Inflammation in Urological Malignancies: The Silent Killer
Abstract
:1. Introduction
2. Inflammation as Risk Factor in Genitourinary Cancers
2.1. Infectious Agents
2.1.1. Parasites
2.1.2. Bacterial Infections
2.1.3. Viruses
2.2. Noninfectious Causes
3. Inflammation-Related Pathways in Genitourinary Cancers
3.1. VEGF Pathway
3.2. VHL Pathway
3.3. mTOR Pathway
3.4. TNF Pathway
3.5. STAT Pathway
3.6. Nuclear Factor-Kappa B Pathway
3.7. Others
4. Inflammation-Related Molecules in Genitourinary Cancers
4.1. Cytokines
4.2. Chemokines
4.3. Others
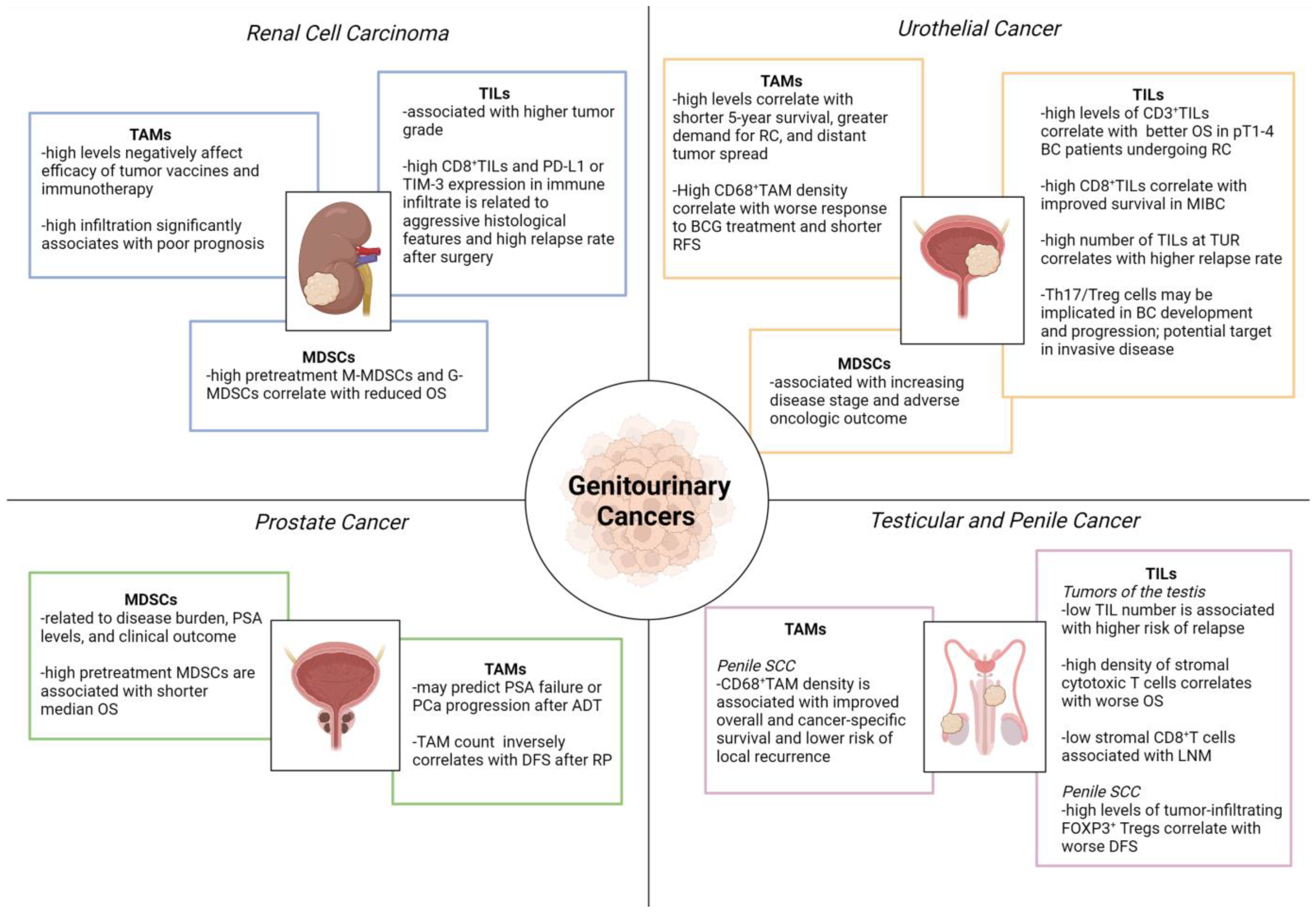
5. Inflammatory Cells in Genitourinary Cancers
5.1. Tumor-Associated Macrophages (TAMs)
5.2. Myeloid-Derived Suppressor Cells (MDSCs)
5.3. Tumor Infiltrating Lymphocytes (TILs)
6. Inflammation as a Therapeutic Target in Genitourinary Cancers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leibovici, D.; Grossman, H.B.; Dinney, C.P.; Millikan, R.E.; Lerner, S.; Wang, Y.; Gu, J.; Dong, Q.; Wu, X. Polymorphisms in inflammation genes and bladder cancer: From initiation to recurrence, progression, and survival. J. Clin. Oncol. 2005, 23, 5746–5756. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Ngabire, D.; Kim, G.D. Autophagy and inflammatory response in the tumor microenvironment. Int. J. Mol. Sci. 2017, 18, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porta, C.; Larghi, P.; Rimoldi, M.; Totaro, M.G.; Allavena, P.; Mantovani, A.; Sica, A. Cellular and molecular pathways linking inflammation and cancer. Immunobiology 2009, 214, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Nesi, G.; Nobili, S.; Cai, T.; Caini, S.; Santi, R. Chronic inflammation in urothelial bladder cancer. Virchows Arch. 2015, 467, 623–633. [Google Scholar] [CrossRef]
- Cai, T.; Santi, R.; Tamanini, I.; Galli, I.C.; Perletti, G.; Bjerklund Johansen, T.E.; Nesi, G. Current knowledge of the potential links between inflammation and prostate cancer. Int. J. Mol. Sci. 2019, 20, 3833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuper, H.; Adami, H.O.; Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 2000, 248, 171–183. [Google Scholar] [CrossRef]
- Rebbeck, T.R. Prostate cancer genetics: Variation by race, ethnicity, and geography. Semin. Radiat. Oncol. 2017, 27, 3–10. [Google Scholar] [CrossRef]
- Modena, A.; Iacovelli, R.; Scarpa, A.; Brunelli, M.; Ciccarese, C.; Fantinel, E.; Bimbatti, D.; Massari, F.; Martignoni, G.; Tortora, G. Investigating BRCA mutations: A breakthrough in precision medicine of castration-resistant prostate cancer. Target. Oncol. 2016, 11, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Boehm, K.; Valdivieso, R.; Meskawi, M.; Larcher, A.; Schiffmann, J.; Sun, M.; Graefen, M.; Saad, F.; Parent, M.É.; Karakiewicz, P.I. Prostatitis, other genitourinary infections and prostate cancer: Results from a population-based case-control study. World J. Urol. 2016, 34, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, S.; Fiorentino, M.; Andrén, O.; Fang, F.; Mucci, L.A.; Varenhorst, E.; Fall, K.; Rider, J.R. Inflammation, focal atrophic lesions, and prostatic intraepithelial neoplasia with respect to risk of lethal prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 2280–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Wang, K.; Xiong, Z.; Yuan, C.; Wang, C.; Cao, Q.; Yu, H.; Meng, X.; Xie, K.; Cheng, Z.; et al. Impact of inflammation and immunotherapy in renal cell carcinoma. Oncol. Lett. 2020, 20, 272. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.J. Emerging avenues linking inflammation and cancer. Free Radic. Biol. Med. 2012, 52, 2013–2037. [Google Scholar] [CrossRef]
- Hannan, T.J.; Mysorekar, I.U.; Hung, C.S.; Isaacson-Schmid, M.L.; Hultgren, S.J. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010, 6, e1001042. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Abraham, S.N. Innate and adaptive immune responses in the urinary tract. Eur. J. Clin. Investig. 2008, 38 (Suppl. 2), 21–28. [Google Scholar] [CrossRef]
- Fried, B.; Reddy, A.; Mayer, D. Helminths in human carcinogenesis. Cancer Lett. 2011, 305, 239–249. [Google Scholar] [CrossRef]
- Abol-Enein, H. Infection: Is it a cause of bladder cancer? Scand. J. Urol. Nephrol. Suppl. 2008, 218, 79–84. [Google Scholar] [CrossRef]
- Botelho, M.C.; Oliveira, P.A.; Lopes, C.; Correia da Costa, J.M.; Machado, J.C. Urothelial dysplasia and inflammation induced by Schistosoma haematobium total antigen instillation in mice normal urothelium. Urol. Oncol. 2011, 29, 809–814. [Google Scholar] [CrossRef]
- Botelho, M.C.; Machado, J.C.; da Costa, J.M. Schistosoma haematobium and bladder cancer: What lies beneath? Virulence 2010, 1, 84–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohshima, H.; Tatemichi, M.; Sawa, T. Chemical basis of inflammation-induced carcinogenesis. Arch. Biochem. Biophys. 2003, 417, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantor, A.F.; Hartge, P.; Hoover, R.N.; Narayana, A.S.; Sullivan, J.W.; Fraumeni, J.F., Jr. Urinary tract infection and risk of bladder cancer. Am. J. Epidemiol. 1984, 119, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.H.; Hanum, N.; Grotenhuis, A.J.; Castaño-Vinyals, G.; van der Heijden, A.G.; Aben, K.K.; Mysorekar, I.U.; Kiemeney, L.A. Recurrent urinary tract infection and risk of bladder cancer in the Nijmegen bladder cancer study. Br. J. Cancer 2015, 112, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Castelao, J.E.; Groshen, S.; Cortessis, V.K.; Shibata, D.; Conti, D.V.; Yuan, J.M.; Pike, M.C.; Gago-Dominguez, M. Urinary tract infections and reduced risk of bladder cancer in Los Angeles. Br. J. Cancer 2009, 100, 834–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Videčnik Zorman, J.; Matičič, M.; Jeverica, S.; Smrkolj, T. Diagnosis and treatment of bacterial prostatitis. Acta Dermatovenerol. Alp. Pannonica Adriat. 2015, 24, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Pandareesh, M.D.; Kameshwar, V.H.; Byrappa, K. Prostate carcinogenesis: Insights in relation to epigenetics and inflammation. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Lax, A.J. Opinion: Bacterial toxins and cancer—A case to answer? Nat. Rev. Microbiol. 2005, 3, 343–349. [Google Scholar] [CrossRef]
- Kidd, L.C.; Chaing, S.; Chipollini, J.; Giuliano, A.R.; Spiess, P.E.; Sharma, P. Relationship between human papillomavirus and penile cancer-implications for prevention and treatment. Transl. Androl. Urol. 2017, 6, 791–802. [Google Scholar] [CrossRef]
- Wei, L.; Gravitt, P.E.; Song, H.; Maldonado, A.M.; Ozbun, M.A. Nitric oxide induces early viral transcription coincident with increased DNA damage and mutation rates in human papillomavirus-infected cells. Cancer Res. 2009, 69, 4878–4884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guma, S.; Maglantay, R.; Lau, R.; Wieczorek, R.; Melamed, J.; Deng, F.M.; Zhou, M.; Makarov, D.; Lee, P.; Pincus, M.R.; et al. Papillary urothelial carcinoma with squamous differentiation in association with human papilloma virus: Case report and literature review. Am. J. Clin. Exp. Urol. 2016, 4, 12–16. [Google Scholar] [PubMed]
- Li, N.; Yang, L.; Zhang, Y.; Zhao, P.; Zheng, T.; Dai, M. Human papillomavirus infection and bladder cancer risk: A meta-analysis. J. Infect. Dis. 2011, 204, 217–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, R.E.; Davidson, D.D.; Lopez-Beltran, A.; Montironi, R.; MacLennan, G.T.; Compérat, E.; Idrees, M.T.; Emerson, R.E.; Cheng, L. Human papillomavirus is not an etiologic agent of urothelial inverted papillomas. Am. J. Surg. Pathol. 2013, 37, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Youshya, S.; Purdie, K.; Breuer, J.; Proby, C.; Sheaf, M.T.; Oliver, R.T.; Baithun, S. Does human papillomavirus play a role in the development of bladder transitional cell carcinoma? A comparison of PCR and immunohistochemical analysis. J. Clin. Pathol. 2005, 58, 207–210. [Google Scholar] [CrossRef]
- Lawson, J.S.; Glenn, W.K. Multiple pathogens and prostate cancer. Infect. Agent. Cancer 2022, 17, 23. [Google Scholar] [CrossRef]
- Yin, B.; Liu, W.; Yu, P.; Liu, C.; Chen, Y.; Duan, X.; Liao, Z.; Chen, Y.; Wang, X.; Pan, X.; et al. Association between human papillomavirus and prostate cancer: A meta-analysis. Oncol. Lett. 2017, 14, 1855–1865. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Huang, Z.; Jian, Z.; Wei, X. The association between hepatitis C virus infection and renal cell cancer, prostate cancer, and bladder cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 10833. [Google Scholar] [CrossRef]
- Garolla, A.; Vitagliano, A.; Muscianisi, F.; Valente, U.; Ghezzi, M.; Andrisani, A.; Ambrosini, G.; Foresta, C. Role of viral infections in testicular cancer etiology: Evidence from a systematic review and meta-analysis. Front. Endocrinol. 2019, 10, 355. [Google Scholar] [CrossRef]
- Clouston, D.; Lawrentschuk, N. Metaplastic conditions of the bladder. BJU Int. 2013, 112 (Suppl. 2), 27–31. [Google Scholar] [CrossRef]
- Groah, S.L.; Weitzenkamp, D.A.; Lammertse, D.P.; Whiteneck, G.G.; Lezotte, D.C.; Hamman, R.F. Excess risk of bladder cancer in spinal cord injury: Evidence for an association between indwelling catheter use and bladder cancer. Arch. Phys. Med. Rehabil. 2002, 83, 346–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalisvaart, J.F.; Katsumi, H.K.; Ronningen, L.D.; Hovey, R.M. Bladder cancer in spinal cord injury patients. Spinal Cord. 2010, 48, 257–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, D.A.; Cummings, J.M.; Longo, W.E.; Virgo, K.S.; Johnson, F.E.; Parra, R.O. Role of chronic catheterization in the development of bladder cancer in patients with spinal cord injury. Urology 1999, 53, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Sun, L.M.; Lin, C.L.; Liang, J.A.; Chang, Y.J.; Sung, F.C.; Kao, C.H. Risk of prostate and bladder cancers in patients with spinal cord injury: A population-based cohort study. Urol. Oncol. 2014, 32, 51.e1–51.e7. [Google Scholar] [CrossRef]
- Moreira, D.M.; Nickel, J.C.; Gerber, L.; Muller, R.L.; Andriole, G.L.; Castro-Santamaria, R.; Freedland, S.J. Smoking is associated with acute and chronic prostatic inflammation: Results from the REDUCE study. Cancer Prev. Res. 2015, 8, 312–317. [Google Scholar] [CrossRef] [Green Version]
- DeMarzo, A.M.; Nelson, W.G.; Isaacs, W.B.; Epstein, J.I. Pathological and molecular aspects of prostate cancer. Lancet 2003, 361, 955–964. [Google Scholar] [CrossRef]
- Vignozzi, L.; Gacci, M.; Cellai, I.; Santi, R.; Corona, G.; Morelli, A.; Rastrelli, G.; Comeglio, P.; Sebastanelli, A.; Maneschi, E.; et al. Fat boosts, while androgen receptor activation counteracts, BPH-associated prostate inflammation. Prostate 2013, 73, 789–800. [Google Scholar] [CrossRef]
- Vykhovanets, E.V.; Resnick, M.I.; MacLennan, G.T.; Gupta, S. Experimental rodent models of prostatitis: Limitations and potential. Prostate Cancer Prostatic Dis. 2007, 10, 15–29. [Google Scholar] [CrossRef]
- Jia, Y.L.; Liu, X.; Yan, J.Y.; Chong, L.M.; Li, L.; Ma, A.C.; Zhou, L.; Sun, Z.Y. The alteration of inflammatory markers and apoptosis on chronic prostatitis induced by estrogen and androgen. Int. Urol. Nephrol. 2015, 47, 39–46. [Google Scholar] [CrossRef]
- Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002, 20, 4368–4380. [Google Scholar] [CrossRef]
- Zachary, I.; Gliki, G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc. Res. 2001, 49, 568–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaelin, W.G., Jr. Treatment of kidney cancer: Insights provided by the VHL tumor-suppressor protein. Cancer 2009, 115, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kim, W.Y. Two sides to every story: The HIF-dependent and HIF-independent functions of pVHL. J. Cell. Mol. Med. 2011, 15, 187–195. [Google Scholar] [CrossRef]
- Staller, P.; Sulitkova, J.; Lisztwan, J.; Moch, H.; Oakeley, E.J.; Krek, W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature 2003, 425, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Schermer, B.; Ghenoiu, C.; Bartram, M.; Müller, R.U.; Kotsis, F.; Höhne, M.; Kühn, W.; Rapka, M.; Nitschke, R.; Zentgraf, H.; et al. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J. Cell Biol. 2006, 175, 547–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, J.P.; Nayak, B.; Shanmugasundaram, K.; Friedrichs, W.; Sudarshan, S.; Eid, A.A.; DeNapoli, T.; Parekh, D.J.; Gorin, Y.; Block, K. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PLoS ONE 2012, 7, e30712. [Google Scholar] [CrossRef]
- Oh, W.J.; Jacinto, E. mTOR complex 2 signaling and functions. Cell Cycle 2011, 10, 2305–2316. [Google Scholar] [CrossRef] [Green Version]
- Pantuck, A.J.; Seligson, D.B.; Klatte, T.; Yu, H.; Leppert, J.T.; Moore, L.; O’Toole, T.; Gibbons, J.; Belldegrun, A.S.; Figlin, R.A. Prognostic relevance of the mTOR pathway in renal cell carcinoma: Implications for molecular patient selection for targeted therapy. Cancer 2007, 109, 2257–2267. [Google Scholar] [CrossRef]
- Dodd, K.M.; Yang, J.; Shen, M.H.; Sampson, J.R.; Tee, A.R. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene 2015, 34, 2239–2250. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Han, L.Z.; Liang, H.; Mi, C.; Shi, H.; Lee, J.J.; Jin, X. Celastrol inhibits the HIF-1α pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells. Oncol. Rep. 2014, 32, 235–242. [Google Scholar] [CrossRef]
- Tan, P.; Shi, M.; Lai, L.; Tang, Z.; Xie, N.; Xu, H.; Wei, Q.; Zhang, X.; Yang, L.; Wu, L. Regulative role of the CXCL13-CXCR5 axis in the tumor microenvironment. Precis. Clin. Med. 2018, 1, 49–56. [Google Scholar] [CrossRef]
- Azizi, M.; Tang, D.H.; Verduzco, D.; Peyton, C.C.; Chipollini, J.; Yuan, Z.; Schaible, B.J.; Zhou, J.M.; Johnstone, P.A.; Giuliano, A.; et al. Impact of PI3K-AKT-mTOR signaling pathway up-regulation on prognosis of penile squamous-cell carcinoma: Results from a tissue microarray study and review of the literature. Clin. Genitourin. Cancer 2019, 17, e80–e91. [Google Scholar] [CrossRef] [PubMed]
- Al-Lamki, R.S.; Sadler, T.J.; Wang, J.; Reid, M.J.; Warren, A.Y.; Movassagh, M.; Lu, W.; Mills, I.G.; Neal, D.E.; Burge, J.; et al. Tumor necrosis factor receptor expression and signaling in renal cell carcinoma. Am. J. Pathol. 2010, 177, 943–954. [Google Scholar] [CrossRef]
- Elsässer-Beile, U.; Rindsfüser, M.; Grussenmeyer, T.; Schultze-Seemann, W.; Wetterauer, U. Enhanced expression of IFN-gamma mRNA in CD4(+)or CD8(+)tumour-infiltrating lymphocytes compared to peripheral lymphocytes in patients with renal cell cancer. Br. J. Cancer 2000, 83, 637–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, M.J.; Sun, K.H.; Tang, S.J.; Deng, M.W.; Wu, Y.H.; Sung, J.S.; Cha, T.L.; Sun, G.H. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci. 2008, 99, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Tang, S.J.; Chuang, M.J.; Cha, T.L.; Li, J.Y.; Sun, G.H.; Sun, K.H. TNF-α induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol. Cancer Res. 2012, 10, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Van Boxel-Dezaire, A.H.; Rani, M.R.; Stark, G.R. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 2006, 25, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Buettner, R.; Mora, L.B.; Jove, R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002, 8, 945–954. [Google Scholar]
- Bowman, T.; Broome, M.A.; Sinibaldi, D.; Wharton, W.; Pledger, W.J.; Sedivy, J.M.; Irby, R.; Yeatman, T.; Courtneidge, S.A.; Jove, R. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 7319–7324. [Google Scholar] [CrossRef] [Green Version]
- Aoki, Y.; Feldman, G.M.; Tosato, G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 2003, 101, 1535–1542. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, Z.; Hou, Y.; Hu, J.; Wang, C. The effects of STAT3 and survivin silencing on the growth of human bladder carcinoma cells. Tumour Biol. 2014, 35, 5401–5407. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Liao, Y.; Wang, M.; Wang, Y.; Wang, K.; Guo, J.; Wu, P.; Zhong, B.; Guo, T.; Wu, C. BUB1 drives the occurrence and development of bladder cancer by mediating the STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2021, 40, 378. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Kontos, S.; Kominea, A.; Melachrinou, M.; Balampani, E.; Sotiropoulou-Bonikou, G. Inverse expression of estrogen receptor-beta and nuclear factor-kappaB in urinary bladder carcinogenesis. Int. J. Urol. 2010, 17, 801–809. [Google Scholar] [CrossRef]
- Duan, W.; Wang, E.; Zhang, F.; Wang, T.; You, X.; Qiao, B. Association between the NFKB1-94ins/del ATTG polymorphism and cancer risk: An updated meta-analysis. Cancer Investig. 2014, 32, 311–320. [Google Scholar] [CrossRef]
- Rani, A.; Dasgupta, P.; Murphy, J.J. Prostate Cancer: The role of inflammation and chemokines. Am. J. Pathol. 2019, 189, 2119–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioni, B.; Nevedomskaya, E.; Melis, M.H.M.; van Burgsteden, J.; Stelloo, S.; Hodel, E.; Spinozzi, D.; de Jong, J.; van der Poel, H.; de Boer, J.P.; et al. Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration. Mol. Oncol. 2018, 12, 1308–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Q.; Xin, L. Notch signaling in prostate cancer: Refining a therapeutic opportunity. Histol. Histopathol. 2016, 31, 149–157. [Google Scholar] [CrossRef]
- De Bono, J.S.; Piulats, J.M.; Pandha, H.S.; Petrylak, D.P.; Saad, F.; Aparicio, L.M.; Sandhu, S.K.; Fong, P.; Gillessen, S.; Hudes, G.R.; et al. Phase II randomized study of figitumumab plus docetaxel and docetaxel alone with crossover for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.J.; Chudow, J.; DeNunzio, M.; Slovin, S.F.; Danila, D.C.; Morris, M.J.; Scher, H.I.; Rathkopf, D.E. A phase I trial of IGF-1R inhibitor cixutumumab and mTOR inhibitor temsirolimus in metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 2020, 8, 171–178.e2. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Gilbert, D.; Goddard, N.; Looijenga, L.; Shipley, J. Genes, chromosomes and the development of testicular germ cell tumors of adolescents and adults. Genes Chromosomes Cancer 2008, 47, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kemmer, K.; Corless, C.L.; Fletcher, J.A.; McGreevey, L.; Haley, A.; Griffith, D.; Cummings, O.W.; Wait, C.; Town, A.; Heinrich, M.C. KIT mutations are common in testicular seminomas. Am. J. Pathol. 2004, 164, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilcek, J.; Feldmann, M. Historical review: Cytokines as therapeutics and targets of therapeutics. Trends Pharmacol. Sci. 2004, 25, 201–209. [Google Scholar] [CrossRef]
- De Vivar Chevez, A.R.; Finke, J.; Bukowski, R. The role of inflammation in kidney cancer. Adv. Exp. Med. Biol. 2014, 816, 197–234. [Google Scholar] [CrossRef]
- Tsui, K.H.; Wang, S.W.; Chung, L.C.; Feng, T.H.; Lee, T.Y.; Chang, P.L.; Juang, H.H. Mechanisms by which interleukin-6 attenuates cell invasion and tumorigenesis in human bladder carcinoma cells. Biomed. Res. Int. 2013, 2013, 791212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.F.; Lin, P.Y.; Wu, C.F.; Chen, W.C.; Wu, C.T. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS ONE 2013, 8, e61901. [Google Scholar] [CrossRef] [Green Version]
- Pignot, G.; Bieche, I.; Vacher, S.; Güet, C.; Vieillefond, A.; Debré, B.; Lidereau, R.; Amsellem-Ouazana, D. Large-scale real-time reverse transcription-PCR approach of angiogenic pathways in human transitional cell carcinoma of the bladder: Identification of VEGFA as a major independent prognostic marker. Eur. Urol. 2009, 56, 678–688. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, Y.K.; Chung, C.J.; Huang, C.Y.; Pu, Y.S.; Shiue, H.S.; Lai, L.A.; Lin, Y.C.; Su, C.T.; Hsueh, Y.M. Polymorphism of inflammatory genes and arsenic methylation capacity are associated with urothelial carcinoma. Toxicol. Appl. Pharmacol. 2013, 272, 30–36. [Google Scholar] [CrossRef]
- Chikazawa, M.; Inoue, K.; Fukata, S.; Karashima, T.; Shuin, T. Expression of angiogenesis-related genes regulates different steps in the process of tumor growth and metastasis in human urothelial cell carcinoma of the urinary bladder. Pathobiology 2008, 75, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.T.; Leite, K.R.; Piovesan, L.F.; Pontes-Junior, J.; Viana, N.I.; Abe, D.K.; Crippa, A.; Moura, C.M.; Adonias, S.P.; Srougi, M.; et al. Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of bladder cancer. BMC Urol. 2012, 12, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Park, S.S.; Lee, U.S.; Kim, W.J.; Moon, S.K. Signaling pathway for TNF-alpha-induced MMP-9 expression: Mediation through p38 MAP kinase, and inhibition by anti-cancer molecule magnolol in human urinary bladder cancer 5637 cells. Int. Immunopharmacol. 2008, 8, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.C.; Wang, P.H.; Ding, Q.; Guan, M.; Zhang, Y.F.; Jiang, H.W.; Wen, H.; Wu, Z. Expression of pigment epithelium-derived factor and tumor necrosis factor-α is correlated in bladder tumor and is related to tumor angiogenesis. Urol. Oncol. 2013, 31, 241–246. [Google Scholar] [CrossRef]
- Stadler, W.M.; Halabi, S.; Rini, B.; Ernstoff, M.S.; Davila, E.; Picus, J.; Barrier, R.; Small, E.J.; Cancer and Leukemia Group B. A phase II study of gemcitabine and capecitabine in metastatic renal cancer: A report of Cancer and Leukemia Group B protocol 90008. Cancer 2006, 107, 1273–1279. [Google Scholar] [CrossRef]
- Wang, L.; Huang, T.; Chen, W.; Gao, X.; Zhou, T.; Wu, Z.; Sun, Y. Silencing of CXCR4 by RNA interference inhibits cell growth and metastasis in human renal cancer cells. Oncol. Rep. 2012, 28, 2043–2048. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Cheng, G.; Hao, M.; Zheng, J.; Zhou, X.; Zhang, J.; Taichman, R.S.; Pienta, K.J.; Wang, J. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010, 29, 709–722. [Google Scholar] [CrossRef] [Green Version]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Jöhrer, K.; Zelle-Rieser, C.; Perathoner, A.; Moser, P.; Hager, M.; Ramoner, R.; Gander, H.; Höltl, L.; Bartsch, G.; Greil, R.; et al. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin. Cancer Res. 2005, 11, 2459–2465. [Google Scholar] [CrossRef] [Green Version]
- Darash-Yahana, M.; Gillespie, J.W.; Hewitt, S.M.; Chen, Y.Y.; Maeda, S.; Stein, I.; Singh, S.P.; Bedolla, R.B.; Peled, A.; Troyer, D.A.; et al. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS ONE 2009, 4, e6695. [Google Scholar] [CrossRef]
- Maeda, K.; Das, D.; Nakata, H.; Mitsuya, H. CCR5 inhibitors: Emergence, success, and challenges. Expert Opin. Emerg. Drugs 2012, 17, 135–145. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, S.; Liu, J.; Cui, Y.; Fan, Y.; Lv, T.; Liu, L.; Li, J.; He, Q.; Han, W.; et al. CD8+ T cells promote proliferation of benign prostatic hyperplasia epithelial cells under low androgen level via modulation of CCL5/STAT5/CCND1 signaling pathway. Sci. Rep. 2017, 7, 42893. [Google Scholar] [CrossRef]
- Conley-LaComb, M.K.; Semaan, L.; Singareddy, R.; Li, Y.; Heath, E.I.; Kim, S.; Cher, M.L.; Chinni, S.R. Pharmacological targeting of CXCL12/CXCR4 signaling in prostate cancer bone metastasis. Mol. Cancer 2016, 15, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sbrissa, D.; Semaan, L.; Govindarajan, B.; Li, Y.; Caruthers, N.J.; Stemmer, P.M.; Cher, M.L.; Sethi, S.; Vaishampayan, U.; Shisheva, A.; et al. A novel cross-talk between CXCR4 and PI4KIIIα in prostate cancer cells. Oncogene 2019, 38, 332–344. [Google Scholar] [CrossRef]
- Miyake, M.; Lawton, A.; Goodison, S.; Urquidi, V.; Gomes-Giacoia, E.; Zhang, G.; Ross, S.; Kim, J.; Rosser, C.J. Chemokine (C-X-C) ligand 1 (CXCL1) protein expression is increased in aggressive bladder cancers. BMC Cancer 2013, 13, 322. [Google Scholar] [CrossRef] [Green Version]
- Batsi, O.; Giannopoulou, I.; Nesseris, I.; Valavanis, C.; Gakiopoulou, H.; Patsouris, E.S.; Arapandoni-Dadioti, P.; Lazaris, A.C. Immunohistochemical evaluation of CXCL12-CXCR4 axis and VEGFR3 expression in primary urothelial cancer and its recurrence. Anticancer Res. 2014, 34, 3537–3542. [Google Scholar] [PubMed]
- Zhang, W.; Wang, H.; Sun, M.; Deng, X.; Wu, X.; Ma, Y.; Li, M.; Shuoa, S.M.; You, Q.; Miao, L. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Commun. 2020, 40, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Murai, R.; Itoh, Y.; Kageyama, S.; Nakayama, M.; Ishigaki, H.; Teramoto, K.; Narita, M.; Yoshida, T.; Tomita, K.; Kobayashi, K.I.; et al. Prediction of intravesical recurrence of non-muscle-invasive bladder cancer by evaluation of intratumoral Foxp3+ T cells in the primary transurethral resection of bladder tumor specimens. PLoS ONE 2018, 13, e0204745. [Google Scholar] [CrossRef] [PubMed]
- Loveland, K.L.; Dias, V.; Meachem, S.; Rajpert-De Meyts, E. The transforming growth factor-beta superfamily in early spermatogenesis: Potential relevance to testicular dysgenesis. Int. J. Androl. 2007, 30, 377–384. [Google Scholar] [CrossRef]
- Kanetsky, P.A.; Mitra, N.; Vardhanabhuti, S.; Li, M.; Vaughn, D.J.; Letrero, R.; Ciosek, S.L.; Doody, D.R.; Smith, L.M.; Weaver, J.; et al. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat. Genet. 2009, 41, 811–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, D.C.; Chandler, I.; McIntyre, A.; Goddard, N.C.; Gabe, R.; Huddart, R.A.; Shipley, J. Clinical and biological significance of CXCL12 and CXCR4 expression in adult testes and germ cell tumours of adults and adolescents. J. Pathol. 2009, 217, 94–102. [Google Scholar] [CrossRef] [PubMed]
- McIver, S.C.; Loveland, K.L.; Roman, S.D.; Nixon, B.; Kitazawa, R.; McLaughlin, E.A. The chemokine CXCL12 and its receptor CXCR4 are implicated in human seminoma metastasis. Andrology 2013, 1, 517–529. [Google Scholar] [CrossRef]
- Mo, M.; Li, Y.; Hu, X. Serum CXCL5 level is associated with tumor progression in penile cancer. Biosci. Rep. 2021, 41, BSR20202133. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.O.; Park, S.J.; Yun, C.H.; Chung, A.S. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J. Biochem. Mol. Biol. 2003, 36, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Petrella, B.L.; Lohi, J.; Brinckerhoff, C.E. Identification of membrane type-1 matrix metalloproteinase as a target of hypoxia-inducible factor-2 alpha in von Hippel-Lindau renal cell carcinoma. Oncogene 2005, 24, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Kallakury, B.V.; Karikehalli, S.; Haholu, A.; Sheehan, C.E.; Azumi, N.; Ross, J.S. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin. Cancer Res. 2001, 7, 3113–3119. [Google Scholar] [PubMed]
- Yang, B.; Zhang, D.; Qian, J.; Cheng, Y. Chelerythrine suppresses proliferation and metastasis of human prostate cancer cells via modulating MMP/TIMP/NF-κB system. Mol. Cell. Biochem. 2020, 474, 199–208. [Google Scholar] [CrossRef]
- Margulis, V.; Shariat, S.F.; Ashfaq, R.; Thompson, M.; Sagalowsky, A.I.; Hsieh, J.T.; Lotan, Y. Expression of cyclooxygenase-2 in normal urothelium, and superficial and advanced transitional cell carcinoma of bladder. J. Urol. 2007, 177, 1163–1168. [Google Scholar] [CrossRef]
- Tadin, T.; Krpina, K.; Stifter, S.; Babarović, E.; Fučkar, Z.; Jonjić, N. Lower cyclooxygenase-2 expression is associated with recurrence of solitary non-muscle invasive bladder carcinoma. Diagn. Pathol. 2012, 7, 152. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, D.C.; Baxter, E.W.; Loadman, P.M.; Hull, M.A.; Knowles, M.A. FGFR1-induced epithelial to mesenchymal transition through MAPK/PLCγ/COX-2-mediated mechanisms. PLoS ONE 2012, 7, e38972. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, M.A.; Hausladen, D.A.; Yoon, J.H.; Weiss, R.M. Prostaglandin E2 production and cyclooxygenase-2 induction in human urinary tract infections and bladder cancer. J. Urol. 2002, 168 Pt 1, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Von der Emde, L.; Goltz, D.; Latz, S.; Müller, S.C.; Kristiansen, G.; Ellinger, J.; Syring, I. Prostaglandin receptors EP1-4 as a potential marker for clinical outcome in urothelial bladder cancer. Am. J. Cancer Res. 2014, 4, 952–962. [Google Scholar] [PubMed]
- Ehsan, A.; Sommer, F.; Schmidt, A.; Klotz, T.; Koslowski, J.; Niggemann, S.; Jacobs, G.; Engelmann, U.; Addicks, K.; Bloch, W. Nitric oxide pathways in human bladder carcinoma. The distribution of nitric oxide synthases, soluble guanylyl cyclase, cyclic guanosine monophosphate, and nitrotyrosine. Cancer 2002, 95, 2293–2301. [Google Scholar] [CrossRef]
- Gecit, I.; Aslan, M.; Gunes, M.; Pirincci, N.; Esen, R.; Demir, H.; Ceylan, K. Serum prolidase activity, oxidative stress, and nitric oxide levels in patients with bladder cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Bingle, L.; Brown, N.J.; Lewis, C.E. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J. Pathol. 2002, 196, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Forssell, J.; Oberg, A.; Henriksson, M.L.; Stenling, R.; Jung, A.; Palmqvist, R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin. Cancer Res. 2007, 13, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- Kawai, O.; Ishii, G.; Kubota, K.; Murata, Y.; Naito, Y.; Mizuno, T.; Aokage, K.; Saijo, N.; Nishiwaki, Y.; Gemma, A.; et al. Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 2008, 113, 1387–1395. [Google Scholar] [CrossRef]
- Welsh, T.J.; Green, R.H.; Richardson, D.; Waller, D.A.; O’Byrne, K.J.; Bradding, P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 8959–8967. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Komohara, Y.; Hasita, H.; Ohnishi, K.; Fujiwara, Y.; Suzu, S.; Eto, M.; Takeya, M. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011, 102, 1424–1431. [Google Scholar] [CrossRef]
- Dufresne, M.; Dumas, G.; Asselin, E.; Carrier, C.; Pouliot, M.; Reyes-Moreno, C. Pro-inflammatory type-1 and anti-inflammatory type-2 macrophages differentially modulate cell survival and invasion of human bladder carcinoma T24 cells. Mol. Immunol. 2011, 48, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, C.; Kim, M.J.; Schwendener, R.A.; Alitalo, K.; Heston, W.; Kim, I.; Kim, W.J.; Koh, G.Y. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol. Cancer 2011, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Hanada, T.; Nakagawa, M.; Emoto, A.; Nomura, T.; Nasu, N.; Nomura, Y. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int. J. Urol. 2000, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Ayari, C.; LaRue, H.; Hovington, H.; Caron, A.; Bergeron, A.; Têtu, B.; Fradet, V.; Fradet, Y. High level of mature tumor-infiltrating dendritic cells predicts progression to muscle invasion in bladder cancer. Hum. Pathol. 2013, 44, 1630–1637. [Google Scholar] [CrossRef]
- Masson-Lecomte, A.; Rava, M.; Real, F.X.; Hartmann, A.; Allory, Y.; Malats, N. Inflammatory biomarkers and bladder cancer prognosis: A systematic review. Eur. Urol. 2014, 66, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Yuri, P.; Shigemura, K.; Kitagawa, K.; Hadibrata, E.; Risan, M.; Zulfiqqar, A.; Soeroharjo, I.; Hendri, A.Z.; Danarto, R.; Ishii, A.; et al. Increased tumor-associated macrophages in the prostate cancer microenvironment predicted patients’ survival and responses to androgen deprivation therapies in Indonesian patients cohort. Prostate Int. 2020, 8, 62–69. [Google Scholar] [CrossRef]
- Schmieder, A.; Michel, J.; Schönhaar, K.; Goerdt, S.; Schledzewski, K. Differentiation and gene expression profile of tumor-associated macrophages. Semin. Cancer Biol. 2012, 22, 289–297. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [Green Version]
- Zahreddine, H.; Borden, K.L. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Chahoud, J.; Netto, F.; Lazcano Segura, R.; Parra Cuentas, E.R.; Lu, X.; Rao, P.; Wistuba, I.I.; Pickering, C.R.; Pettaway, C.A. Tumor immune microenvironment alterations in penile squamous cell carcinoma using multiplex immunofluorescence and image analysis approaches. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Ottenhof, S.R.; Djajadiningrat, R.S.; Thygesen, H.H.; Jakobs, P.J.; Jóźwiak, K.; Heeren, A.M.; de Jong, J.; Sanders, J.; Horenblas, S.; Jordanova, E.S. The prognostic value of immune factors in the tumor microenvironment of penile squamous cell carcinoma. Front. Immunol. 2018, 9, 1253. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat. Rev. Immunol. 2004, 4, 941–952. [Google Scholar] [CrossRef]
- Peranzoni, E.; Zilio, S.; Marigo, I.; Dolcetti, L.; Zanovello, P.; Mandruzzato, S.; Bronte, V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 2010, 22, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Sippel, T.R.; White, J.; Nag, K.; Tsvankin, V.; Klaassen, M.; Kleinschmidt-DeMasters, B.K.; Waziri, A. Neutrophil degranulation and immunosuppression in patients with GBM: Restoration of cellular immune function by targeting arginase I. Clin. Cancer Res. 2011, 17, 6992–7002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youn, J.I.; Collazo, M.; Shalova, I.N.; Biswas, S.K.; Gabrilovich, D.I. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J. Leukoc. Biol. 2012, 91, 167–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, S.; Weinschenk, T.; Stenzl, A.; Zdrojowy, R.; Pluzanska, A.; Szczylik, C.; Staehler, M.; Brugger, W.; Dietrich, P.Y.; Mendrzyk, R.; et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012, 18, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.; Daurkin, I.; Vieweg, J.; Daaka, Y.; Kusmartsev, S. Aberrant PGE₂ metabolism in bladder tumor microenvironment promotes immunosuppressive phenotype of tumor-infiltrating myeloid cells. Int. Immunopharmacol. 2011, 11, 848–855. [Google Scholar] [CrossRef] [Green Version]
- Fridlender, Z.G.; Sun, J.; Singhal, S.; Kapoor, V.; Cheng, G.; Suzuki, E.; Albelda, S.M. Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Mol. Ther. 2010, 18, 1947–1959. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; McGuire, T.R.; Britton, H.C.; Schwarz, J.K.; Loberiza, F.R., Jr.; Meza, J.L.; Talmadge, J.E. Lenalidomide and cyclophosphamide immunoregulation in patients with metastatic, castration-resistant prostate cancer. Clin. Exp. Metastasis 2015, 32, 111–124. [Google Scholar] [CrossRef]
- Brusa, D.; Simone, M.; Gontero, P.; Spadi, R.; Racca, P.; Micari, J.; Degiuli, M.; Carletto, S.; Tizzani, A.; Matera, L. Circulating immunosuppressive cells of prostate cancer patients before and after radical prostatectomy: Profile comparison. Int. J. Urol. 2013, 20, 971–978. [Google Scholar] [CrossRef]
- Koinis, F.; Xagara, A.; Chantzara, E.; Leontopoulou, V.; Aidarinis, C.; Kotsakis, A. Myeloid-derived suppressor cells in prostate cancer: Present knowledge and future perspectives. Cells 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Santegoets, S.J.; Stam, A.G.; Lougheed, S.M.; Gall, H.; Jooss, K.; Sacks, N.; Hege, K.; Lowy, I.; Scheper, R.J.; Gerritsen, W.R.; et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J. Immunother. Cancer 2014, 2, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, N.; Moriya, F.; Waki, K.; Yamada, A.; Itoh, K.; Noguchi, M. Immunological efficacy of herbal medicines in prostate cancer patients treated by personalized peptide vaccine. Cancer Sci. 2017, 108, 2326–2332. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, M.; Moriya, F.; Koga, N.; Matsueda, S.; Sasada, T.; Yamada, A.; Kakuma, T.; Itoh, K. A randomized phase II clinical trial of personalized peptide vaccination with metronomic low-dose cyclophosphamide in patients with metastatic castration-resistant prostate cancer. Cancer Immunol. Immunother. 2016, 65, 151–160. [Google Scholar] [CrossRef]
- Chi, N.; Tan, Z.; Ma, K.; Bao, L.; Yun, Z. Increased circulating myeloid-derived suppressor cells correlate with cancer stages, interleukin-8 and -6 in prostate cancer. Int. J. Clin. Exp. Med. 2014, 7, 3181–3192. [Google Scholar] [PubMed]
- Hossain, D.M.; Pal, S.K.; Moreira, D.; Duttagupta, P.; Zhang, Q.; Won, H.; Jones, J.; D’Apuzzo, M.; Forman, S.; Kortylewski, M. TLR9-Targeted STAT3 silencing abrogates immunosuppressive activity of myeloid-derived suppressor cells from prostate cancer patients. Clin. Cancer Res. 2015, 21, 3771–3782. [Google Scholar] [CrossRef] [Green Version]
- Baine, M.K.; Turcu, G.; Zito, C.R.; Adeniran, A.J.; Camp, R.L.; Chen, L.; Kluger, H.M.; Jilaveanu, L.B. Characterization of tumor infiltrating lymphocytes in paired primary and metastatic renal cell carcinoma specimens. Oncotarget 2015, 6, 24990–25002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Li, C.; Cai, X.; Xie, Z.; Zhou, L.; Cheng, B.; Zhong, R.; Xiong, S.; Li, J.; Chen, Z.; et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. EClinicalMedicine 2021, 41, 101134. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Frigola, X.; Bonne-Annee, S.; Mercader, M.; Kuntz, S.M.; Krambeck, A.E.; Sengupta, S.; Dong, H.; Cheville, J.C.; Lohse, C.M.; et al. Tumor-infiltrating Foxp3-CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin. Cancer Res. 2007, 13, 2075–2081. [Google Scholar] [CrossRef]
- Granier, C.; Dariane, C.; Combe, P.; Verkarre, V.; Urien, S.; Badoual, C.; Roussel, H.; Mandavit, M.; Ravel, P.; Sibony, M.; et al. Tim-3 expression on tumor-infiltrating PD-1+CD8+T cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res. 2017, 77, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Liakou, C.I.; Narayanan, S.; Ng Tang, D.; Logothetis, C.J.; Sharma, P. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human bladder cancer. Cancer Immun. 2007, 7, 10. [Google Scholar] [PubMed]
- Winerdal, M.E.; Marits, P.; Winerdal, M.; Hasan, M.; Rosenblatt, R.; Tolf, A.; Selling, K.; Sherif, A.; Winqvist, O. FOXP3 and survival in urinary bladder cancer. BJU Int. 2011, 108, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krpina, K.; Babarović, E.; Dorđević, G.; Fuckar, Z.; Jonjić, N. The association between the recurrence of solitary non-muscle invasive bladder cancer and tumor infiltrating lymphocytes. Croat. Med. J. 2012, 53, 598–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, L.J.; Lu, H.T.; Li, G.L.; Wang, X.M.; Su, Y.; Xu, W.H.; Shen, B.Z. Involvement of T helper type 17 and regulatory T cell activity in tumour immunology of bladder carcinoma. Clin. Exp. Immunol. 2010, 161, 480–489. [Google Scholar] [CrossRef]
- Ebelt, K.; Babaryka, G.; Frankenberger, B.; Stief, C.G.; Eisenmenger, W.; Kirchner, T.; Schendel, D.J.; Noessner, E. Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7-H1+ lymphocyte clusters. Eur. J. Cancer 2009, 45, 1664–1672. [Google Scholar] [CrossRef]
- Yunger, S.; Bar El, A.; Zeltzer, L.A.; Fridman, E.; Raviv, G.; Laufer, M.; Schachter, J.; Markel, G.; Itzhaki, O.; Besser, M.J. Tumor-infiltrating lymphocytes from human prostate tumors reveal anti-tumor reactivity and potential for adoptive cell therapy. Oncoimmunology 2019, 8, e1672494. [Google Scholar] [CrossRef] [Green Version]
- Bols, B.; Jensen, L.; Jensen, A.; Braendstrup, O. Immunopathology of in situ seminoma. Int. J. Exp. Pathol. 2000, 81, 211–217. [Google Scholar] [CrossRef]
- Parker, C.; Milosevic, M.; Panzarella, T.; Banerjee, D.; Jewett, M.; Catton, C.; Tew-George, B.; Gospodarowicz, M.; Warde, P. The prognostic significance of the tumour infiltrating lymphocyte count in stage I testicular seminoma managed by surveillance. Eur. J. Cancer 2002, 38, 2014–2019. [Google Scholar] [CrossRef]
- Lohneis, P.; Boral, S.; Kaufmann, A.M.; Lehmann, A.; Schewe, C.; Dietel, M.; Anagnostopoulos, I.; Jöhrens, K. Human papilloma virus status of penile squamous cell carcinoma is associated with differences in tumour-infiltrating T lymphocytes. Virchows Arch. 2015, 466, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Sennepin, A.; Real, F.; Duvivier, M.; Ganor, Y.; Henry, S.; Damotte, D.; Revol, M.; Cristofari, S.; Bomsel, M. The human penis is a genuine immunological effector site. Front. Immunol. 2017, 8, 1732. [Google Scholar] [CrossRef] [Green Version]
- Wouters, M.C.A.; Nelson, B.H. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin. Cancer Res. 2018, 24, 6125–6135. [Google Scholar] [CrossRef] [Green Version]
- Vassallo, J.; Rodrigues, A.F.; Campos, A.H.; Rocha, R.M.; da Cunha, I.W.; Zequi, S.C.; Guimarães, G.C.; da Fonseca, F.P.; Lopes, A.; Cubilla, A.; et al. Pathologic and imunohistochemical characterization of tumoral inflammatory cell infiltrate in invasive penile squamous cell carcinomas: Fox-P3 expression is an independent predictor of recurrence. Tumour Biol. 2015, 36, 2509–2516. [Google Scholar] [CrossRef]
- Hladek, L.; Bankov, K.; von der Grün, J.; Filmann, N.; Demes, M.; Vallo, S.; Wild, P.J.; Winkelmann, R. Tumor-associated immune cell infiltrate density in penile squamous cell carcinomas. Virchows Arch. 2022, 480, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.; de Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-inflammatory drugs as anticancer agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [Green Version]
- Neganova, M.; Liu, J.; Aleksandrova, Y.; Klochkov, S.; Fan, R. Therapeutic influence on important targets associated with chronic inflammation and oxidative stress in cancer treatment. Cancers 2021, 13, 6062. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.C. COX-2 inhibitors in cancer treatment and prevention, a recent development. Anticancer Drugs 2002, 13, 127–137. [Google Scholar] [CrossRef]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.; Craig, B.A.; Cheng, L.; Snyder, P.W.; Mohammed, S.I.; Stewart, J.C.; Zheng, R.; Loman, R.A.; Foster, R.S.; Knapp, D.W. Effects of short-term celecoxib treatment in patients with invasive transitional cell carcinoma of the urinary bladder. Mol. Cancer Ther. 2010, 9, 1371–1377. [Google Scholar] [CrossRef] [Green Version]
- Sabichi, A.L.; Lee, J.J.; Grossman, H.B.; Liu, S.; Richmond, E.; Czerniak, B.A.; De la Cerda, J.; Eagle, C.; Viner, J.L.; Palmer, J.L.; et al. A randomized controlled trial of celecoxib to prevent recurrence of nonmuscle-invasive bladder cancer. Cancer Prev. Res. 2011, 4, 1580–1589. [Google Scholar] [CrossRef] [Green Version]
- Rosser, C.J.; Nix, J.; Ferguson, L.; Hernandez, L.; Wong, H.C. Phase Ib trial of ALT-803, an IL-15 superagonist, plus BCG for the treatment of BCG-naive patients with non-muscle-invasive bladder cancer. J. Clin. Oncol. 2018, 36 (Suppl. 6), 510. [Google Scholar] [CrossRef]
- Sonpavde, G.; Rosser, C.J.; Pan, C.; Parikh, R.A.; Nix, J.; Gingrich, J.R.; Hernandez, L.; Huang, B.; Wong, H.C. Phase I trial of ALT-801, a first-in-class T-cell receptor (TCR)-interleukin (IL)-2 fusion molecule, plus gemcitabine (G) for Bacillus Calmette Guerin (BCG)-resistant non-muscle-invasive bladder cancer (NMIBC). J. Clin. Oncol. 2016, 34, 451-451. [Google Scholar] [CrossRef]
- Hatano, K.; Fujita, K.; Nonomura, N. Application of anti-inflammatory agents in prostate cancer. J. Clin. Med. 2020, 9, 2680. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z. Proinflammatory cytokine interleukin-6 in prostate carcinogenesis. Am. J. Clin. Exp. Urol. 2014, 2, 231–238. [Google Scholar]
- Karkera, J.; Steiner, H.; Li, W.; Skradski, V.; Moser, P.L.; Riethdorf, S.; Reddy, M.; Puchalski, T.; Safer, K.; Prabhakar, U.; et al. The anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I study. Prostate 2011, 71, 1455–1465. [Google Scholar] [CrossRef]
- Liu, C.; Lou, W.; Zhu, Y.; Nadiminty, N.; Schwartz, C.T.; Evans, C.P.; Gao, A.C. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 3198–3210. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.; Hu, J.; Yang, L.; Tan, P.; Tang, Z.; West, B.L.; Bollag, G.; Xu, H.; Wu, L. Inhibition of TAMs improves the response to docetaxel in castration-resistant prostate cancer. Endocr. Relat. Cancer 2019, 26, 131–140. [Google Scholar] [CrossRef]
- Loberg, R.D.; Ying, C.; Craig, M.; Yan, L.; Snyder, L.A.; Pienta, K.J. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia 2007, 9, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loberg, R.D.; Ying, C.; Craig, M.; Day, L.L.; Sargent, E.; Neeley, C.; Wojno, K.; Snyder, L.A.; Yan, L.; Pienta, K.J. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007, 67, 9417–9424. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Kaneda, M.M.; Tsujikawa, T.; Nguyen, A.V.; Affara, N.I.; Ruffell, B.; Gorjestani, S.; Liudahl, S.M.; Truitt, M.; Olson, P.; et al. Bruton Tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016, 6, 270–285. [Google Scholar] [CrossRef] [Green Version]
- Molina-Cerrillo, J.; Alonso-Gordoa, T.; Gajate, P.; Grande, E. Bruton’s tyrosine kinase (BTK) as a promising target in solid tumors. Cancer Treat. Rev. 2017, 58, 41–50. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Oliver, T.; Rowe, M.; Thomas, S.; Zakharia, Y.; Gilman, P.B.; Muller, A.J.; Prendergast, G.C. Indoximod: An immunometabolic adjuvant that empowers T cell activity in cancer. Front. Oncol. 2018, 8, 370. [Google Scholar] [CrossRef] [Green Version]
- Saïd-Sadier, N.; Ojcius, D.M. Alarmins, inflammasomes and immunity. Biomed. J. 2012, 35, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Tang, Y.; Fang, J.; Zhou, Z.; Xing, Z.; Guo, Z.; Guo, X.; Wang, W.; Jiao, W.; Xu, Z.; et al. Simvastatin inhibits renal cancer cell growth and metastasis via AKT/mTOR, ERK and JAK2/STAT3 pathway. PLoS ONE 2013, 8, e62823. [Google Scholar] [CrossRef] [Green Version]
- Guleria, R.S.; Choudhary, R.; Tanaka, T.; Baker, K.M.; Pan, J. Retinoic acid receptor-mediated signaling protects cardiomyocytes from hyperglycemia induced apoptosis: Role of the renin-angiotensin system. J. Cell. Physiol. 2011, 226, 292–307. [Google Scholar] [CrossRef] [Green Version]
- Sourbier, C.; Lindner, V.; Lang, H.; Agouni, A.; Schordan, E.; Danilin, S.; Rothhut, S.; Jacqmin, D.; Helwig, J.J.; Massfelder, T. The phosphoinositide 3-kinase/Akt pathway: A new target in human renal cell carcinoma therapy. Cancer Res. 2006, 66, 5130–5142. [Google Scholar] [CrossRef] [Green Version]
- Daurkin, I.; Eruslanov, E.; Stoffs, T.; Perrin, G.Q.; Algood, C.; Gilbert, S.M.; Rosser, C.J.; Su, L.M.; Vieweg, J.; Kusmartsev, S. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res. 2011, 71, 6400–6409. [Google Scholar] [CrossRef] [PubMed]

| Study | Type of Study | No. of Patients | Population | Experimental Drugs | Primary Endpoint | Results | AEs |
|---|---|---|---|---|---|---|---|
| Dhawan et al. [182] | Prospective study | 13 | Invasive BC | Celecoxib | Tumor cell apoptosis | Apoptosis more frequent in celecoxib vs. controls (p < 0.04) | Mild symptoms (e.g., fatigue, stomach and back pain) in 2 cases |
| Sabichi et al. [183] | Phase II | 146 | High-risk NMIBC | Celecoxib | TTR | Average TTR delayed from 1.74 to 2.86 yrs | 449 AEs, including 60 G ≥ 3 events; no deaths |
| Rosser et al. [184] | Phase I | 9 | BCG-naïve NMIBC | ALT-803 (IL-15 super-agonist) plus BCG | Antitumor activity | All pts disease-free at 24 mos | Hematuria and urinary tract pain related to BCG in all pts; hypertension (G3) in 1 case; no G4 AEs or deaths |
| NCT03022825 | Phase II | 200 | BCG unresponsive high-grade NMIBC | ALT-803 plus BCG | CR; DFR | NA | NA |
| NCT00433446 | Phase II | 62 | mCRPC | Siltuximab (IL-6 inhibitor) | PSA response | PSA RR 3.8% | DIC and cerebral ischemia (G4) in 1 case; elevated AST/ALT; gastritis; hematologic toxicity (G3) |
| NCT00385827 | Phase II | 106 | HRPC | Siltuximab + mitoxantrone/prednisone | AEs; SAEs | NA | NA |
| NCT03123978 | Phase I | 12 | mCRPC | Niclosamide plus enzalutamide | AEs; RP2D | NA | NA |
| NCT02807805 | Phase II | 37 | CRPC | Abiraterone plus niclosamide | PSA response | NA | NA |
| NCT01560923 | Phase II | 63 | mCRPC | Indoximod after sipuleucel-T | Immune response to sipuleucel-T | No difference in PSA progression; higher median rPFS with indoximod (10.3 vs. 4.1 mos, p = 0.011) | No significant difference between the two arms |
| NCT02472275 | Phase I | 8 | Unfavorable risk PCa | Pexidartinib | AEs; MTD | NA | NA |
| NCT02643667 | Phase II | 27 | Localized PCa | Ibrutinib | DLT | NA | NA |
| NCT01234311 | Phase III | 1245 | mCRPC | Tasquinimod | PFS | Higher median rPFS with tasquinimod (7.0 vs. 4.4 mos, p < 0.001) | G ≥ 3 AEs 42.8% |
| NCT00992186 | Phase II | 46 | mCRPC | CNTO 888 (mAb anti-CCL2) | Composite response | 1 SD > 6 mos; 14 SD ≥ 3 mos; median OS 10.2 mos; no PSA or radiological response | G ≥ 3 AEs 67%; SAEs including pneumonia, spinal cord compression, back pain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, M.; Roviello, G.; Santi, R.; Villari, D.; Spatafora, P.; Galli, I.C.; Sessa, F.; Conte, F.L.; Mini, E.; Cai, T.; et al. Inflammation in Urological Malignancies: The Silent Killer. Int. J. Mol. Sci. 2023, 24, 866. https://doi.org/10.3390/ijms24010866
Catalano M, Roviello G, Santi R, Villari D, Spatafora P, Galli IC, Sessa F, Conte FL, Mini E, Cai T, et al. Inflammation in Urological Malignancies: The Silent Killer. International Journal of Molecular Sciences. 2023; 24(1):866. https://doi.org/10.3390/ijms24010866
Chicago/Turabian StyleCatalano, Martina, Giandomenico Roviello, Raffaella Santi, Donata Villari, Pietro Spatafora, Ilaria Camilla Galli, Francesco Sessa, Francesco Lupo Conte, Enrico Mini, Tommaso Cai, and et al. 2023. "Inflammation in Urological Malignancies: The Silent Killer" International Journal of Molecular Sciences 24, no. 1: 866. https://doi.org/10.3390/ijms24010866
APA StyleCatalano, M., Roviello, G., Santi, R., Villari, D., Spatafora, P., Galli, I. C., Sessa, F., Conte, F. L., Mini, E., Cai, T., & Nesi, G. (2023). Inflammation in Urological Malignancies: The Silent Killer. International Journal of Molecular Sciences, 24(1), 866. https://doi.org/10.3390/ijms24010866










