Natural Products as the Potential to Improve Alzheimer’s and Parkinson’s Disease
Abstract
1. Introduction
2. Neurodegenerative Diseases
2.1. Main Cause of Alzheimer’s and Parkinson’s Disease
2.2. Mitochondrial Dysfunction
2.3. Inflammation
3. Current Medications for Alzheimer’s and Parkinson’s Disease
3.1. Donepezil
3.2. Galantamine
3.3. Rivastigmine
3.4. Memantine
3.5. Levodopa
3.6. Catechol O Methyltransferase Inhibitor (COMT)
3.7. Monoamine Oxidase-B Inhibitor
3.8. Dopamine Agonist
| Target Disease | Medication Name | Mechanism | Adverse Effect | Ref. |
|---|---|---|---|---|
| Alzheimer’s disease | Donepezil | Acetylcholinesterase inhibitor | Nausea, vomiting, diarrhea, dizziness, trouble sleeping | [35,36] |
| Galantamine | Acetylcholinesterase inhibitor and allosteric modulator on nicotinic acetylcholine receptors | Nausea, stomach cramps, vomiting, irregular breathing, confusion, muscle weakness | [37,38] | |
| Rivastigmine | Acetylcholinesterase inhibitor and butyrylcholinesterase inhibitor | Abdominal pain, weight loss, diarrhea, loss of appetite, nausea, irregular breathing, chest pain, irregular heartbeat | [39,40] | |
| Memantine | NMDA receptor agonist | Pain, headache, fatigue, increased blood pressure, vomiting, drowsiness, cough, shortness of breath | [41] | |
| Parkinson’s disease | Levodopa | Supplement of dopamine | Fluctuations, dyskinesias, dystonias, autonomic dysfunction, mood control disorders, cognitive decline | [42,43] |
| Catechol O methyltransferase inhibitor | Prolongation of levodopa action | Levodopa-related adverse effects, confusion, hallucinations, urine discoloration, diarrhea | [44,45,46] | |
| Monoamine oxidase-B inhibitor | Preventation of dopamine breakdown | Nausea, dizziness, constipation, confusion, hallucinations | [47,48] | |
| Dopamine agonist | Inducement of dopamine-like effects | Nausea, vomiting, orthostatic hypotension, hallucinations, delusions | [49,50,51,52] |
4. Natural Materials—Compounds Derived from Natural Products
- A.
- Papers that did not contain the specified keywords.
- B.
- Review articles that covered multiple diseases.
- C.
- Case reports, clinical trial studies, and literature review studies.
- D.
- Abstracts and dissertations that were not relevant to the study.
4.1. Reynoutria multiflora Moldenke
4.2. Achillea fragrantissima Sch.Bip.
4.3. Theobroma cacao L.
4.4. Salvia miltiorrhiza Bunge
4.5. Asparagus racemosus Willd.
4.6. Opuntia ficus-indica (L.) Mill.
4.7. Gardenia jasminoides J.Ellis
4.8. Vitis labrusca L.
4.9. Paullinia cupana Kunth
4.10. Tussilago farfara L.
4.11. Panax ginseng C.A.Mey.
4.12. Polygala tenuifolia Willd.
4.13. Alpinia oxyphylla Miq.
4.14. Paeonia suffruticosa Andrews
4.15. Paeonia lactiflora Pall.
4.16. Cynanchum otophyllum C.K.Schneid.
5. Conclusions and Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorman, A.M. Neuronal cell death in neurodegenerative diseases: Recurring themes around protein handling. J. Cell. Mol. Med. 2008, 12, 2263–2280. [Google Scholar] [CrossRef]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef]
- Onyike, C.U.; Diehl-Schmid, J. The epidemiology of frontotemporal dementia. Int. Rev. Psychiatry 2013, 25, 130–137. [Google Scholar] [CrossRef]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Leray, E.; Moreau, T.; Fromont, A.; Edan, G. Epidemiology of multiple sclerosis. Rev. Neurol. 2016, 172, 3–13. [Google Scholar] [CrossRef]
- Rawlins, M.D.; Wexler, N.S.; Wexler, A.R.; Tabrizi, S.J.; Douglas, I.; Evans, S.J.; Smeeth, L. The Prevalence of Huntington’s Disease. Neuroepidemiology 2016, 46, 144–153. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Prim. 2021, 7, 33. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim. 2021, 7, 47. [Google Scholar] [CrossRef]
- Onyango, I.G.; Khan, S.M.; Bennett, J.P., Jr. Mitochondria in the pathophysiology of Alzheimer’s and Parkinson’s diseases. Front. Biosci. 2017, 22, 854–872. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. New therapeutic strategy for Parkinson’s and Alzheimer’s disease. Curr. Med. Chem. 2010, 17, 2764–2774. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Alpha-synuclein and beta-amyloid—different targets, same players: Calcium, free radicals and mitochondria in the mechanism of neurodegeneration. Biochem. Biophys. Res. Commun. 2017, 483, 1110–1115. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimer’s Dis. JAD 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef]
- Penke, B.; Bogár, F.; Fülöp, L. β-Amyloid and the Pathomechanisms of Alzheimer’s Disease: A Comprehensive View. Molecules 2017, 22, 1692. [Google Scholar] [CrossRef]
- van der Lee, S.J.; Wolters, F.J.; Ikram, M.K.; Hofman, A.; Ikram, M.A.; Amin, N.; van Duijn, C.M. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: A community-based cohort study. Lancet. Neurol. 2018, 17, 434–444. [Google Scholar] [CrossRef]
- Wong, Y.C.; Krainc, D. α-synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat. Med. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Fujikake, N.; Shin, M.; Shimizu, S. Association Between Autophagy and Neurodegenerative Diseases. Front. Neurosci. 2018, 12, 255. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, T.; Zhang, L.; Zhou, Y.; Luo, H.; Xu, H.; Wang, X. Dysregulation of Ubiquitin-Proteasome System in Neurodegenerative Diseases. Front. Aging Neurosci. 2016, 8, 303. [Google Scholar] [CrossRef]
- Galloway, D.A.; Phillips, A.E.M.; Owen, D.R.J.; Moore, C.S. Phagocytosis in the Brain: Homeostasis and Disease. Front. Immunol. 2019, 10, 790. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Area-Gomez, E.; Guardia-Laguarta, C.; Schon, E.A.; Przedborski, S. Mitochondria, OxPhos, and neurodegeneration: Cells are not just running out of gas. J. Clin. Investig. 2019, 129, 34–45. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Kitazawa, M.; Yamasaki, T.R.; LaFerla, F.M. Microglia as a potential bridge between the amyloid beta-peptide and tau. Ann. N. Y. Acad. Sci. 2004, 1035, 85–103. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Allen Reish, H.E.; Standaert, D.G. Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J. Park. Dis. 2015, 5, 1–19. [Google Scholar] [CrossRef]
- Beers, D.R.; Henkel, J.S.; Zhao, W.; Wang, J.; Appel, S.H. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl. Acad. Sci. USA 2008, 105, 15558–15563. [Google Scholar] [CrossRef]
- Rostami, J.; Mothes, T.; Kolahdouzan, M.; Eriksson, O.; Moslem, M.; Bergström, J.; Ingelsson, M.; O’Callaghan, P.; Healy, L.M.; Falk, A.; et al. Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates. J. Neuroinflamm. 2021, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.E.; Blurton-Jones, M. Examining the mechanisms that link β-amyloid and α-synuclein pathologies. Alzheimer’s Res. Ther. 2012, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Jiang, Q.; McDermott, J.; Han, J.J. Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell 2018, 17, e12802. [Google Scholar] [CrossRef] [PubMed]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 6, Cd001190. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, V.; Sharma, S. Donepezil; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Olin, J.; Schneider, L. Galantamine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2002, 1, Cd001747. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Inglis, F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int. J. Clin. Pract. Suppl. 2002, 127, 45–63. [Google Scholar]
- Birks, J.S.; Grimley Evans, J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015, 10, CD001191. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, H. Efficacy and adverse effects of memantine treatment for Alzheimer’s disease from randomized controlled trials. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2015, 36, 1633–1641. [Google Scholar] [CrossRef]
- de Bie, R.M.A.; Clarke, C.E.; Espay, A.J.; Fox, S.H.; Lang, A.E. Initiation of pharmacological therapy in Parkinson’s disease: When, why, and how. Lancet. Neurol. 2020, 19, 452–461. [Google Scholar] [CrossRef]
- Thanvi, B.R.; Lo, T.C. Long term motor complications of levodopa: Clinical features, mechanisms, and management strategies. Postgrad. Med. J. 2004, 80, 452–458. [Google Scholar] [CrossRef]
- Zhu, B.T.; Patel, U.K.; Cai, M.X.; Conney, A.H. O-Methylation of tea polyphenols catalyzed by human placental cytosolic catechol-O-methyltransferase. Drug Metab. Dispos. Biol. Fate Chem. 2000, 28, 1024–1030. [Google Scholar]
- Bonifácio, M.J.; Palma, P.N.; Almeida, L.; Soares-da-Silva, P. Catechol-O-methyltransferase and its inhibitors in Parkinson’s disease. CNS Drug Rev. 2007, 13, 352–379. [Google Scholar] [CrossRef]
- Kaakkola, S. Problems with the present inhibitors and a relevance of new and improved COMT inhibitors in Parkinson’s disease. Int. Rev. Neurobiol. 2010, 95, 207–225. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53 (Suppl. S3), S26–S36, discussion S36–S38. [Google Scholar] [CrossRef]
- Finberg, J.P.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology. Front. Pharmacol. 2016, 7, 340. [Google Scholar] [CrossRef]
- Reichmann, H.; Bilsing, A.; Ehret, R.; Greulich, W.; Schulz, J.B.; Schwartz, A.; Rascol, O. Ergoline and non-ergoline derivatives in the treatment of Parkinson’s disease. J. Neurol. 2006, 253 (Suppl. S4), iv36–iv38. [Google Scholar] [CrossRef]
- Oertel, W.H.; Quinn, N.P. Parkinson’s disease: Drug therapy. Bailliere’s Clin. Neurol. 1997, 6, 89–108. [Google Scholar]
- Rascol, O.; Brooks, D.J.; Korczyn, A.D.; De Deyn, P.P.; Clarke, C.E.; Lang, A.E. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N. Engl. J. Med. 2000, 342, 1484–1491. [Google Scholar] [CrossRef]
- Brooks, D.J. Dopamine agonists: Their role in the treatment of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2000, 68, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lang, X.-Y.; Li, X.-X.; Gu, R.-Z.; Liu, Q.-S.; Lan, R.; Qin, X.-Y. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside attenuates MPP+/MPTP-induced neurotoxicity in vitro and in vivo by restoring the BDNF-TrkB and FGF2-Akt signaling axis and inhibition of apoptosis. Food Funct. 2019, 10, 6009–6019. [Google Scholar] [CrossRef] [PubMed]
- Elmann, A.; Telerman, A.; Mordechay, S.; Erlank, H.; Rindner, M.; Ofir, R.; Kashman, Y. 3,5,4′-Trihydroxy-6,7,3′-trimethoxyflavone protects astrocytes against oxidative stress via interference with cell signaling and by reducing the levels of intracellular reactive oxygen species. Neurochem. Int. 2014, 78, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cimini, A.; Gentile, R.; D’Angelo, B.; Benedetti, E.; Cristiano, L.; Avantaggiati, M.L.; Giordano, A.; Ferri, C.; Desideri, G. Cocoa powder triggers neuroprotective and preventive effects in a human Alzheimer’s disease model by modulating BDNF signaling pathway. J. Cell. Biochem. 2013, 114, 2209–2220. [Google Scholar] [CrossRef]
- Chong, C.-M.; Zhou, Z.-Y.; Razmovski-Naumovski, V.; Cui, G.-Z.; Zhang, L.-Q.; Sa, F.; Hoi, P.-M.; Chan, K.; Lee, S.M.-Y. Danshensu protects against 6-hydroxydopamine-induced damage of PC12 cells in vitro and dopaminergic neurons in zebrafish. Neurosci. Lett. 2013, 543, 121–125. [Google Scholar] [CrossRef]
- Parihar, M.; Hemnani, T. Experimental excitotoxicity provokes oxidative damage in mice brain and attenuation by extract of Asparagus racemosus. J. Neural Transm. 2004, 111, 1–12. [Google Scholar] [CrossRef]
- Briffa, M.; Ghio, S.; Neuner, J.; Gauci, A.J.; Cacciottolo, R.; Marchal, C.; Caruana, M.; Cullin, C.; Vassallo, N.; Cauchi, R.J. Extracts from two ubiquitous Mediterranean plants ameliorate cellular and animal models of neurodegenerative proteinopathies. Neurosci. Lett. 2017, 638, 12–20. [Google Scholar] [CrossRef]
- Zang, C.; Liu, H.; Shang, J.; Yang, H.; Wang, L.; Sheng, C.; Zhang, Z.; Bao, X.; Yu, Y.; Yao, X. Gardenia jasminoides J. Ellis extract GJ-4 alleviated cognitive deficits of APP/PS1 transgenic mice. Phytomedicine 2021, 93, 153780. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, N.; Chen, M.; Jin, H.; Nie, J.; Shi, J.; Jin, F. Procyanidin protects against 6-hydroxydopamine-induced dopaminergic neuron damage via the regulation of the PI3K/Akt signalling pathway. Biomed. Pharmacother. 2019, 114, 108789. [Google Scholar] [CrossRef]
- Boasquívis, P.F.; Silva, G.M.M.; Paiva, F.A.; Cavalcanti, R.M.; Nunez, C.V.; de Paula Oliveira, R. Guarana (Paullinia cupana) extract protects Caenorhabditis elegans models for Alzheimer disease and Huntington disease through activation of antioxidant and protein degradation pathways. Oxidative Med. Cell. Longev. 2018, 2018, 9241308. [Google Scholar] [CrossRef]
- Lee, J.; Song, K.; Huh, E.; Oh, M.S.; Kim, Y.S. Neuroprotection against 6-OHDA toxicity in PC12 cells and mice through the Nrf2 pathway by a sesquiterpenoid from Tussilago farfara. Redox Biol. 2018, 18, 6–15. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Liu, Y.; Liu, X.; Chang, Q.; Liao, Y.; Pan, R. Neuroprotective effect of water extract of Panax ginseng on corticosterone-induced apoptosis in PC12 cells and its underlying molecule mechanisms. J. Ethnopharmacol. 2015, 159, 102–112. [Google Scholar] [CrossRef]
- Liang, Z.; Shi, F.; Wang, Y.; Lu, L.; Zhang, Z.; Wang, X.; Wang, X. Neuroprotective effects of tenuigenin in a SH-SY5Y cell model with 6-OHDA-induced injury. Neurosci. Lett. 2011, 497, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Chen, Y.; Wang, X.; Cui, G.; Ung, C.O.L.; Lu, J.-H.; Cong, W.; Tang, B.; Lee, S.M.-Y. Oxyphylla A ameliorates cognitive deficits and alleviates neuropathology via the Akt-GSK3β and Nrf2-Keap1-HO-1 pathways in vitro and in vivo murine models of Alzheimer’s disease. J. Adv. Res. 2021, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-T.; Hsu, Y.-Y.; Shih, Y.-T.; Lo, Y.-C. Paeonol attenuates microglia-mediated inflammation and oxidative stress–induced neurotoxicity in rat primary microglia and cortical neurons. Shock 2012, 37, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, K.; Wu, D.; Li, X.; Ou, Y. Protective effect of paeoniflorin against glutamate-induced neurotoxicity in PC12 cells via Bcl-2/Bax signal pathway. Folia Neuropathol. 2012, 50, 270–276. [Google Scholar] [CrossRef]
- Iyaswamy, A.; Krishnamoorthi, S.K.; Zhang, H.; Sreenivasmurthy, S.G.; Zhu, Z.; Liu, J.; Su, C.-F.; Guan, X.-J.; Wang, Z.-Y.; Cheung, K.-H. Qingyangshen mitigates amyloid-β and Tau aggregate defects involving PPARα-TFEB activation in transgenic mice of Alzheimer’s disease. Phytomedicine 2021, 91, 153648. [Google Scholar] [CrossRef]
- Gomolin, I.H.; Smith, C.; Jeitner, T.M. Donepezil dosing strategies: Pharmacokinetic considerations. J. Am. Med. Dir. Assoc. 2011, 12, 606–608. [Google Scholar] [CrossRef]
- Metz, C.N.; Pavlov, V.A. Treating disorders across the lifespan by modulating cholinergic signaling with galantamine. J. Neurochem. 2021, 158, 1359–1380. [Google Scholar] [CrossRef]
- Haake, A.; Nguyen, K.; Friedman, L.; Chakkamparambil, B.; Grossberg, G.T. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Drug Saf. 2020, 19, 147–157. [Google Scholar] [CrossRef]
- MartInez-Coria, H.; Arrieta-Cruz, I.; Cruz, M.E.; López-Valdés, H.E. Physiopathology of ischemic stroke and its modulation using memantine: Evidence from preclinical stroke. Neural Regen. Res. 2021, 16, 433–439. [Google Scholar] [CrossRef]
- Ahlskog, J.E. Common Myths and Misconceptions That Sidetrack Parkinson Disease Treatment, to the Detriment of Patients. Mayo Clin. Proc. 2020, 95, 2225–2234. [Google Scholar] [CrossRef]
- Artusi, C.A.; Sarro, L.; Imbalzano, G.; Fabbri, M.; Lopiano, L. Safety and efficacy of tolcapone in Parkinson’s disease: Systematic review. Eur. J. Clin. Pharmacol. 2021, 77, 817–829. [Google Scholar] [CrossRef]
- Rome, B.N.; Egilman, A.C.; Patel, N.G.; Kesselheim, A.S. Using Multiple Authorized Generics to Maintain High Prices: The Example of Entacapone. Value Health 2023, 26, 370–377. [Google Scholar] [CrossRef]
- Craft, B.M.; Baker, D.E.; Levien, T.L. Opicapone: Once-Daily COMT Inhibitor for the Treatment of Wearing Off in Parkinson’s Disease. Sr. Care Pharm. 2022, 37, 55–61. [Google Scholar] [CrossRef]
- Niu, Z.-X.; Wang, Y.-T.; Zhang, S.-N.; Li, Y.; Chen, X.-B.; Wang, S.-Q.; Liu, H.-M. Application and synthesis of thiazole ring in clinically approved drugs. Eur. J. Med. Chem. 2023, 250, 115172. [Google Scholar] [CrossRef]
- Wilson, S.M.; Wurst, M.G.; Whatley, M.F.; Daniels, R.N. Classics in Chemical Neuroscience: Pramipexole. ACS Chem. Neurosci. 2020, 11, 2506–2512. [Google Scholar] [CrossRef]
- Thach, A.; Kirson, N.; Zichlin, M.L.; Dieye, I.; Pappert, E.; Williams, G.R. Cost-Effectiveness of Apomorphine Sublingual Film as an “On-Demand” Treatment for “OFF” Episodes in Patients with Parkinson’s Disease. J. Health Econ. Outcomes Res. 2021, 8, 82–92. [Google Scholar] [CrossRef]
- Haas, L.T.; Salazar, S.V.; Smith, L.M.; Zhao, H.R.; Cox, T.O.; Herber, C.S.; Degnan, A.P.; Balakrishnan, A.; Macor, J.E.; Albright, C.F.; et al. Silent Allosteric Modulation of mGluR5 Maintains Glutamate Signaling while Rescuing Alzheimer’s Mouse Phenotypes. Cell Rep. 2017, 20, 76–88. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Wang, H.; Kelly, T.; Rush, R.; Nguyen, R.; Bisen, S.; Yamashita, Y.; Sloan, N.; Dang, B.; Sigmon, A.; et al. The c-Abl inhibitor IkT-148009 suppresses neurodegeneration in mouse models of heritable and sporadic Parkinson’s disease. Sci. Transl. Med. 2023, 15, eabp9352. [Google Scholar] [CrossRef]
- Chen, C.M.; Wu, C.C.; Huang, C.L.; Chang, M.Y.; Cheng, S.H.; Lin, C.T.; Tsai, Y.C. Lactobacillus plantarum PS128 Promotes Intestinal Motility, Mucin Production, and Serotonin Signaling in Mice. Probiotics Antimicrob. Proteins 2022, 14, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.; Huntwork-Rodriguez, S.; Vissers, M.; Daryani, V.M.; Diaz, D.; Goo, M.S.; Chen, J.J.; Maciuca, R.; Fraser, K.; Mabrouk, O.S.; et al. LRRK2 Inhibition by BIIB122 in Healthy Participants and Patients with Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2023, 38, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Rishton, G.M.; Look, G.C.; Ni, Z.-J.; Zhang, J.; Wang, Y.; Huang, Y.; Wu, X.; Izzo, N.J.; LaBarbera, K.M.; Limegrover, C.S.; et al. Discovery of Investigational Drug CT1812, an Antagonist of the Sigma-2 Receptor Complex for Alzheimer’s Disease. ACS Med. Chem. Lett. 2021, 12, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
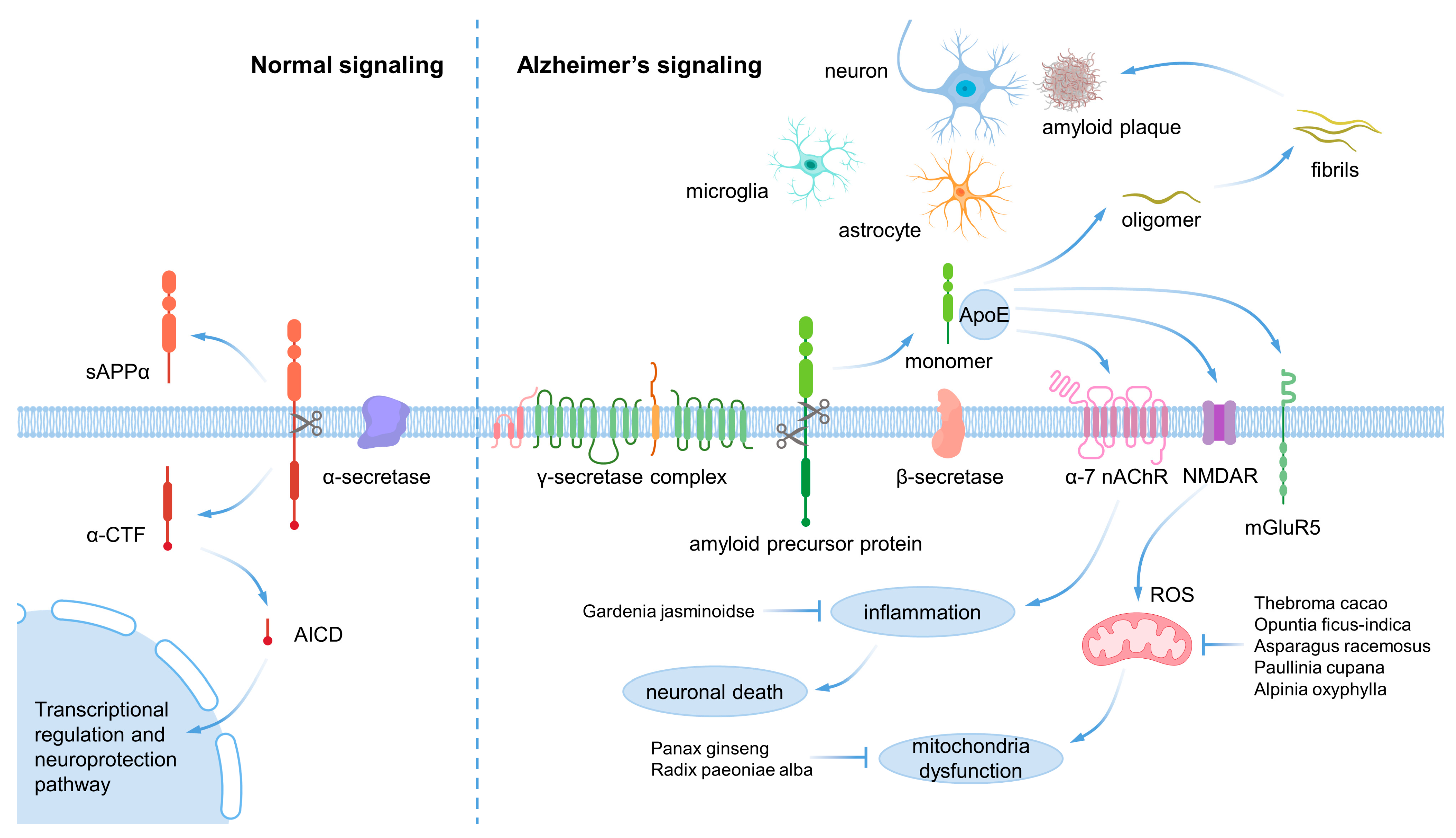
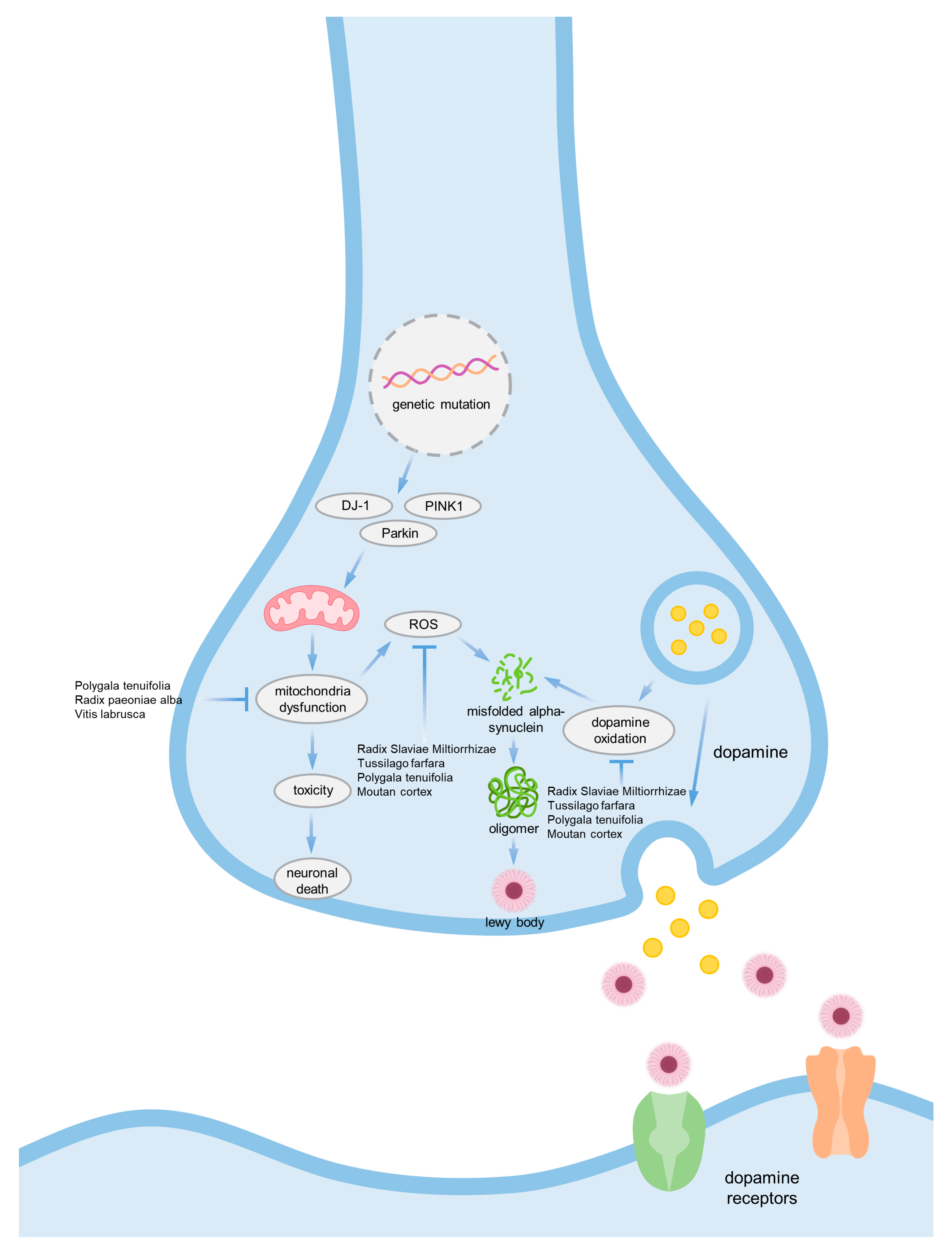

| Natural Products | Part of the Natural Products | Model | Dose | Effects | Ref. |
|---|---|---|---|---|---|
| Reynoutria multiflora Moldenke | Purified compound | Mesencephalic dopamine neurons and SH-SY5Y cell | 100, 200 μM (in vitro) 20 mg/kg (in vivo) | Anti-oxidative, anti-aging, and anti-inflammatory effects Restoration of the expression of FGF2 and BDNF, which inhibits apoptosis. Activation of the FGF2-Akt and BDNF-TrkB signaling pathways in the substantia nigra and corpus striatum, leading to the recovery of dopaminergic neurons. | [53] |
| Achillea fragrantissima Sch.Bip. | - | H2O2-treated astrocytes and neuron | Up to 34.7 μM (in vitro) | The inhibition of phosphorylation of stress-activated protein kinase/c-Jun N-terminal kinase (ERK 1/2), mitogen-activated protein kinase kinase (MEK1), kinase (SAPK/JNK), and the transcription factor cyclic AMP response element-binding protein (CREB) was observed. | [54] |
| Theobroma cacao L. | Commercial cocoa powder | β-amyloid-treated SH-SY5Y | Mixture of 30 μg/mL of epicatechin, 10 μg/mL catechin and 170 μg total polyphenols (in vitro) | Antioxidant, activating the BDNF survival pathway. | [55] |
| Salvia miltiorrhiza Bunge | - | 6-OHDA-treated PC12 and zebrafish | 100, 200, 400 μM | Activated the nuclear translocation of Nrf2 to increase heme oxygenase-1 (HO-1), conferring protection against ROS. Induced the phosphorylation of Akt. | [56] |
| Asparagus racemosus Willd. | Root | Intra-hippocampal and intra-striatal administration of kainic acid | 18 mg/kg | Reduction of membranal lipid peroxidation and protein carbonyl following improvement in GPx activity and GSH contents. | [57] |
| Opuntia ficus-indica (L.) Mill. | Fruit skin | AD fly model with brain-specific expression of Aβ42 and PD fly model based on transgenic expression of the human α-syn A53T mutant | 1 mg/mL (in yeast) 0.06% (in drosophila) 100, 400, 800, 2000 μg/mL (in vitro) | Inhibition of the fibrillogenesis of both Aβ42 and α-syn Accumulation of remodeled oligomeric aggregates that are less effective at disrupting lipid membrane integrity. | [58] |
| Gardenia jasminoides J.Ellis | Fruit | APP/PS1 transgenic mice | 10, 20, 50 mg/kg | Suppressed neuroinflammatory responses in the brain through regulating phosphatidylinositide 3-kinase/AKT (PI3K/AKT) signaling pathway activation, expression of inflammatory proteins and release of inflammatory cytokines. | [59] |
| Vitis labrusca L. | Purified compound | 6-OHDA-treated PC12 and rats | 12.5, 25, 50 μM (in vitro) 60 mg/kg (in vivo) | Neuroprotection against 6-OHDA-induced neurotoxicity. Reduction oxidative stress and improvement in mitochondrial dysfunction. Activation of the PI3K/Akt signaling pathway. | [60] |
| Paullinia cupana Kunth | - | Aβ42-induced ad model of Caenorhabditis elegans | 10, 50 mg/mL | Antioxidant activity and modulation of proteostasis. Intracellular ROS and the accumulation of autophagosomes reduction. Increased the expression of SOD-3 and HSP-16.2. | [61] |
| Tussilago farfara L. | Buds | 6-OHDA-treated PC12 and mice | 1.25, 2.5, 5, 10 μM (in vitro) 5 mg/kg (in vivo) | Activating the Nrf2/HO-1 signaling pathway. | [62] |
| Panax ginseng C.A.Mey. | Root | PC12 cells were treated with 250 μmol/L corticosterone | 6.25, 12.5, 25, 50, 100, 200 μg/mL | Neuroprotection against corticosterone-induced damage in PC12 cells, and the intervening of HDAC6 and HSP90 of the GR-related function proteins, and subsequent restoration of ER and mitochondria functions. | [63] |
| Polygala tenuifolia Willd. | - | 6-OHDA-treated SH-SY5Y | 12.5, 25, 50, 100 μM | Antioxidative effects, maintenance of mitochondrial function, and regulation of caspase-3 and tyrosine hydroxylase expression and activity. | [64] |
| Alpinia oxyphylla Miq. | Purified compound | N2a/APP cells and SAMP8 mice | 12, 25, 50, 100, 200, 400 μM (in vitro) 10, 20 mg/kg (in vivo) | Antioxidative effect through the Akt-GSK3b and Nrf2-Keap1-HO-1 pathways. | [65] |
| Paeonia × suffruticosa Andrews | Purified compound | 6-OHDA-treated cortical neurons | 0.75, 1, 1.5 μM | Decreased reactive oxygen species production. Increased cell viability, superoxide dismutase activity, and the anti-apoptotic protein expression. | [66] |
| Paeonia lactiflora Pall. | - | Glutamate-treated PC12 cell | 0.1, 1, 10 μM | Neuroprotective effect on glutamate-induced apoptosis in PC12 cells by regulating the mitochondrial membrane potential and Bcl-2/Bax signal pathway. | [67] |
| Cynanchum otophyllum C.K.Schneid. | 3XTg AD mice | 6.5, 12.5, 25 μg/mL (in vitro) 25, 50, 100 mg/kg (in vivo) | Activation of PPARα-TFEB pathway. | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.W.; Lee, J.H.; Kim, B.; Yang, G.; Kim, J.U. Natural Products as the Potential to Improve Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 8827. https://doi.org/10.3390/ijms24108827
Kim SW, Lee JH, Kim B, Yang G, Kim JU. Natural Products as the Potential to Improve Alzheimer’s and Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(10):8827. https://doi.org/10.3390/ijms24108827
Chicago/Turabian StyleKim, Sung Wook, Jun Ho Lee, Bumjung Kim, Gabsik Yang, and Jong Uk Kim. 2023. "Natural Products as the Potential to Improve Alzheimer’s and Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 10: 8827. https://doi.org/10.3390/ijms24108827
APA StyleKim, S. W., Lee, J. H., Kim, B., Yang, G., & Kim, J. U. (2023). Natural Products as the Potential to Improve Alzheimer’s and Parkinson’s Disease. International Journal of Molecular Sciences, 24(10), 8827. https://doi.org/10.3390/ijms24108827





