Connecting Circuits with Networks in Addiction Neuroscience: A Salience Network Perspective
Abstract
1. Introduction
2. Functions and Connectivity of the Nodes of the Salience Network
2.1. The Anterior Insular Cortex (AIC)
2.1.1. Anatomy of the AIC
2.1.2. Functions of the AIC
2.2. Dorsal Anterior Cingulate Cortex (dACC)
2.2.1. Anatomy of dACC
2.2.2. Functions of dACC
2.3. Additional Regions
2.4. Relationship with the Cingulo-Opercular Network
3. The Salience Network in Addiction
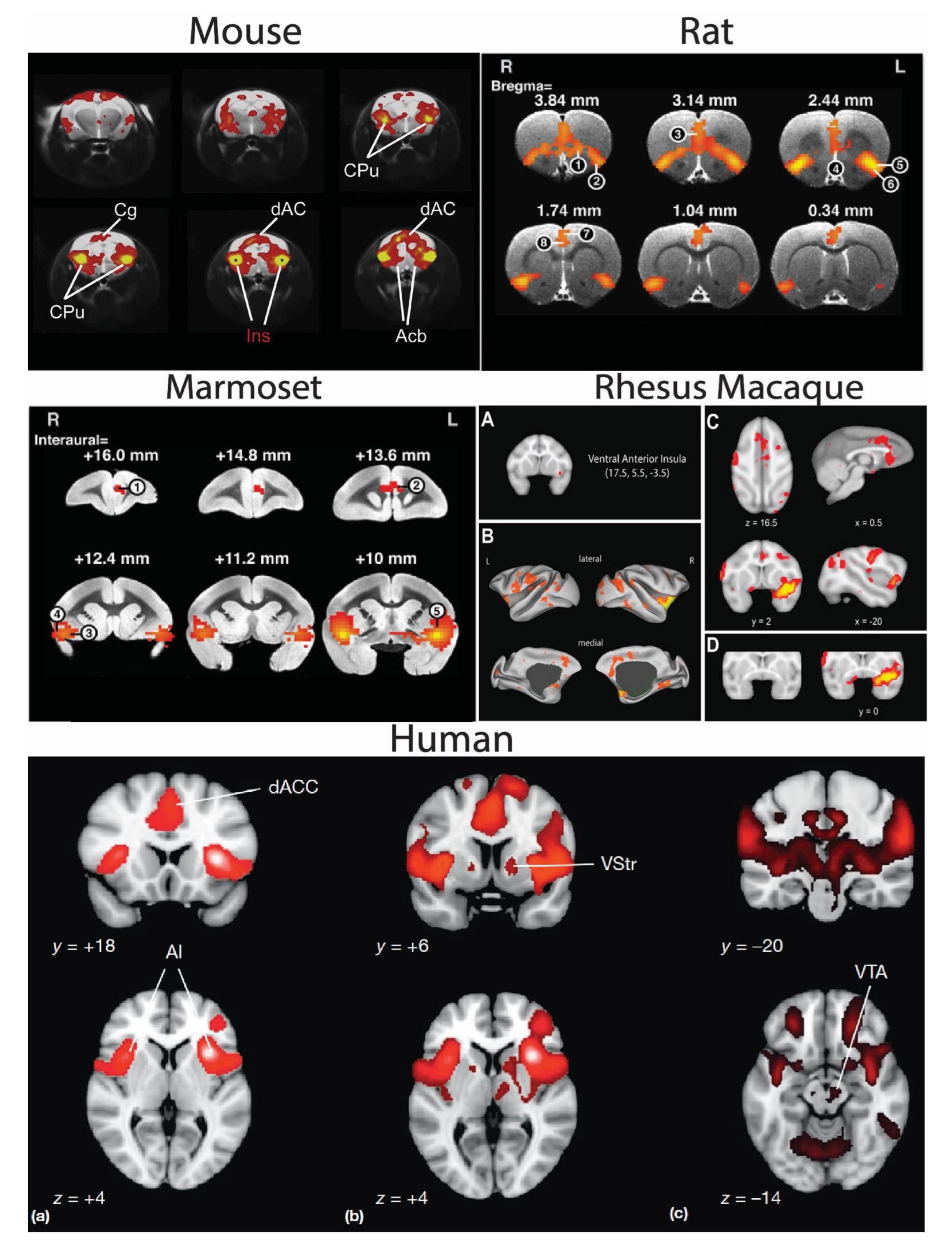
4. Cross-Species Salience Network
Circuits/Manipulations of the SN
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, M.D.; Raichle, M.E. Spontaneous Fluctuations in Brain Activity Observed with Functional Magnetic Resonance Imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.S.; Greicius, M.D. Greater than the Sum of Its Parts: A Review of Studies Combining Structural Connectivity and Resting-State Functional Connectivity. Anat. Embryol. 2009, 213, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Ross, K.; Uddin, L.Q.; Sklar, A.B.; Castellanos, F.X.; Milham, M.P. Functional Brain Correlates of Social and Nonsocial Processes in Autism Spectrum Disorders: An Activation Likelihood Estimation Meta-Analysis. Biol. Psychiatry 2009, 65, 63–74. [Google Scholar] [CrossRef]
- Thomas Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The Organization of the Human Cerebral Cortex Estimated by intrinsic Functional Connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, X.; He, X.; Wang, L.; Wang, K.; Qiu, B. Aberrant Structural and Functional Connectivity in the Salience Network and Central Executive Network Circuit in Schizophrenia. Neurosci. Lett. 2016, 627, 178–184. [Google Scholar] [CrossRef]
- Mueller, S.; Wang, D.; Fox, M.D.; Yeo, B.T.; Sepulcre, J.; Sabuncu, M.R.; Shafee, R.; Lu, J.; Liu, H. Individual Variability in Functional Connectivity Architecture of the Human Brain. Neuron 2013, 77, 586–595. [Google Scholar] [CrossRef]
- Langs, G.; Golland, P.; Ghosh, S.S. Predicting Activation across individuals with Resting-State Functional Connectivity Based Multi-Atlas Label Fusion. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015; Lecture Notes in Computer Science; Navab, N., Hornegger, J., Wells, W.M., Frangi, A., Eds.; Springer international Publishing: Cham, Switzerland, 2015; Volume 9350, pp. 313–320. [Google Scholar] [CrossRef]
- Bergmann, E.; Gofman, X.; Kavushansky, A.; Kahn, I. Individual Variability in Functional Connectivity Architecture of the Mouse Brain. Commun. Biol. 2020, 3, 738. [Google Scholar] [CrossRef]
- Cole, M.W.; Bassett, D.S.; Power, J.D.; Braver, T.S.; Petersen, S.E. Intrinsic and Task-Evoked Network Architectures of the Human Brain. Neuron 2014, 83, 238–251. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. From the Cover: The Human Brain Is intrinsically Organized into Dynamic, Anticorrelated Functional Networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Finn, E.S.; Shen, X.; Scheinost, D.; Rosenberg, M.; Huang, J.; Chun, M.; Papademetris, X.; Constable, R. Functional Connectome Fingerprinting: Identifying individuals Using Patterns of Brain Connectivity. Nat. Neurosci. 2015, 18, 1664–1671. [Google Scholar] [CrossRef]
- Bassett, D.S.; Bullmore, E.T. Human Brain Networks in Health and Disease. Curr. Opin. Neurol. 2009, 22, 340–347. [Google Scholar] [CrossRef]
- Nelson, S.M.; Dosenbach, N.U.F.; Cohen, A.L.; Wheeler, M.E.; Schlaggar, B.L.; Petersen, S.E. Role of the Anterior insula in Task-Level Control and Focal Attention. Anat. Embryol. 2010, 214, 669–680. [Google Scholar] [CrossRef]
- Fornito, A.; Zalesky, A.; Breakspear, M. The Connectomics of Brain Disorders. Nat. Rev. Neurosci. 2015, 16, 159–172. [Google Scholar] [CrossRef]
- Sutherland, M.T.; McHugh, M.J.; Pariyadath, V.; Stein, E.A. Resting State Functional Connectivity in Addiction: Lessons Learned and a Road Ahead. Neuroimage 2012, 62, 2281–2295. [Google Scholar] [CrossRef]
- Peterson, A.; Thome, J.; Frewen, P.; Lanius, R.A. Resting-State Neuroimaging Studies: A New Way of Identifying Differences and Similarities among the Anxiety Disorders? Can. J. Psychiatry 2014, 59, 294–300. [Google Scholar] [CrossRef]
- Liang, X.; He, Y.; Salmeron, B.J.; Gu, H.; Stein, E.A.; Yang, Y. Interactions between the Salience and Default-Mode Networks Are Disrupted in Cocaine Addiction. J. Neurosci. 2015, 35, 8081–8090. [Google Scholar] [CrossRef]
- Grodin, E.; Cortes, C.R.; Spagnolo, P.A.; Momenan, R. Structural Deficits in Salience Network Regions Are Associated with increased Impulsivity and Compulsivity in Alcohol Dependence. Drug Alcohol Depend. 2017, 179, 100–108. [Google Scholar] [CrossRef]
- Zhang, R.; Volkow, N.D. Brain Default-Mode Network Dysfunction in Addiction. Neuroimage 2019, 200, 313–331. [Google Scholar] [CrossRef]
- Buckner, R.L.; Vincent, J.L. Unrest at Rest: Default Activity and Spontaneous Network Correlations. Neuroimage 2007, 37, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Supekar, K.; Menon, V.; Dougherty, R.F. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cereb. Cortex 2008, 19, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Spreng, R.N.; Stevens, W.D.; Chamberlain, J.P.; Gilmore, A.W.; Schacter, D.L. Default Network Activity, Coupled with the Frontoparietal Control Network, Supports Goal-Directed Cognition. Neuroimage 2010, 53, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Dinicola, L.M. The Brain’s Default Network: Updated Anatomy, Physiology and Evolving insights. Nat. Rev. Neurosci. 2019, 20, 593–608. [Google Scholar] [CrossRef]
- Gozzi, A.; Schwarz, A.J. Large-Scale Functional Connectivity Networks in the Rodent Brain. Neuroimage 2016, 127, 496–509. [Google Scholar] [CrossRef]
- Cole, M.; Schneider, W. The Cognitive Control Network: Integrated Cortical Regions with Dissociable Functions. Neuroimage 2007, 37, 343–360. [Google Scholar] [CrossRef]
- Grandjean, J.; Canella, C.; Anckaerts, C.; Ayrancı, G.; Bougacha, S.; Bienert, T.; Buehlmann, D.; Coletta, L.; Gallino, D.; Gass, N.; et al. Common Functional Networks in the Mouse Brain Revealed by Multi-Centre Resting-State Fmri Analysis. Neuroimage 2020, 205, 116278. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, Switching, Attention and Control: A Network Model of insula Function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Menon, V. Large-Scale Functional Brain Organization. Brain Mapp. Encycl. Ref. 2015, 2, 449–459. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Lu, H.; Zou, Q.; Gu, H.; Raichle, M.E.; Stein, E.A.; Yang, Y. Rat brains also have a default mode network. Proc. Natl. Acad. Sci. USA 2012, 109, 3979–3984. [Google Scholar] [CrossRef]
- Goulden, N.; Khusnulina, A.; Davis, N.J.; Bracewell, R.M.; Bokde, A.L.; McNulty, J.P.; Mullins, P.G. The Salience Network Is Responsible for Switching between the Default Mode Network and the Central Executive Network: Replication from DCM. Neuroimage 2014, 99, 180–190. [Google Scholar] [CrossRef]
- Bolton, T.A.; Wotruba, D.; Buechler, R.; Theodoridou, A.; Michels, L.; Kollias, S.; Rössler, W.; Heekeren, K.; Van De Ville, D. Triple Network Model Dynamically Revisited: Lower Salience Network State Switching in Pre-psychosis. Front Physiol. 2020, 11, 66. [Google Scholar] [CrossRef]
- Mandino, F.; Vrooman, R.M.; Foo, H.E.; Yeow, L.Y.; Bolton, T.A.W.; Salvan, P.; Teoh, C.L.; Lee, C.Y.; Beauchamp, A.; Luo, S.; et al. A Triple-Network Organization for the Mouse Brain. Mol. Psychiatry 2021, 27, 865–872. [Google Scholar] [CrossRef]
- Touroutoglou, A.; Bliss-Moreau, E.; Zhang, J.; Mantini, D.; Vanduffel, W.; Dickerson, B.C.; Barrett, L.F. A Ventral Salience Network in the Macaque Brain. Neuroimage 2016, 132, 190–197. [Google Scholar] [CrossRef]
- Das, A.; Menon, V. Spatiotemporal integrity and spontaneous nonlinear dynamic properties of the salience network revealed by human intracranial electrophysiology: A multicohort replication. Cereb. Cortex 2020, 30, 5309–5321. [Google Scholar] [CrossRef]
- Corbetta, M.; Patel, G.; Shulman, G.L. The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron 2008, 58, 306–324. [Google Scholar] [CrossRef]
- Bartra, O.; McGuire, J.T.; Kable, J.W. The Valuation System: A Coordinate-Based Meta-Analysis of Bold Fmri Experiments Examining Neural Correlates of Subjective Value. Neuroimage 2013, 76, 412–427. [Google Scholar] [CrossRef]
- Ham, T.; Leff, A.; de Boissezon, X.; Joffe, A.; Sharp, D.J. Cognitive Control and the Salience Network: An investigation of Error Processing and Effective Connectivity. J. Neurosci. 2013, 33, 7091–7098. [Google Scholar] [CrossRef]
- Craig, A.D. How Do You Feel? interoception: The Sense of the Physiological Condition of the Body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef]
- Craig, A.D.B. How Do You Feel—Now? The Anterior insula and Human Awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, C.B.; Larsen, J.T.; Cohen, J.D. Context Dependence of the Event-Related Brain Potential Associated with Reward and Punishment. Psychophysiology 2004, 41, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, B.; Adhikari, B.M.; Dhamala, M. The Activity in the Anterior insulae Is Modulated by Perceptual Decision-Making Difficulty. Neuroscience 2016, 327, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, D.; Daniel, J.L.; Vinod, M. A Critical Role for the Right Fronto-insular Cortex in Switching between Central-Executive and Default-Mode Networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef]
- Bonnelle, V.; Ham, T.E.; Leech, R.; Kinnunen, K.M.; Mehta, M.A.; Greenwood, R.J.; Sharp, D.J. Salience Network integrity Predicts Default Mode Network Function after Traumatic Brain injury. Proc. Natl. Acad. Sci. USA 2012, 109, 4690–4695. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef]
- Jilka, S.R.; Scott, G.; Ham, T.; Pickering, A.; Bonnelle, V.; Braga, R.M.; Leech, R.; Sharp, D.J. Damage to the Salience Network and interactions with the Default Mode Network. J. Neurosci. 2014, 34, 10798–10807. [Google Scholar] [CrossRef]
- Mutschler, I.; Wieckhorst, B.; Kowalevski, S.; Derix, J.; Wentlandt, J.; Schulze-Bonhage, A.; Ball, T. Functional organization of the human anterior insular cortex. Neurosci. Lett. 2009, 457, 66–70. [Google Scholar] [CrossRef]
- Gasquoine, P.G. Contributions of the insula to Cognition and Emotion. Neuropsychol. Rev. 2014, 24, 77–87. [Google Scholar] [CrossRef]
- Labrakakis, C. The Role of the insular Cortex in Pain. Int. J. Mol. Sci. 2023, 24, 5736. [Google Scholar] [CrossRef]
- Molnar-Szakacs, I.; Uddin, L.Q. Anterior insula as a gatekeeper of executive control. Neurosci. Biobehav. Rev. 2022, 139, 104736. [Google Scholar] [CrossRef]
- Evrard, H.C. The Organization of the Primate insular Cortex. Front. Neuroanat. 2019, 13, 43. [Google Scholar] [CrossRef]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef]
- Uddin, L. Salience Processing and insular Cortical Function and Dysfunction. Nat. Rev. Neurosci. 2014, 16, 55–61. [Google Scholar] [CrossRef]
- Holroyd, C.B.; Verguts, T. The best laid plans: Computational principles of anterior cingulate cortex. Trends Cogn. Sci. 2021, 25, 316–329. [Google Scholar] [CrossRef]
- Centanni, S.W.; Janes, A.C.; Haggerty, D.L.; Atwood, B.; Hopf, F.W. Better living through understanding the insula: Why subregions can make all the difference. Neuropharmacology 2021, 198, 108765. [Google Scholar] [CrossRef]
- Grier, M.D.; Zimmermann, J.; Heilbronner, S.R. Estimating Brain Connectivity with Diffusion-Weighted Magnetic Resonance Imaging: Promise and Peril. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 846–854. [Google Scholar] [CrossRef]
- Gallay, D.S.; Gallay, M.N.; Jeanmonod, D.; Rouiller, E.M.; Morel, A. The insula of Reil Revisited: Multiarchitectonic Organization in Macaque Monkeys. Cereb. Cortex 2011, 22, 175–190. [Google Scholar] [CrossRef]
- Gogolla, N. The insular Cortex. Curr. Biol. 2017, 27, R580–R586. [Google Scholar] [CrossRef]
- Evrard, H.C.; Logothetis, N.K.; Craig, A.B. Modular Architectonic Organization of the insula in the Macaque Monkey. J. Comp. Neurol. 2013, 522, 64–97. [Google Scholar] [CrossRef]
- Augustine, R. Circuitry and Fimctional Aspects of the insular Lobe in Primates including Humans. Brain Res. Rev. 1996, 22, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Mesulam, M.-M. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J. Comp. Neurol. 1982, 212, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.; Mesulam, M.-M.; Pandya, D. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience 1981, 6, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Krockenberger, M.T.O.S.; Logothetis, N.K.; Evrard, H.C. Connection ‘S Tripes’ in the Primate insula. bioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Chang, L.; Yarkoni, T.; Khaw, M.W.; Sanfey, A.G. Decoding the Role of the insula in Human Cognition: Functional Parcellation and Large-Scale Reverse inference. Cereb. Cortex 2012, 23, 739–749. [Google Scholar] [CrossRef]
- Saleeba, C.; Dempsey, B.; Le, S.; Goodchild, A.; McMullan, S. A Student’s Guide to Neural Circuit Tracing. Front. Neurosci. 2019, 13, 897. [Google Scholar] [CrossRef]
- Tang, W.; Choi, E.Y.; Heilbronner, S.R.; Haber, S.N. Nonhuman Primate Meso-Circuitry Data: A Translational Tool to Understand Brain Networks across Species. Anat. Embryol. 2020, 226, 1–11. [Google Scholar] [CrossRef]
- Menon, V. Brain networks and cognitive impairment in psychiatric disorders. World Psychiatry 2020, 19, 309–310. [Google Scholar] [CrossRef]
- Nieuwenhuys, R. The insular cortex. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 195, pp. 123–163. [Google Scholar] [CrossRef]
- Ogawa, H. Gustatory Cortex of Primates: Anatomy and Physiology. Neurosci. Res. 1994, 20, 1–13. [Google Scholar] [CrossRef]
- Mesulam, M.-M.; Mufson, E.J. The insula of Reil in Man and Monkey. In Association and Auditory Cortices; Springer: Boston, MA, USA, 1985; pp. 179–226. [Google Scholar] [CrossRef]
- Friedman, D.P.; Murray, E.A.; O’Neill, J.B.; Mishkin, M. Cortical Connections of the Somatosensory Fields of the Lateral Sulcus of Macaques: Evidence for A Corticolimbic Pathway for Touch. J. Comp. Neurol. 1986, 252, 323–347. [Google Scholar] [CrossRef]
- Cerliani, L.; Thomas, R.M.; Jbabdi, S.; Siero, J.C.; Nanetti, L.; Crippa, A.; Gazzola, V.; D’Arceuil, H.; Keysers, C. Probabilistic Tractography Recovers A Rostrocaudal Trajectory of Connectivity Variability in the Human insular Cortex. Hum. Brain Mapp. 2011, 33, 2005–2034. [Google Scholar] [CrossRef]
- Cloutman, L.L.; Binney, R.J.; Drakesmith, M.; Parker, G.J.; Ralph, M.A.L. The Variation of Function across the Human insula Mirrors Its Patterns of Structural Connectivity: Evidence From in Vivo Probabilistic Tractography. Neuroimage 2012, 59, 3514–3521. [Google Scholar] [CrossRef]
- Das, A.; Myers, J.; Mathura, R.; Shofty, B.; Metzger, B.A.; Bijanki, K.; Wu, C.; Jacobs, J.; Sheth, S.A. Spontaneous Neuronal Oscillations in the Human insula Are Hierarchically Organized Traveling Waves. eLife 2022, 11, e76702. [Google Scholar] [CrossRef]
- Deen, B.; Pitskel, N.B.; Pelphrey, K.A. Three Systems of insular Functional Connectivity Identified with Cluster Analysis. Cereb. Cortex 2010, 21, 1498–1506. [Google Scholar] [CrossRef]
- Kelly, C.; Toro, R.; Di Martino, A.; Cox, C.L.; Bellec, P.; Castellanos, F.X.; Milham, M.P. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage 2012, 61, 1129–1142. [Google Scholar] [CrossRef]
- Nomi, J.S.; Farrant, K.; Damaraju, E.; Rachakonda, S.; Calhoun, V.D.; Uddin, L.Q. Dynamic Functional Network Connectivity Reveals Unique and Overlapping Profiles of insula Subdivisions. Hum. Brain Mapp. 2016, 37, 1770–1787. [Google Scholar] [CrossRef]
- Nomi, J.; Schettini, E.; Broce, I.; Dick, A.; Uddin, L. Structural Connections of Functionally Defined Human insular Subdivisions. Cereb. Cortex 2017, 28, 3445–3456. [Google Scholar] [CrossRef]
- Vogt, B.A.; Pandya, D.N.; Rosene, D.L. Cingulate Cortex of the Rhesus Monkey: I. Cytoarchitecture and thalamic afferents. J. Comp. Neurol. 1987, 262, 256–270. [Google Scholar] [CrossRef]
- Vogt, B.A.; Finch, D.M.; Olson, C.R. Functional Heterogeneity in Cingulate Cortex: The Anterior Executive and Posterior Evaluative Regions. Cereb. Cortex 1992, 2, 435–443. [Google Scholar] [CrossRef]
- Devinsky, O.; Morrell, M.J.; Vogt, B.A. Contributions of Anterior Cingulate Cortex to Behaviour. Brain J. Neurol. 1995, 118 Pt 1, 279–306. [Google Scholar] [CrossRef]
- Bush, G.; Vogt, B.A.; Holmes, J.; Dale, A.M.; Greve, D.; Jenike, M.A.; Rosen, B.R. Dorsal Anterior Cingulate Cortex: A Role in Reward-Based Decision Making. Proc. Natl. Acad. Sci. USA 2001, 99, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Margulies, D.S.; Kelly, A.C.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Mapping the Functional Connectivity of Anterior Cingulate Cortex. Neuroimage 2007, 37, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Allman, J.M.; Tetreault, N.A.; Hakeem, A.Y.; Manaye, K.F.; Semendeferi, K.; Erwin, J.M.; Park, S.; Goubert, V.; Hof, P.R. The von Economo neurons in the frontoinsular and anterior cingulate cortex. Ann. N. Y. Acad. Sci. 2011, 1225, 59–71. [Google Scholar] [CrossRef]
- Evrard, H.C.; Forro, T.; Logothetis, N.K. Von Economo Neurons in the Anterior insula of the Macaque Monkey. Neuron 2012, 74, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Butti, C.; Santos, M.; Uppal, N.; Hof, P.R. Von Economo neurons: Clinical and Evolutionary Perspectives. Cortex 2013, 49, 312–326. [Google Scholar] [CrossRef]
- Eckert, M.A.; Menon, V.; Walczak, A.; Ahlstrom, J.; Denslow, S.; Horwitz, A.; Dubno, J.R. At the Heart of the Ventral Attention System: The Right Anterior insula. Hum. Brain Mapp. 2008, 30, 2530–2541. [Google Scholar] [CrossRef]
- Kurth, F.; Zilles, K.; Fox, P.; Laird, A.; Eickhoff, S.B. A Link Between the Systems: Functional Differentiation and integration within the Human insula Revealed by Meta-Analysis. Anat. Embryol. 2010, 214, 519–534. [Google Scholar] [CrossRef]
- Lamm, C.; Singer, T. The Role of Anterior insular Cortex in Social Emotions. Anat. Embryol. 2010, 214, 579–591. [Google Scholar] [CrossRef]
- Royer, J.; Paquola, C.; Larivière, S.; de Wael, R.V.; Tavakol, S.; Lowe, A.J.; Benkarim, O.; Evans, A.C.; Bzdok, D.; Smallwood, J.; et al. Myeloarchitecture Gradients in the Human insula: Histological Underpinnings and Association to intrinsic Functional Connectivity. Neuroimage 2020, 216, 116859. [Google Scholar] [CrossRef]
- Chiarello, C.; Vazquez, D.; Felton, A.; Leonard, C.M. Structural asymmetry of anterior insula: Behavioral correlates and individual differences. Brain Lang. 2013, 126, 109–122. [Google Scholar] [CrossRef]
- Kober, H.; Barrett, L.F.; Joseph, J.; Bliss-Moreau, E.; Lindquist, K.; Wager, T.D. Functional Grouping and Cortical–Subcortical interactions in Emotion: A Meta-Analysis of Neuroimaging Studies. Neuroimage 2008, 42, 998–1031. [Google Scholar] [CrossRef]
- Gu, X.; Hof, P.R.; Friston, K.J.; Fan, J. Anterior insular cortex and emotional awareness: Anterior Insular Cortex and Emotional Awareness. J. Comp. Neurol. 2013, 521, 3371–3388. [Google Scholar] [CrossRef]
- Gu, X.; Wang, X.; Hula, A.; Wang, S.; Xu, S.; Lohrenz, T.M.; Knight, R.T.; Gao, Z.; Dayan, P.; Montague, P.R. Necessary, Yet Dissociable Contributions of the Insular and Ventromedial Prefrontal Cortices to Norm Adaptation: Computational and Lesion Evidence in Humans. J. Neurosci. 2015, 35, 467–473. [Google Scholar] [CrossRef]
- Auvray, M.; Myin, E.; Spence, C. The Sensory-Discriminative and Affective-Motivational Aspects of Pain. Neurosci. Biobehav. Rev. 2010, 34, 214–223. [Google Scholar] [CrossRef]
- Talbot, K.; Madden, V.; Jones, S.; Moseley, G. The Sensory and Affective Components of Pain: Are They Differentially Modifiable Dimensions or inseparable Aspects of a Unitary Experience? A Systematic Review. Br. J. Anaesth. 2019, 123, e263–e272. [Google Scholar] [CrossRef]
- Singer, T.; Seymour, B.; O’Doherty, J.; Kaube, H.; Dolan, R.J.; Frith, C.D. Empathy for Pain involves the Affective but not Sensory Components of Pain. Science 2004, 303, 1157–1162. [Google Scholar] [CrossRef]
- Wicker, B.; Keysers, C.; Plailly, J.; Royet, J.-P.; Gallese, V.; Rizzolatti, G. Both of Us Disgusted in My insula: The Common Neural Basis of Seeing and Feeling Disgust. Neuron 2003, 40, 655–664. [Google Scholar] [CrossRef]
- Jabbi, M.; Bastiaansen, J.; Keysers, C. A Common Anterior insula Representation of Disgust Observation, Experience and Imagination Shows Divergent Functional Connectivity Pathways. PLoS ONE 2008, 3, e2939. [Google Scholar] [CrossRef]
- Medford, N.; Critchley, H.D. Conjoint Activity of Anterior insular and Anterior Cingulate Cortex: Awareness and Response. Brain Struct. Funct. 2010, 214, 535–549. [Google Scholar] [CrossRef]
- Namkung, H.; Kim, S.-H.; Sawa, A. The insula: An Underestimated Brain Area in Clinical Neuroscience, Psychiatry, and Neurology. Trends Neurosci. 2017, 40, 200–207. [Google Scholar] [CrossRef]
- Livneh, Y.; Sugden, A.U.; Madara, J.C.; Essner, R.A.; Flores, V.I.; Sugden, L.A.; Resch, J.M.; Lowell, B.B.; Andermann, M.L. Estimation of Current and Future Physiological States in insular Cortex. Neuron 2020, 105, 1094–1111.e10. [Google Scholar] [CrossRef] [PubMed]
- Namboodiri, V.M.K.; Stuber, G.D. Interoceptive inception in insula. Neuron 2020, 105, 959–960. [Google Scholar] [CrossRef] [PubMed]
- Cechetto, D.F.; Saper, C.B. Evidence for a Viscerotopic Sensory Representation in the Cortex and Thalamus in the Rat. J. Comp. Neurol. 1987, 262, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, C.; Rubin-Kahana, D.S.; Pushparaj, A.; Musiol, M.; Blumberger, D.M.; Daskalakis, Z.J.; Zangen, A.; Le Foll, B. The insula: A Brain Stimulation Target for the Treatment of Addiction. Front. Pharmacol. 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, T.; Ryali, S.; Kochalka, J.; Li, C.-S.R.; Menon, V. Causal interactions within a Frontal-Cingulate-Parietal Network During Cognitive Control: Convergent Evidence from a Multisite–Multitask investigation. Cereb. Cortex 2015, 26, 2140–2153. [Google Scholar] [CrossRef]
- Baleydier, C.; Mauguiere, F. The Duality of the Cingulate Gyrus in Monkey: Neuroanatomical Study and Functional Hypothesis. Brain 1980, 103, 525–554. [Google Scholar] [CrossRef]
- Vogt, B.A.; Pandya, D.N. Cingulate Cortex of the Rhesus Monkey: II. Cortical afferents. J. Comp. Neurol. 1987, 262, 271–289. [Google Scholar] [CrossRef]
- Vogt, B.A. Pain and Emotion interactions in Subregions of the Cingulate Gyrus. Nat. Rev. Neurosci. 2005, 6, 533–544. [Google Scholar] [CrossRef]
- Picard, N.; Strick, P.L. Motor Areas of the Medial Wall: A Review of Their Location and Functional Activation. Cereb. Cortex 1996, 6, 342–353. [Google Scholar] [CrossRef]
- Paus, T. Primate Anterior Cingulate Cortex: Where Motor Control, Drive and Cognition interface. Nat. Rev. Neurosci. 2001, 2, 417–424. [Google Scholar] [CrossRef]
- Tang, W.; Jbabdi, S.; Zhu, Z.; Cottaar, M.; Grisot, G.; Lehman, J.F.; Yendiki, A.; Haber, S.N. A Connectional Hub in the Rostral Anterior Cingulate Cortex Links Areas of Emotion and Cognitive Control. eLife 2019, 8, e43761. [Google Scholar] [CrossRef]
- Botvinick, M.M.; Cohen, J.D.; Carter, C.S. Conflict Monitoring and Anterior Cingulate Cortex: An Update. Trends Cogn. Sci. 2004, 8, 539–546. [Google Scholar] [CrossRef]
- Amiez, C.; Joseph, J.; Procyk, E. Reward Encoding in the Monkey Anterior Cingulate Cortex. Cereb. Cortex 2005, 16, 1040–1055. [Google Scholar] [CrossRef]
- Kennerley, S.W.; Wallis, J.D. Encoding of Reward and Space During a Working Memory Task in the Orbitofrontal Cortex and Anterior Cingulate Sulcus. J. Neurophysiol. 2009, 102, 3352–3364. [Google Scholar] [CrossRef]
- Williams, Z.M.; Bush, G.; Rauch, S.L.; Cosgrove, G.R.; Eskandar, E.N. Human Anterior Cingulate Neurons and the integration of Monetary Reward with Motor Responses. Nat. Neurosci. 2004, 7, 1370–1375. [Google Scholar] [CrossRef]
- Heilbronner, S.R.; Hayden, B.Y. Dorsal Anterior Cingulate Cortex: A Bottom-Up View. Annu. Rev. Neurosci. 2016, 39, 149–170. [Google Scholar] [CrossRef]
- Kolling, N.; Wittmann, M.K.; Behrens, T.E.J.; Boorman, E.D.; Mars, R.B.; Rushworth, M.F.S. Value, Search, Persistence and Model Updating in Anterior Cingulate Cortex. Nat. Neurosci. 2016, 19, 1280–1285. [Google Scholar] [CrossRef]
- Shenhav, A.; Cohen, J.D.; Botvinick, M.M. Dorsal Anterior Cingulate Cortex and the Value of Control. Nat. Neurosci. 2016, 19, 1286–1291. [Google Scholar] [CrossRef]
- Rainville, P.; Duncan, G.H.; Price, D.D.; Carrier, B.; Bushnell, M.C. Pain Affect Encoded in Human Anterior Cingulate but Not Somatosensory Cortex. Science 1997, 277, 968–971. [Google Scholar] [CrossRef]
- Krishnan, A.; Woo, C.-W.; Chang, L.J.; Ruzic, L.; Gu, X.; López-Solà, M.; Jackson, P.L.; Pujol, J.; Fan, J.; Wager, T.D. Somatic and Vicarious Pain Are Represented by Dissociable Multivariate Brain Patterns. eLife 2016, 5, e15166. [Google Scholar] [CrossRef]
- Lee, J.-J.; Kim, H.J.; Čeko, M.; Park, B.-Y.; Lee, S.A.; Park, H.; Roy, M.; Kim, S.-G.; Wager, T.D.; Woo, C.-W. A Neuroimaging Biomarker for Sustained Experimental and Clinical Pain. Nat. Med. 2021, 27, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Rangarajan, V.; Shirer, W.R.; Desai, N.; Greicius, M.D. The Will to Persevere induced by Electrical Stimulation of the Human Cingulate Gyrus. Neuron 2013, 80, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Shackman, A.J.; Salomons, T.V.; Slagter, H.; Fox, A.S.; Winter, J.; Davidson, R. The integration of Negative Affect, Pain and Cognitive Control in the Cingulate Cortex. Nat. Rev. Neurosci. 2011, 12, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.D.; Atlas, L.Y.; Botvinick, M.M.; Chang, L.J.; Coghill, R.C.; Davis, K.D.; Iannetti, G.D.; Poldrack, R.A.; Shackman, A.J.; Yarkoni, T. Pain in the ACC? Proc. Natl. Acad. Sci. USA 2016, 113, E2474–E2475. [Google Scholar] [CrossRef]
- Etkin, A.; Büchel, C.; Gross, J.J. The Neural Bases of Emotion Regulation. Nat. Rev. Neurosci. 2015, 16, 693–700. [Google Scholar] [CrossRef]
- Hur, J.; Stockbridge, M.D.; Fox, A.S.; Shackman, A.J. Dispositional Negativity, Cognition, and Anxiety Disorders: An integrative Translational Neuroscience Framework. Prog. Brain Res. 2019, 247, 375–436. [Google Scholar] [CrossRef]
- Trambaiolli, L.R.; Peng, X.; Lehman, J.F.; Linn, G.; Russ, B.E.; Schroeder, C.E.; Liu, H.; Haber, S.N. Anatomical and functional connectivity support the existence of a salience network node within the caudal ventrolateral prefrontal cortex. eLife 2022, 11, e76334. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Damien, A.F.; Francis, M.M.; Alexander, L.C.; Kristin, K.W.; Ronny, A.T.D.; Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E.; et al. Distinct Brain Networks for Adaptive and Stable Task Control in Humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11073–11078. [Google Scholar] [CrossRef]
- Dosenbach, N.U.; Visscher, K.M.; Palmer, E.D.; Miezin, F.M.; Wenger, K.K.; Kang, H.C.; Burgund, E.D.; Grimes, A.L.; Schlaggar, B.L.; Petersen, S.E. A Core System for the Implementation of Task Sets. Neuron 2006, 50, 799–812. [Google Scholar] [CrossRef]
- Cocchi, L.; Zalesky, A.; Fornito, A.; Mattingley, J.B. Dynamic Cooperation and Competition Between Brain Systems During Cognitive Control. Trends Cogn. Sci. 2013, 17, 493–501. [Google Scholar] [CrossRef]
- Sadaghiani, S.; D’Esposito, M. Functional Characterization of the Cingulo-Opercular Network in the Maintenance of Tonic Alertness. Cereb. Cortex 2014, 25, 2763–2773. [Google Scholar] [CrossRef]
- Coste, C.P.; Kleinschmidt, A. Cingulo-Opercular Network Activity Maintains Alertness. Neuroimage 2016, 128, 264–272. [Google Scholar] [CrossRef]
- Gratton, C.; Dworetsky, A.; Adeyemo, B.; Seitzman, B.A.; Smith, D.M.; Petersen, S.E.; Neta, M. The Cingulo-Opercular Network Is Composed of Two Distinct Sub-Systems. bioRxiv 2022. [Google Scholar] [CrossRef]
- Sadaghiani, S.; Hesselmann, G.; Friston, K.J.; Kleinschmidt, A. The Relation of Ongoing Brain Activity, Evoked Neural Responses, and Cognition. Front. Syst. Neurosci. 2010, 4, 20. [Google Scholar] [CrossRef]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L.; et al. Functional Network Organization of the Human Brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef]
- Han, S.W.; Eaton, H.P.; Marois, R. Functional Fractionation of the Cingulo-opercular Network: Alerting insula and Updating Cingulate. Cereb. Cortex 2018, 29, 2624–2638. [Google Scholar] [CrossRef]
- Tomiyama, H.; Murayama, K.; Nemoto, K.; Tomita, M.; Hasuzawa, S.; Mizobe, T.; Kato, K.; Ohno, A.; Tsuruta, S.; Togao, O.; et al. Increased Functional Connectivity Between Presupplementary Motor Area and inferior Frontal Gyrus Associated with the Ability of Motor Response inhibition in Obsessive–Compulsive Disorder. Hum. Brain Mapp. 2021, 43, 974–984. [Google Scholar] [CrossRef]
- Tsai, P.-J.; Keeley, R.J.; Carmack, S.A.; Vendruscolo, J.C.; Lu, H.; Gu, H.; Vendruscolo, L.F.; Koob, G.F.; Lin, C.-P.; Stein, E.A.; et al. Converging Structural and Functional Evidence for a Rat Salience Network. Biol. Psychiatry 2020, 88, 867–878. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Bechara, A. The hidden island of addiction: The insula. Trends Neurosci. 2009, 32, 56–67. [Google Scholar] [CrossRef]
- Durazzo, T.C.; Tosun, D.; Buckley, S.; Gazdzinski, S.; Mon, A.; Fryer, S.L.; Meyerhoff, D.J. Cortical Thickness, Surface Area, and Volume of the Brain Reward System in Alcohol Dependence: Relationships to Relapse and Extended Abstinence. Alcohol. Clin. Exp. Res. 2011, 35, 1187–1200. [Google Scholar] [CrossRef]
- Peters, S.K.; Dunlop, K.; Downar, J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front. Syst. Neurosci. 2016, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.J.; Lawrence, A.J. It’s more than just interoception: The insular Cortex involvement in Alcohol Use Disorder. J. Neurochem. 2021, 157, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Samantha, N.S.; Cui, H.; Zeng, N.; Du, J.; Yuan, T.; Li, D.; De Ridder, D.; Zhang, C. Anterior Cingulate Cortex in Addiction: New insights for Neuromodulation. Neuromodulation 2021, 24, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, G.; Zhang, W.; Han, Y.; Zhang, L.; Xu, H.; Meng, S.; Lu, L.; Xue, Y.; Shi, J. An orbitofrontal cortex–anterior insular cortex circuit gates compulsive cocaine use. Sci. Adv. 2022, 8, eabq5745. [Google Scholar] [CrossRef]
- Padula, C.B.; Tenekedjieva, L.-T.; McCalley, D.M.; Al-Dasouqi, H.; Hanlon, C.A.; Williams, L.M.; Kozel, F.A.; Knutson, B.; Durazzo, T.C.; Yesavage, J.A.; et al. Targeting the Salience Network: A Mini-Review on a Novel Neuromodulation Approach for Treating Alcohol Use Disorder. Front. Psychiatry 2022, 13, 893833. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.D. Converging findings from linkage and association analyses on susceptibility genes for smoking and other addictions. Mol. Psychiatry 2016, 21, 992–1008. [Google Scholar] [CrossRef]
- Durazzo, T.C.; Meyerhoff, D.; Yoder, K.K. Cigarette Smoking Is Associated with Cortical Thinning in Anterior Frontal Regions, insula and Regions Showing Atrophy in Early Alzheimer’s Disease. Drug Alcohol Depend. 2018, 192, 277–284. [Google Scholar] [CrossRef]
- Demirakca, T.; Ende, G.; Kämmerer, N.; Welzel-Marquez, H.; Hermann, D.; Heinz, A.; Mann, K. Effects of Alcoholism and Continued Abstinence on Brain Volumes in Both Genders. Alcohol. Clin. Exp. Res. 2011, 35, 1678–1685. [Google Scholar] [CrossRef]
- Sullivan, E.V.; Müller-Oehring, E.; Pitel, A.-L.; Chanraud, S.; Shankaranarayanan, A.; Alsop, D.C.; Rohlfing, T.; Pfefferbaum, A. A Selective insular Perfusion Deficit Contributes to Compromised Salience Network Connectivity in Recovering Alcoholic Men. Biol. Psychiatry 2013, 74, 547–555. [Google Scholar] [CrossRef]
- Butcher, T.J.; Chumin, E.J.; West, J.D.; Dzemidzic, M.; Yoder, K.K. Cerebral Blood Flow in the Salience Network of individuals with Alcohol Use Disorder. Alcohol Alcohol. 2021, 57, 445–451. [Google Scholar] [CrossRef]
- Senatorov, V.V.; Damadzic, R.; Mann, C.L.; Schwandt, M.L.; George, D.T.; Hommer, D.W.; Heilig, M.; Momenan, R. Reduced Anterior insula, Enlarged Amygdala in Alcoholism and Associated Depleted Von Economo Neurons. Brain 2014, 138, 69–79. [Google Scholar] [CrossRef]
- Ersche, K.D.; Barnes, A.; Jones, P.S.; Morein-Zamir, S.; Robbins, T.W.; Bullmore, E.T. Abnormal Structure of Frontostriatal Brain Systems Is Associated with Aspects of Impulsivity and Compulsivity in Cocaine Dependence. Brain 2011, 134, 2013–2024. [Google Scholar] [CrossRef]
- Moreno-López, L.; Catena, A.; Fernández-Serrano, M.J.; Delgado-Rico, E.; Stamatakis, E.A.; Pérez-García, M.; Verdejo-García, A. Trait Impulsivity and Prefrontal Gray Matter Reductions in Cocaine Dependent individuals. Drug Alcohol Depend. 2012, 125, 208–214. [Google Scholar] [CrossRef]
- Harris, G.J.; Jaffin, S.K.; Hodge, S.M.; Kennedy, D.; Caviness, V.S.; Marinkovic, K.; Papadimitriou, G.M.; Makris, N.; Berman, M.O. Frontal White Matter and Cingulum Diffusion Tensor Imaging Deficits in Alcoholism. Alcohol. Clin. Exp. Res. 2008, 32, 1001–1013. [Google Scholar] [CrossRef]
- Liu, H.; Li, L.; Hao, Y.; Cao, D.; Xu, L.; Rohrbaugh, R.; Xue, Z.; Hao, W.; Shan, B.; Liu, Z. Disrupted White Matter integrity in Heroin Dependence: A Controlled Study Utilizing Diffusion Tensor Imaging. Am. J. Drug Alcohol Abus. 2008, 34, 562–575. [Google Scholar] [CrossRef]
- Romero, M.J.; Asensio, S.; Palau, C.; Sanchez, A.; Romero, F.J. Cocaine Addiction: Diffusion Tensor Imaging Study of the inferior Frontal and Anterior Cingulate White Matter. Psychiatry Res. Neuroimaging 2010, 181, 57–63. [Google Scholar] [CrossRef]
- Goodkind, M.; Eickhoff, S.B.; Oathes, D.; Jiang, Y.; Chang, A.; Jones-Hagata, L.B.; Ortega, B.N.; Zaiko, Y.V.; Roach, E.L.; Korgaonkar, M.; et al. Identification of a Common Neurobiological Substrate for Mental Illness. JAMA Psychiatry 2015, 72, 305–315. [Google Scholar] [CrossRef]
- Garavan, H.; Stout, J.C. Neurocognitive insights into substance abuse. Trends Cogn. Sci. 2005, 9, 195–201. [Google Scholar] [CrossRef]
- Garavan, H. Insula and drug cravings. Brain Struct. Funct. 2010, 214, 593–601. [Google Scholar] [CrossRef]
- Droutman, V.; Read, S.J.; Bechara, A. Revisiting the role of the insula in addiction. Trends Cogn. Sci. 2015, 19, 414–420. [Google Scholar] [CrossRef]
- Dunlop, K.; Hanlon, C.A.; Downar, J. Noninvasive brain stimulation treatments for addiction and major depression. Ann. N. Y. Acad. Sci. 2017, 1394, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.L.; May, A.C.; Poppa, T.; Davenport, P.W.; Tapert, S.F.; Paulus, M.P. You are the danger: Attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug Alcohol Depend. 2014, 142, 110–119. [Google Scholar] [CrossRef]
- Stewart, J.L.; Connolly, C.G.; May, A.C.; Tapert, S.F.; Wittmann, M.; Paulus, M.P. Striatum and insula dysfunction during reinforcement learning differentiates abstinent and relapsed methamphetamine-dependent individuals. Addiction 2014, 109, 460–471. [Google Scholar] [CrossRef]
- Kim, Y.T.; Song, H.J.; Seo, J.H.; Lee, J.J.; Lee, J.; Kwon, D.H.; Yoo, D.S.; Lee, H.J.; Suh, K.J.; Chang, Y. The differences in neural network activity between methamphetamine abusers and healthy subjects performing an emotion-matching task: Functional MRI study. NMR Biomed. 2011, 24, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.L.; Luks, T.L.; Dryden, W.Y.; Rait, M.A.; Simpson, G.V. Adolescent Smokers Show Decreased Brain Responses to Pleasurable Food Images Compared with Nonsmokers. Nicotine Tob. Res. 2011, 13, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Janes, A.C.; Pizzagalli, D.A.; Richardt, S.; Frederick, B.D.; Chuzi, S.; Pachas, G.; Culhane, M.A.; Holmes, A.J.; Fava, M.; Evins, A.E.; et al. Brain Reactivity to Smoking Cues Prior to Smoking Cessation Predicts Ability to Maintain Tobacco Abstinence. Biol. Psychiatry 2010, 67, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Janes, A.C.; Farmer, S.; Peechatka, A.L.; Frederick, B.D.B.; Lukas, S.E. Insula–Dorsal Anterior Cingulate Cortex Coupling is Associated with Enhanced Brain Reactivity to Smoking Cues. Neuropsychopharmacology 2015, 40, 1561–1568. [Google Scholar] [CrossRef]
- Janes, A.; Gilman, J.; Radoman, M.; Pachas, G.; Fava, M.; Evins, A. Revisiting the Role of the insula and Smoking Cue-Reactivity in Relapse: A Replication and Extension of Neuroimaging Findings. Drug Alcohol Depend. 2017, 179, 8–12. [Google Scholar] [CrossRef]
- Kühn, S.; Gallinat, J. Common biology of craving across legal and illegal drugs—A quantitative meta-analysis of cue-reactivity brain response. Eur. J. Neurosci. 2011, 33, 1318–1326. [Google Scholar] [CrossRef]
- Canessa, N.; Basso, G.; Carne, I.; Poggi, P.; Gianelli, C. Increased decision latency in alcohol use disorder reflects altered resting-state synchrony in the anterior salience network. Sci. Rep. 2021, 11, 19581. [Google Scholar] [CrossRef]
- Kaufman, J.N.; Ross, T.J.; Stein, E.A.; Garavan, H. Cingulate Hypoactivity in Cocaine Users During a GO-NOGO Task as Revealed by Event-Related Functional Magnetic Resonance Imaging. J. Neurosci. 2003, 23, 7839–7843. [Google Scholar] [CrossRef]
- Bolla, K.; Ernst, M.; Kiehl, K.; Mouratidis, M.; Eldreth, D.; Contoreggi, C.; Matochik, J.; Kurian, V.; Cadet, J.; Kimes, A.; et al. Prefrontal Cortical Dysfunction in Abstinent Cocaine Abusers. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 456–464. [Google Scholar] [CrossRef]
- Kübler, A.; Murphy, K.; Garavan, H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur. J. Neurosci. 2005, 21, 1984–1992. [Google Scholar] [CrossRef]
- Ma, N.; Liu, Y.; Li, N.; Wang, C.X.; Zhang, H.; Jiang, X.F.; Xu, H.S.; Fu, X.M.; Hu, X.; Zhang, D.R. Addiction related alteration in resting-state brain connectivity. NeuroImage 2010, 49, 738–744. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Bechara, A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Anat. Embryol. 2010, 214, 435–450. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stewart, J.L. Interoception and drug addiction. Neuropharmacology 2014, 76, 342–350. [Google Scholar] [CrossRef]
- Sutherland, M.T.; Carroll, A.J.; Salmeron, B.J.; Ross, T.J.; Hong, L.E.; Stein, E.A. Down-Regulation of Amygdala and insula Functional Circuits by Varenicline and Nicotine in Abstinent Cigarette Smokers. Biol. Psychiatry 2013, 74, 538–546. [Google Scholar] [CrossRef]
- Xie, C.; Li, S.-J.; Shao, Y.; Fu, L.; Goveas, J.; Ye, E.; Li, W.; Cohen, A.D.; Chen, G.; Zhang, Z. Identification of Hyperactive intrinsic Amygdala Network Connectivity Associated with Impulsivity in Abstinent Heroin Addicts. Behav. Brain Res. 2011, 216, 639–646. [Google Scholar] [CrossRef]
- Gu, H.; Salmeron, B.J.; Ross, T.J.; Geng, X.; Zhan, W.; Stein, E.A.; Yang, Y. Mesocorticolimbic Circuits Are Impaired in Chronic Cocaine Users As Demonstrated by Resting-State Functional Connectivity. Neuroimage 2010, 53, 593–601. [Google Scholar] [CrossRef]
- Lerman, C.; Gu, H.; Loughead, J.; Ruparel, K.; Yang, Y.; Stein, E.A. Large-Scale Brain Network Coupling Predicts Acute Nicotine Abstinence Effects on Craving and Cognitive Function. JAMA Psychiatry 2014, 71, 523–530. [Google Scholar] [CrossRef]
- Sutherland, M.T.; Stein, E.A. Functional Neurocircuits and Neuroimaging Biomarkers of Tobacco Use Disorder. Trends Mol. Med. 2018, 24, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, N.H.; Rudrauf, D.; Damasio, H.; Bechara, A. Damage to the insula Disrupts Addiction to Cigarette Smoking. Science 2007, 315, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Suñer-Soler, R.; Grau, A.; Gras, M.E.; Font-Mayolas, S.; Silva, Y.; Dávalos, A.; Cruz, V.; Rodrigo, J.; Serena, J. Smoking Cessation 1 Year Poststroke and Damage to the insular Cortex. Stroke 2012, 43, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, A.; Williams, G.C.; Benesch, C.G.; Wang, H.Z.; Spitzer, E.M.; Scott, B.E.; Block, R.C.; van Wijngaarden, E. Damage to the insula Leads to Decreased Nicotine withdrawal during Abstinence. Addiction 2015, 110, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Joutsa, J.; Moussawi, K.; Siddiqi, S.H.; Abdolahi, A.; Drew, W.; Cohen, A.L.; Ross, T.J.; Deshpande, H.U.; Wang, H.Z.; Bruss, J.; et al. Brain Lesions Disrupting Addiction Map to a Common Human Brain Circuit. Nat. Med. 2022, 28, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Gaznick, N.; Tranel, D.; McNutt, A.; Bechara, A. Basal Ganglia Plus insula Damage Yields Stronger Disruption of Smoking Addiction Than Basal Ganglia Damage Alone. Nicotine Tob. Res. 2013, 16, 445–453. [Google Scholar] [CrossRef]
- Schultz, W.; Dayan, P.; Montague, P.R. A Neural Substrate of Prediction and Reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef]
- Howes, O.D.; Nour, M.M. Dopamine and the Aberrant Salience Hypothesis of Schizophrenia. World Psychiatry 2016, 15, 3–4. [Google Scholar] [CrossRef]
- Feltenstein, M.W.; See, R.E. The neurocircuitry of addiction: An overview. Br. J. Pharmacol. 2008, 154, 261–274. [Google Scholar] [CrossRef]
- Taber, K.H.; Black, D.N.; Porrino, L.J.; Hurley, R.A. Neuroanatomy of Dopamine: Reward and Addiction. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 1–4. [Google Scholar] [CrossRef]
- Solinas, M.; Belujon, P.; Fernagut, P.O.; Jaber, M.; Thiriet, N. Dopamine and addiction: What have we learned from 40 years of research. J. Neural. Transm. 2019, 126, 481–516. [Google Scholar] [CrossRef]
- Wise, R.A.; Robble, M.A. Dopamine and Addiction. Annu. Rev. Psychol. 2020, 71, 79–106. [Google Scholar] [CrossRef]
- Wise, R.A.; Jordan, C.J. Dopamine, behavior, and addiction. J. Biomed. Sci. 2021, 28, 83. [Google Scholar] [CrossRef]
- Imperato, A.; Chiara, G.D. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J. Pharmacol. Exp. Ther. 1986, 239, 219–228. [Google Scholar]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of Addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Suzuki, M.; Sedvall, G.C. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J. Chem. Neuroanat. 2001, 22, 127–137. [Google Scholar] [CrossRef]
- Arsenault, J.T.; Rima, S.; Stemmann, H.; Vanduffel, W. Role of the Primate Ventral Tegmental Area in Reinforcement and Motivation. Curr. Biol. 2014, 24, 1347–1353. [Google Scholar] [CrossRef]
- Kayser, A.S.; Allen, D.C.; Navarro-Cebrian, A.; Mitchell, J.M.; Fields, H.L. Dopamine, Corticostriatal Connectivity, and intertemporal Choice. J. Neurosci. 2012, 32, 9402–9409. [Google Scholar] [CrossRef]
- Malik, S.; Jacobs, M.; Cho, S.-S.; Boileau, I.; Blumberger, D.; Heilig, M.; Wilson, A.; Daskalakis, Z.J.; Strafella, A.P.; Zangen, A.; et al. Deep TMS of the insula using the H-coil modulates dopamine release: A crossover [11C] PHNO-PET pilot trial in healthy humans. Brain Imaging Behav. 2017, 12, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Nour, M.M.; Dahoun, T.; Jauhar, S.; Pepper, F.; Expert, P.; Veronese, M.; Adams, R.A.; Turkheimer, F.; Mehta, M.A.; et al. Mesolimbic Dopamine Function Is Related to Salience Network Connectivity: An integrative Positron Emission Tomography and Magnetic Resonance Study. Biol. Psychiatry 2018, 85, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Paulus, M.P.; Tapert, S.F.; Schulteis, G. The role of interoception and alliesthesia in addiction. Pharmacol. Biochem. Behav. 2009, 94, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Olsen, V.V.; Lugo, R.G.; Sütterlin, S. The somatic marker theory in the context of addiction: Contributions to understanding development and maintenance. Psychol. Res. Behav. Manag. 2015, 8, 187–200. [Google Scholar]
- Touroutoglou, A.; Hollenbeck, M.; Dickerson, B.C.; Barrett, L.F. Dissociable Large-Scale Networks Anchored in the Right Anterior insula Subserve Affective Experience and Attention. Neuroimage 2012, 60, 1947–1958. [Google Scholar] [CrossRef]
- Belcher, A.M.; Yen, C.C.; Stepp, H.; Gu, H.; Lu, H.; Yang, Y.; Silva, A.C.; Stein, E.A. Large-scale brain networks in the awake, truly resting marmoset monkey. J. Neurosci. 2013, 33, 16796–16804. [Google Scholar] [CrossRef]
- Belcher, A.M.; Yen, C.C.C.; Notardonato, L.; Ross, T.J.; Volkow, N.D.; Yang, Y.; Stein, E.A.; Silva, A.C.; Tomasi, D. Functional Connectivity Hubs and Networks in the Awake Marmoset Brain. Front. Integr. Neurosci. 2016, 10, 9. [Google Scholar] [CrossRef]
- Sforazzini, F.; Schwarz, A.J.; Galbusera, A.; Bifone, A.; Gozzi, A. Distributed BOLD and CBV-Weighted Resting-State Networks in the Mouse Brain. Neuroimage 2014, 87, 403–415. [Google Scholar] [CrossRef]
- Burman, K.J.; Rosa, M.G.P. Architectural subdivisions of medial and orbital frontal cortices in the marmoset monkey (Callithrix jacchus). J. Comp. Neurol. 2009, 514, 11–29. [Google Scholar] [CrossRef]
- Reser, D.H.; Majka, P.; Snell, S.; Chan, J.M.; Watkins, K.; Worthy, K.; Quiroga, M.D.M.; Rosa, M.G. Topography of claustrum and insula projections to medial prefrontal and anterior cingulate cortices of the common marmoset (Callithrix jacchus). J. Comp. Neurol. 2017, 525, 1421–1441. [Google Scholar] [CrossRef]
- Paxinos, G. The Marmoset Brain in Stereotaxic Coordinates, 1st ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Saper, C.B. Convergence of Autonomic and Limbic Connections in the insular Cortex of the Rat. J. Comp. Neurol. 1982, 210, 163–173. [Google Scholar] [CrossRef]
- Maffei, A.; Haley, M.; Fontanini, A. Neural Processing of Gustatory information in insular Circuits. Curr. Opin. Neurobiol. 2012, 22, 709–716. [Google Scholar] [CrossRef]
- Gehrlach, D.A.; Weiand, C.; Gaitanos, T.N.; Cho, E.; Klein, A.S.; Hennrich, A.A.; Conzelmann, K.-K.; Gogolla, N.; Max Planck Institute of Neurobiology; Circuits for Emotion Research Group; et al. A Whole-Brain Connectivity Map of Mouse insular Cortex. eLife 2020, 9, e55585. [Google Scholar] [CrossRef]
- Schilman, E.A.; Uylings, H.B.; Graaf, Y.G.-D.; Joel, D.; Groenewegen, H.J. The Orbital Cortex in Rats Topographically Projects to Central Parts of the Caudate–Putamen Complex. Neurosci. Lett. 2008, 432, 40–45. [Google Scholar] [CrossRef]
- Procyk, E.; Wilson, C.R.E.; Stoll, F.M.; Faraut, M.C.M.; Petrides, M.; Amiez, C. Midcingulate Motor Map and Feedback Detection: Converging Data from Humans and Monkeys. Cereb. Cortex 2014, 26, 467–476. [Google Scholar] [CrossRef]
- Vogt, B.A.; Paxinos, G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct. 2014, 219, 185–192. [Google Scholar] [CrossRef]
- Van Heukelum, S.; Mars, R.B.; Guthrie, M.; Buitelaar, J.K.; Beckmann, C.F.; Tiesinga, P.H.; Vogt, B.A.; Glennon, J.C.; Havenith, M.N. Where is Cingulate Cortex? A Cross-Species View. Trends Neurosci. 2020, 43, 285–299. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics. Nat. Methods 2011, 8, 26–29. [Google Scholar] [CrossRef]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef]
- Galvan, X.A.; Stauffer, X.W.R.; Acker, L.; El-shamayleh, X.Y.; Inoue, X.K.; Ohayon, X.S.; Schmid, X.M.C. Nonhuman Primate Optogenetics: Recent Advances and Future Directions. J. Neurosci. 2017, 37, 10894–10903. [Google Scholar] [CrossRef]
- Kim, C.; Adhikari, A.; Deisseroth, A.A.K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci. 2017, 18, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Urban, D.J.; Roth, B.L. DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic Tools with Therapeutic Utility. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L. DREADDs for Neuroscientists. Neuron 2016, 89, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Bucci, D.J.; Luikart, B.W.; Mahler, S.V. DREADDs: Use and application in behavioral neuroscience. Behav. Neurosci. 2016, 130, 137–155. [Google Scholar] [CrossRef]
- Whissell, P.D.; Tohyama, S.; Martin, L.J. The use of DREADDs to deconstruct behavior. Front. Genet. 2016, 7, 70. [Google Scholar] [CrossRef]
- Gomez, J.L.; Bonaventura, J.; Lesniak, W.; Mathews, W.B.; Sysa-Shah, P.; Rodriguez, L.A.; Ellis, R.J.; Richie, C.T.; Harvey, B.K.; Dannals, R.F.; et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017, 357, 503–507. [Google Scholar] [CrossRef]
- Bonaventura, J.; Eldridge, M.A.G.; Hu, F.; Gomez, J.L.; Sanchez-Soto, M.; Abramyan, A.M.; Lam, S.; Boehm, M.A.; Ruiz, C.; Farrell, M.R.; et al. High-potency ligands for dreadd imaging and activation in rodents and monkeys. Nat. Commun. 2019, 10, 4627. [Google Scholar] [CrossRef]
- Goutaudier, R.; Coizet, V.; Carcenac, C.; Carnicella, S. DREADDs: The power of the lock, the weakness of the key. favoring the pursuit of specific conditions rather than specific ligands. ENeuro 2019, 6, 5. [Google Scholar] [CrossRef]
- Cushnie, A.K.; Bullock, D.N.; Manea, A.M.G.; Tang, W.; Zimmermann, J.; Heilbronner, S.R. The use of chemogenetic actuator ligands in nonhuman primate DREADDS-fMRI. Curr. Res. Neurobiol. 2023, 4, 100072. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Deisseroth, K. Recent advances in optogenetics and pharmacogenetics. Brain Res. 2013, 1511, 1–5. [Google Scholar] [CrossRef]
- Jiang, J.; Cui, H.; Rahmouni, K. Optogenetics and pharmacogenetics: Principles and applications. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R633–R645. [Google Scholar] [CrossRef]
- Vlasov, K.; Van Dort, C.J.; Solt, K. Optogenetics and Chemogenetics. Methods Enzymol. 2018, 603, 181–196. [Google Scholar]
- Inoue, K.; Matsumoto, M.; Takada, M. Nonhuman Primate Optogenetics: Current Status and Future Prospects. In Optogenetics: Light-Sensing Proteins and Their Applications in Neuroscience and Beyond; Advances in Experimental Medicine and Biology; Yawo, H., Kandori, H., Koizumi, A., Kageyama, R., Eds.; Springer: Singapore, 2021; pp. 345–358. [Google Scholar]
- Lee, C.; Lavoie, A.; Liu, J.; Chen, S.X.; Liu, B.-H. Light Up the Brain: The Application of Optogenetics in Cell-Type Specific Dissection of Mouse Brain Circuits. Front. Neural Circuits 2020, 14, 18. [Google Scholar] [CrossRef]
- Navabpour, S.; Kwapis, J.L.; Jarome, T.J. A neuroscientist’s guide to transgenic mice and other genetic tools. Neurosci. Biobehav. Rev. 2020, 108, 732–748. [Google Scholar] [CrossRef]
- Clark, P.J.; Brodnik, Z.D.; España, R.A. Chemogenetic Signaling in Space and Time: Considerations for Designing Neuroscience Experiments Using DREADDs. Neuroscientist 2022. [Google Scholar] [CrossRef]
- Galvan, A.; Caiola, M.J.; Albaugh, D.L. Advances in optogenetic and chemogenetic methods to study brain circuits in non-human primates. J. Neural Transm. 2017, 125, 547–563. [Google Scholar] [CrossRef]
- Campbell, C.E.J.; Marchant, N.J.; Florey, T.; Health, M.; Brain, M.; Campbell, E.J.; Marchant, N.J. The use of chemogenetics in behavioural neuroscience: Receptor variants, targeting approaches and caveats. Br. J. Pharmacol. 2018, 175, 994–1003. [Google Scholar] [CrossRef]
- Yamamori, T. Functional visualization and manipulation in the marmoset brain using viral vectors. Curr. Opin. Pharmacol. 2021, 60, 11–16. [Google Scholar] [CrossRef]
- Deffains, M.; Nguyen, T.H.; Orignac, H.; Biendon, N.; Dovero, S.; Bezard, E.; Boraud, T. In vivo electrophysiological validation of DREADD-based modulation of pallidal neurons in the non-human primate. Eur. J. Neurosci. 2020, 53, 2192–2204. [Google Scholar] [CrossRef]
- Jazayeri, M.; Lindbloom-Brown, Z.; Horwitz, G.D. Saccadic eye movements evoked by optogenetic activation of primate VNat. Nat. Neurosci. 2012, 15, 1368–1370. [Google Scholar] [CrossRef]
- Ohayon, S.; Grimaldi, P.; Schweers, N.; Tsao, D.Y. Saccade Modulation by Optical and Electrical Stimulation in the Macaque Frontal Eye Field. J. Neurosci. 2013, 33, 16684–16697. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Evrard, H.C.; Shapcott, K.A.; Haverkamp, S.; Logothetis, N.K.; Schmid, M.C. Cell-Targeted Optogenetics and Electrical Microstimulation Reveal the Primate Koniocellular Projection to Supra-granular Visual Cortex. Neuron 2016, 90, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Rajalingham, R.; Sorenson, M.; Azadi, R.; Bohn, S.; DiCarlo, J.J.; Afraz, A. Chronically implantable LED arrays for behavioral optogenetics in primates. Nat. Methods 2021, 18, 1112–1116. [Google Scholar] [CrossRef]
- Galvan, A.; Raper, J.; Hu, X.; Paré, J.; Bonaventura, J.; Richie, C.T.; Michaelides, M.; Mueller, S.A.L.; Roseboom, P.H.; Oler, J.A.; et al. Ultrastructural localization of DREADD s in monkeys. Eur. J. Neurosci. 2019, 50, 2801–2813. [Google Scholar] [CrossRef] [PubMed]
- Raper, J.; Galvan, A. Applications of chemogenetics in non-human primates. Curr. Opin. Pharmacol. 2022, 64, 102204. [Google Scholar] [CrossRef]
- Cushnie, A.K.; El-Nahal, H.G.; Bohlen, M.O.; May, P.J.; Basso, M.A.; Grimaldi, P.; Wang, M.Z.; de Velasco Ezequiel, M.F.; Sommer, M.A.; Heilbronner, S.R. Using rAAV2-Retro in Rhesus Macaques: Promise and Caveats for Circuit Manipulation. J. Neurosci. Methods 2020, 345, 108859. [Google Scholar] [CrossRef]
- Azzinnari, D.; Sigrist, H.; Staehli, S.; Palme, R.; Hildebrandt, T.; Leparc, G.; Hengerer, B.; Seifritz, E.; Pryce, C.R. Mouse Social Stress induces increased Fear Conditioning, Helplessness and Fatigue to Physical Challenge Together with Markers of Altered Immune and Dopamine Function. Neuropharmacology 2014, 85, 328–341. [Google Scholar] [CrossRef]
- Grandjean, J.; Azzinnari, D.; Seuwen, A.; Sigrist, H.; Seifritz, E.; Pryce, C.R.; Rudin, M. Chronic psychosocial Stress in Mice Leads to Changes in Brain Functional Connectivity and Metabolite Levels Comparable to Human Depression. Neuroimage 2016, 142, 544–552. [Google Scholar] [CrossRef]
- Conio, B.; Martino, M.; Magioncalda, P.; Escelsior, A.; Inglese, M.; Amore, M.; Northoff, G. Opposite effects of dopamine and serotonin on resting-state networks: Review and implications for psychiatric disorders. Mol. Psychiatry 2019, 25, 82–93. [Google Scholar] [CrossRef]
- Van der Veen, B.; Kapanaiah, S.K.; Kilonzo, K.; Steele-Perkins, P.; Jendryka, M.M.; Schulz, S.; Tasic, B.; Yao, Z.; Zeng, H.; Akam, T.; et al. Control of impulsivity by Gi-protein signalling in layer-5 pyramidal neurons of the anterior cingulate cortex. Commun. Biol. 2021, 4, 662. [Google Scholar] [CrossRef]
- Menon, V.; Cerri, D.; Lee, B.; Yuan, R.; Lee, S.H.; Shih, Y.Y.I. Optogenetic stimulation of anterior insular cortex neurons in male rats reveals causal mechanisms underlying suppression of the default mode network by the salience network. Nat. Commun. 2023, 14, 866. [Google Scholar] [CrossRef]
- Cai, W.; Ryali, S.; Pasumarthy, R.; Talasila, V.; Menon, V. Dynamic Causal Brain Circuits During Working Memory and Their Functional Controllability. Nat. Commun. 2021, 12, 3314. [Google Scholar] [CrossRef]
- Chao, T.-H.H.; Lee, B.; Hsu, L.-M.; Cerri, D.H.; Zhang, W.-T.; Wang, T.-W.W.; Ryali, S.; Menon, V.; Shih, Y.-Y.I. Neuronal dynamics of the default mode network and anterior insular cortex: Intrinsic properties and modulation by salient stimuli. Sci. Adv. 2023, 9, eade5732. [Google Scholar] [CrossRef]
- Peeters, L.M.; Hinz, R.; Detrez, J.R.; Missault, S.; De Vos, W.H.; Verhoye, M.; Van der Linden, A.; Keliris, G.A. Chemogenetic silencing of neurons in the mouse anterior cingulate area modulates neuronal activity and functional connectivity. NeuroImage 2020, 220, 117088. [Google Scholar] [CrossRef]
- Rocchi, F.; Canella, C.; Noei, S.; Gutierrez-Barragan, D.; Coletta, L.; Galbusera, A.; Stuefer, A.; Vassanelli, S.; Pasqualetti, M.; Iurilli, G.; et al. Increased fMRI connectivity upon chemogenetic inhibition of the mouse prefrontal cortex. Nat. Commun. 2022, 13, 1056. [Google Scholar] [CrossRef]
- Alstott, J.; Breakspear, M.; Hagmann, P.; Cammoun, L.; Sporns, O. Modeling the Impact of Lesions in the Human Brain. PLoS Comput. Biol. 2009, 5, e1000408. [Google Scholar] [CrossRef]
- Suárez, L.E.; Markello, R.D.; Betzel, R.F.; Misic, B. Linking Structure and Function in Macroscale Brain Networks. Trends Cogn. Sci. 2020, 24, 302–315. [Google Scholar] [CrossRef]
- Grayson, D.S.; Bliss-Moreau, E.; Machado, C.J.; Bennett, J.; Shen, K.; Grant, K.A.; Fair, D.A.; Amaral, D.G. The Rhesus Monkey Connectome Predicts Disrupted Functional Networks Resulting from Pharmacogenetic inactivation of the Amygdala. Neuron 2016, 91, 453–466. [Google Scholar] [CrossRef]
- Nestler, E.J. Molecular neurobiology of addiction. Am. J. Addict. 2001, 10, 201–217. [Google Scholar] [CrossRef]
- Uhl, G.R.; Koob, G.F.; Cable, J. The neurobiology of addiction. Ann. N. Y. Acad. Sci. 2019, 1451, 5–28. [Google Scholar] [CrossRef]
- Degiorgis, L.; Arefin, T.M.; Ben-Hamida, S.; Noblet, V.; Antal, C.; Bienert, T.; Reisert, M.; von Elverfeldt, D.; Kieffer, B.L.; Harsan, L.A. Translational Structural and Functional Signatures of Chronic Alcohol Effects in Mice. Biol. Psychiatry 2022, 91, 1039–1050. [Google Scholar] [CrossRef]
- Cosme, C.V.; Gutman, A.L.; LaLumiere, R.T. The dorsal agranular insular cortex regulates the cued reinstatement of cocaine-seeking, but not food-seeking, behavior in rats. Neuropsychopharmacology 2015, 40, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Matsushima, Y.; Niikura, K.; Narita, M.; Takagi, S.; Nakahara, K.; Kurahashi, K.; Abe, M.; Saeki, M.; Asato, M.; et al. Implication of dopaminergic projection from the ventral tegmental area to the anterior cingulate cortex in μ-opioid-induced place preference. Addict. Biol. 2010, 15, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Ceric, F.; Torrealba, F. inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 2007, 318, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Pich, E.M.; Pagliusi, S.R.; Tessari, M.; Talabot-Ayer, D.; van Huijsduijnen, R.H.; Chiamulera, C. Common neural substrates for the addictive properties of nicotine and cocaine. Science 1997, 275, 83–86. [Google Scholar] [CrossRef]
- Haaranen, M.; Scuppa, G.; Tambalo, S.; Järvi, V.; Bertozzi, S.M.; Armirotti, A.; Sommer, W.H.; Bifone, A.; Hyytiä, P. Anterior insula stimulation suppresses appetitive behavior while inducing forebrain activation in alcohol-preferring rats. Transl. Psychiatry 2020, 10, 150. [Google Scholar] [CrossRef]
- Venniro, M.; Caprioli, D.; Zhang, M.; Whitaker, L.R.; Zhang, S.; Warren, B.L.; Cifani, C.; Marchant, N.J.; Yizhar, O.; Bossert, J.M.; et al. The Anterior Insular Cortex→Central Amygdala Glutamatergic Pathway Is Critical to Relapse after Contingency Management. Neuron 2017, 96, 414–427.e8. [Google Scholar] [CrossRef]
- Scarlata, M.J.; Keeley, R.J.; Stein, E.A. Nicotine addiction: Translational insights from circuit neuroscience. Pharmacol. Biochem. Behav. 2021, 204, 173171. [Google Scholar] [CrossRef]
- Sommer, W.H.; Canals, S.; Bifone, A.; Heilig, M.; Hyytiä, P. From a systems view to spotting a hidden island: A narrative review implicating insula function in alcoholism. Neuropharmacology 2022, 209, 108989. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cushnie, A.K.; Tang, W.; Heilbronner, S.R. Connecting Circuits with Networks in Addiction Neuroscience: A Salience Network Perspective. Int. J. Mol. Sci. 2023, 24, 9083. https://doi.org/10.3390/ijms24109083
Cushnie AK, Tang W, Heilbronner SR. Connecting Circuits with Networks in Addiction Neuroscience: A Salience Network Perspective. International Journal of Molecular Sciences. 2023; 24(10):9083. https://doi.org/10.3390/ijms24109083
Chicago/Turabian StyleCushnie, Adriana K., Wei Tang, and Sarah R. Heilbronner. 2023. "Connecting Circuits with Networks in Addiction Neuroscience: A Salience Network Perspective" International Journal of Molecular Sciences 24, no. 10: 9083. https://doi.org/10.3390/ijms24109083
APA StyleCushnie, A. K., Tang, W., & Heilbronner, S. R. (2023). Connecting Circuits with Networks in Addiction Neuroscience: A Salience Network Perspective. International Journal of Molecular Sciences, 24(10), 9083. https://doi.org/10.3390/ijms24109083





