The DmeRF System Is Involved in Maintaining Cobalt Homeostasis in Vibrio parahaemolyticus
Abstract
:1. Introduction
2. Results
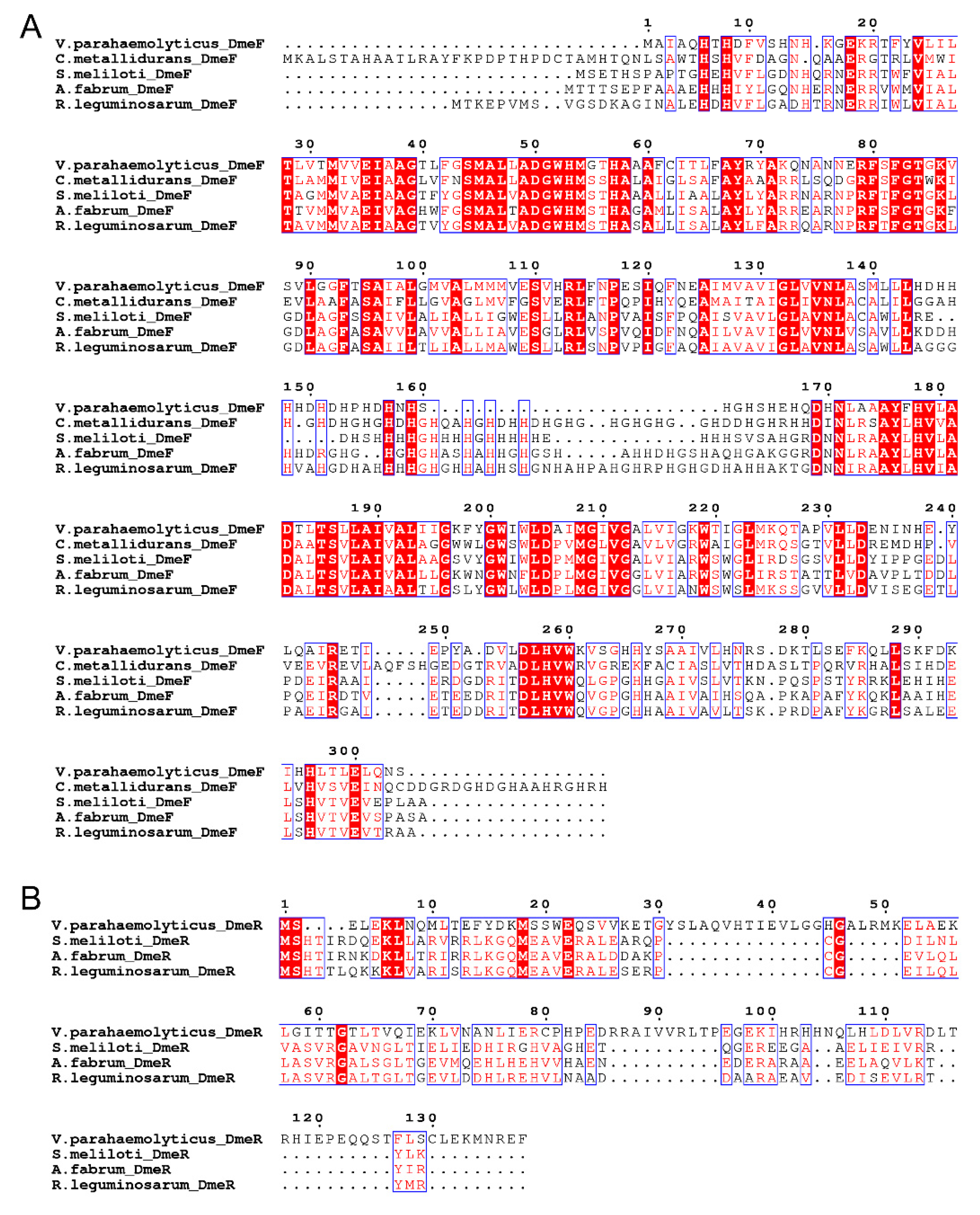
2.1. Identification of the DmeRF System in V. parahaemolyticus
2.2. The V. parahaemolyticus dmeF Gene Is Inducible by Zinc, Copper, and Cobalt
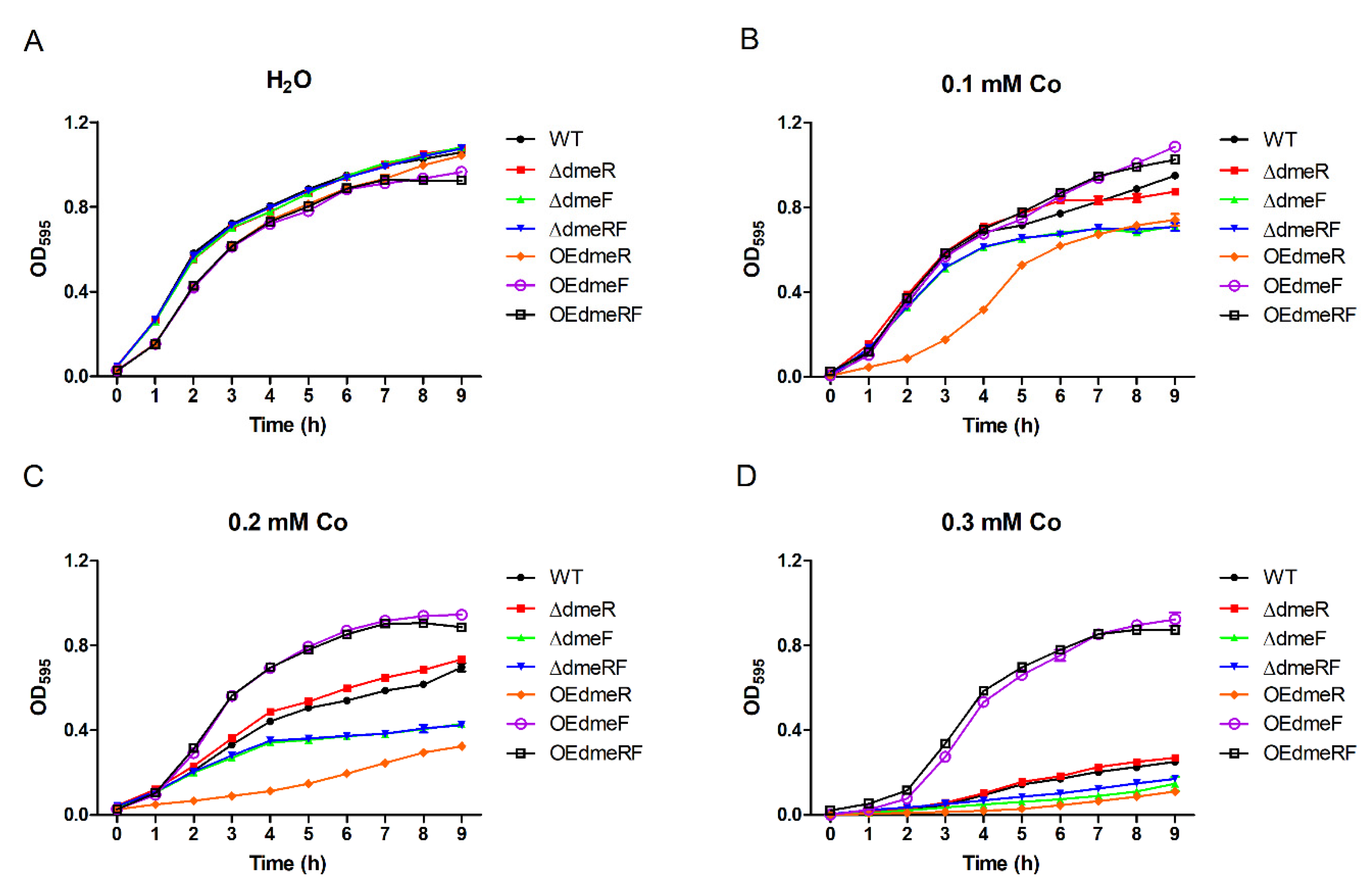
2.3. DmeF Contributes to V. parahaemolyticus Growth under High-Cobalt Conditions
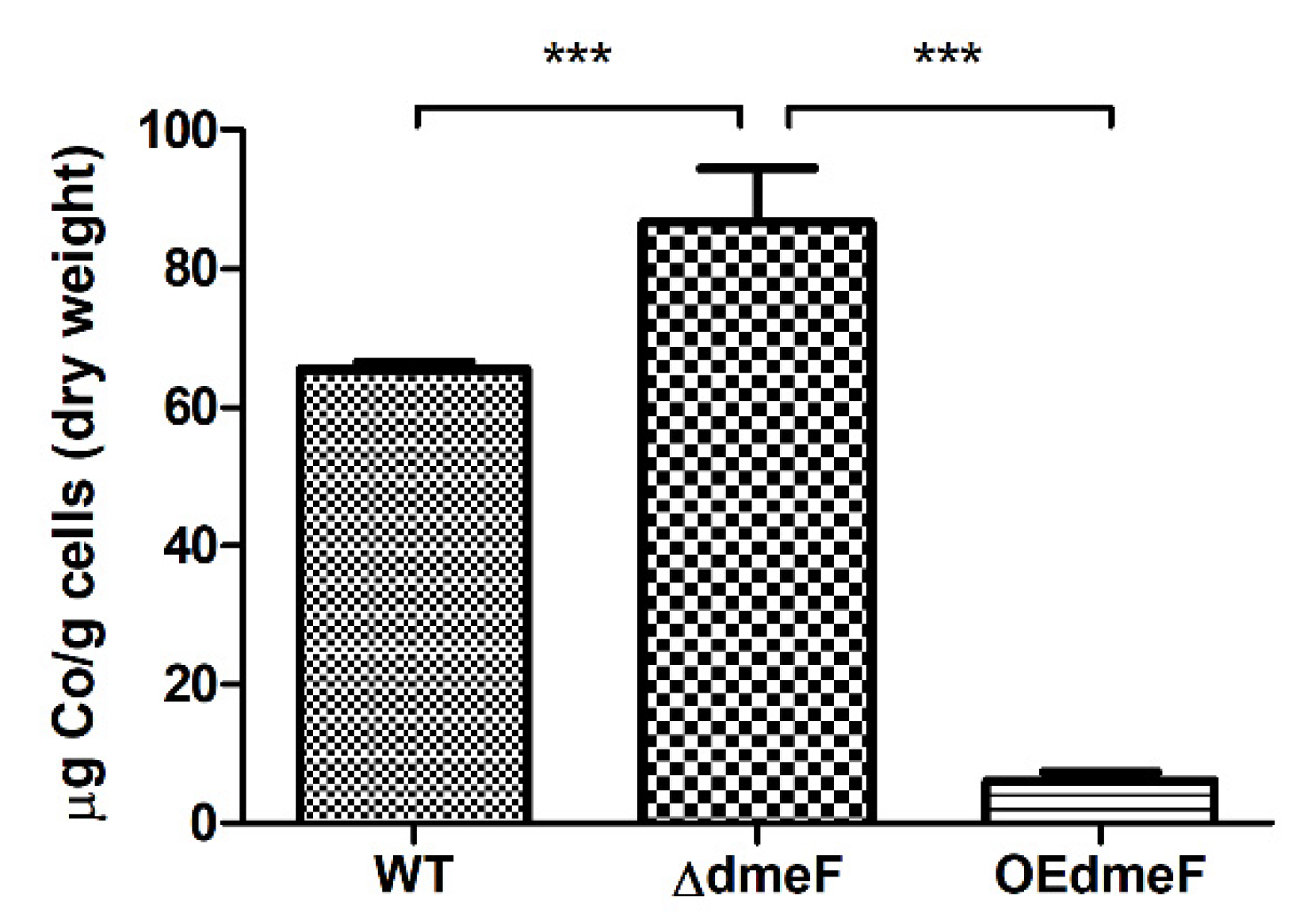
2.4. The ΔdmeF Mutant Accumulated Increased Levels of Cellular Cobalt Content
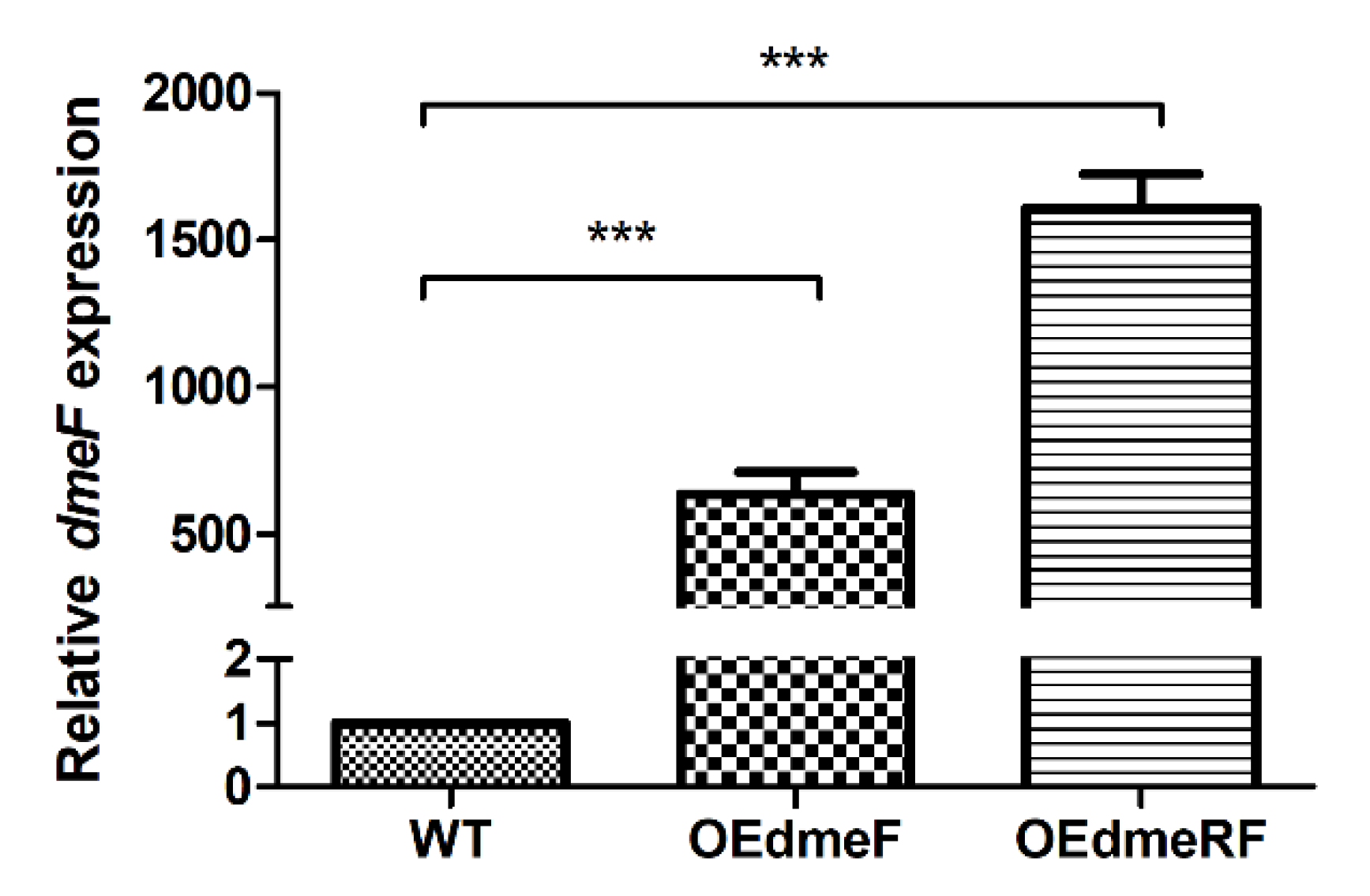
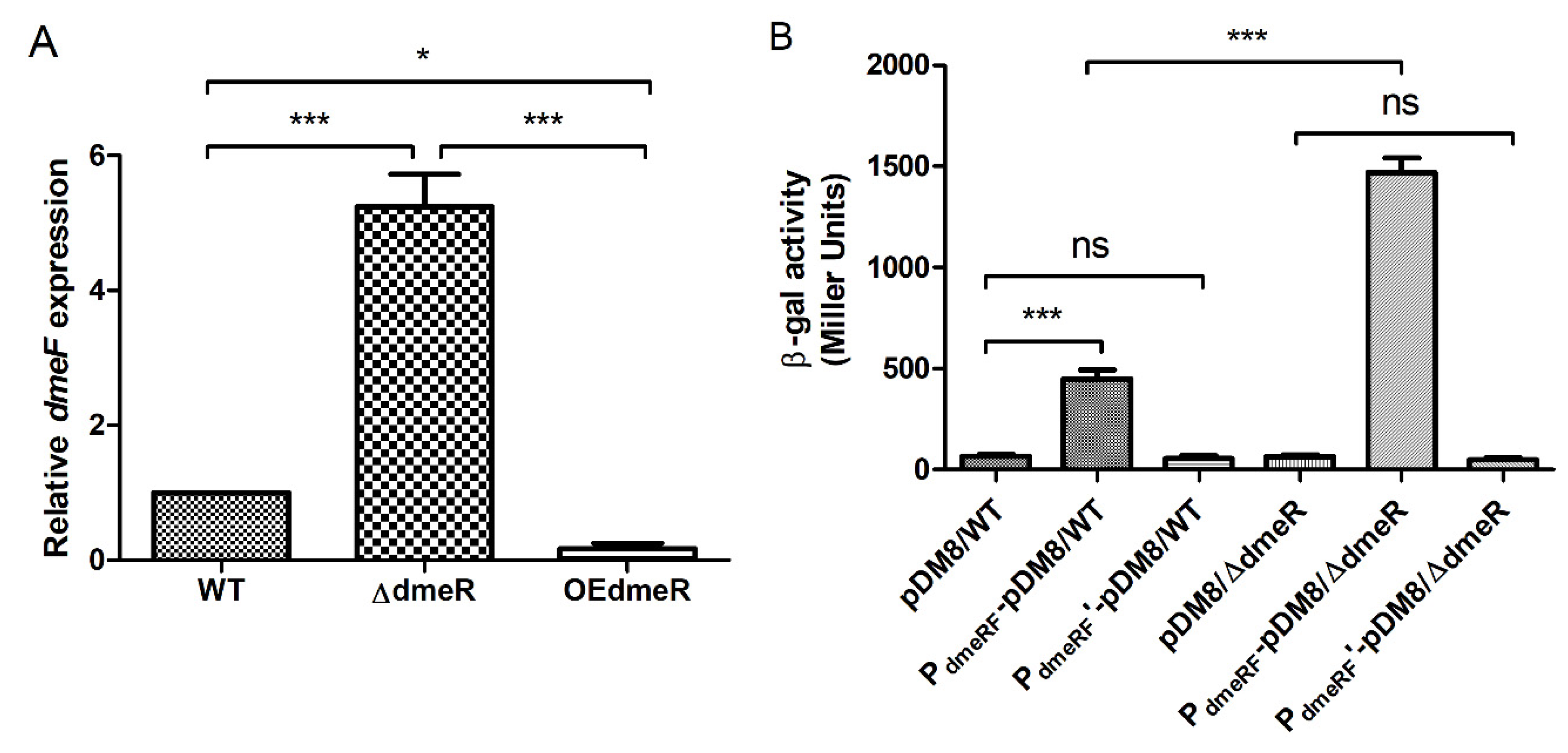
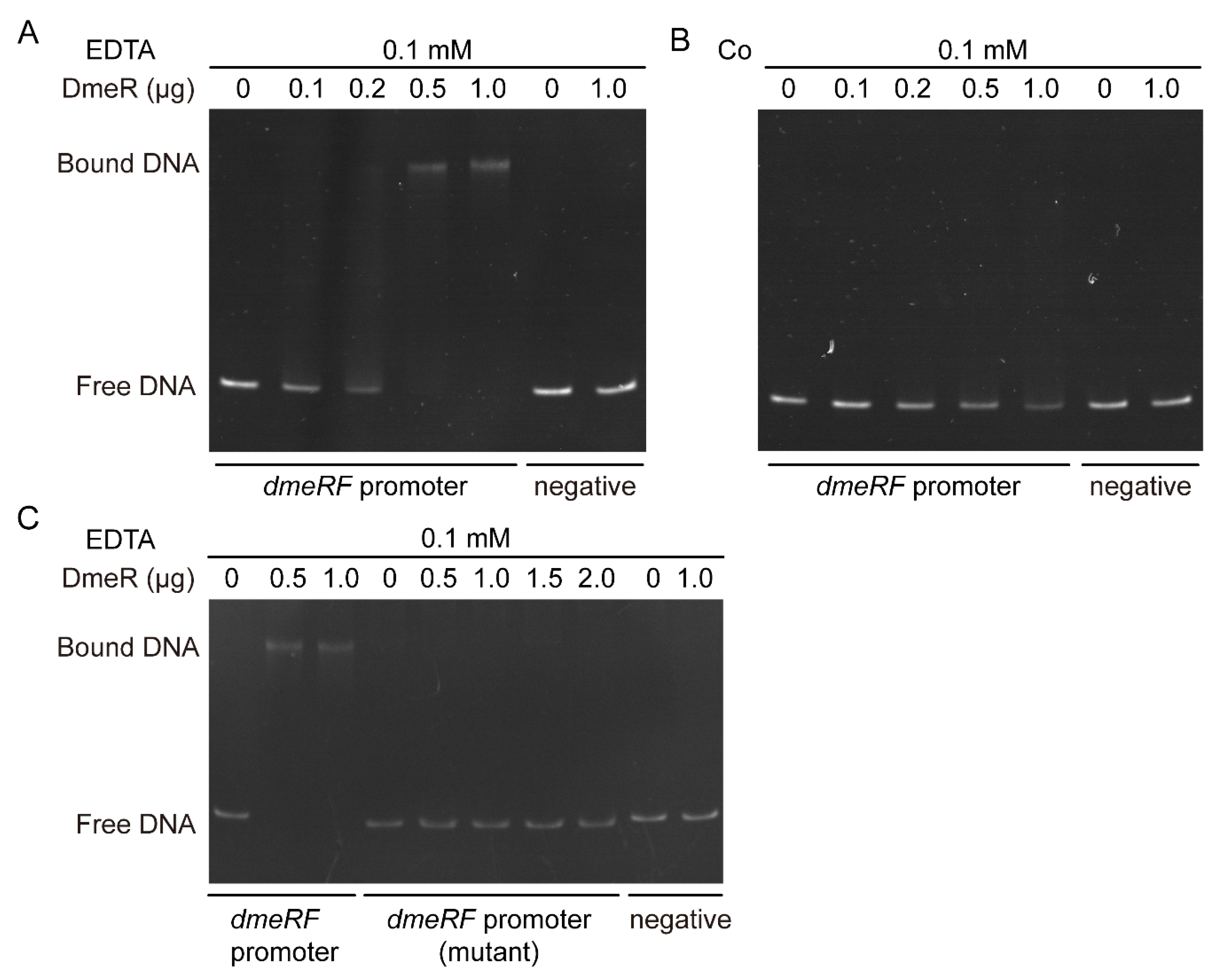
2.5. DmeR Negatively Regulates the dmeRF Operon by Binding Directly to the Promoter While Cobalt Inhibits the Interaction
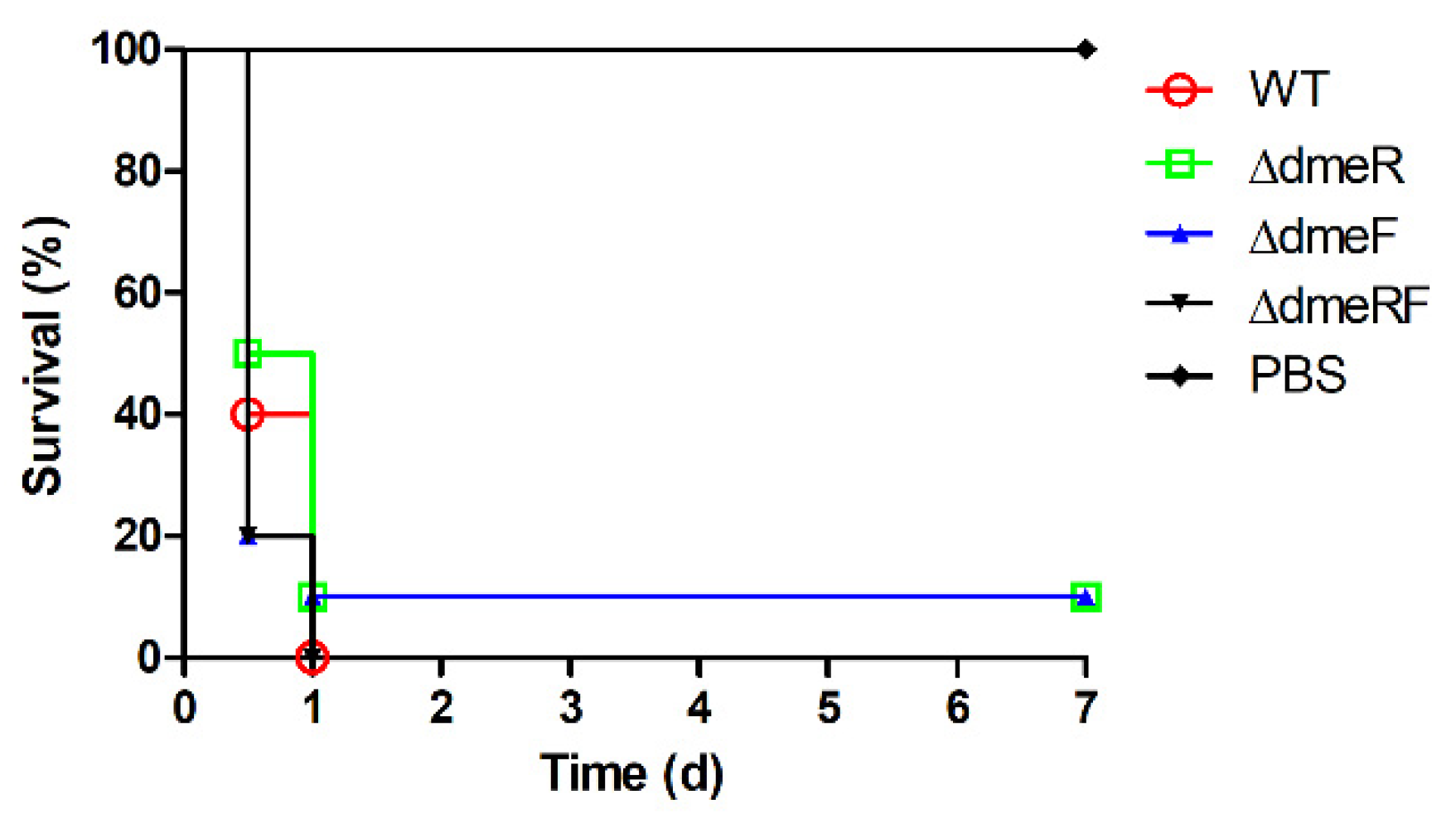
2.6. The DmeRF System Is Not Required for V. parahaemolyticus Virulence in Mice
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Culture Conditions, Plasmids, and Primers
4.2. RNA Extraction and qRT-PCR Analysis
4.3. Construction of Gene Deletion and Overexpression Strains
4.4. Growth Evaluation
4.5. Intracellular Metal Content Analysis
4.6. Construction of LacZ Fusion Strains and β-galactosidase Activity Assays
4.7. rDmeR Expression, Purification, and EMSAs
4.8. Mouse Infection Experiment
4.9. Bioinformatic and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foster, A.W.; Young, T.R.; Chivers, P.T.; Robinson, N.J. Protein metalation in biology. Curr. Opin. Chem. Biol. 2022, 66, 102095. [Google Scholar] [CrossRef] [PubMed]
- Skaar, E.P.; Raffatellu, M. Metals in infectious diseases and nutritional immunity. Metallomics 2015, 7, 926–928. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrangsu, P.; Rensing, C.; Helmann, J.D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 2017, 15, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Djoko, K.Y.; Ong, C.L.; Walker, M.J.; McEwan, A.G. The Role of Copper and Zinc Toxicity in Innate Immune Defense against Bacterial Pathogens. J. Biol. Chem. 2015, 290, 18954–18961. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, J.R.; Skaar, E.P. Metals as phagocyte antimicrobial effectors. Curr. Opin. Immunol. 2019, 60, 1–9. [Google Scholar] [CrossRef]
- Akbari, M.S.; Doran, K.S.; Burcham, L.R. Metal Homeostasis in Pathogenic Streptococci. Microorganisms 2022, 10, 1501. [Google Scholar] [CrossRef]
- Osman, D.; Cooke, A.; Young, T.R.; Deery, E.; Robinson, N.J.; Warren, M.J. The requirement for cobalt in vitamin B12: A paradigm for protein metalation. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118896. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, S. Cobalt proteins. Eur. J. Biochem. 1999, 261, 1–9. [Google Scholar] [CrossRef]
- Leonard, S.; Gannett, P.M.; Rojanasakul, Y.; Schwegler-Berry, D.; Castranova, V.; Vallyathan, V.; Shi, X. Cobalt-mediated generation of reactive oxygen species and its possible mechanism. J. Inorg. Biochem. 1998, 70, 239–244. [Google Scholar] [CrossRef]
- Majtan, T.; Frerman, F.E.; Kraus, J.P. Effect of cobalt on Escherichia coli metabolism and metalloporphyrin formation. Biometals 2011, 24, 335–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barras, F.; Fontecave, M. Cobalt stress in Escherichia coli and Salmonella enterica: Molecular bases for toxicity and resistance. Metallomics 2011, 3, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Thorgersen, M.P.; Downs, D.M. Cobalt targets multiple metabolic processes in Salmonella enterica. J. Bacteriol. 2007, 189, 7774–7781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokpikul, T.; Chaoprasid, P.; Saninjuk, K.; Sirirakphaisarn, S.; Johnrod, J.; Nookabkaew, S.; Sukchawalit, R.; Mongkolsuk, S. Regulation of the Cobalt/Nickel Efflux Operon dmeRF in Agrobacterium tumefaciens and a Link between the Iron-Sensing Regulator RirA and Cobalt/Nickel Resistance. Appl. Environ. Microbiol. 2016, 82, 4732–4742. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Jia, M.; Gao, M.; Lu, T.; Li, L.; Zhou, P. PmtA functions as a ferrous iron and cobalt efflux pump in Streptococcus suis. Emerg. Microbes. Infect. 2019, 8, 1254–1264. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, J.C.; Cavet, J.S.; Robinson, N.J. Cobalt-dependent transcriptional switching by a dual-effector MerR-like protein regulates a cobalt-exporting variant CPx-type ATPase. J. Biol. Chem. 1999, 274, 25827–25832. [Google Scholar] [CrossRef] [Green Version]
- Raimunda, D.; Long, J.E.; Sassetti, C.M.; Argüello, J.M. Role in metal homeostasis of CtpD, a Co2+ transporting P(1B4)-ATPase of Mycobacterium smegmatis. Mol. Microbiol. 2012, 84, 1139–1149. [Google Scholar] [CrossRef] [Green Version]
- Munkelt, D.; Grass, G.; Nies, D.H. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J. Bacteriol. 2004, 186, 8036–8043. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Song, X.; Wang, J.; Bai, X.; Gao, E.; Wei, G. Nickel and cobalt resistance properties of Sinorhizobium meliloti isolated from Medicago lupulina growing in gold mine tailing. PeerJ 2018, 6, e5202. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Sanz, L.; Prieto, R.I.; Imperial, J.; Palacios, J.M.; Brito, B. Functional and expression analysis of the metal-inducible dmeRF system from Rhizobium leguminosarum bv. viciae. Appl. Environ. Microbiol. 2013, 79, 6414–6422. [Google Scholar] [CrossRef]
- Rubio-Sanz, L.; Brito, B.; Palacios, J. Analysis of metal tolerance in Rhizobium leguminosarum strains isolated from an ultramafic soil. FEMS Microbiol. Lett. 2018, 365, fny010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letchumanan, V.; Chan, K.G.; Lee, L.H. Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Front. Microbiol. 2014, 5, 705. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, S.; Hiyoshi, H.; Tandhavanant, S.; Kodama, T. Advances on Vibrio parahaemolyticus research in the postgenomic era. Microbiol. Immunol. 2020, 64, 167–181. [Google Scholar] [CrossRef]
- Zhang, L.; Orth, K. Virulence determinants for Vibrio parahaemolyticus infection. Curr. Opin. Microbiol. 2013, 16, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Broberg, C.A.; Calder, T.J.; Orth, K. Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect. 2011, 13, 992–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.T.; Chen, I.T.; Yang, Y.T.; Ko, T.P.; Huang, Y.T.; Huang, J.Y.; Huang, M.F.; Lin, S.J.; Chen, C.Y.; Lin, S.S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef] [Green Version]
- Prachumwat, A.; Taengchaiyaphum, S.; Mungkongwongsiri, N.; Aldama-Cano, D.J.; Flegel, T.W.; Sritunyalucksana, K. Update on early mortality syndrome/acute hepatopancreatic necrosis disease by April 2018. J. World Aquacult. Soc. 2019, 50, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Makino, K.; Oshima, K.; Kurokawa, K.; Yokoyama, K.; Uda, T.; Tagomori, K.; Iijima, Y.; Najima, M.; Nakano, M.; Yamashita, A.; et al. Genome sequence of Vibrio parahaemolyticus: A pathogenic mechanism distinct from that of V cholerae. Lancet 2003, 361, 743–749. [Google Scholar] [CrossRef]
- Milton, D.L.; O’Toole, R.; Horstedt, P.; Wolf-Watz, H. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 1996, 178, 1310–1319. [Google Scholar] [CrossRef] [Green Version]
- Morales, V.M.; Bäckman, A.; Bagdasarian, M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 1991, 97, 39–47. [Google Scholar] [CrossRef]
- Croxatto, A.; Chalker, V.J.; Lauritz, J.; Jass, J.; Hardman, A.; Williams, P.; Cámara, M.; Milton, D.L. VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J. Bacteriol. 2002, 184, 1617–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Gu, D.; Lu, X.; Zhou, X.; Xia, X. Transcriptomic analysis of PhoR reveals its role in regulation of swarming motility and T3SS expression in Vibrio parahaemolyticus. Microbiol. Res. 2020, 235, 126448. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Pinochet-Barros, A.; Gaballa, A.; Patel, S.J.; Arguello, J.M.; Helmann, J.D. PfeT, a P1B4 -type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication. Mol. Microbiol. 2015, 98, 787–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.K.; Wei, M.; Qiu, J.; Jia, M.D.; Zhou, X.H.; Jiao, X.A. TroR Negatively Regulates the TroABCD System and Is Required for Resistance to Metal Toxicity and Virulence in Streptococcus suis. Appl. Environ. Microbiol. 2021, 87, e0137521. [Google Scholar] [CrossRef]
- Gu, D.; Zhang, Y.; Wang, Q.; Zhou, X. S-nitrosylation-mediated activation of a histidine kinase represses the type 3 secretion system and promotes virulence of an enteric pathogen. Nat. Commun. 2020, 11, 5777. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, H.; Nie, L.; He, M.; Peng, Q.; Zhu, W.; Nie, H.; Chen, W.; Huang, Q. Identification of c-di-GMP/FleQ-Regulated New Target Genes, Including cyaA, Encoding Adenylate Cyclase, in Pseudomonas putida. mSystems 2021, 6, e00295-21. [Google Scholar] [CrossRef]
- Zheng, C.; Qiu, J.; Zhao, X.; Yu, S.; Wang, H.; Wan, M.; Wei, M.; Jiao, X. The AdcR-regulated AdcA and AdcAII contribute additively to zinc acquisition and virulence in Streptococcus suis. Vet. Microbiol. 2022, 269, 109418. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic. Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]


| Strain or Plasmid | Relevant Characteristics 1 | Source or Reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α λpir | Cloning host for pDM4, pMMB207, and pDM8 | Laboratory collection |
| S17-1 λpir | Conjugal donor for pDM4, pMMB207, and pDM8 | Laboratory collection |
| DH5α | Cloning host for pET-30a | Laboratory collection |
| BL21(DE3) | Expression host for pET-30a | Laboratory collection |
| V. parahaemolyticus | ||
| RIMD 2,210,633 (WT) | Clinical isolate, CarbR | [28] |
| ΔdmeR | dmeR deletion mutant of RIMD 2210633 | This study |
| ΔdmeF | dmeF deletion mutant of RIMD 2210633 | This study |
| ΔdmeRF | dmeR and dmeF double mutant of RIMD 2210633 | This study |
| OEdmeR | dmeR overexpression strain in ΔdmeR background | This study |
| OEdmeF | dmeF overexpression strain in ΔdmeF background | This study |
| OEdmeRF | dmeRF overexpression strain in ΔdmeRF background | This study |
| pDM8/WT | RIMD 2,210,633 harboring pDM8 | This study |
| PdmeRF-pDM8/WT | RIMD 2,210,633 harboring PdmeRF-pDM8 | This study |
| PdmeRF’-pDM8/WT | RIMD 2,210,633 harboring PdmeRF’-pDM8 | This study |
| pDM8/ΔdmeR | ΔdmeR harboring pDM8 | This study |
| PdmeRF-pDM8/ΔdmeR | ΔdmeR harboring PdmeRF-pDM8 | This study |
| PdmeRF’-pDM8/ΔdmeR | ΔdmeR harboring PdmeRF’-pDM8 | This study |
| Plasmids | ||
| pDM4 | Suicide vector containing a sacB counterselectable marker; CmR | [29] |
| pDM4-ΔdmeR | Knockout vector for dmeR deletion | This study |
| pDM4-ΔdmeF | Knockout vector for dmeF deletion | This study |
| pDM4-ΔdmeRF | Knockout vector for dmeRF deletion | This study |
| pMMB207 | Wide-host-range low-copy-number vector; CmR | [30] |
| pMMB207-dmeR | pMMB207 containing dmeR and an additional ribosome-binding site | This study |
| pMMB207-dmeF | pMMB207 containing dmeF and an additional ribosome-binding site | This study |
| pMMB207-dmeRF | pMMB207 containing dmeRF and an additional ribosome-binding site | This study |
| pDM8 | Plasmid containing the promoterless lacZ gene; CmR | [31] |
| PdmeRF-pDM8 | pDM8 containing the promoter of dmeRF | This study |
| PdmeRF’-pDM8 | pDM8 containing the mutant promoter of dmeRF | This study |
| pET-30a | Expression vector; KanR | Novagen |
| pET30a-dmeR | pET-30a containing dmeR | This study |
| Primer | Sequence (5′-3′) 1 | Size (bp) | Target Gene |
|---|---|---|---|
| QdmeF-F | CACCAAAGCACCAACGATCC | 151 | An internal region of dmeF |
| QdmeF-R | CGAGCACCAGGACCACAATC | ||
| QgyrB-F | GGTGGTATTCAAGCGTTCGTTC | 116 | An internal region of gyrB |
| QgyrB-R | TGCATTGCCACTTCTACCGAG | ||
| dmeR-LA-F | TCCCCCGGGCCACCACAAACGCTCTCTG | 738 | The left arm of dmeR |
| dmeR-LA-R | GATGAGCGAACCGGGAATTCTAGGTTTCA | ||
| dmeR-RA-F | ATTCCCGGTTCGCTCATCGTCAGCTATTT | 732 | The right arm of dmeR |
| dmeR-RA-R | CCGCTCGAGTGTTCGCTTATCGCTATGCT | ||
| dmeR-in-F | AAAGGTTGATTGCTGCTCTG | 325 | An internal region of dmeR |
| dmeR-in-R | TTCTTGGGAACAGTCAGTCG | ||
| dmeR-out-F | GCATTTTGTGTTGGTGTGACT | 338/733 | A fragment containing dmeR |
| dmeR-out-R | CGATTGAGCCATACGCAG | ||
| OEdmeR-F | TCCCCCGGGTAAGGAGGTAGGATAATAATGAGCGAACTAGAAAAGTTGAA | 417 | dmeR and an additional ribosome-binding site |
| OEdmeR-R | AACTGCAGCTAGAATTCCCGGTTCATTTT | ||
| dmeF-LA-F | TCCCCCGGGATTATGCTTGCCACCGCT | 714 | The left arm of dmeF |
| dmeF-LA-R | CACGCACGATAAATAGCTGACGATGAGCGA | ||
| dmeF-RA-F | TCAGCTATTTATCGTGCGTGTGTTGTGC | 723 | The right arm of dmeF |
| dmeF-RA-R | CCGCTCGAGAGCACGCCATTACGATAGAG | ||
| OEdmeF-F | TCCCCCGGGTAAGGAGGTAGGATAATAATGGCAATAGCACAACACAC | 929 | dmeF and an additional ribosome-binding site |
| OEdmeF-R | AACTGCAGTAGTTCGCTCATCGTCAGC | ||
| dmeF-in-F | CATCTAACCAAATCCATCCG | 291 | An internal region of dmeF |
| dmeF-in-R | AATCCGTTCACCGTTTGTT | ||
| dmeF-out-F | CAGCACTTCAATGGTATGGAC | 279/1157 | A fragment containing dmeF |
| dmeF-out-R | AGTGATGAACGCCTTTCTTAGT | ||
| dmeR-LA-R2 | ACGCACGATACCGGGAATTCTAGGTTTCA | ||
| dmeF-RA-F2 | ATTCCCGGTATCGTGCGTGTGTTGTGC | ||
| OEdmeRF-F | TCCCCCGGGTAAGGAGGTAGGATAATAATGGCAATAGCACAACACAC | 1344 | dmeRF and an additional ribosome-binding site |
| OEdmeRF-R | AACTGCAGCGGATGAAACCTAGAATTCC | ||
| PdmeRF-F | CGGATCCGGGGAATTCCCGGGTAAGCGGCTGATTCCCAAAC | 208 | The promoter of dmeRF, for β-galactosidase activity assays |
| PdmeRF-R | AAGCTTATCGATTCGCCCGGGGCGTGTGTTGTGCTATTGCC | ||
| dmeR-F | CGCGGATCCATGAGCGAACTAGAAAAGTTGAA | 417 | The dmeR gene |
| dmeR-R | CCGCTCGAGCTAG AATTCCCGGTTCATTTT | ||
| PdmeRF-F2 | TAAGCGGCTGATTCCCAAAC | 208 | The promoter of dmeRF, for EMSAs |
| PdmeRF-R2 | GCGTGTGTTGTGCTATTGCC | ||
| PgyrB-F | CAAGGGCAACATCTTACAGC | 215 | The promoter of gyrB |
| PgyrB-R | TCTATCCTGCCATGTTCCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Kong, M.; Yang, J.; Zhao, X.; Shi, Y.; Zhai, Y.; Qiu, J.; Zheng, C. The DmeRF System Is Involved in Maintaining Cobalt Homeostasis in Vibrio parahaemolyticus. Int. J. Mol. Sci. 2023, 24, 414. https://doi.org/10.3390/ijms24010414
Zhao Y, Kong M, Yang J, Zhao X, Shi Y, Zhai Y, Qiu J, Zheng C. The DmeRF System Is Involved in Maintaining Cobalt Homeostasis in Vibrio parahaemolyticus. International Journal of Molecular Sciences. 2023; 24(1):414. https://doi.org/10.3390/ijms24010414
Chicago/Turabian StyleZhao, Yuxuan, Mengyao Kong, Jiaxue Yang, Xiaoxian Zhao, Yiran Shi, Yimeng Zhai, Jun Qiu, and Chengkun Zheng. 2023. "The DmeRF System Is Involved in Maintaining Cobalt Homeostasis in Vibrio parahaemolyticus" International Journal of Molecular Sciences 24, no. 1: 414. https://doi.org/10.3390/ijms24010414
APA StyleZhao, Y., Kong, M., Yang, J., Zhao, X., Shi, Y., Zhai, Y., Qiu, J., & Zheng, C. (2023). The DmeRF System Is Involved in Maintaining Cobalt Homeostasis in Vibrio parahaemolyticus. International Journal of Molecular Sciences, 24(1), 414. https://doi.org/10.3390/ijms24010414






