Piriformospora indica Increases Resistance to Fusarium pseudograminearum in Wheat by Inducing Phenylpropanoid Pathway
Abstract
:1. Introduction
2. Results
2.1. P. indica Promote Root Growth and Plant Development
2.2. P. indica Induces Disease Resistance to F. pseudograminearum
2.3. Seeds Precolonized by P. indica Reduced the Colonization of Pathogen F. pseudograminearum
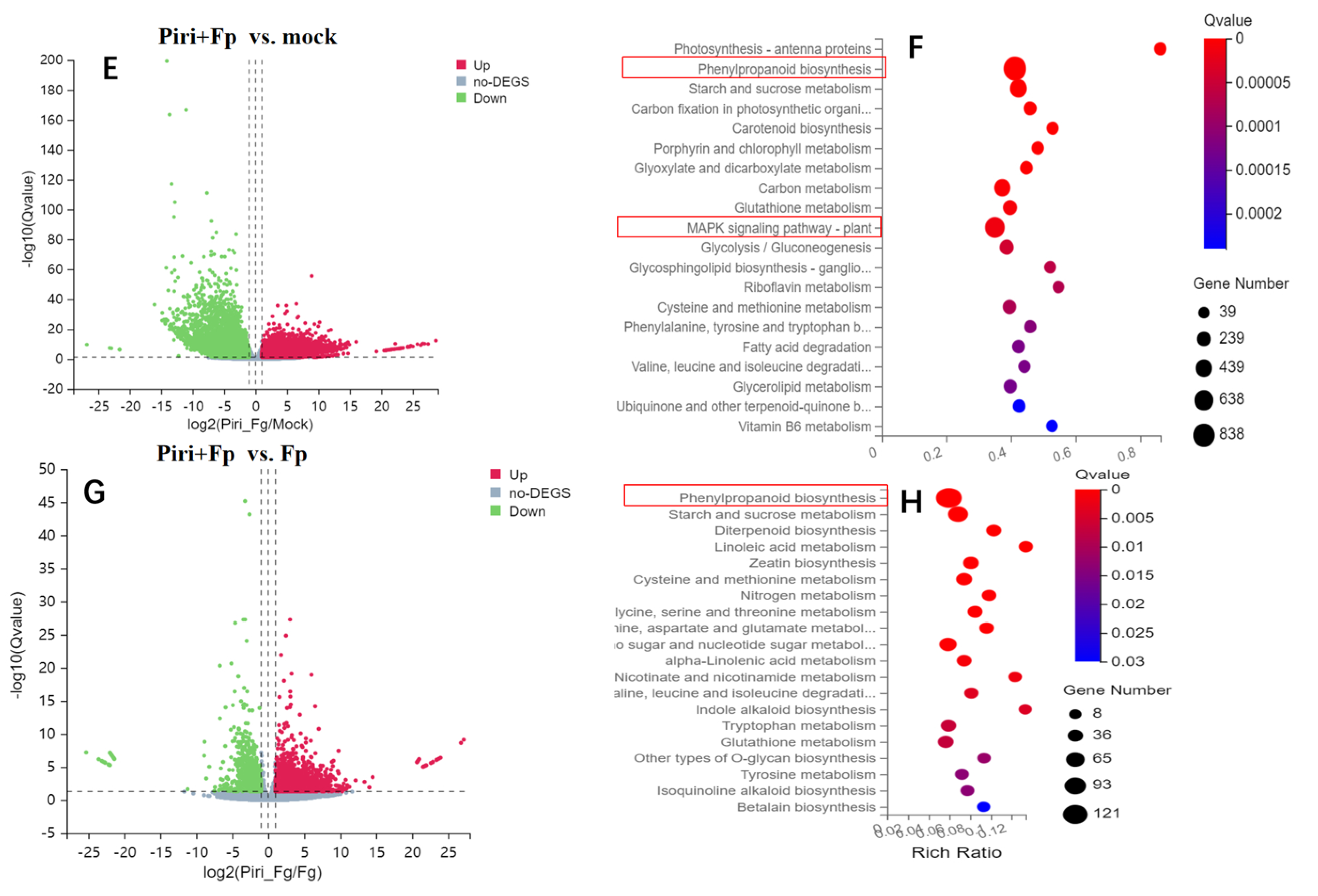
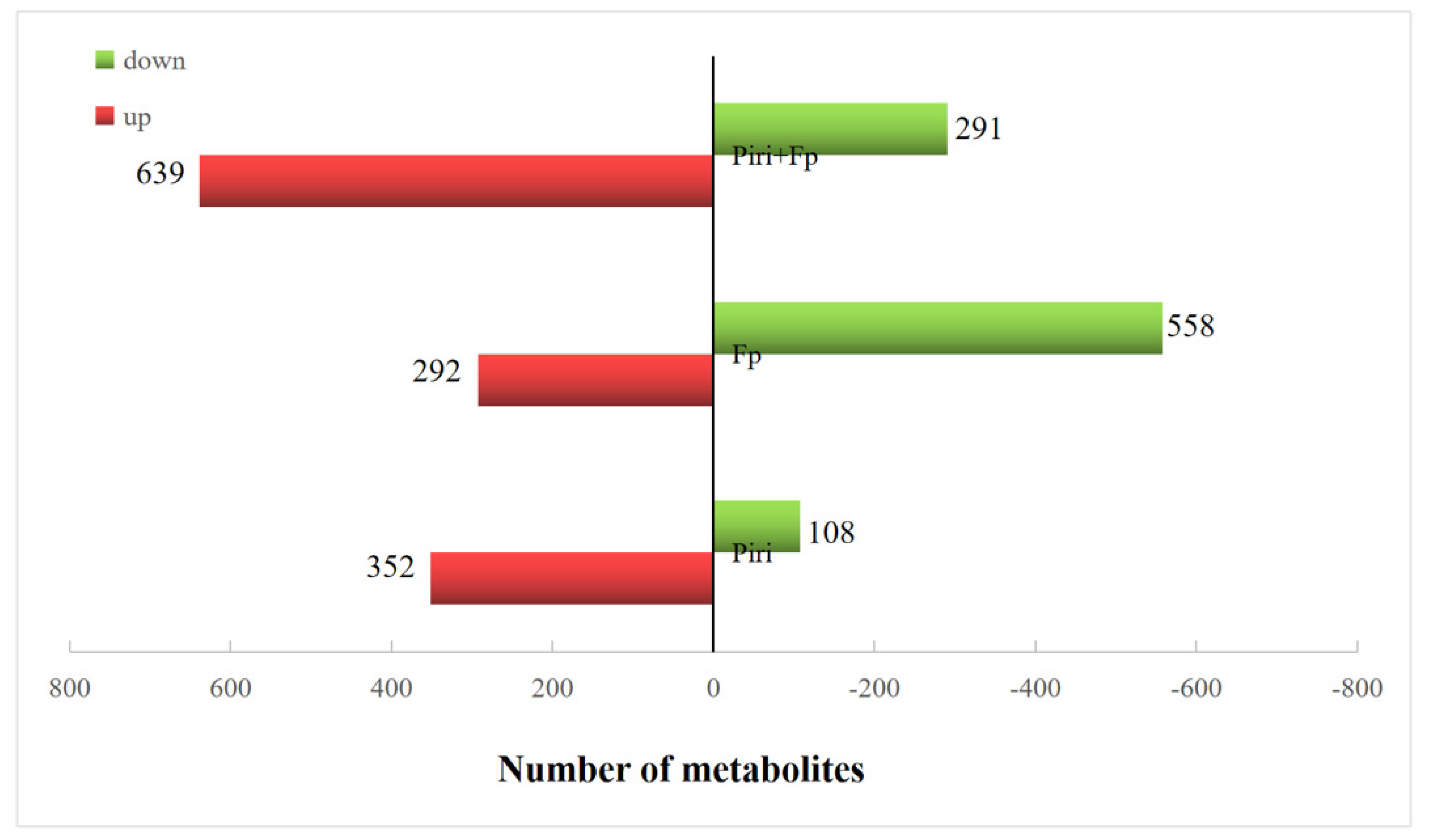
2.4. Expression Profile Analysis in Wheat Responsive to Piriformospora indica and F. pseudograminearum Colonization
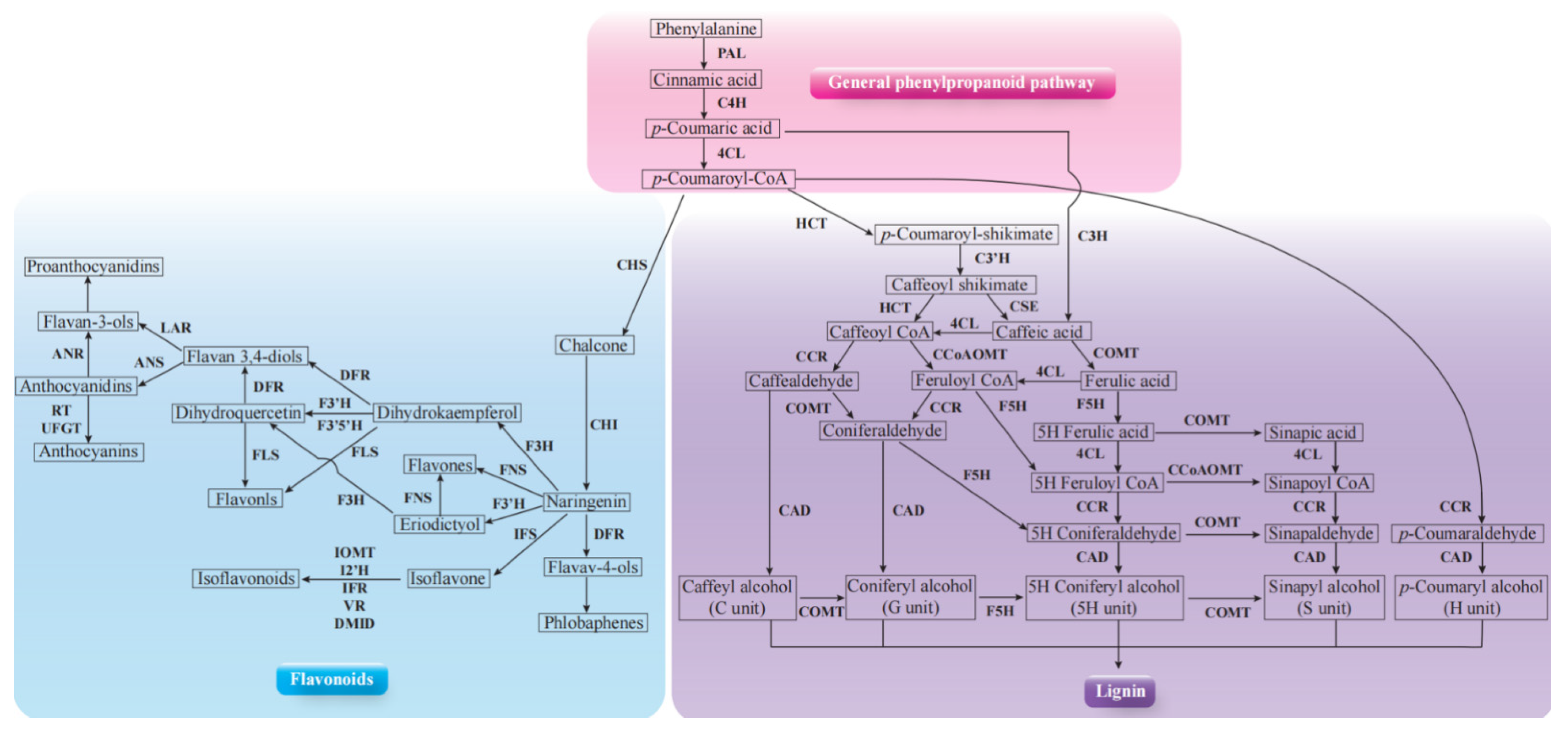
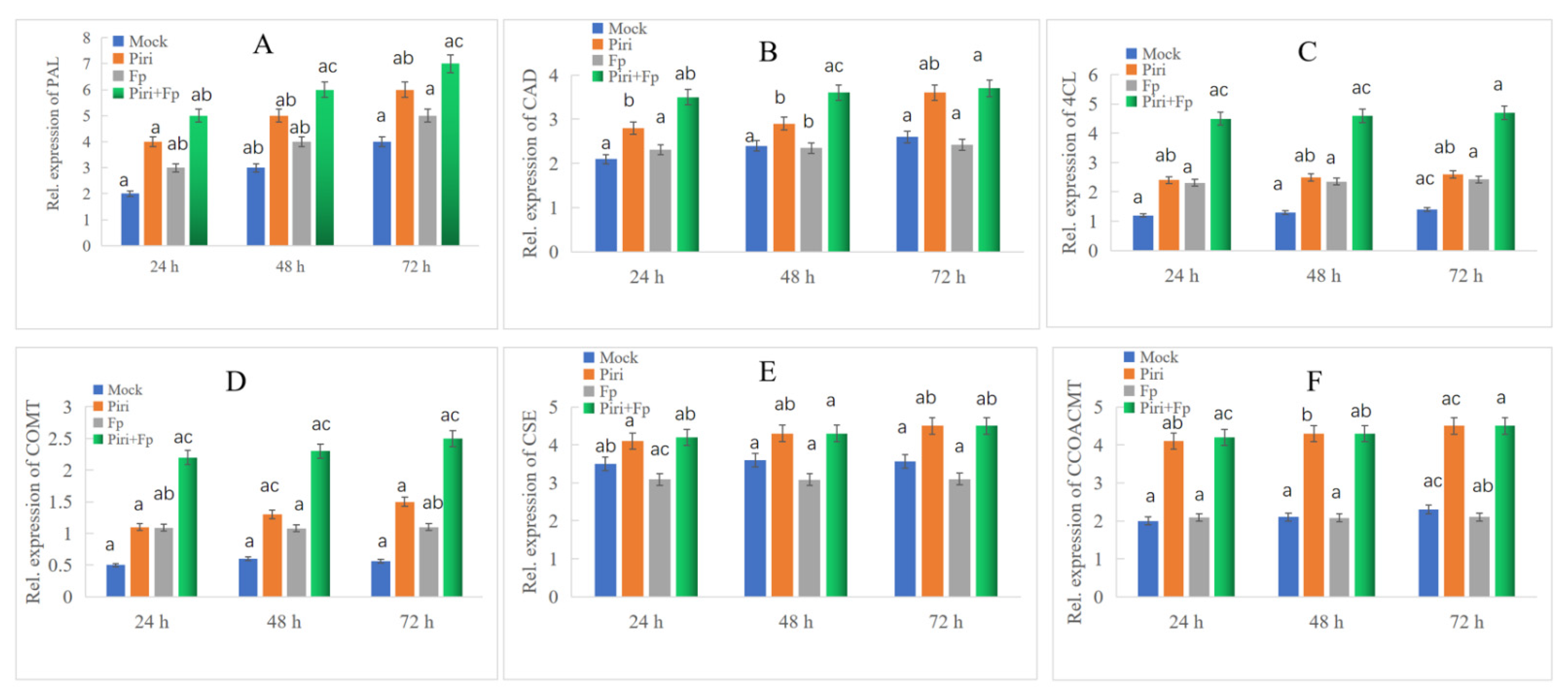
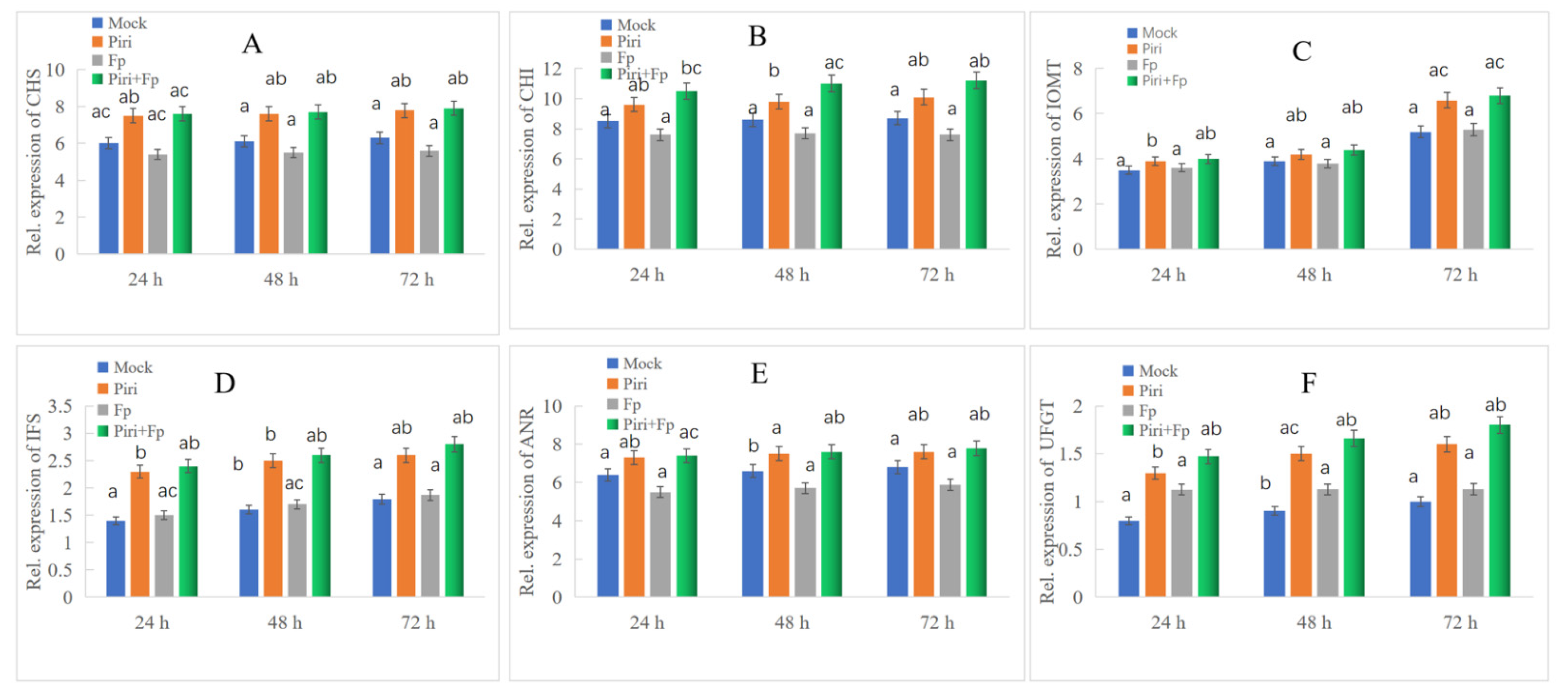
2.5. Key DEGs Involved in Phenylpropanoid Biosynthesis Pathway Expression Identification by QPCR
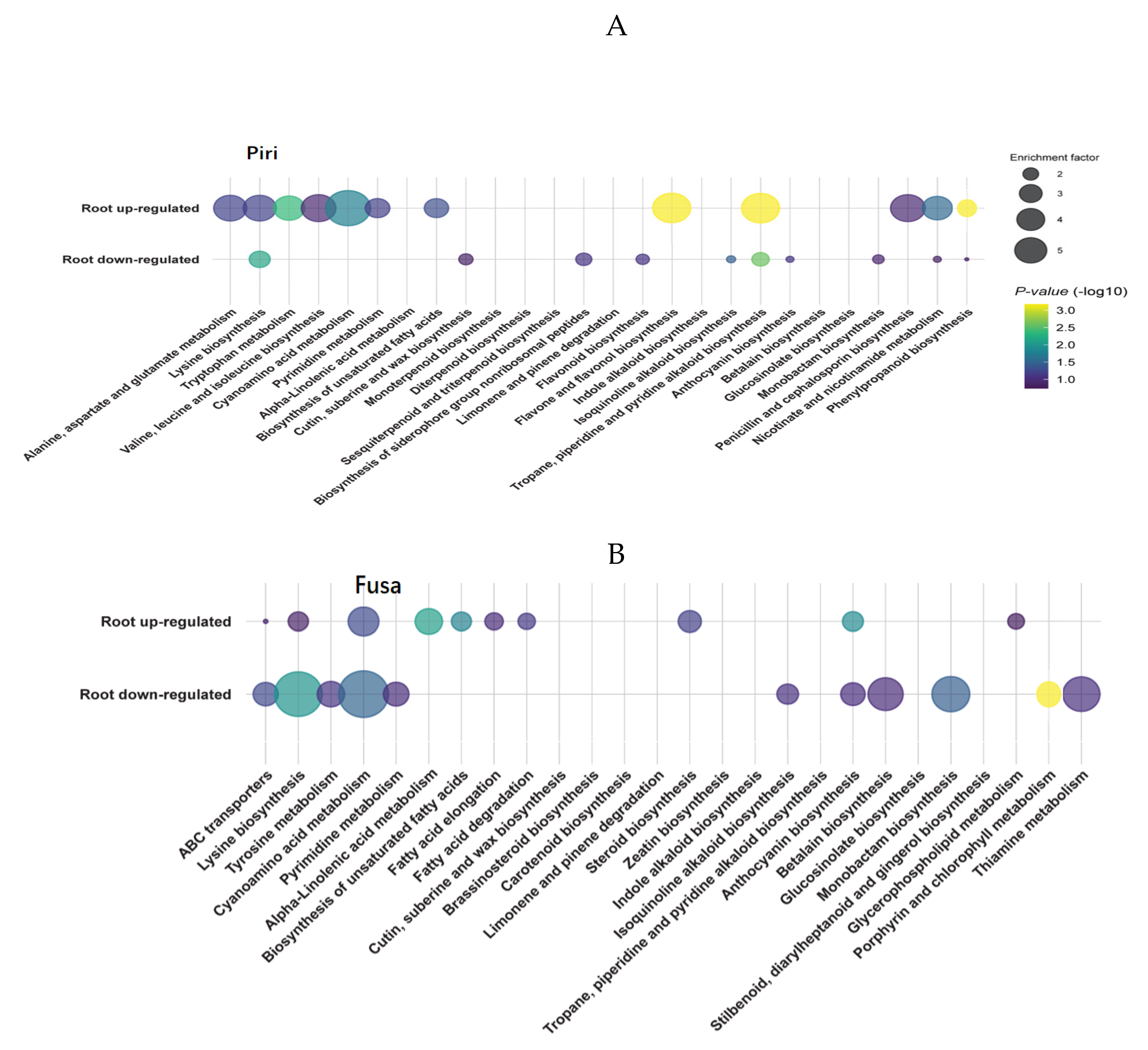
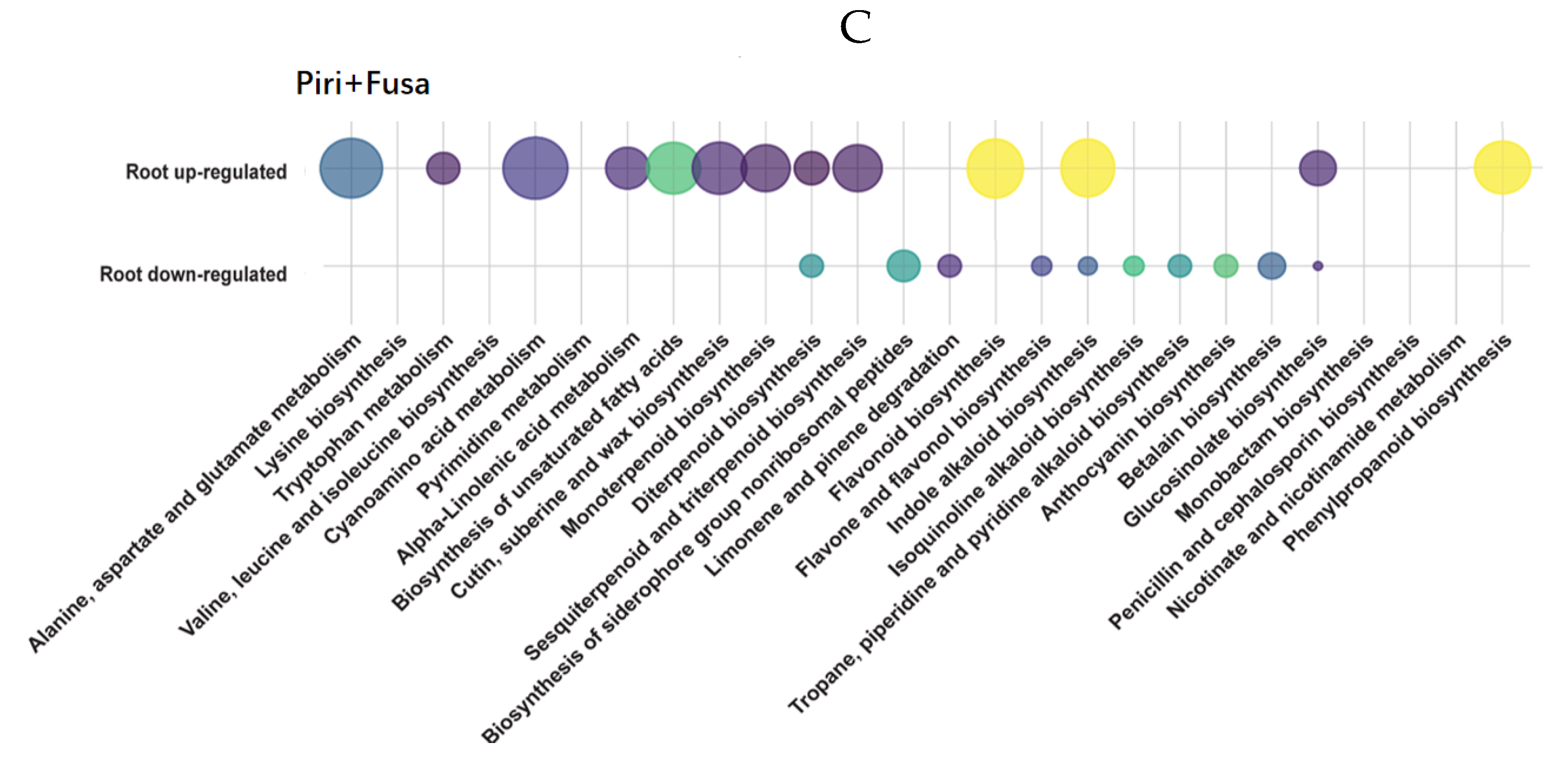
2.6. Metabolic Profiles of Wheat Root in Response to P. indica Colonization
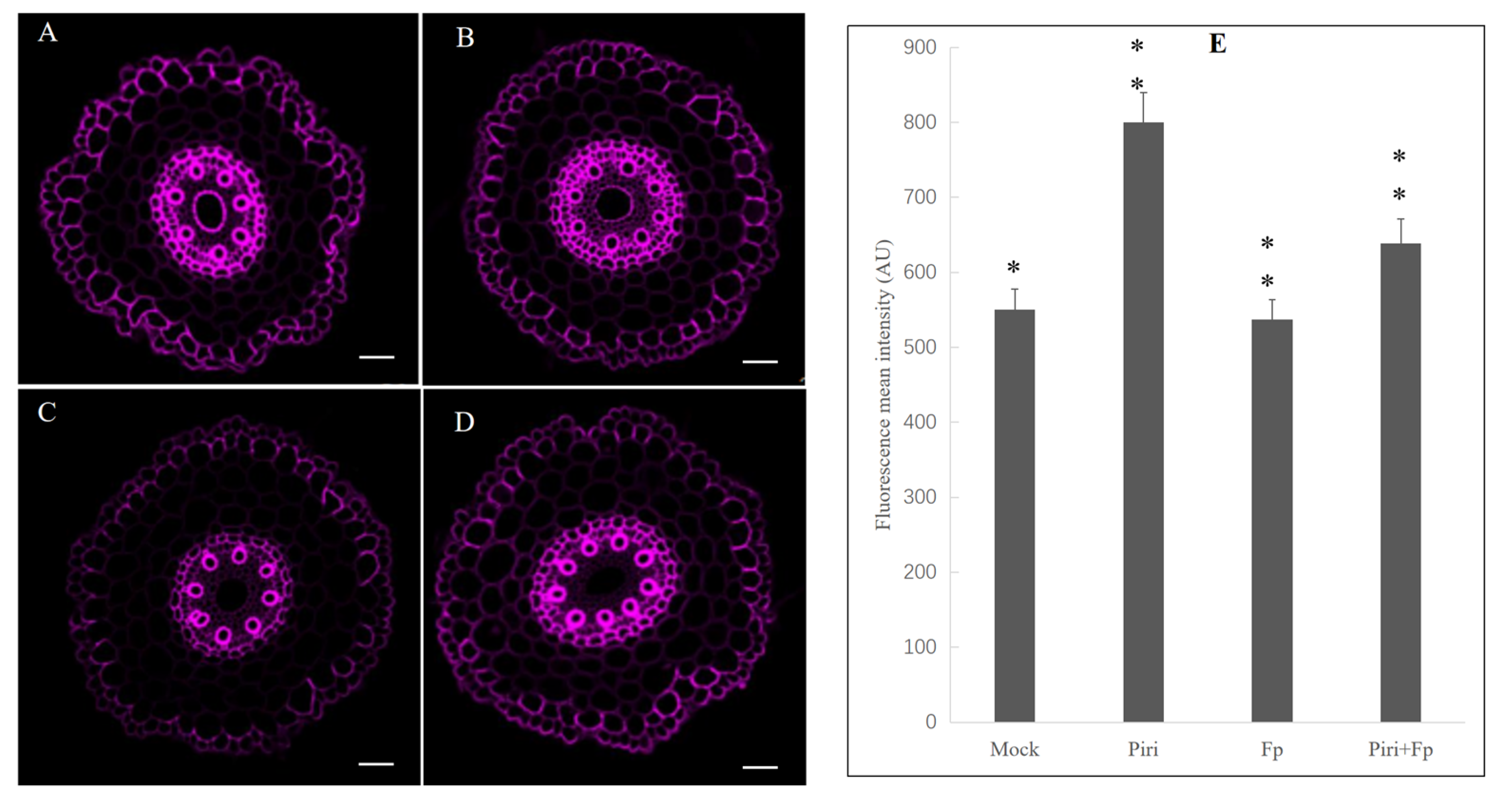
2.7. Lignin Staining of Wheat Roots among Different Treatments
3. Discussion
4. Materials and Methods
4.1. Fungal Inoculums Preparation and Inoculation
4.2. Colonization Observation of P. indica
4.3. Plant Disease Evaluation
4.4. Deoxynivalenol (DON) Assay
4.5. Quantification of F. pseudograminearum Colonization by QPCR
4.6. Histochemical Detection of Lignin
4.7. mRNA Library Construction
4.8. GO and KEGG Enrichment Analysis of Differentially Expressed Genes
4.9. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR) for Validation of Sequencing Results
4.10. Metabolic Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smiley, R.W.; Gourlie, J.A.; Easley, S.A.; Patterson, L.M.; Whittaker, R.G. Crop Damage Estimates for Crown Rot of Wheat and Barley in the Pacific Northwest. Plant Dis. 2005, 89, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, S.; Simpfendorfer, S.; Bentley, A.R.; Hickey, L.T. Crown rot of wheat in Australia: Fusarium pseudograminearum taxonomy, population biology and disease management. Australas. Plant Pathol. 2018, 47, 285–299. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Li, W.; Zhang, P.; Sun, H.Y.; Zhang, X.X.; Zhang, A.X.; Chen, H.G. Fusarium pseudograminearum as an emerging pathogen of crown rot of wheat in eastern China. Plant Pathol. 2020, 69, 240–248. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Fan, Z.; Wu, J.; Wang, L.; He, D.; Mohamed, S.R.; Dawood, D.H.; Shi, J.; Gao, T.; et al. Antifungal activities of metconazole against the emerging wheat pathogen Fusarium pseudograminearum. Pestic. Biochem. Physiol. 2023, 190, 105298. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.F.; Yan, Z.; Yang, M.X.; Waalwijk, C.; Van der Lee, T.; Van Diepeningen, A.; Brankovics, B.; Chen, W.; Feng, J.; Zhang, H. Contamination and translocation of deoxynivalenol and its derivatives associated with Fusarium Crown Rot of wheat in Northern China. Plant Dis. 2021, 105, 3397–3406. [Google Scholar] [CrossRef]
- Duan, Y.; Xiao, X.; Li, T.; Chen, W.; Wang, J.; Fraaije, B.A.; Zhou, M. Impact of epoxiconazole on Fusarium head blight control, grain yield and deoxynivalenol accumulation in wheat. Pestic. Biochem. Physiol. 2018, 152, 138–147. [Google Scholar] [CrossRef]
- Bai, G.H.; Plattner, R.; Desjardins, A.; Kolb, F.; Jones, S.S. Resistance to fusarium head blight and deoxynivalenol accumulation in wheat. Plant Breed. 2001, 120, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yu, J.J.; Zhang, Y.N.; Zhang, X.; Cheng, C.J.; Wang, J.X.; Hollomon, D.W.; Fan, P.-S.; Zhou, M.-G. Effect of carbendazim resistance on trichothecene production and aggressiveness of Fusarium graminearum. MPMI 2009, 22, 1143–1150. [Google Scholar] [CrossRef]
- Li, L.; Guo, N.; Feng, Y.; Duan, M.; Li, C. Effect of Piriformospora indica-induced systemic resistance and basal immunity against Rhizoctonia cerealis and Fusarium graminearum in Wheat. Front. Plant Sci. 2022, 13, 836940. [Google Scholar] [CrossRef]
- Verma, S.; Varma, A.; Rexer, K.H.; Hassel, A.; Kost, G.; Sarbhoy, A.; Bisen, P.; Bütehorn, B.; Franken, P. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 1998, 90, 896–903. [Google Scholar] [CrossRef]
- Qiang, X.; Weiss, M.; Kogel, K.-H.; Schäfer, P. Piriformospora indica—A mutualistic basidiomycete with an exceptionally large plant host range. Mol. Plant Pathol. 2012, 13, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Zuccaro, A.; Basiewicz, M.; Zurawska, M.; Biedenkopf, D.; Kogel, K.H. Karyotype analysis, genome organization, and stable genetic transformation of the root colonizing fungus Piriformospora indica. Fungal Genet. Biol. 2009, 46, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Mishra, S.; Kundu, P.; Jogawat, A.; Vadassery, J. Piriformospora indica recruits host-derived putrescine for growth promotion in plants. Plant Physiol. 2021, 188, 2289–2307. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.; Molitor, A.; Kogel, K.H.; Waller, F. Systemic resistance in Arabidopsis conferred by the mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1. Plant Cell Physiol. 2008, 49, 1747–1751. [Google Scholar] [CrossRef]
- Fakhro, A.; Andrade-Linares, D.R.; Von Bargen, S.; Bandte, M.; Büttner, C.; Grosch, R.; Schwarz, D.; Franken, P. Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 2010, 20, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kuo, Y.W.; Lin, K.H.; Huang, W.; Deng, C.; Yeh, K.W.; Chen, S.P. Piriformospora indica colonization increases the growth, development, and herbivory resistance of sweet potato (Ipomoea batatas L.). Plant Cell Rep. 2021, 40, 339–350. [Google Scholar] [CrossRef]
- Sahay, N.S.; Varma, A. Piriformospora indica: A new biological hardening tool for micropropagated plants. FEMS Microbiol. Lett. 2006, 181, 297–302. [Google Scholar] [CrossRef]
- Deshmukh, S.D.; Kogel, K.-H. Piriformospora indica protects barley from root rot caused by Fusarium graminearum. J. Plant Dis. Prot. 2007, 114, 263–268. [Google Scholar] [CrossRef]
- Sun, C.; Shao, Y.; Vahabi, K.; Lu, J.; Bhattacharya, S.; Dong, S.; Yeh, K.-W.; Sherameti, I.; Lou, B.; Baldwin, I.T.; et al. The beneficial fungus Piriformospora indica protects Arabidopsis from Verticillium dahliae infection by downregulation plant defense responses. BMC Plant Biol. 2014, 14, 268. [Google Scholar] [CrossRef]
- Panda, S.; Busatto, N.; Hussain, K.; Kamble, A. Piriformospora indica-primed transcriptional reprogramming induces defense response against early blight in tomato. Sci. Hortic. 2019, 255, 209–219. [Google Scholar] [CrossRef]
- Jacobs, S.; Zechmann, B.; Molitor, A.; Trujillo, M.; Petutschnig, E.; Lipka, V.; Kogel, K.H.; Schäfer, P. Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica. Plant Physiol. 2011, 156, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Waller, F.; Mukherjee, K.; Deshmukh, S.D.; Achatz, B.; Sharma, M.; Schäfer, P.; Kogel, K.H. Systemic and local modulation of plant responses by Piriformospora indica and related Sebacinales species. J. Plant Physiol. 2008, 165, 60–70. [Google Scholar] [CrossRef]
- Ding, Y.; Kalo, P.; Yendrek, C.; Sun, J.; Liang, Y.; Marsh, J.F.; Harris, J.M.; Oldroyd, G.E.D. Abscisic acid coordinates nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell. 2008, 20, 2681–2695. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Saxena, P.; Choudhary, D.K.; Abdin, M.Z.; Varma, A. Dual symbiosis between Piriformospora indica and Azotobacter chroococcum enhances the artemisinin content in Artemisia annua L. World J. Microbiol. Biotechnol. 2016, 32, 19. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Kamal, S.; Sharma, P.K.; Oelmüller, R.; Varma, A. Root endophyte Piriformospora indica DSM 11827 alters plant morphology, enhances biomass and antioxidant activity of medicinal plant Bacopa monniera. J. Basic Microbiol. 2013, 53, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Tripathi, S.; Varma, A. In vitro plant development and root colonization of Coleus forskohlii by Piriformospora indica. World J. Microbiol. Biotechnol. 2014, 30, 1075–1084. [Google Scholar] [CrossRef]
- Bajaj, R.; Agarwal, A.; Rajpal, K.; Asthana, S.; Kumar, R.; Prasad, R.; Kharkwal, A.; Sherameti, I.; Oelmüller, R.; Varma, A. Co-cultivation of Curcuma longa with Piriformospora indica enhances the yield and active ingredients. Am. J. Curr. Microbiol. 2014, 2, 12. [Google Scholar]
- Dong, N.-Q.; Lin, H.-X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Cocetta, G.; Ferrante, A. The antioxidants changes in ornamental flowers during development and senescence. Antioxidants 2013, 2, 132–155. [Google Scholar] [CrossRef]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From Biosynthesis to Health Benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Grotewold, E. Plant specialized metabolism. Plant Sci. 2020, 298, 110579. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Z.; Jianxu, L.; Jianhua, L.; Lv, H.; Yang, J.; Li, W.; Xie, D.; Xiong, Z.; Zhang, P. Diterpenoid UDP-Glycosyltransferases from Chinese Sweet Tea and Ashitaba Complete the Biosynthesis of Rubusoside. Mol. Plant 2018, 11, 1308–1311. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, J.; Zhang, G.; Ding, W.; Duan, L.; Yang, J.; Kui, L.; Cheng, X.; Ruan, J.; Fan, W.; et al. Engineering yeast for the production of breviscapine by genomic analysis and synthetic biology approaches. Nat. Commun. 2018, 9, 448. [Google Scholar] [CrossRef]
- Halpin, C. Lignin engineering to improve saccharification and digestibility in grasses. Curr. Opin. Biotechnol. 2019, 56, 223–229. [Google Scholar] [CrossRef]
- Chanoca, A.; De Vries, L.; Boerjan, W. Lignin Engineering in Forest Trees. Front. Plant Sci. 2019, 10, 912. [Google Scholar] [CrossRef]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef]
- Burgess, B.A.; Backhouse, D.; Summerell, B.A.; Swan, L.J. Crown rot of wheat. In Fusarium: Paul E. Nelson Memorial Symposium; Summerell, B.A., Leslie, J.F., Backhouse, D., Bryden, W.L., Burgess, L.W., Eds.; APS Press: St. Paul, MN, USA, 2001; pp. 271–294. [Google Scholar]
- Kazan, K.; Gardiner, D.M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef]
- Beccari, G.; Covarelli, L.; Nicholson, P. Infection processes and soft wheat response to root rot and crown rot caused by Fusarium culmorum: Root rot and crown rot caused by F. culmorum on soft wheat. Plant Pathol. 2011, 60, 671–684. [Google Scholar] [CrossRef]
- Backhouse, D.; Burgess, L.W. Climatic analysis of the distribution of Fusarium graminearum, F. pseudograminearum and F. culmorum on cereals in Australia. Australas. Plant Pathol. 2002, 31, 321–327. [Google Scholar]
- Saad, A.; Christopher, J.; Martin, A.; McDonald, S.; Percy, C. Fusarium pseudograminearum and F. culmorum affect the root system architecture of bread wheat. Crop J. 2023, 11, 316–321. [Google Scholar] [CrossRef]
- Yan, C.; Rizwan, M.H.; Liang, D.; Reichelt, M.; Mithöfer, A.; Scholz, S.S.; Oelmüller, R.; Chen, F. The effect of the root-colonizing Piriformospora indica on passion fruit (Passiflora edulis) development: Initial defense shifts to fitness benefits and higher fruit quality. Food Chem. 2021, 359, 129671. [Google Scholar] [CrossRef]
- Jahandideh Mahjen Abadi, V.A.; Sepehri, M.; Khatabi, B.; Rezaei, M. Alleviation of zinc deficiency in wheat inoculated with root endophytic fungus Piriformospora indica and rhizobacterium Pseudomonas putida. Rhizosphere 2021, 17, 100311. [Google Scholar] [CrossRef]
- Sharma, M.; Schmid, M.; Rothballer, M.; Hause, G.; Zuccaro, A.; Imani, J.; Kämpfer, P.; Domann, E.; Schäfer, P.; Hartmann, A.; et al. Detection and identification of bacteria intimately associated with fungi of the order Sebacinales. Cell. Microbiol. 2008, 10, 2235–2246. [Google Scholar] [CrossRef]
- Gray, J.; Caparrós-Ruiz, D.; Grotewold, E. Grass phenylpropanoids: Regulate before using! Plant Sci. 2012, 184, 112–120. [Google Scholar] [CrossRef]
- Lanot, A.; Hodge, D.; Lim, E.-K.; Vaistij, F.E.; Bowles, D.J. Redirection of flux through the phenylpropanoid pathway by increased glucosylation of soluble intermediates. Planta 2008, 228, 609–616. [Google Scholar] [CrossRef]
- Xu, W.; Xu, X.; Han, R.; Wang, X.; Wang, K.; Qi, G.; Ma, P.; Komatsuda, T.; Liu, C. Integrated transcriptome and metabolome analysis reveals that flavonoids function in wheat resistance to powdery mildew. Front. Plant Sci. 2023, 14, 1125194. [Google Scholar] [CrossRef]
- Nassimi, Z.; Taheri, P. Endophytic fungus Piriformospora indica induced systemic resistance against rice sheath blight via affecting hydrogen peroxide and antioxidants. Biocontrol Sci. Technol. 2017, 27, 252–267. [Google Scholar] [CrossRef]
- Saad, A.; Macdonald, B.; Martin, A.; Knight, N.L.; Percy, C. Comparison of disease severity caused by four soil-borne pathogens in winter cereal seedlings. Crop Pasture Sci. 2021, 5, 325–334. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Wang, X.; Zhu, P.; Wu, H.; Qi, S. Plant growth-promoting endophyte Piriformospora indica alleviates salinity stress in Medicago truncatula. Plant Physiol. Biochem. 2017, 119, 211–223. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Chen, Y. Pathogenicity of Fusarium species on different plant parts of spring wheat under controlled conditions. Plant Dis. 2005, 89, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ursache, R.; Andersen, T.G.; Marhavý, P.; Geldner, N. A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J. 2018, 93, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Sexauer, M.; Shen, D.; Schön, M.; Andersen, T.G.; Markmann, K. Visualizing polymeric components that define distinct root barriers across plant lineages. Development 2021, 148, dev199820. [Google Scholar] [CrossRef]
- Basheer, J.; Vadovič, P.; Šamajová, O.; Melicher, P.; Komis, G.; Křenek, P.; Králová, M.; Pechan, T.; Ovečka, M.; Takáč, T.; et al. Knockout of MITOGEN-ACTIVATED PROTEIN KINASE 3 causes barley root resistance against Fusarium graminearum. Plant Physiol. 2022, 190, 2847–2867. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
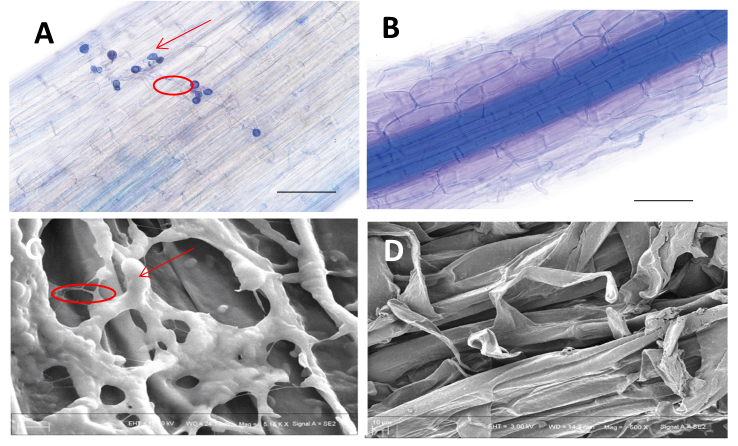
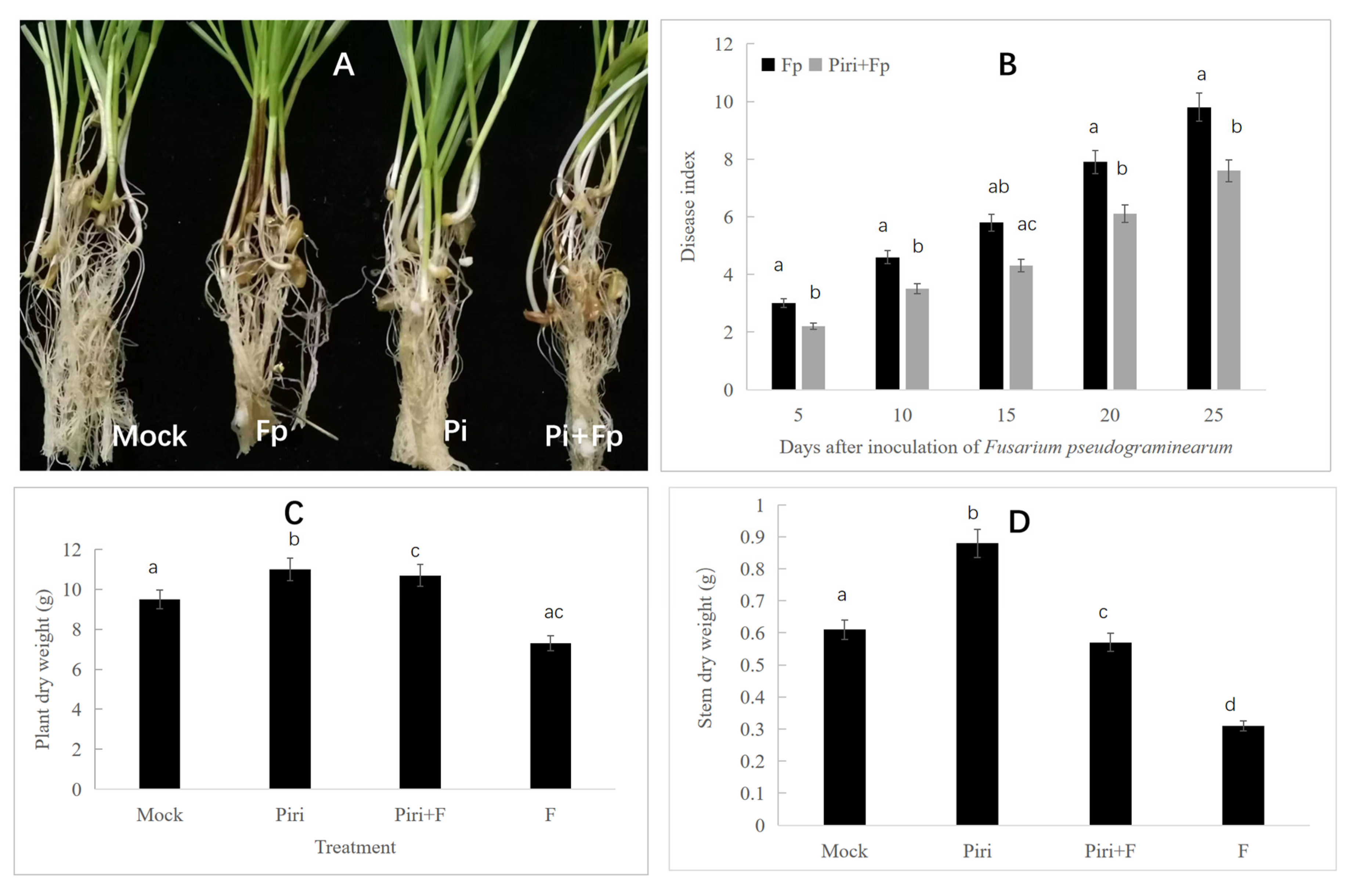

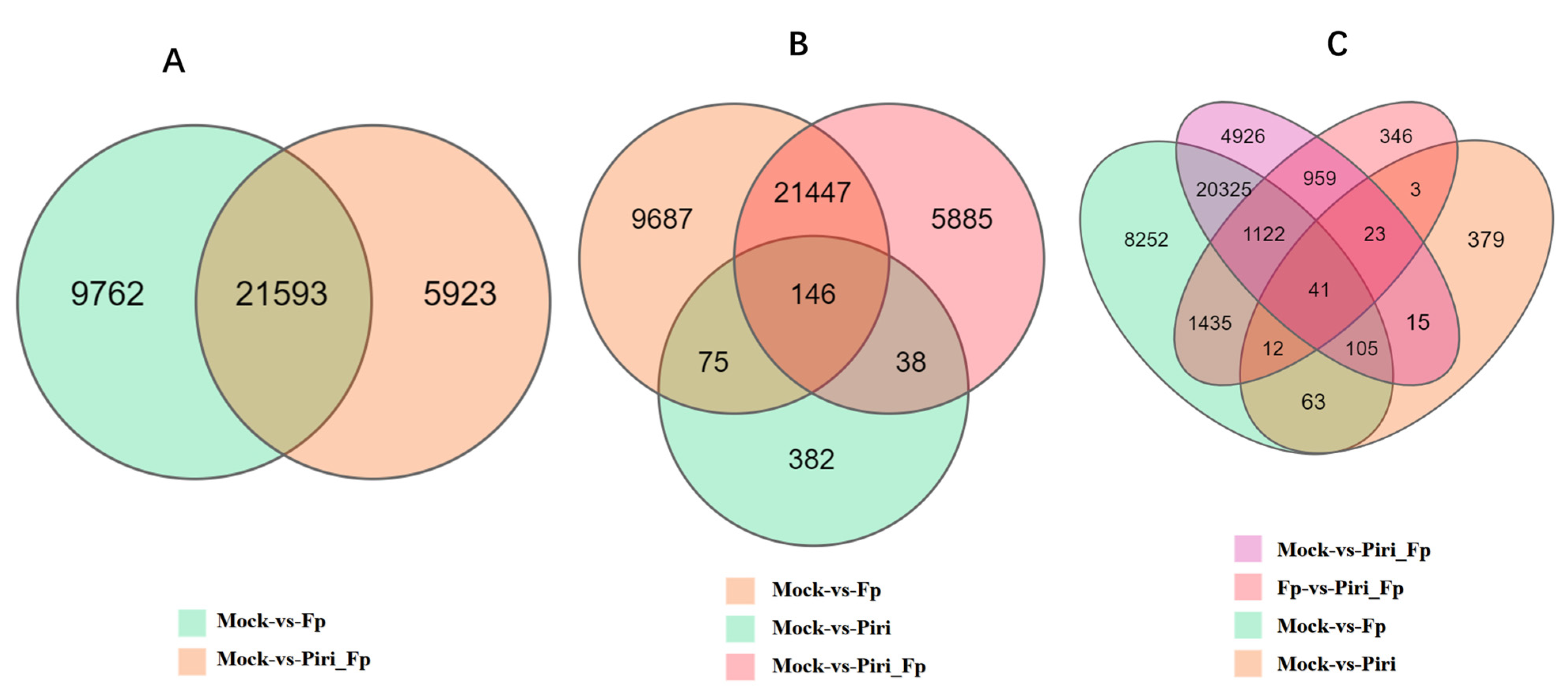
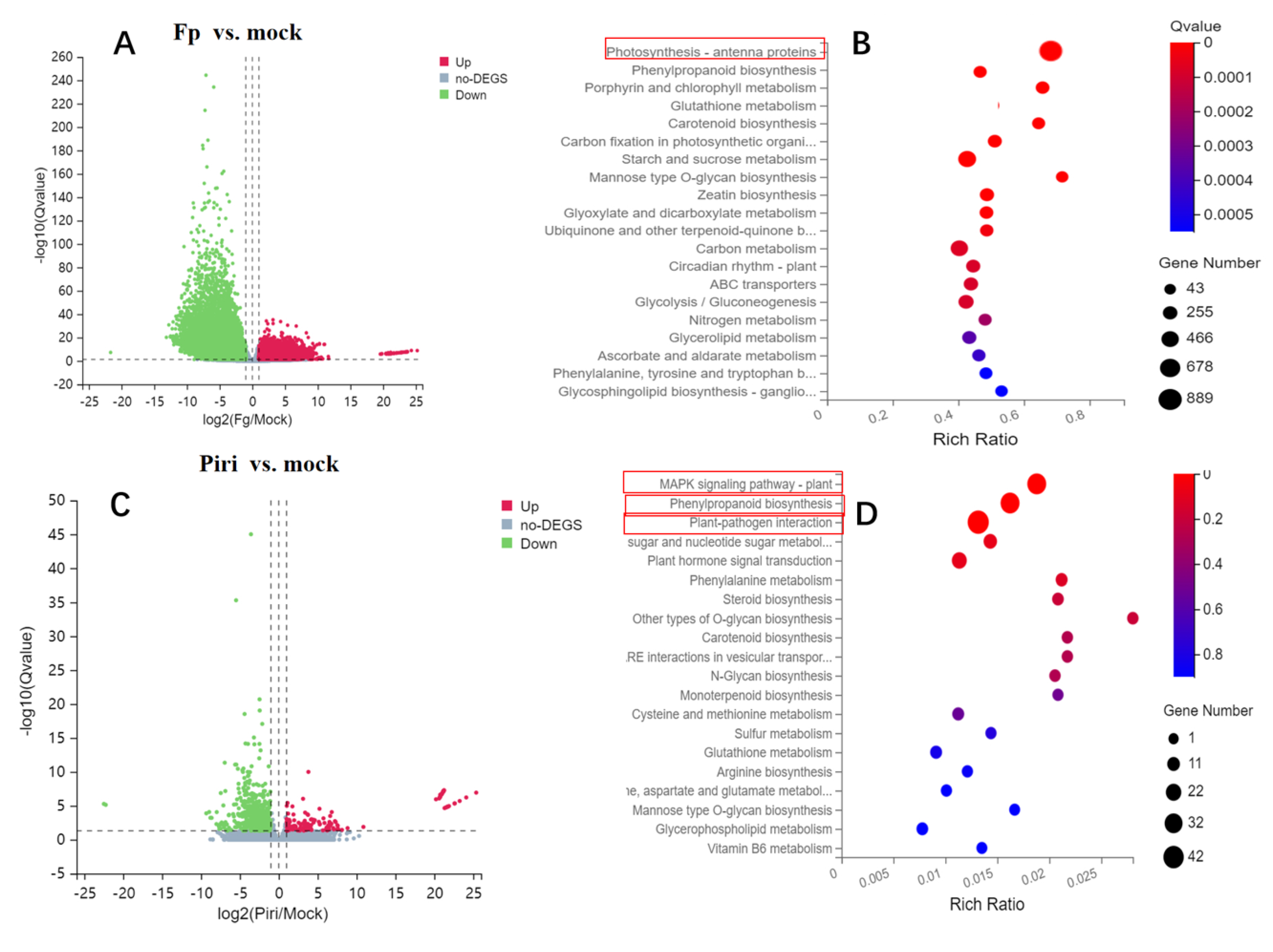
| ID | NCBI Accession | Description | GO | KEGG Pathway |
|---|---|---|---|---|
| TraesCS2D02G377200 | XM_044477614.1 | Triticum aestivum phenylalanine ammonia-lyase-like (PAL) | GO: 0045548 phenylalanine ammonia-lyase activity GO: 0006559 L-phenylalanine catabolic process GO: 0009800 cinnamic acid biosynthetic process | 00360 Phenylalanine metabolism 00940 Phenylpropanoid biosynthesis |
| TraesCS5A02G213900 | XM_044525426.1 | Triticum aestivum probable cinnamyl alcohol dehydrogenase 8D | GO: 0008270 zinc ion binding GO: 0016616 oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor GO: 0045551 cinnamyl-alcohol dehydrogenase activity GO: 0052747 inapyl alcohol dehydrogenase activity GO: 0009809 lignin biosynthetic process | 00940 Phenylpropanoid biosynthesis |
| TraesCS7A02G084600 | XM_044570301.1 | Triticum aestivum probable O-methyltransferase 2 | GO: 0008171 O-methyltransferase activity GO: 0008757 S-adenosylmethionine-dependent methyltransferase activity GO: 0046983 protein dimerization activity GO: 0019438 aromatic compound biosynthetic process GO: 0032259 methylation | 00945 Stilbenoid, diarylheptanoid, and gingerol biosynthesis |
| TraesCS2B02G395400 | XM_044469367.1 | Triticum aestivum caffeoyl shikimate esterase-like | 00561 Glycerolipid metabolism | |
| (4CL) TraesCS6B02G294100 | XM_044557718.1 | Triticum aestivum 4-coumarate-oA ligase 2-like | GO: 0016207 4-coumarate-CoA ligase activity GO: 0009698 phenylpropanoid metabolic process | 00130 Ubiquinone and other terpenoid-quinone biosynthesis 00940 Phenylpropanoid biosynthesis |
| TraesCS7D02G239400 | XM_044583893.1 | Triticum aestivum tricin synthase 2 | GO: 0042409 caffeoyl-CoA O-methyltransferase activity CCOACMT GO: 0046872 metal ion binding GO: 0009809 lignin biosynthetic process GO: 0032259 methylation | |
| TraesCS5B02G268300 | XM_044533418.1 | Triticum aestivum cinnamoyl-CoA reductase 1-like | GO: 0016491 oxidoreductase activity GO: 0016616 oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor GO: 0016621 cinnamoyl-CoA reductase activity GO: 0050662 coenzyme binding GO: 0007623 circadian rhythm GO: 0009809 lignin biosynthetic process | 00940 Phenylpropanoid biosynthesis |
| ID | NCBI Accession | Description | GO | KEGG Pathway |
|---|---|---|---|---|
| TraesCS5D02G488700 | XM_044543986.1 | Triticum aestivum chalcone synthase 2-like(CHS) | GO: 0016210 naringenin-chalcone synthase activity GO: 0009813 flavonoid biosynthetic process | 00941/Flavonoid biosynthesis 04712 Circadian rhythm—plant |
| TraesCS5D02G489000 | XM_044546410.1 | Triticum aestivum chalcone--flavanone isomerase-like | GO: 0045430 chalcone isomerase activity GO: 0009813 flavonoid biosynthetic process (CHI) | 00941 Flavonoid biosynthesis |
| TraesCS7A02G333900 | XM_044567041.1 | Triticum aestivum flavone O-methyltransferase 1-like | GO: 0008171 O-methyltransferase activity GO: 0008757 S-adenosylmethionine-dependent methyltransferase activity GO: 0046983 protein dimerization activity GO: 0009809 lignin biosynthetic process GO: 0009813 flavonoid biosynthetic GO: 0019438 aromatic compound biosynthetic process GO: 0032259 methylation | |
| TraesCS2B02G103600 | XM_044466025.1 | Triticum aestivum isoflavone reductase homolog (IFR) | GO: 0016491 oxidoreductase activity | 00998 Biosynthesis of various secondary metabolites—part 2 |
| TraesCS7D02G152300 | XM_044587211.1 | Triticum aestivum putative anthocyanidin reductase(ANR) | GO: 0003824 catalytic activity GO: 0050662 coenzyme binding | |
| TraesCS4D02G227300 | XM_044519958.1 | Triticum aestivum UDP-glycosyltransferase 83A1-like | GO: 0016758 transferase activity, transferring hexosyl groups |
| Number | Treatments | Abbreviation | Repeats | P. indica Pre-Inoculation | Harvest at () day |
|---|---|---|---|---|---|
| 1 | Mock | Mock | 6 | - | 140 + 140 |
| 2 | Piriformospora indica | Piri | 6 | + | 14pi + 140 |
| 3 | F.pseudograminearum | Fp | 6 | - | 140 + 14fp |
| 4 | P. indica + F. pseudograminearum | Piri + Fp | 6 | + | 14pi + 14fp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Hao, R.; Yang, X.; Feng, Y.; Bi, Z. Piriformospora indica Increases Resistance to Fusarium pseudograminearum in Wheat by Inducing Phenylpropanoid Pathway. Int. J. Mol. Sci. 2023, 24, 8797. https://doi.org/10.3390/ijms24108797
Li L, Hao R, Yang X, Feng Y, Bi Z. Piriformospora indica Increases Resistance to Fusarium pseudograminearum in Wheat by Inducing Phenylpropanoid Pathway. International Journal of Molecular Sciences. 2023; 24(10):8797. https://doi.org/10.3390/ijms24108797
Chicago/Turabian StyleLi, Liang, Ruiying Hao, Xiurong Yang, Yu Feng, and Zhenghui Bi. 2023. "Piriformospora indica Increases Resistance to Fusarium pseudograminearum in Wheat by Inducing Phenylpropanoid Pathway" International Journal of Molecular Sciences 24, no. 10: 8797. https://doi.org/10.3390/ijms24108797





