; Si, W.; Dong, X. Hydrogen Peroxide Responsive Iron-Based Nanoplatform for Multimodal Imaging-Guided Cancer Therapy. Small 2019, 15, e1803791. [Google Scholar] [CrossRef] [PubMed]
- Ranji-Burachaloo, H.; Karimi, F.; Xie, K.; Fu, Q.; Gurr, P.A.; Dunstan, D.E.; Qiao, G.G. MOF-Mediated Destruction of Cancer Using the Cell’s Own Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2017, 9, 33599–33608. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, W.; Qing, Z.; Zheng, J.; Xiao, Y.; Yang, S.; Wang, L.; Li, Y.; Yang, R. In Vivo Lighted Fluorescence via Fenton Reaction: Approach for Imaging of Hydrogen Peroxide in Living Systems. Anal. Chem. 2016, 88, 3998–4003. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Wang, L.; Zhang, H.; Zhang, L.; Chen, Y.; Shi, J. Construction of Single-Iron-Atom Nanocatalysts for Highly Efficient Catalytic Antibiotics. Small 2019, 15, e1901834. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Z.; Tian, R.; Lu, C. Efficient bacteria inactivation by ligand-induced continuous generation of hydroxyl radicals in Fenton-like reaction. J. Hazard. Mater. 2019, 369, 408–415. [Google Scholar] [CrossRef]
- Mao, L.; Yin, Y.; Zhang, L.; Chen, X.; Wang, X.; Chen, F.; Liu, C. Regulation of Inflammatory Response and Osteogenesis to Citrate-Based Biomaterials through Incorporation of Alkaline Fragments. Adv. Healthc. Mater. 2022, 11, e2101590. [Google Scholar] [CrossRef]
- Yao, M.; Cheng, S.; Zhong, G.; Zhou, J.; Shao, H.; Ma, L.; Du, C.; Peng, F.; Zhang, Y. Enhanced osteogenesis of titanium with nano-Mg(OH)2 film and a mechanism study via whole genome expression analysis. Bioact. Mater. 2021, 6, 2729–2741. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, Z.; Huang, Y.; Jia, Z.; Cui, Z.; Li, Z.; Wu, S.; Liang, Y.; Zhu, S.; Yang, X.; et al. Unraveling the osteogenesis of magnesium by the activity of osteoblasts in vitro. J. Mater. Chem. B 2018, 6, 6615–6621. [Google Scholar] [CrossRef]
- Wang, C.X.; Ma, T.; Wang, M.Y.; Guo, H.Z.; Ge, X.Y.; Zhang, Y.; Lin, Y. Facile distribution of an alkaline microenvironment improves human bone marrow mesenchymal stem cell osteogenesis on a titanium surface through the ITG/FAK/ALP pathway. Int. J. Implant Dent. 2021, 7, 56. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Yin, Y.; Jian, L.; Han, X.; Zhao, X.; Wang, D. Enhanced Bactericidal Effect of Calcinated Mg–Fe Layered Double Hydroxide Films Driven by the Fenton Reaction. Int. J. Mol. Sci. 2023, 24, 272. https://doi.org/10.3390/ijms24010272
Chen L, Yin Y, Jian L, Han X, Zhao X, Wang D. Enhanced Bactericidal Effect of Calcinated Mg–Fe Layered Double Hydroxide Films Driven by the Fenton Reaction. International Journal of Molecular Sciences. 2023; 24(1):272. https://doi.org/10.3390/ijms24010272
Chicago/Turabian StyleChen, Lei, Yijia Yin, Linjia Jian, Xianglong Han, Xuefeng Zhao, and Donghui Wang. 2023. "Enhanced Bactericidal Effect of Calcinated Mg–Fe Layered Double Hydroxide Films Driven by the Fenton Reaction" International Journal of Molecular Sciences 24, no. 1: 272. https://doi.org/10.3390/ijms24010272
APA StyleChen, L., Yin, Y., Jian, L., Han, X., Zhao, X., & Wang, D. (2023). Enhanced Bactericidal Effect of Calcinated Mg–Fe Layered Double Hydroxide Films Driven by the Fenton Reaction. International Journal of Molecular Sciences, 24(1), 272. https://doi.org/10.3390/ijms24010272




