Metformin Attenuates Slow-to-Fast Fiber Shift and Proteolysis Markers Increase in Rat Soleus after 7 Days of Rat Hindlimb Unloading
Abstract
:1. Introduction
2. Results
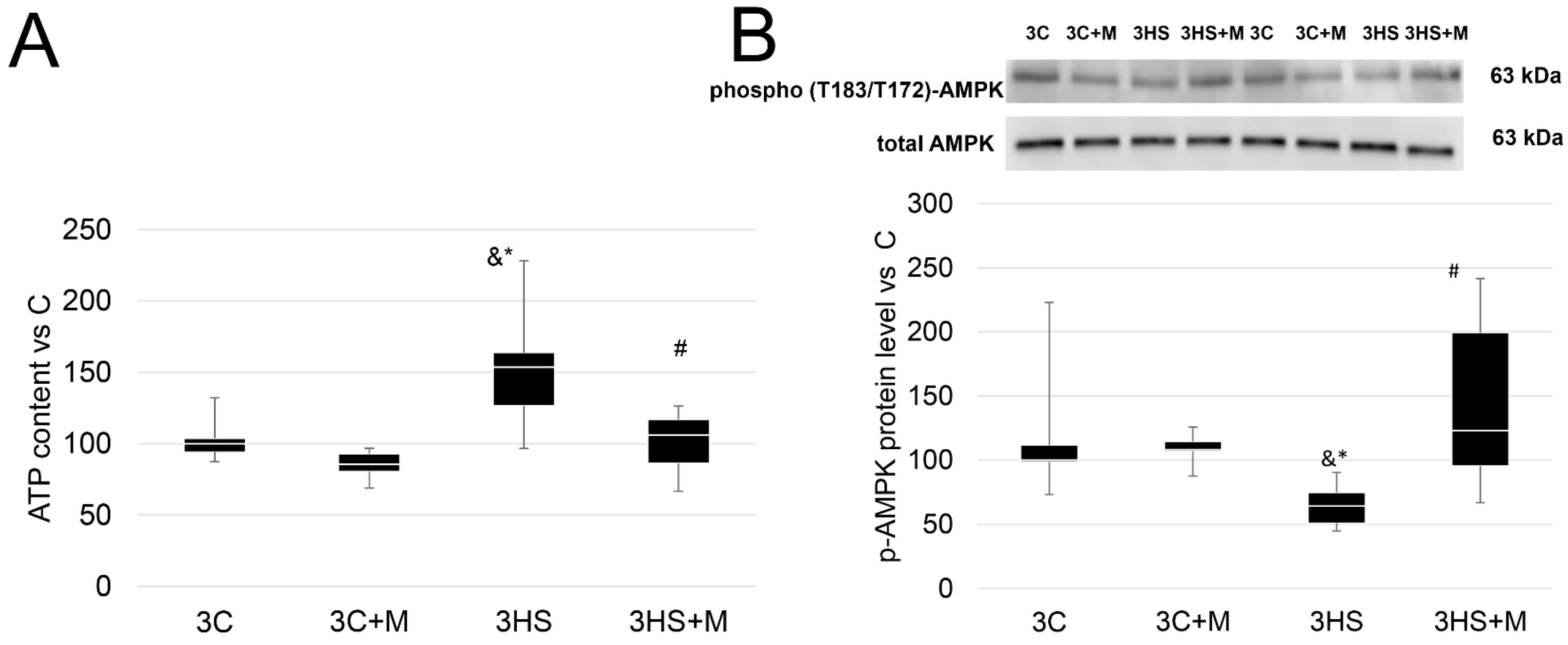
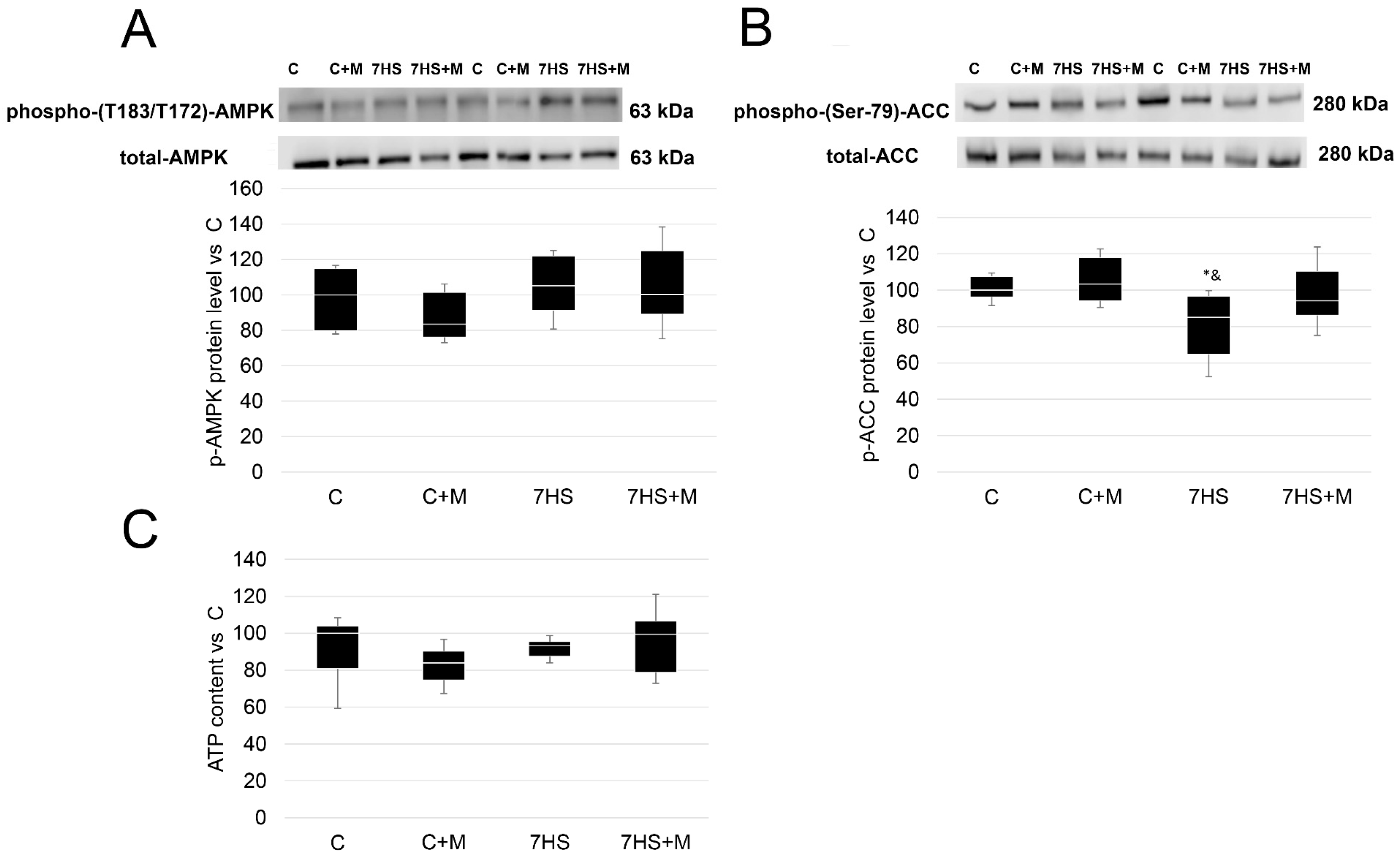
2.1. ATP Content and p-ACC/p-AMPK Protein Levels after 3 Days and 7 Days of Rat Hindlimb Unloading
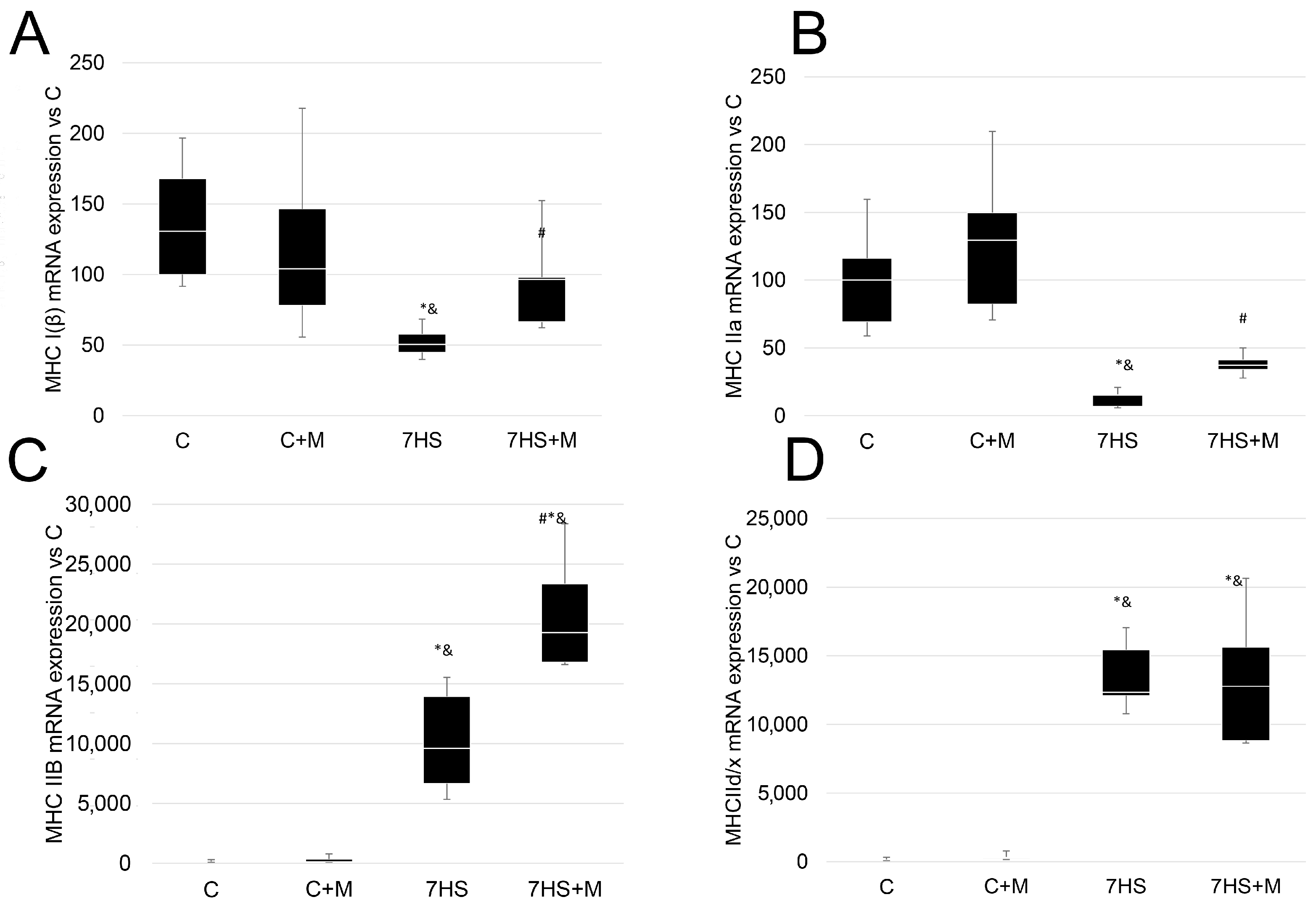
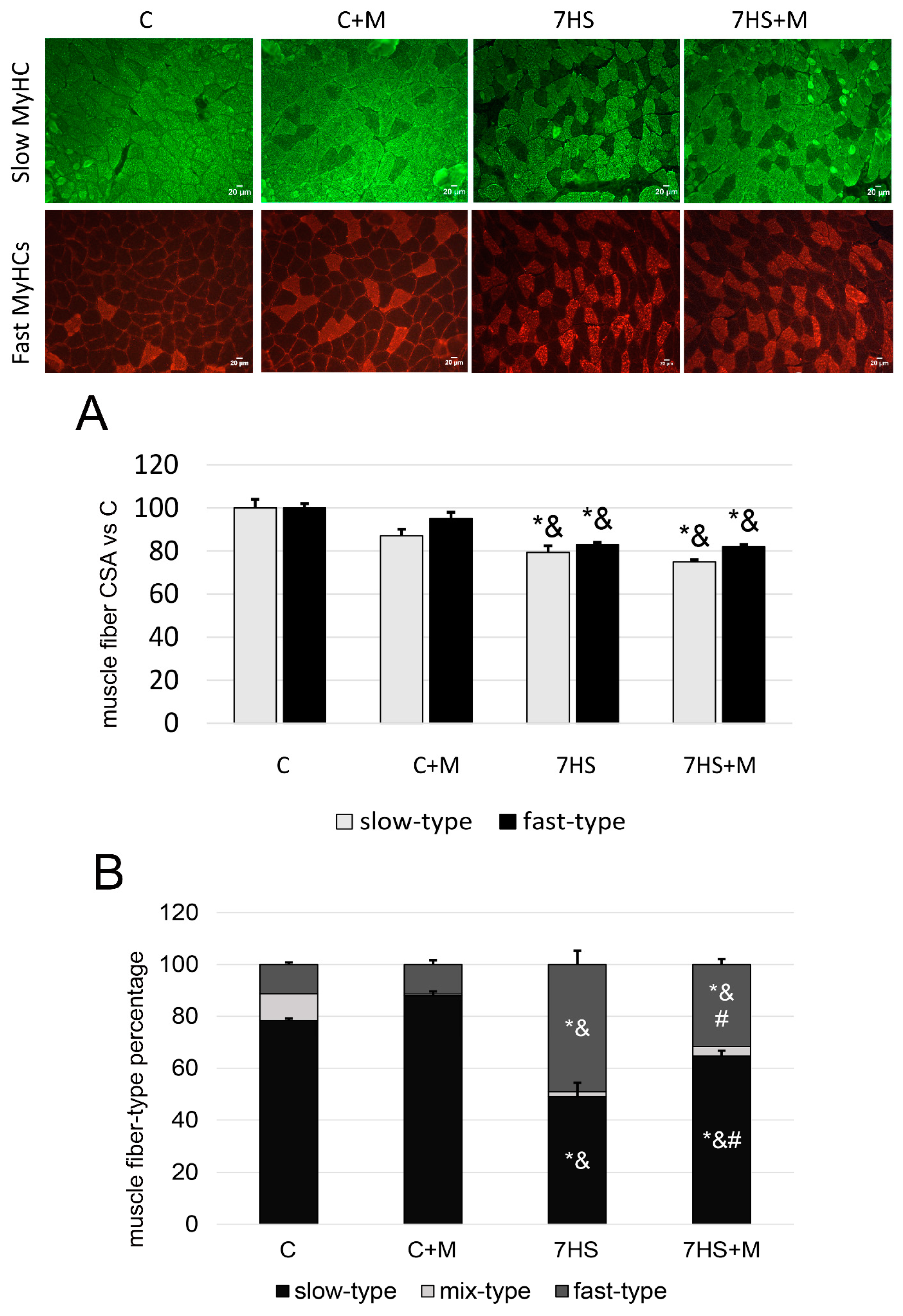
2.2. Influence of Metformin Administration on MyHC Expression and Fiber-Type Ratio
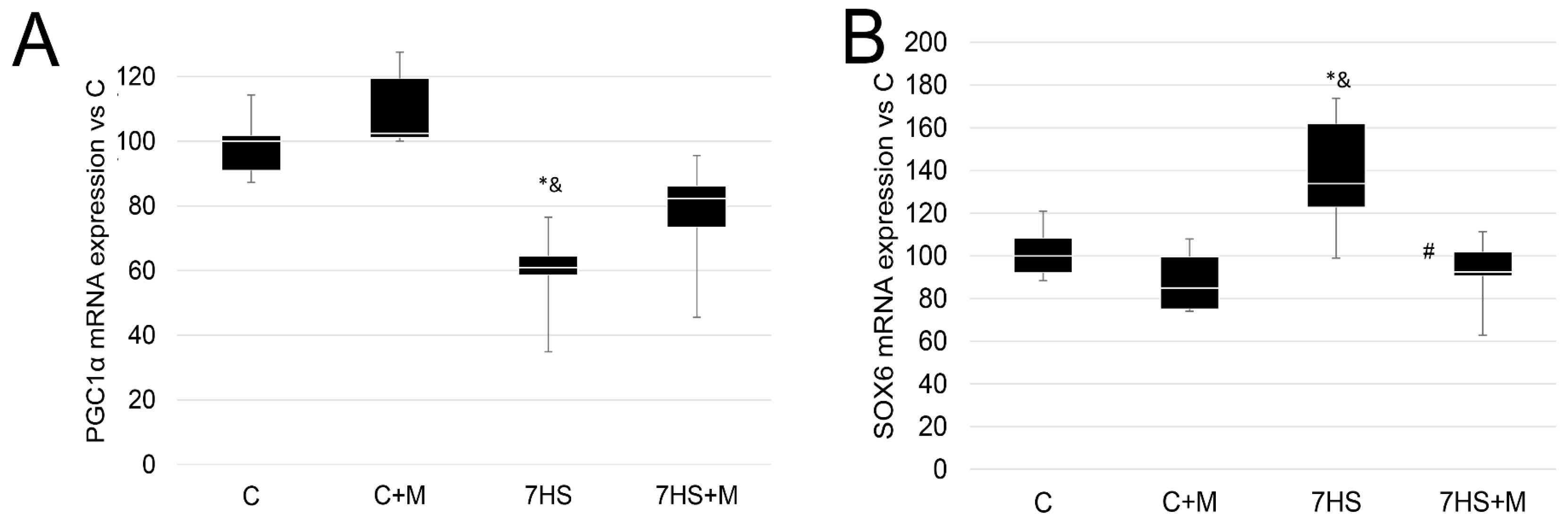
2.3. Effect of Metformin Administration on MEF-2D and HAT p300 Nuclear Protein Level S
2.4. Influence of Metformin Administration on Calcineurin/NFAT Pathway upon Hindlimb Suspension
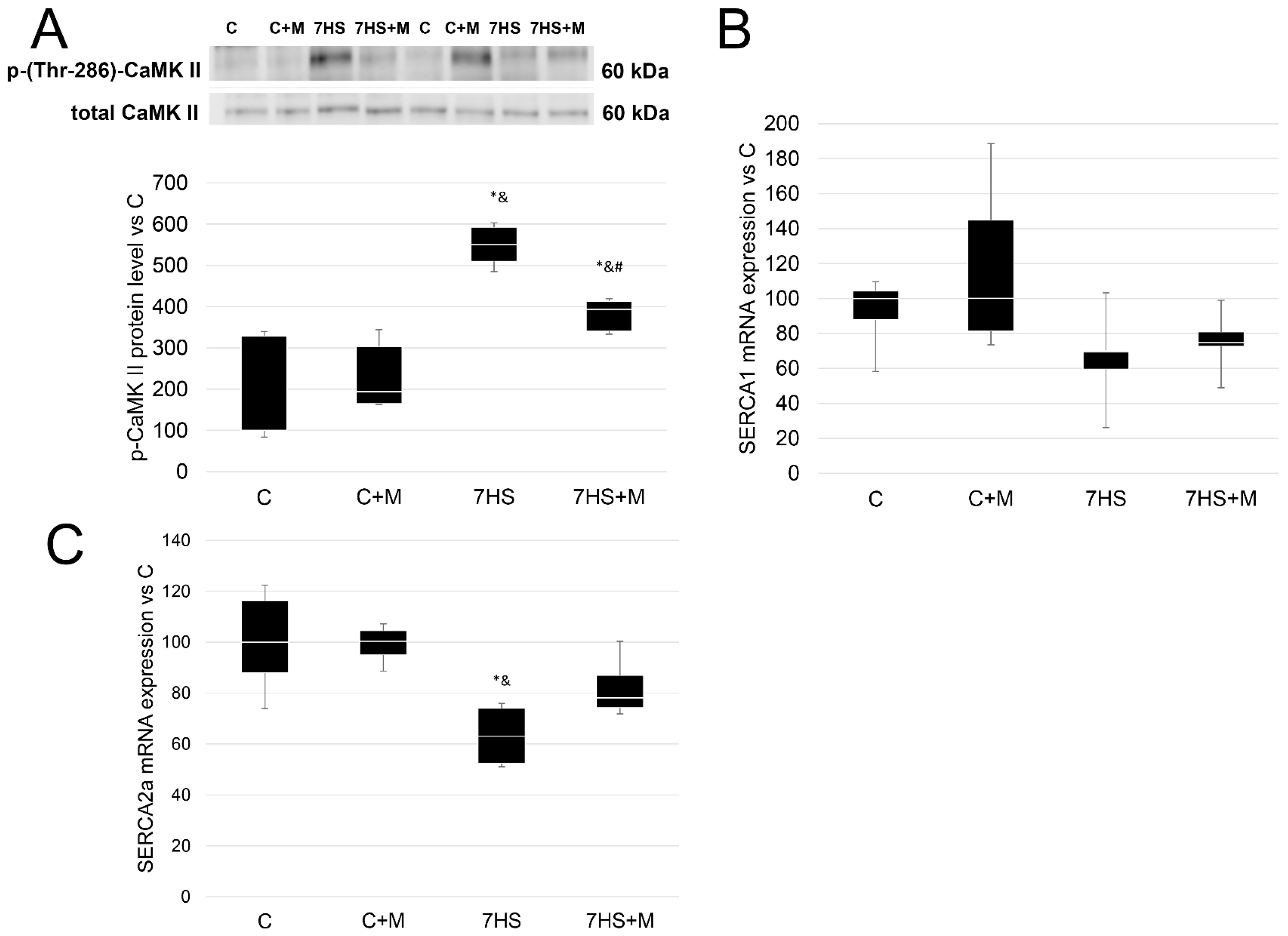
2.5. Metformin Affects Calcium-Dependent Signaling Markers
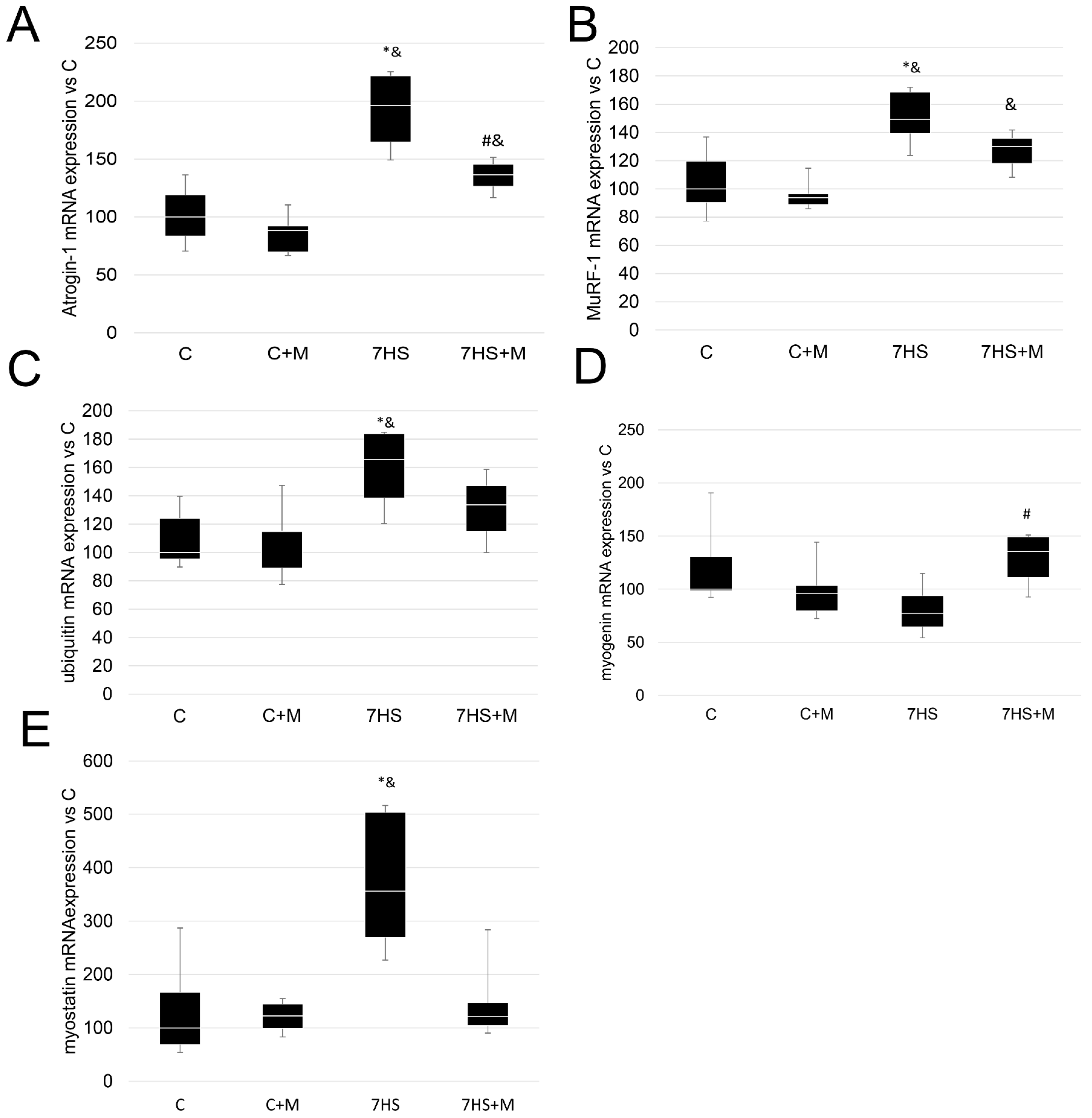
2.6. Influence of Metformin Administration on Ubiquitin–Proteasome Signaling Markers
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. ATP Content Evaluation
4.3. Immunohistochemistry
4.4. Protein Extraction
4.5. Western Blot Analysis
4.6. RNA Isolation and Reverse Transcription
4.7. Quantitative PCR Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharlo, K.; Tyganov, S.A.; Tomilovskaya, E.; Popov, D.V.; Saveko, A.A.; Shenkman, B.S. Effects of Various Muscle Disuse States and Countermeasures on Muscle Molecular Signaling. Int. J. Mol. Sci. 2021, 23, 468. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.S. How Postural Muscle Senses Disuse? Early Signs and Signals. Int. J. Mol. Sci. 2020, 21, 5037. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.S.; Kozlovskaya, I.B. Cellular Responses of Human Postural Muscle to Dry Immersion. Front. Physiol. 2019, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, T.L.; Gongol, B.; Zhang, F.; Martin, M.; Johnson, D.A.; Xiao, H.; Wang, Y.; Subramaniam, S.; Chien, S.; Shyy, J.Y. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci. Signal. 2017, 10, eaaf7478. [Google Scholar] [CrossRef] [Green Version]
- Mirzoev, T.; Tyganov, S.; Vilchinskaya, N.; Lomonosova, Y.; Shenkman, B. Key Markers of mTORC1-Dependent and mTORC1-Independent Signaling Pathways Regulating Protein Synthesis in Rat Soleus Muscle During Early Stages of Hindlimb Unloading. Cell. Physiol. Biochem. 2016, 39, 1011–1020. [Google Scholar] [CrossRef]
- Vilchinskaya, N.A.; Krivoi, I.I.; Shenkman, B.S. AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling. Int. J. Mol. Sci. 2018, 19, 3558. [Google Scholar] [CrossRef] [Green Version]
- Vilchinskaya, N.A.; Mochalova, E.P.; Nemirovskaya, T.L.; Mirzoev, T.M.; Turtikova, O.V.; Shenkman, B.S. Rapid decline in MyHC I(beta) mRNA expression in rat soleus during hindlimb unloading is associated with AMPK dephosphorylation. J. Physiol. 2017, 595, 7123–7134. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.C.; Misulis, K.E.; Dettbarn, W.D. Activity dependent characteristics of fast and slow muscle: Biochemical and histochemical considerations. Neurochem. Res. 1989, 14, 647–655. [Google Scholar] [CrossRef]
- Sharlo, K.A.; Paramonova, I.I.; Lvova, I.D.; Mochalova, E.P.; Kalashnikov, V.E.; Vilchinskaya, N.A.; Tyganov, S.A.; Konstantinova, T.S.; Shevchenko, T.F.; Kalamkarov, G.R.; et al. Plantar Mechanical Stimulation Maintains Slow Myosin Expression in Disused Rat Soleus Muscle via NO-Dependent Signaling. Int. J. Mol. Sci. 2021, 22, 1372. [Google Scholar] [CrossRef]
- Desaphy, J.F.; Pierno, S.; Liantonio, A.; De Luca, A.; Didonna, M.P.; Frigeri, A.; Nicchia, G.P.; Svelto, M.; Camerino, C.; Zallone, A.; et al. Recovery of the soleus muscle after short- and long-term disuse induced by hindlimb unloading: Effects on the electrical properties and myosin heavy chain profile. Neurobiol. Dis. 2005, 18, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Lomonosova, Y.N.; Turtikova, O.V.; Shenkman, B.S. Reduced expression of MyHC slow isoform in rat soleus during unloading is accompanied by alterations of endogenous inhibitors of calcineurin/NFAT signaling pathway. J. Muscle. Res. Cell. Motil. 2016, 37, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Sharlo, K.; Paramonova, I.; Turtikova, O.; Tyganov, S.; Shenkman, B. Plantar mechanical stimulation prevents calcineurin-NFATc1 inactivation and slow-to-fast fiber type shift in rat soleus muscle under hindlimb unloading. J. Appl. Physiol. 2019, 126, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Ingalls, C.P.; Warren, G.L.; Armstrong, R.B. Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J. Appl. Physiol. 1999, 87, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Shenkman, B.S.; Nemirovskaya, T.L. Calcium-dependent signaling mechanisms and soleus fiber remodeling under gravitational unloading. J. Muscle. Res. Cell. Motil. 2008, 29, 221–230. [Google Scholar] [CrossRef]
- Zaripova, K.A.; Kalashnikova, E.P.; Belova, S.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Role of Pannexin 1 ATP-Permeable Channels in the Regulation of Signaling Pathways during Skeletal Muscle Unloading. Int. J. Mol. Sci. 2021, 22, 10444. [Google Scholar] [CrossRef]
- Kravtsova, V.V.; Vilchinskaya, N.A.; Rozlomii, V.L.; Shenkman, B.S.; Krivoi, I.I. Low Ouabain Doses and AMP-Activated Protein Kinase as Factors Supporting Electrogenesis in Skeletal Muscle. Biochem. (Mosc) 2019, 84, 1085–1092. [Google Scholar] [CrossRef]
- Krivoi, I.I.; Kravtsova, V.V.; Altaeva, E.G.; Kubasov, I.V.; Prokof’ev, A.V.; Drabkina, T.M.; Nikol’skii, E.E.; Shenkman, B.S. Decrease in the electrogenic contribution of Na,K-ATPase and resting membrane potential as a possible mechanism of calcium ion accumulation in filaments of the rat musculus soleus subjected to the short-term gravity unloading. Biofizika 2008, 53, 1051–1057. [Google Scholar]
- Andersson, D.C.; Betzenhauser, M.J.; Reiken, S.; Meli, A.C.; Umanskaya, A.; Xie, W.; Shiomi, T.; Zalk, R.; Lacampagne, A.; Marks, A.R. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell. Metab. 2011, 14, 196–207. [Google Scholar] [CrossRef] [Green Version]
- Andersson, D.C.; Meli, A.C.; Reiken, S.; Betzenhauser, M.J.; Umanskaya, A.; Shiomi, T.; D’Armiento, J.; Marks, A.R. Leaky ryanodine receptors in beta-sarcoglycan deficient mice: A potential common defect in muscular dystrophy. Skelet Muscle 2012, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Nemirovskaya, T.L.; Sharlo, K.A. Roles of ATP and SERCA in the Regulation of Calcium Turnover in Unloaded Skeletal Muscles: Current View and Future Directions. Int. J. Mol. Sci. 2022, 23, 6937. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Belova, S.P.; Lomonosova, Y.N.; Shenkman, B.S.; Nemirovskaya, T.L. The blockade of dihydropyridine channels prevents an increase in mu-calpain level under m. soleus unloading. Dokl. Biochem. Biophys. 2015, 460, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.S.; Belova, S.P.; Lomonosova, Y.N.; Kostrominova, T.Y.; Nemirovskaya, T.L. Calpain-dependent regulation of the skeletal muscle atrophy following unloading. Arch. Biochem. Biophys. 2015, 584, 36–41. [Google Scholar] [CrossRef]
- Raney, M.A.; Turcotte, L.P. Evidence for the involvement of CaMKII and AMPK in Ca2+-dependent signaling pathways regulating FA uptake and oxidation in contracting rodent muscle. J. Appl. Physiol. 2008, 104, 1366–1373. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, A.; Hatano, N.; Fujiwara, Y.; Sha’ri, A.; Takabatake, S.; Akano, H.; Kanayama, N.; Magari, M.; Nozaki, N.; Tokumitsu, H. AMP-activated protein kinase-mediated feedback phosphorylation controls the Ca(2+)/calmodulin (CaM) dependence of Ca(2+)/CaM-dependent protein kinase kinase beta. J. Biol. Chem. 2017, 292, 19804–19813. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Randall, W.R.; Schneider, M.F. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J. Cell. Biol. 2005, 168, 887–897. [Google Scholar] [CrossRef] [Green Version]
- MacDonnell, S.M.; Weisser-Thomas, J.; Kubo, H.; Hanscome, M.; Liu, Q.; Jaleel, N.; Berretta, R.; Chen, X.; Brown, J.H.; Sabri, A.K.; et al. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ. Res. 2009, 105, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Shen, T.; Liu, Y.; Randall, W.R.; Schneider, M.F. Parallel mechanisms for resting nucleo-cytoplasmic shuttling and activity dependent translocation provide dual control of transcriptional regulators HDAC and NFAT in skeletal muscle fiber type plasticity. J. Muscle. Res. Cell. Motil. 2006, 27, 405–411. [Google Scholar] [CrossRef]
- Meissner, J.D.; Umeda, P.K.; Chang, K.C.; Gros, G.; Scheibe, R.J. Activation of the beta myosin heavy chain promoter by MEF-2D, MyoD, p300, and the calcineurin/NFATc1 pathway. J. Cell. Physiol. 2007, 211, 138–148. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. Arginine promotes skeletal muscle fiber type transformation from fast-twitch to slow-twitch via Sirt1/AMPK pathway. J. Nutr. Biochem. 2018, 61, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.F.; Sparling, D.P.; Olson, A.L.; Winder, W.W.; Dohm, G.L. Regulation of muscle GLUT4 enhancer factor and myocyte enhancer factor 2 by AMP-activated protein kinase. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E1071–E1076. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Cheng, X.; Cui, Y.; Xia, Q.; Yan, X.; Zhang, M.; Lan, G.; Liu, J.; Shan, T.; Huang, Y. Resveratrol regulates skeletal muscle fibers switching through the AdipoR1-AMPK-PGC-1alpha pathway. Food Funct. 2019, 10, 3334–3343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drenning, J.A.; Lira, V.A.; Simmons, C.G.; Soltow, Q.A.; Sellman, J.E.; Criswell, D.S. Nitric oxide facilitates NFAT-dependent transcription in mouse myotubes. Am. J. Physiol. Cell. Physiol. 2008, 294, C1088–C1095. [Google Scholar] [CrossRef] [Green Version]
- Braz, J.C.; Bueno, O.F.; Liang, Q.; Wilkins, B.J.; Dai, Y.S.; Parsons, S.; Braunwart, J.; Glascock, B.J.; Klevitsky, R.; Kimball, T.F.; et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Investig. 2003, 111, 1475–1486. [Google Scholar] [CrossRef] [Green Version]
- Sharlo, K.A.; Mochalova, E.P.; Belova, S.P.; Lvova, I.D.; Nemirovskaya, T.L.; Shenkman, B.S. The role of MAP-kinase p38 in the m. soleus slow myosin mRNA transcription regulation during short-term functional unloading. Arch. Biochem. Biophys. 2020, 695, 108622. [Google Scholar] [CrossRef] [PubMed]
- Tavi, P.; Westerblad, H. The role of in vivo Ca(2)(+) signals acting on Ca(2)(+)-calmodulin-dependent proteins for skeletal muscle plasticity. J. Physiol. 2011, 589, 5021–5031. [Google Scholar] [CrossRef]
- Wright, D.C.; Geiger, P.C.; Han, D.H.; Jones, T.E.; Holloszy, J.O. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J. Biol. Chem. 2007, 282, 18793–18799. [Google Scholar] [CrossRef] [Green Version]
- Cohen, T.J.; Choi, M.C.; Kapur, M.; Lira, V.A.; Yan, Z.; Yao, T.P. HDAC4 regulates muscle fiber type-specific gene expression programs. Mol. Cells 2015, 38, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Rockl, K.S.; Hirshman, M.F.; Brandauer, J.; Fujii, N.; Witters, L.A.; Goodyear, L.J. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 2007, 56, 2062–2069. [Google Scholar] [CrossRef] [Green Version]
- Yamakuchi, M.; Higuchi, I.; Masuda, S.; Ohira, Y.; Kubo, T.; Kato, Y.; Maruyama, I.; Kitajima, I. Type I muscle atrophy caused by microgravity-induced decrease of myocyte enhancer factor 2C (MEF2C) protein expression. FEBS Lett. 2000, 477, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Rothermel, B.; Vega, R.B.; Yang, J.; Wu, H.; Bassel-Duby, R.; Williams, R.S. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J. Biol. Chem. 2000, 275, 8719–8725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Rothermel, B.; Vega, R.B.; Frey, N.; McKinsey, T.A.; Olson, E.N.; Bassel-Duby, R.; Williams, R.S. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ. Res. 2000, 87, E61–E68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, J.; Bissa, B.; Brecht, L.; Allers, L.; Choi, S.W.; Gu, Y.; Zbinden, M.; Burge, M.R.; Timmins, G.; Hallows, K.; et al. AMPK, a Regulator of Metabolism and Autophagy, Is Activated by Lysosomal Damage via a Novel Galectin-Directed Ubiquitin Signal Transduction System. Mol. Cell. 2020, 77, 951–969. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belova, S.P.; Zaripova, K.; Sharlo, K.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Metformin attenuates an increase of calcium-dependent and ubiquitin-proteasome markers in unloaded muscle. J. Appl. Physiol. 2022, 133, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Sharlo, K.A.; Paramonova, I.I.; Lvova, I.D.; Vilchinskaya, N.A.; Bugrova, A.E.; Shevchenko, T.F.; Kalamkarov, G.R.; Shenkman, B.S. NO-Dependent Mechanisms of Myosin Heavy Chain Transcription Regulation in Rat Soleus Muscle After 7-Days Hindlimb Unloading. Front. Physiol. 2020, 11, 814. [Google Scholar] [CrossRef]
- Stevens, L.; Sultan, K.R.; Peuker, H.; Gohlsch, B.; Mounier, Y.; Pette, D. Time-dependent changes in myosin heavy chain mRNA and protein isoforms in unloaded soleus muscle of rat. Am. J. Physiol. 1999, 277, C1044–C1049. [Google Scholar] [CrossRef]
- Baldwin, K.M.; Haddad, F.; Pandorf, C.E.; Roy, R.R.; Edgerton, V.R. Alterations in muscle mass and contractile phenotype in response to unloading models: Role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013, 4, 284. [Google Scholar] [CrossRef] [Green Version]
- Lomonosova, Y.N.; Kalamkarov, G.R.; Bugrova, A.E.; Shevchenko, T.F.; Kartashkina, N.L.; Lysenko, E.A.; Shvets, V.I.; Nemirovskaya, T.L. Protective effect of L-Arginine administration on proteins of unloaded m. soleus. Biochemistry (Mosc) 2011, 76, 571–580. [Google Scholar] [CrossRef]
- Giger, J.M.; Bodell, P.W.; Zeng, M.; Baldwin, K.M.; Haddad, F. Rapid muscle atrophy response to unloading: Pretranslational processes involving MHC and actin. J. Appl. Physiol. 2009, 107, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Shalaby, S.M.; El-Gendy, J.; Abdelghany, E.M.A. Beneficial effects of metformin on muscle atrophy induced by obesity in rats. J. Cell. BioChem. 2019, 120, 5677–5686. [Google Scholar] [CrossRef] [PubMed]
- Suwa, M.; Egashira, T.; Nakano, H.; Sasaki, H.; Kumagai, S. Metformin increases the PGC-1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J. Appl. Physiol. 2006, 101, 1685–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handschin, C.; Chin, S.; Li, P.; Liu, F.; Maratos-Flier, E.; Lebrasseur, N.K.; Yan, Z.; Spiegelman, B.M. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J. Biol. Chem. 2007, 282, 30014–30021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr. Opin. Genet. Dev. 2008, 18, 426–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessels, B.; Ciapaite, J.; van den Broek, N.M.; Nicolay, K.; Prompers, J.J. Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a dose-dependent manner. PLoS ONE 2014, 9, e100525. [Google Scholar] [CrossRef] [Green Version]
- Langone, F.; Cannata, S.; Fuoco, C.; Lettieri Barbato, D.; Testa, S.; Nardozza, A.P.; Ciriolo, M.R.; Castagnoli, L.; Gargioli, C.; Cesareni, G. Metformin protects skeletal muscle from cardiotoxin induced degeneration. PLoS ONE 2014, 9, e114018. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Chen, X.; Chen, D.; Yu, B.; Li, M.; He, J.; Huang, Z. MicroRNA-499-5p regulates skeletal myofiber specification via NFATc1/MEF2C pathway and Thrap1/MEF2C axis. Life Sci. 2018, 215, 236–245. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Wu, H.; Arnold, M.A.; Shelton, J.M.; Backs, J.; McAnally, J.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J. Clin. Investig. 2007, 117, 2459–2467. [Google Scholar] [CrossRef] [Green Version]
- Sharlo, K.; Lvova, I.; Turtikova, O.; Tyganov, S.; Kalashnikov, V.; Shenkman, B. Plantar stimulation prevents the decrease in fatigue resistance in rat soleus muscle under one week of hindlimb suspension. Arch. BioChem. Biophys. 2022, 718, 109150. [Google Scholar] [CrossRef] [PubMed]
- Miska, E.A.; Karlsson, C.; Langley, E.; Nielsen, S.J.; Pines, J.; Kouzarides, T. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 1999, 18, 5099–5107. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chan, J.K.; Zhu, G.; Wu, Z. Myocyte enhancer factor 2 acetylation by p300 enhances its DNA binding activity, transcriptional activity, and myogenic differentiation. Mol. Cell. Biol. 2005, 25, 3575–3582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramonova, I.I.; Sharlo., K.A.; Vilchinskaya, N.A.; Shenkman, B.S. The Time Course of Muscle Nuclear Content of Transcription Factors Regulating the MyHC I(β) Expression in the Rat Soleus Muscle under Gravitational Unloading. Biochem. Moscow. Suppl. Ser. A 2020, 14, 242–248. [Google Scholar] [CrossRef]
- Afinanisa, Q.; Cho, M.K.; Seong, H.A. AMPK Localization: A Key to Differential Energy Regulation. Int. J. Mol. Sci. 2021, 22, 10921. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ruegg, U.T.; Takeda, S. ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. Int. J. Mol. Sci. 2018, 19, 2804. [Google Scholar] [CrossRef] [Green Version]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.C.; Hadj Sassi, A.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. CMLS 2014, 71, 4361–4371. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Gomes-Marcondes, M.C. Metformin treatment modulates the tumour-induced wasting effects in muscle protein metabolism minimising the cachexia in tumour-bearing rats. BMC Cancer 2016, 16, 418. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Song, L.; Wang, R.; Hu, S.; Yang, Z.; Zhang, Z.; Sun, B.; Cui, W. Maternal Metformin Treatment during Gestation and Lactation Improves Skeletal Muscle Development in Offspring of Rat Dams Fed High-Fat Diet. Nutrients 2021, 13, 3417. [Google Scholar] [CrossRef]
- Krawiec, B.J.; Nystrom, G.J.; Frost, R.A.; Jefferson, L.S.; Lang, C.H. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am. J. Physiology. Endocrinol. Metab. 2007, 292, E1555–E1567. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Moon, J.W.; Lee, J.O.; Kim, J.H.; Jung, E.J.; Kim, S.J.; Oh, J.Y.; Wu, S.W.; Lee, P.R.; Park, S.H.; et al. Metformin induces muscle atrophy by transcriptional regulation of myostatin via HDAC6 and FoxO3a. J. Cachexia Sarcopenia Muscle 2022, 13, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Calixto, M.C.; Lintomen, L.; Andre, D.M.; Leiria, L.O.; Ferreira, D.; Lellis-Santos, C.; Anhe, G.F.; Bordin, S.; Landgraf, R.G.; Antunes, E. Metformin attenuates the exacerbation of the allergic eosinophilic inflammation in high fat-diet-induced obesity in mice. PLoS ONE 2013, 8, e76786. [Google Scholar] [CrossRef] [PubMed]
- Mackey, A.L.; Kjaer, M.; Charifi, N.; Henriksson, J.; Bojsen-Moller, J.; Holm, L.; Kadi, F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve 2009, 40, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic. Acids. Res. 2001, 29, e45. [Google Scholar] [CrossRef]


| Group | Animal Weight, g | M Soleus Weight, mg |
|---|---|---|
| C | 208.7 ± 33 | 91.2 ± 17 |
| C+M | 202.8 ± 30 | 93.2 ± 16 |
| 7HS | 187.4 ± 30 | 56.1 ± 7 *& |
| 7HS+M | 174.6 ± 24 *& | 52.7 ± 10 *& |
| Gene Description | Primer Sequence |
|---|---|
| Myh7 (MyHC I(β)) | 5′-ACAGAGGAAGACAGGAAGAACCTAC-3′ 5′-GGGCTTCACAGGCATCCTTAG-3′ |
| Myh2 (MyHC IIa) | 5′-TATCCTCAGGCTTCAAGATTTG-3′ 5′-TAAATAGAATCACATGGGGACA-3′ |
| Myh4 (MyHC IIb) | 5′-CTGAGGAACAATCCAACGTC-3′ 5′-TTGTGTGATTTCTTCTGTCACCT-3′ |
| Myh1 (MyHC IId/x) | 5′-CGCGAGGTTCACACCAAA-3′ 5′-TCCCAAAGTCGTAAGTACAAAATGG-3′ |
| SERCA1 | 5′-GACTGAGTTTGGGGAACAGCT-3′ 5′-GAGGTGGTGATGACAGCAGG-3′ |
| SERCA2 | 5′-GAAGCAGTTCATCCGCTACCTCA-3′ 5′-GCAGACCATCCGTCACCAGA-3′ |
| PGC1alpha | 5′-GTGCAGCCAAGACTCTGTATGG-3′ 5′-GTCCAGGTCATTCACATCAAGTTC-3′ |
| SOX6 | 5′-TCAAAGGCGATTTACCAGTGAC-3′ 5′-TTGTTGTGCATTATGGGGTGC-3′ |
| Rcan1 (MCIP1.4) | 5′-CCGTTGGCTGGAAACAAG-3′ 5′-GGTCACTCTCACACACGTGG-3′ |
| RPL19 | 5′-GTACCCTTCCTCTTCCCTATGC-3′ 5′-CAATGCCAACTCTCGTCAACAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharlo, K.A.; Lvova, I.D.; Belova, S.P.; Zaripova, K.A.; Shenkman, B.S.; Nemirovskaya, T.L. Metformin Attenuates Slow-to-Fast Fiber Shift and Proteolysis Markers Increase in Rat Soleus after 7 Days of Rat Hindlimb Unloading. Int. J. Mol. Sci. 2023, 24, 503. https://doi.org/10.3390/ijms24010503
Sharlo KA, Lvova ID, Belova SP, Zaripova KA, Shenkman BS, Nemirovskaya TL. Metformin Attenuates Slow-to-Fast Fiber Shift and Proteolysis Markers Increase in Rat Soleus after 7 Days of Rat Hindlimb Unloading. International Journal of Molecular Sciences. 2023; 24(1):503. https://doi.org/10.3390/ijms24010503
Chicago/Turabian StyleSharlo, Kristina A., Irina D. Lvova, Svetlana P. Belova, Ksenia A. Zaripova, Boris S. Shenkman, and Tatiana L. Nemirovskaya. 2023. "Metformin Attenuates Slow-to-Fast Fiber Shift and Proteolysis Markers Increase in Rat Soleus after 7 Days of Rat Hindlimb Unloading" International Journal of Molecular Sciences 24, no. 1: 503. https://doi.org/10.3390/ijms24010503





