The Anti-Acne Potential and Chemical Composition of Knautia drymeia Heuff. and Knautia macedonica Griseb Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Extracts
2.1.1. Liquid Chromatography–Mass Spectrometry Analysis (LC-MS)
2.1.2. Gas Chromatography–Mass Spectrometry Analysis (GC-MS)
2.2. Biological Activity
2.2.1. Antioxidant Activity
2.2.2. Enzyme Inhibitory Activity
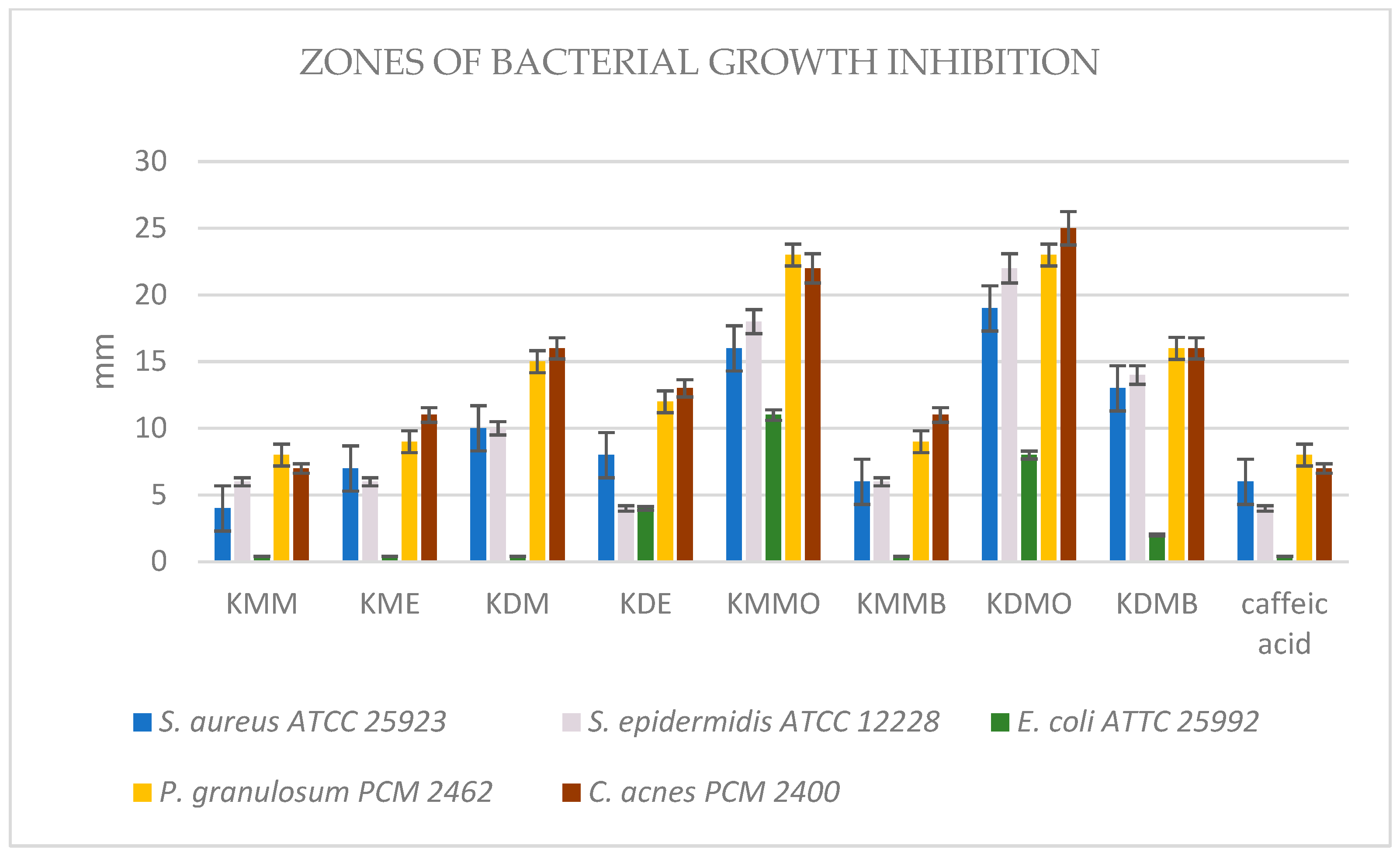
2.2.3. Antibacterial Activity
2.2.4. Cytotoxic Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Preparation of the Extracts
3.4. LC-MS Analysis
3.5. GC-MS Analysis
3.6. Antioxidant Activity
3.6.1. DPPH• Assay
3.6.2. ABTS●+ Assay
3.6.3. Metal Chelating Activity (CHEL)
3.7. Enzyme Inhibitory Activity
3.7.1. Cyclooxygenase-1 (COX-1) and Cyclooxygenase-2 (COX-2) Inhibitory Activity
3.7.2. Lipoxygenase Inhibitory Activity
3.8. Antibacterial Activity
3.8.1. Bacterial Conditions
3.8.2. Disc Diffusion Method
3.8.3. MIC and MBC Determination
3.9. Cytotoxic Activity
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonart, T. Newer approaches to the treatment of acne vulgaris. Am. J. Clin. Dermatol. 2012, 13, 357–364. [Google Scholar] [CrossRef]
- Chrząszcz, M.; Miazga-Karska, M.; Klimek, K.; Granica, S.; Tchórzewska, D.; Ginalska, G.; Szewczyk, K. Extracts from Cephalaria uralensis (Murray) Roem. & Schult. and Cephalaria gigantea (Ledeb.) Bobrov as potential agents for treatment of acne vulgaris: Chemical characterization and in vitro biological evaluation. Antioxidants 2020, 9, 796. [Google Scholar] [CrossRef]
- Knutsen-Larson, S.; Dawson, A.L.; Dunnick, C.A.; Dellavalle, R.P. Acne vulgaris: Pathogenesis, treatment, and needs assessment. Dermatol. Clin. 2012, 30, 99–106. [Google Scholar] [CrossRef]
- Aktan, S.; Özmen, E.; Berna, S. Anxiety, depression, and nature of acne vulgaris in adolescents. Int. J. Dermatol. 2000, 39, 354–357. [Google Scholar] [CrossRef]
- Tahir, C.M. Pathogenesis of acne vulgaris: Simplified. J. Pak. Assoc. Dermatol. 2010, 20, 93–97. [Google Scholar]
- Global Acne Market Report for 2016–2026. Available online: https://www.reportlinker.com/p05251482 (accessed on 10 April 2023).
- Harper, J.C. An update on the pathogenesis and management of acne vulgaris. J. Am. Acad. Dermatol. 2004, 51, 36–38. [Google Scholar] [CrossRef]
- Cong, T.-X.; Hao, D.; Wen, X.; Li, X.-H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef]
- Sahib, A.S.; Al-Anbari, H.H.; Raghif, A.R.A. Oxidative stress in acne vulgaris: An important therapeutic target. J. Mol. Pathophysiol. 2013, 2, 27–31. [Google Scholar] [CrossRef]
- Arican, O.; Kurutas, E.B.; Sasmaz, S. Oxidative stress in patients with acne vulgaris. Mediators Inflamm. 2005, 6, 380–384. [Google Scholar] [CrossRef]
- Chrząszcz, M.; Szewczyk, K.; Tchórzewska, D. Biotechnological potential of Cephalaria uralensis (Murray) Roem. & Schult. And C. gigantea (Ledeb.) Bobrov—Comparative analysis of plant anatomy and the content of biologically active substances. Plants 2021, 10, 986. [Google Scholar] [CrossRef]
- Levy, S.B. Multidrug resistance—A sign of the times. N. Engl. J. Med. 1998, 338, 1376–1378. [Google Scholar] [CrossRef]
- Patwardhan, B.; Mashelkar, R.A. Traditional medicine—Inspired approaches to drug discovery: Can Ayurveda show the way forward? Drug Discov. 2009, 14, 804–811. [Google Scholar] [CrossRef]
- Vange, V.; Heuch, I.; Vandvik, V. Do seed mass and family affect germination and juvenile performance in Knautia arvensis? A study using failure-time methods. Acta Oecol. 2004, 25, 169–178. [Google Scholar] [CrossRef]
- Karalija, E.; Ćavar Zeljković, S.; Tarkowski, P.; Muratović, E.; Parić, A. The effect of cytokinins on growth, phenolics, antioxidants and antimicrobial potential in liquid agitated shoot cultures of Knautia sarajevensis. Plant Cell Tissue Organ Cult. 2017, 131, 347–357. [Google Scholar] [CrossRef]
- Karalija, E.; Zeljković, S.Ć.; Tarkowski, P.; Muratović, E.; Parić, A. Media composition affects seed dormancy, apical dominance and phenolic profile of Knautia sarajevensis (Dipsacaceae), Bosnian endemic. Acta Bot. Croat. 2018, 77, 70–79. [Google Scholar] [CrossRef]
- Chrząszcz, M.; Dos Santos Szewczyk, K.; Dąbrowska, A.; Tchórzewska, D. Comparative analysis of the biotechnological potential of Knautia drymeia Heuff. and K. macedonica Griseb. Acta Sci. Pol. Hortorum Cultus. 2023, 22, 99–117. [Google Scholar] [CrossRef]
- Ghosh, V.K.; Nagore, D.H.; Kadbhane, K.P.; Patil, M.J. Different approaches of alternative medicines in acne vulgaris treatment. Orient. Pharm. Exp. Med. 2011, 11, 1–9. [Google Scholar] [CrossRef]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Mugwena, N.W. A Synopsis of Medicinally Important Indigenous Species of the Genus Scabiosa (Caprifoliaceae), an Evaluation of Their Biological Activity and Synergistic Properties of Scabiosa columbaria. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2020; pp. 1–98. [Google Scholar]
- Moldoch, J.; Szajwaj, B.; Masullo, M.; Pecio, L.; Oleszek, W.; Piacente, S.; Stochmal, A. Phenolic constituents of Knautia arvensis aerial parts. Nat. Prod. Commun. 2011, 6, 1627–1630. [Google Scholar] [CrossRef]
- Karalija, E.; Muratović, E.; Tarkowski, P.; Zeljković, S.Ć. Variation in phenolic composition of Knautia arvensis in correlation with geographic area and plant organ. Nat. Prod. Commun. 2017, 12, 545–548. [Google Scholar] [CrossRef]
- Czaban, J.; Mołdoch, J.; Wróblewska, B.; Szumacher-Strabel, M.; Cieślak, A.; Oleszek, W.; Stochmal, A. Effects of triterpenoid saponins of field scabious (Knautia arvensis L. Coult.), alfalfa, red clover and common soapwort on growth of Gaeumannomyces graminis var. tritici and Fusarium culmorum. Allelopath. J. 2013, 32, 79–89. [Google Scholar]
- Plouvier, V.; Favre-Bonvin, J. Les iridoïdes et séco-iridoïdes: Répartition, structure, propriétés, biosynthèse. Phytochemistry 1971, 10, 1697–1722. [Google Scholar] [CrossRef]
- Kopyt’ko, Y.F.; Dargaeva, T.D.; Rendyuk, T.D. Composition of the field scabious (Knautia arvensis L.). Pharm. Chem. J. 2020, 54, 725–733. [Google Scholar] [CrossRef]
- Karalija, E.; Zeljković, S.Ć.; Parić, A. Harvest time-related changes in biomass, phenolics and antioxidant potential in Knautia sarajevensis shoot cultures after elicitation with salicylic acid and yeast. In Vitr. Cell. Dev. Biol. Plant. 2019, 56, 177–183. [Google Scholar] [CrossRef]
- Giambanelli, E.; Filippo D’Antuono, L.; Romero-González, R.; Garrido Frenich, A. Identification and quantification of phenolic compounds in edible wild leafy vegetables by UHPLC/Orbitrap-MS. J. Sci. Food Agric. 2017, 98, 945–954. [Google Scholar] [CrossRef]
- Boyarskih, I.G.; Syso, A.I.; Siromlya, T.I. Variability of chemical elements and biologically active polyphenols in Lonicera caerulea subsp. Altaica (Caprifoliaceae) plant organs along an altitudinal gradient. Contemp. Probl. Ecol. 2019, 12, 594–606. [Google Scholar] [CrossRef]
- Pawłowska-Ćwięk, L. Flawonoidy wybranych gatunków z rodzin Caprifoliaceae i Cornaceae. Fragm. Flor. Geobot. Ser. Polonica. 1997, 2, 297–309. [Google Scholar]
- Movsumov, I.S.; Yusifova, D.Y.; Garaev, E.A.; Isaev, M.I. Flavonoids from Knautia montana flowers growing in Azerbaijan. Chem. Nat. Compd. 2011, 47, 438–439. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Choi, J.M.; Lee, E.O.; Lee, H.J.; Kim, K.H.; Ahn, K.S.; Shim, B.S.; Kim, N.I.; Song, M.C.; Baek, N.I.; Kim, S.H. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phytother. Res. 2007, 21, 954–959. [Google Scholar] [CrossRef]
- Chrząszcz, M.; Krzemińska, B.; Celiński, R.; Szewczyk, K. Phenolic composition and antioxidant activity of plants belonging to the Cephalaria (Caprifoliaceae) genus. Plants 2021, 10, 952. [Google Scholar] [CrossRef]
- Krzemińska, B.; Dybowski, M.P.; Klimek, K.; Typek, R.; Miazga-Karska, M.; Dos Santos Szewczyk, K. The anti-acne potential and chemical composition of two cultivated Cotoneaster species. Cells 2022, 11, 367. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Krzyżanowska, J. Preliminary anti-fungal activity of some Dipsacaceae family plants. Herba Pol. 1999, 2, 101–107. [Google Scholar]
- Sarıkahya, N.B.; Pekmez, M.; Arda, N.; Kayce, P.; Yavaşoğlu, N.Ü.K.; Kırmızıgül, S. Isolation and characterization of biologically active glycosides from endemic Cephalaria species in Anatolia. Phytochem. Lett. 2011, 4, 415–420. [Google Scholar] [CrossRef]
- Besbes, M.; Omri, A.; Cheraif, I.; Daami, M.; Jannet, H.B.; Mastouri, M.; Aouni, M.; Selmi, B. Chemical composition and antimicrobial activity of essential oils from Scabiosa arenaria Forssk. growing wild in Tunisia. Chem. Biodiv. 2012, 9, 829–839. [Google Scholar] [CrossRef]
- Chen, J.; Yao, D.; Yuan, H.; Zhang, S.; Tian, J.; Guo, W.; Liang, W.; Li, H.; Zhang, Y. Dipsacus asperoides polysaccharide induces apoptosis in osteosarcoma cells by modulating the PI3K/Akt pathway. Carbohyd. Polym. 2013, 95, 780–784. [Google Scholar] [CrossRef]
- Tabatadze, N.; Elias, R.; Faure, R.; Gerkens, P.; De Pauw-Gillet, M.C.; Kemertelidze, E.; Chea, A.; Ollivier, E. Cytotoxic triterpenoid saponins from the roots of Cephalaria gigantea. Chem. Pharm. Bull. 2007, 55, 102–105. [Google Scholar] [CrossRef]
- Ji, D.; Wu, Y.; Zhang, B.; Zhang, C.F.; Yang, Z.L. Triterpene saponins from the roots of Dipsacus asper and their protective effects against the Aβ25-35 induced cytotoxicity in PC12 cells. Fitoterapia 2012, 83, 834–848. [Google Scholar] [CrossRef]
- Guo, J.T.; Lee, H.L.; Chiang, S.H.; Lin, H.I.; Chang, C.Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- CLSI Performance Standards for Antimicrobial Susceptibility Testing; Eighteenth International Supplement; CLSI document M7-MIC; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2008.
- O’Donnell, F.; Smyth, T.J.P.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Miazga-Karska, M.; Szewczyk, K.; Klimek, K.; Ginalska, G. In vitro activity of peptide fractions from Impatiens glandulifera against caries causing bacteria. Acta Pol. Pharm. 2017, 74, 710–714. [Google Scholar] [PubMed]
- Pitucha, M.; Wo’s, M.; Miazga-Karska, M.; Klimek, K.; Mirosław, B.; Pachuta-Stec, A.; Gładysz, A.; Ginalska, G. Synthesis, antibacterial and antiproliferative potential of some new 1-pyridinecarbonyl-4-substituted thiosemicarbazide derivatives. Med. Chem. Res. 2016, 25, 1666–1677. [Google Scholar] [CrossRef] [PubMed]

| Peak No. | Name of Compound | Calibration Standard | Amounts [μg/g DE] | |||
|---|---|---|---|---|---|---|
| KDE | KDM | KDM-O | KDM-B | |||
| 1 | Gallic acid | Gallic acid | 68.8 ± 2.9 | 86.5 ± 4.1 | 246.0 ± 12.5 | 20.6 ± 0.9 |
| 2 | Neochlorogenic acid | Neochlorogenic acid | 5161.8 ± 247.7 | 3592.4 ± 176.3 | 5032.0 ± 245.2 | 3512.3 ± 161.6 |
| 3 | Chlorogenic acid | Chlorogenic acid | 21,845.2 ± 1133.6 | 24,364.3 ± 974.4 | 26,440.2 ± 1163.4 | 24,044.0 ± 1226.1 |
| 4 | p-Hydroxybenzoic acid | p-Hydroxybenzoic acid | 1212.5 ± 59.4 | 2024.6 ± 88.9 | 4644.9 ± 213.4 | 311.2 ± 13.7 |
| 5 | Cryptochlorogenic acid | Cryptochlorogenic acid | 21,923.6 ± 1052.2 | 19,562.7 ± 919.3 | 17,642.4 ± 846.7 | 18,040.1 ± 811.8 |
| 6 | Caffeic acid | Caffeic acid | 1884.4 ± 77.1 | 3948.3 ± 193.5 | 19,322.1 ± 792.1 | 391.2 ± 18.1 |
| 7 | Syringic acid | Syringic acid | 121.6 ± 5.2 | 146.3 ± 6.6 | 117.6 ± 4.9 | 12.0 ± 0.6 |
| 8 | p-Coumaric acid | p-Coumaric acid | 13.5 ± 0.6 | 30.3 ± 1.5 | 25.5 ± 1.2 | 1.9 ± 0.2 |
| 9 | o-Coumaric acid | p-Coumaric acid | 6.3 ± 0.3 | 25.3 ± 1.2 | 12.8 ± 0.6 | 2.2 ± 0.1 |
| 10 | Ferulic acid | Ferulic acid | 3588.8 ± 165.0 | 2624.7 ± 115.5 | 3208.8 ± 150.8 | 1988.7 ± 91.4 |
| 11 | 3,5-Di-O-caffeoylquinic acid | 3,5-Di-O-caffeoylquinic acid | 14,520.0 ± 653.4 | 9412.3 ± 423.3 | 21,805.9 ± 1068.2 | 31,560.2 ± 1325.5 |
| 12 | Vanillic acid | Vanillic acid | 1780.1 ± 83.7 | 1152.8 ± 55.3 | 1144.3 ± 51.5 | 548.0 ± 22.5 |
| 13 | m-Hydroxybenzoic acid | m-Hydroxybenzoic acid | 504.6 ± 22.5 | 363.6 ± 17.8 | 524.3 ± 23.1 | 178.1 ± 9.3 |
| 14 | Salicylic acid | Salicylic acid | 2220.1 ± 91.2 | 1260.4 ± 64.3 | 3112.2 ± 143.2 | 3072.4 ± 147.5 |
| 15 | Kaempferol-3-O-rutinoside | Rutin | 372.8 ± 17.9 | 892.0 ± 41.9 | 716.8 ± 34.4 | 78.8 ± 3.7 |
| 16 | Rutin | Rutin | 444.3 ± 18.6 | 507.0 ± 23.5 | 332.8 ± 15.6 | 130.7 ± 6.4 |
| 17 | Luteolin-7-O-rutinoside | Rutin | 168.4 ± 7.9 | 330.2 ± 16.8 | 374.8 ± 18.0 | 20.4 ± 0.8 |
| 18 | Quercetin-3-O-β-d-(2″-O-β-d-xylosyl)galactoside | Rutin | 23.4 ± 1.2 | 63.2 ± 3.0 | 50.8 ± 2.2 | 10.2 ± 0.5 |
| 19 | Naringin | Rutin | 54.8 ± 2.5 | 31.1 ± 1.4 | 74.4 ± 3.6 | 14.2 ± 0.7 |
| 20 | Rhoifolin | Rutin | 58.4 ± 2.7 | 80.8 ± 3.9 | 251.6 ± 12.1 | 62.6 ± 2.7 |
| 21 | Isorhoifolin | Rutin | 44.8 ± 2.2 | 74.4 ± 3.5 | 381.6 ± 19.5 | 50.7 ± 2.1 |
| 22 | Hyperoside | Rutin | 988.4 ± 46.4 | 1024.3 ± 43.0 | 2088.3 ± 108.6 | 1912.0 ± 78.4 |
| 23 | Isoquercitrin | Rutin | 148.4 ± 7.1 | 182.4 ± 8.2 | 130.4 ± 6.3 | 49.6 ± 2.2 |
| 24 | Quercitrin | Rutin | 1896.6 ± 79.6 | 1024.0 ± 41.2 | 804.2 ± 39.4 | 892.5 ± 44.6 |
| 25 | Quercetin-3-O-glucuronide | Rutin | 39.2 ± 1.7 | 395.6 ± 18.6 | 70.4 ± 3.3 | 7.6 ± 0.4 |
| 26 | Orientin | Rutin | 8960.0 ± 430.1 | 7480.0 ± 351.6 | 8365.4 ± 367.8 | 7404.4 ± 340.4 |
| 27 | Homoorientin | Rutin | 244.0 ± 10.2 | 399.2 ± 18.4 | 676.0 ± 30.4 | 208.4 ± 10.2 |
| 28 | Luteolin-7-O-glucuronide | Rutin | 305.2 ± 15.4 | 262.4 ± 11.5 | 145.6 ± 7.5 | 210.1 ± 9.2 |
| 29 | Kaempferol-3-O-glucuronide | Rutin | 3105.0 ± 133.3 | 4920.4 ± 236.2 | 2884.3 ± 118.2 | 2928.9 ± 143.5 |
| 30 | Apigenin-7-O-glucuronide | Rutin | 836.0 ± 36.8 | 1332.3 ± 54.6 | 1224.3 ± 58.6 | 608.8 ± 28.6 |
| 31 | Luteolin-7-O-glucoside | Rutin | 504.2 ± 22.5 | 1060.0 ± 51.9 | 1044.2 ± 45.9 | 153.2 ± 7.5 |
| 32 | Kaempferol-7-O-glucoside | Rutin | 99.6 ± 4.7 | 129.2 ± 6.2 | 148.8 ± 6.1 | 48.4 ± 2.3 |
| 33 | Kaempferol-3-O-glucoside | Rutin | 31.2 ± 1.4 | 60.8 ± 2.9 | 111.4 ± 4.8 | 29.3 ± 1.2 |
| 34 | Isovitexin | Rutin | 285.2 ± 12.0 | 262.4 ± 11.5 | 207.6 ± 9.3 | 261.6 ± 12.0 |
| 35 | Vitexin | Rutin | 1152.7 ± 55.3 | 2672.0 ± 136.3 | 2808.3 ± 123.6 | 1424.1 ± 69.8 |
| 36 | Apigetrin | Rutin | 768.8 ± 37.6 | 608.4 ± 28.6 | 327.6 ± 16.1 | 103.2 ± 4.9 |
| 37 | Myricetin | Quercetin | 12.5 ± 0.6 | 15.6 ± 0.6 | 41.6 ± 2.2 | 7.1 ± 0.3 |
| 38 | Rosmarinic acid | Caffeic acid | 772.6 ± 38.6 | 1912.0 ± 80.3 | 608.5 ± 30.4 | 408.0 ± 18.0 |
| 39 | Sinapic acid | Ferulic acid | 4.4 ± 0.2 | 166.0 ± 7.8 | 4.9 ± 0.3 | 0.5 ± 0.1 |
| 40 | Quercetin | Quercetin | 85.2 ± 4.4 | 80.8 ± 3.4 | 141.2 ± 6.4 | 18.2 ± 0.9 |
| 41 | Kaempferol | Kaempferol | 206.8 ± 9.9 | 448.0 ± 21.1 | 528.6 ± 24.8 | 22.1 ± 1.1 |
| 42 | Luteolin | Luteolin | 23.8 ± 1.1 | 30.2 ± 1.5 | 68.2 ± 2.8 | 6.3 ± 0.3 |
| 43 | Apigenin | Apigenin | 181.2 ± 8.2 | 341.6 ± 17.4 | 287.6 ± 12.4 | 28.7 ± 1.4 |
| 44 | Naringenin | Apigenin | 24.4 ± 1.0 | 48.4 ± 2.3 | 90.5 ± 4.1 | 24.6 ± 1.2 |
| 45 | Galangin | Apigenin | 17.1 ± 0.8 | 20.8 ± 0.9 | 8.6 ± 0.4 | 7.5 ± 0.3 |
| 46 | Chrysin | Chrysin | 168.4 ± 8.1 | 177.6 ± 7.8 | 127.2 ± 6.1 | 124.8 ± 5.5 |
| 47 | Pinocembrin | Chrysin | 60.8 ± 2.9 | 302.4 ± 13.6 | 79.2 ± 3.6 | 182.0 ± 7.3 |
| Peak No. | Name of Compound | Calibration Standard | Amounts [μg/g DE] | |||
|---|---|---|---|---|---|---|
| KME | KMM | KMM-O | KMM-B | |||
| 1 | Gallic acid | Gallic acid | 226.2 ± 11.1 | 116.3 ± 5.8 | 476.2 ± 24.3 | 16.4 ± 0.7 |
| 2 | Neochlorogenic acid | Neochlorogenic acid | 2480.6 ± 119.0 | 2304.5 ± 108.3 | 5083.4 ± 223.5 | 3916.4 ± 160.6 |
| 3 | Chlorogenic acid | Chlorogenic acid | 21,256.1 ± 869.2 | 22,326.5± 1071.4 | 27,015.6 ± 1269.0 | 28,043.0 ± 1345.9 |
| 4 | p-Hydroxybenzoic acid | p-Hydroxybenzoic acid | 916.4 ± 44.9 | 1524.5 ± 64.5 | 6165.2 ± 301.8 | 315.6 ± 14.2 |
| 5 | Cryptochlorogenic acid | Cryptochlorogenic acid | 16,923.4 ± 710.6 | 26,083.5 ± 1173.6 | 19,280.7 ± 867.6 | 25,720.3 ± 1131.7 |
| 6 | Caffeic acid | Caffeic acid | 952.0 ± 39.0 | 956.4 ± 39.2 | 16,560.0 ± 844.6 | 308.4 ± 14.5 |
| 7 | Syringic acid | Syringic acid | 303.8 ± 13.5 | 364.4 ± 15.3 | 344.4 ± 13.8 | 40.4 ± 1.7 |
| 8 | p-Coumaric acid | p-Coumaric acid | 38.1 ± 1.6 | 94.4 ± 4.2 | 51.2 ± 2.3 | 4.2 ± 0.2 |
| 9 | o-Coumaric acid | p-Coumaric acid | 34.8 ± 1.7 | 100.4 ± 4.8 | 44.8 ± 2.1 | 3.8 ± 0.2 |
| 10 | Ferulic acid | Ferulic acid | 5960.4 ± 256.3 | 3264.4 ± 156.5 | 3776.3 ± 181.2 | 2812.8 ± 115.3 |
| 11 | 3,5-Di-O-caffeoylquinic acid | 3,5-Di-O-caffeoylquinic acid | 11,123.5 ± 513.4 | 9046.6 ± 387.1 | 18,885.1 ± 925.1 | 2188.5 ± 102.8 |
| 12 | Vanillic acid | Vanillic acid | 1384.0 ± 56.7 | 1812.4 ± 79.2 | 1548.6 ± 65.0 | 688.4 ± 28.2 |
| 13 | m-Hydroxybenzoic acid | m-Hydroxybenzoic acid | 148.5 ± 6.7 | 141.2 ± 6.4 | 404.6 ± 18.2 | 210.4 ± 9.9 |
| 14 | Salicylic acid | Salicylic acid | 370.4 ± 16.7 | 660.0 ± 31.7 | 1792.2 ± 75.3 | 484.4 ± 20.3 |
| 15 | Kaempferol-3-O-rutinoside | Rutin | 1956.0 ± 91.9 | 680.3 ± 30.6 | 796.1 ± 37.4 | 62.8 ± 2.8 |
| 16 | Rutin | Rutin | 328.8 ± 15.8 | 756.5 ± 33.3 | 1668.3 ± 80.1 | 816.0 ± 36.7 |
| 17 | Luteolin-7-O-rutinoside | Rutin | 712.3 ± 30.1 | 329.6 ± 15.5 | 353.6 ± 15.6 | 18.8 ± 0.9 |
| 18 | Quercetin-3-O-β-D-(2″-O-β-D-xylosyl)galactoside | Rutin | 123.2 ± 5.5 | 58.3 ± 2.8 | 60.3 ± 2.5 | 5.3 ± 0.2 |
| 19 | Naringin | Rutin | 70.4 ± 3.1 | 6.6 ± 0.3 | 25.2 ± 1.1 | 4.4 ± 0.2 |
| 20 | Rhoifolin | Rutin | 23.5 ± 1.0 | 58.2 ± 2.7 | 192.8 ± 9.3 | 55.2 ± 2.3 |
| 21 | Isorhoifolin | Rutin | 46.4 ± 2.2 | 34.3 ± 1.5 | 112.4 ± 5.3 | 11.2 ± 0.5 |
| 22 | Hyperoside | Rutin | 1516.0 ± 71.3 | 1368.0 ± 57.5 | 528.8 ± 21.6 | 524.0 ± 23.6 |
| 23 | Isoquercitrin | Rutin | 162.4 ± 7.8 | 215.2 ± 10.3 | 220.1 ± 10.6 | 138.8 ± 5.8 |
| 24 | Quercitrin | Rutin | 904.4 ± 40.7 | 1548.3 ± 72.8 | 948.2 ± 43.6 | 1356.0 ± 57.2 |
| 25 | Quercetin-3-O-glucuronide | Rutin | 111.2 ± 5.2 | 174.8 ± 8.6 | 1832.8 ± 80.6 | 127.2 ± 6.2 |
| 26 | Orientin | Rutin | 7423.0 ± 310.8 | 7680.7 ± 368.6 | 1832.8 ± 80.6 | 8084.6 ± 347.4 |
| 27 | Homoorientin | Rutin | 170.8 ± 7.7 | 852.5 ± 34.9 | 8564.4 ± 385.2 | 66.8 ± 3.2 |
| 28 | Luteolin-7-O-glucuronide | Rutin | 133.2 ± 6.3 | 165.2 ± 7.9 | 880.2 ± 42.2 | 322.6 ± 13.8 |
| 29 | Kaempferol-3-O-glucuronide | Rutin | 4320.4 ± 198.7 | 4360.4 ± 209.3 | 182.4 ± 8.6 | 5280.8 ± 216.5 |
| 30 | Apigenin-7-O-glucuronide | Rutin | 1564.0 ± 65.7 | 302.5 ± 12.4 | 4412.3 ± 198.2 | 2596.3 ± 124.6 |
| 31 | Luteolin-7-O-glucoside | Rutin | 992.4 ± 47.6 | 908.0 ± 42.7 | 4360.2 ± 204.9 | 314.4 ± 14.1 |
| 32 | Kaempferol-7-O-glucoside | Rutin | 146.0 ± 6.9 | 186.8 ± 9.5 | 2904.5 ± 133.6 | 65.2 ± 2.9 |
| 33 | Kaempferol-3-O-glucoside | Rutin | 23.6 ± 1.1 | 26.0 ± 1.2 | 154.5 ± 7.4 | 28.4 ± 1.2 |
| 34 | Isovitexin | Rutin | 83.2 ± 4.0 | 193.2 ± 9.3 | 108.4 ± 5.3 | 154.4 ± 7.3 |
| 35 | Vitexin | Rutin | 1512.0 ± 72.0 | 1668.6 ± 78.4 | 96.4 ± 4.9 | 2116.2 ± 93.1 |
| 36 | Apigetrin | Rutin | 668.3 ± 28.1 | 1532.5 ± 70.5 | 5840.8 ± 274.5 | 580.0 ± 27.8 |
| 37 | Myricetin | Quercetin | 13.3 ± 0.5 | 5.2 ± 0.2 | 1424.5 ± 64.1 | 3.2 ± 0.2 |
| 38 | Rosmarinic acid | Caffeic acid | 1756.5 ± 75.5 | 1938.0 ± 78.2 | 41.2 ± 2.0 | 1024.3 ± 52.2 |
| 39 | Sinapic acid | Ferulic acid | 43.6 ± 2.3 | 8.8 ± 0.5 | 1284.0 ± 59.1 | 1.8 ± 0.1 |
| 40 | Quercetin | Quercetin | 36.3 ± 1.6 | 78.8 ± 3.7 | 62.8 ± 2.8 | 9.5 ± 0.4 |
| 41 | Kaempferol | Kaempferol | 196.4 ± 9.2 | 263.6 ± 11.6 | 250.2 ± 11.8 | 31.7 ± 1.5 |
| 42 | Luteolin | Luteolin | 22.4 ± 1.0 | 22.3 ± 1.0 | 1252.5 ± 63.9 | 5.2 ± 0.2 |
| 43 | Apigenin | Apigenin | 194.4 ± 8.7 | 235.6 ± 11.3 | 78.8 ± 3.2 | 87.6 ± 3.7 |
| 44 | Naringenin | Apigenin | 60.0 ± 2.5 | 26.0 ± 1.1 | 1053.8 ± 45.3 | 31.3 ± 1.4 |
| 45 | Galangin | Apigenin | 25.7 ± 1.2 | 71.6 ± 2.9 | 86.8 ± 3.7 | 18.4 ± 0.9 |
| 46 | Chrysin | Chrysin | 198.4 ± 9.1 | 194.4 ± 8.2 | 20.6 ± 0.8 | 177.2 ± 8.7 |
| 47 | Pinocembrin | Chrysin | 912.5 ± 43.8 | 264.8 ± 11.4 | 116.8 ± 5.3 | 113.6 ± 5.3 |
| Peak No. | Retention Time | Name of Compound | Area [%] | |||
|---|---|---|---|---|---|---|
| KDE | KDM | KDM-O | KDM-B | |||
| 1 | 4.621 | 2-Ethylhexanal | nd | nd | nd | 1.81 |
| 2 | 5.505 | 2-Hydroxy-γ-butyrolactone | nd | nd | nd | 0.37 |
| 3 | 5.915 | 2-Ethyl-1-hexanol | nd | nd | nd | 0.46 |
| 4 | 6.369 | Glycerin | nd | nd | nd | 0.72 |
| 5 | 6.800 | 2,5-Dimethylfuran-3,4(2H,5H)-dione | nd | nd | nd | 0.17 |
| 6 | 6.948 | 1,4-Butanediol | nd | 0.45 | nd | 0.23 |
| 7 | 7.556 | Hexanoic acid, 2-ethyl- | nd | nd | nd | 0.10 |
| 8 | 7.835 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | nd | nd | nd | 0.27 |
| 9 | 8.390 | 1,2-Ethanediamine, N,N’-dibutyl- | nd | nd | nd | 0.48 |
| 10 | 8.722 | Butane, 1,1-dibutoxy- | nd | nd | nd | 0.22 |
| 11 | 9.317 | 1-Butoxy-1-isobutoxy-butane | nd | nd | nd | 0.11 |
| 12 | 9.939 | Pentanoic acid, 2,2,4,4-tetramethyl- | nd | nd | nd | 0.18 |
| 13 | 10.204 | 2-Methoxy-4-vinylphenol | nd | nd | 3.00 | nd |
| 14 | 10.297 | 3-Penten-2-one, 3-(2-furanyl)- | nd | 0.70 | nd | nd |
| 15 | 10.804 | Eugenol | nd | 0.15 | nd | nd |
| 16 | 11.030 | 1-Tridecene | 0.78 | nd | nd | nd |
| 17 | 11.500 | cis-7-Tetradecen-1-ol | 11.06 | nd | nd | nd |
| 18 | 11.547 | Undecane, 3-methylene- | nd | 2.43 | 1.57 | nd |
| 19 | 11.552 | 5-Decen-1-ol, (E)- | nd | nd | 1.96 | 0.87 |
| 20 | 11.905 | Undefined compound | 1.71 | nd | nd | nd |
| 21 | 11.988 | 2,4-Dodecadiene, (E,Z)- | nd | 0.57 | nd | 0.24 |
| 22 | 12.007 | Benzaldehyde, 2-hydroxy-6-methyl- | nd | nd | 13.87 | nd |
| 23 | 12.170 | d-Gluco-heptulosan | 9.58 | 3.70 | nd | nd |
| 24 | 12.677 | 2H-Pyran-3(4H)-one, 6-ethenyldihydro-2,2,6-trimethyl- | nd | 9.78 | nd | nd |
| 25 | 12.799 | 1,1-Diisobutoxy-isobutane | nd | nd | nd | 2.38 |
| 26 | 13.365 | 2-Dodecenal | 11.82 | nd | nd | nd |
| 27 | 13.477 | Cyclohexane, (1,2,2-trimethylbutyl)- | nd | nd | nd | 1.32 |
| 28 | 13.850 | Undefined compound | 6.08 | nd | nd | nd |
| 29 | 13.964 | 5-Dodecenol | nd | 2.85 | nd | nd |
| 30 | 14.115 | β-d-Glucopyranoside, methyl | 34.87 | 56.72 | 31.18 | nd |
| 31 | 15.245 | Benzeneacetic acid, 4-hydroxy-3-methoxy-, methyl ester | 0.35 | nd | nd | nd |
| 32 | 15.355 | (E)-4-(3-Hydroxyprop-1-en-1-yl)-2-methoxyphenol | 2.06 | nd | nd | nd |
| 33 | 15.383 | α-d-Glucopyranoside, methyl | nd | nd | nd | 79.45 |
| 34 | 15.610 | 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one | nd | nd | 0.70 | nd |
| 35 | 15.665 | 1,13-Tetradecadien-3-one | 5.32 | nd | nd | nd |
| 36 | 15.703 | Tridecane, 3-methylene- | nd | 2.17 | nd | nd |
| 37 | 16.015 | Isopropyl myristate | 0.53 | nd | nd | nd |
| 38 | 16.257 | Neophytadiene | nd | 0.29 | 1.43 | nd |
| 39 | 16.315 | 2-Pentadecanone, 6,10,14-trimethyl- | 0.57 | nd | nd | nd |
| 40 | 16.326 | 2-Undecanone, 6,10-dimethyl- | nd | 0.41 | 0.82 | nd |
| 41 | 17.035 | Hexadecanoic acid, methyl ester | 0.98 | 1.18 | 1.51 | 0.68 |
| 42 | 17.526 | Palmitic acid | nd | 0.58 | 1.03 | nd |
| 43 | 18.006 | β-d-Glucosyloxyazoxymethane | nd | nd | nd | 0.54 |
| 44 | 18.724 | trans,trans-9,12-Octadecadienoic acid, propyl ester | nd | nd | 0.19 | nd |
| 45 | 18.781 | Linolenic acid methyl ester | nd | nd | 0.47 | nd |
| 46 | 18.786 | 11(Z),14(Z),17(Z)-Eicosatrienoic acid methyl ester | nd | 0.28 | nd | 0.32 |
| 47 | 18.894 | Phytol | 0.36 | 0.35 | 0.90 | nd |
| 48 | 18.960 | Methyl stearate | 1.74 | 1.38 | 2.04 | 1.23 |
| 49 | 20.140 | Undec-10-ynoic acid, tetradecyl ester | 0.24 | 0.35 | nd | nd |
| 50 | 20.175 | E-10,13,13-Trimethyl-11-tetradecen-1-ol acetate | nd | nd | 1.13 | nd |
| 51 | 20.406 | Benzyl β-d-glucoside | nd | nd | nd | 0.54 |
| 52 | 20.471 | Glycidyl palmitate | nd | nd | 0.17 | nd |
| 53 | 20.744 | Methyl 18-methylnonadecanoate | nd | 0.16 | 0.23 | nd |
| 54 | 22.103 | Octacosanal | nd | nd | 0.75 | nd |
| 55 | 22.155 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 2.01 | 1.53 | 6.42 | 1.38 |
| 56 | 23.691 | Methyl (Z)-5,11,14,17-eicosatetraenoate | nd | nd | 2.16 | nd |
| 57 | 23.785 | Octadecanoic acid, 2,3-dihydroxypropyl ester | 2.20 | 7.11 | 10.18 | 5.10 |
| 58 | 25.310 | Hexacosyl nonyl ether | nd | 0.21 | nd | nd |
| 59 | 26.700 | Cholesta-4,6-dien-3-ol, (3β)- | 0.43 | 0.23 | 0.88 | nd |
| 60 | 27.210 | 2-(Decanoyloxy)propane-1,3-diyl dioctanoate | 0.40 | 0.63 | nd | nd |
| 61 | 27.251 | dl-α-Tocopherol | nd | nd | 0.38 | nd |
| 62 | 27.268 | 5-Octadecenal | nd | 0.20 | nd | nd |
| 63 | 28.013 | Ergost-5-en-3-ol, (3β)- | nd | nd | 0.40 | nd |
| 64 | 28.599 | 1-Pentacosanol | nd | nd | 0.38 | nd |
| 65 | 28.625 | Campesterol | 9.16 | 4.70 | 2.76 | 0.18 |
| 66 | 28.747 | Stigmast-5-ene, 3-methoxy-, (3β)- | nd | nd | nd | 0.06 |
| 67 | 28.811 | Cholesta-4,6-dien-3-one | nd | nd | 0.88 | nd |
| 68 | 29.111 | Acetyl betulinaldehyde | nd | nd | 2.31 | 0.29 |
| 69 | 29.226 | Tricyclo [5.4.3.0(1,8)]tetradecan-3-ol-9-one, 4-ethenyl-6-(2-hydroxyacetoxy)-2,4,7,14-tetramethyl- | nd | nd | 1.48 | nd |
| 70 | 29.227 | Ursolic aldehyde | nd | nd | nd | 0.10 |
| 71 | 29.420 | Stigmast-4-en-3-one | 0.38 | nd | nd | nd |
| 72 | 29.513 | Stigmasta-3,5-dien-7-one | nd | nd | 0.72 | nd |
| 73 | 29.517 | 7-Oxo-5-cholesten-3β-yl benzoate | nd | 0.34 | nd | nd |
| 74 | 29.610 | Betulinaldehyde | nd | nd | 2.79 | nd |
| 75 | 29.765 | Cholest-4-en-26-oic acid, 3-oxo- | 0.37 | nd | nd | nd |
| 76 | 29.851 | 9,19-Cyclolanostan-3-ol, 24-methylene-, (3β)- | nd | 0.26 | nd | nd |
| 77 | 30.901 | Methyl ursa-2,12-dien-28-oate | nd | nd | 2.31 | nd |
| 78 | 30.903 | Olean-12-en-28-oic acid, 2β,3β,23-trihydroxy-, methyl ester | nd | 0.29 | nd | 0.20 |
| 79 | 31.523 | Methyl 3-hydroxyurs-12-en-28-oate | nd | nd | 0.93 | nd |
| 80 | 32.686 | Uvaol | nd | nd | 0.18 | nd |
| 81 | 33.86 | Urs-12-en-28-oic acid, 2,3,19-trihydroxy-, methyl ester, (2α,3β)- | nd | nd | 0.31 | nd |
| 82 | 33.951 | Androst-5-en-7-one, 3-(acetyloxy)-, (3 β)- | nd | nd | 0.24 | nd |
| 83 | 36.743 | 25-Nor-9,19-cyclolanostan-24-one, 3-acetoxy-24-phenyl- | nd | nd | 1.34 | nd |
| Peak No. | Retention Time | Name of Compound | Area [%] | |||
|---|---|---|---|---|---|---|
| KME | KMM | KMM-O | KMM-B | |||
| 1 | 5.567 | 2-Hydroxy-γ-butyrolactone | nd | nd | nd | 0.38 |
| 2 | 6.178 | Glycerin | nd | nd | nd | 1.76 |
| 3 | 6.824 | 2,5-Dimethylfuran-3,4(2H,5H)-dione | nd | nd | nd | 0.1 |
| 4 | 6.952 | 1,4-Butanediol | nd | nd | nd | 0.32 |
| 5 | 8.386 | Hexanoic acid, 3-hydroxy-, methyl ester | nd | nd | nd | 0.55 |
| 6 | 8.965 | Coumaran | nd | nd | 2.88 | nd |
| 7 | 9.101 | Catechol | nd | nd | nd | 1.60 |
| 8 | 9.194 | 2-Pyridinamine, 1-oxide | nd | 1.32 | nd | nd |
| 9 | 9.361 | 1-Oxaspiro[3.5]nona-5,8-dien-7-one, 3-methylene- | 1.73 | nd | nd | nd |
| 10 | 10.195 | 2-Methoxy-4-vinylphenol | nd | nd | 0.85 | 1.23 |
| 11 | 11.329 | 4-Ethylcatechol | nd | nd | 0.83 | 0.45 |
| 12 | 11.536 | Undecanal | 5.18 | nd | nd | nd |
| 13 | 11.548 | Cyclohexene, 1,5,5-trimethyl-6-acetylmethyl- | nd | 3.37 | nd | nd |
| 14 | 11.561 | 5-Decen-1-ol, (Z)- | nd | nd | nd | 0.60 |
| 15 | 11.977 | 2,4-Dodecadiene, (E,Z)- | 0.77 | 0.50 | nd | nd |
| 16 | 12.000 | Benzaldehyde, 2-hydroxy-4-methyl- | nd | nd | 7.62 | nd |
| 17 | 12.580 | d-Gluco-heptulosan | 10.24 | 11.49 | nd | nd |
| 18 | 12.804 | Butane, 1,1-dibutoxy- | nd | nd | nd | 9.10 |
| 19 | 12.804 | 2-Propanone, 1,1-dibutoxy- | nd | nd | nd | 1.32 |
| 20 | 13.450 | Undecane, 3-methylene- | 3.79 | 3.12 | 0.54 | nd |
| 21 | 13.488 | Tridecane, 3-methylene- | 1.81 | 1.86 | nd | 0.81 |
| 22 | 13.896 | 3,7,7-Trimethylbicyclo [4.1.0]heptan-2-ol | nd | nd | 1.54 | nd |
| 23 | 13.955 | (Z)6-Pentadecen-1-ol | 4.03 | nd | nd | nd |
| 24 | 13.961 | Cyclopropaneacetic acid, 2-hexyl- | nd | 1.17 | nd | nd |
| 25 | 14.202 | β-d-Glucopyranoside, methyl | 28.26 | 45.90 | nd | 64.31 |
| 26 | 14.359 | 1,2,3,5-Cyclohexanetetrol, (1α,2β,3α,5β)- | 11.82 | nd | 21.7 | nd |
| 27 | 15.352 | 3-Hydroxymethylene-1,7,7-trimethylbicyclo [2.2.1]heptan-2-one | nd | nd | 1.1 | 1.32 |
| 28 | 15.609 | 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one | 6.08 | nd | 0.6 | nd |
| 29 | 16.255 | Neophytadiene | nd | 0.32 | 1.82 | nd |
| 30 | 16.318 | 2-Undecanone, 6,10-dimethyl- | nd | nd | 1.81 | nd |
| 31 | 16.330 | 6-Methyl-2-tridecanone | nd | 0.74 | nd | nd |
| 32 | 17.131 | Hexadecanoic acid, methyl ester | 2.97 | 1.44 | 2.38 | 1.03 |
| 33 | 17.524 | Palmitic acid | nd | 0.62 | 1.93 | nd |
| 34 | 18.614 | n-Heptadecanol-1 | nd | nd | 0.72 | nd |
| 35 | 18.718 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | nd | nd | 0.26 | nd |
| 36 | 18.729 | 9,12-Octadecadienoic acid, methyl ester | nd | nd | nd | 0.09 |
| 37 | 18.730 | Linoleic acid methyl ester | 0.90 | 0.14 | nd | 0.27 |
| 38 | 18.776 | 11(Z),14(Z),17(Z)-Eicosatrienoic acid methyl ester | 1.35 | 0.22 | 0.52 | nd |
| 39 | 18.904 | Phytol | 0.91 | 0.45 | 1.09 | nd |
| 40 | 19.011 | Methyl stearate | 7.80 | 4.73 | 2.77 | 2.98 |
| 41 | 19.156 | 2-Methyl-Z,Z-3,13-octadecadienol | nd | nd | 0.35 | nd |
| 42 | 19.360 | Octadecanoic acid | nd | nd | 0.20 | nd |
| 43 | 20.172 | Undec-10-ynoic acid, tetradecyl ester | nd | 0.53 | 1.70 | nd |
| 44 | 20.735 | Methyl 18-methylnonadecanoate | 0.49 | 0.24 | 0.47 | 0.14 |
| 45 | 20.962 | 4,8,12,16-Tetramethylheptadecan-4-olide | nd | nd | 0.15 | nd |
| 46 | 21.312 | Oxalic acid, 3,5-difluorophenyl undecyl ester | nd | nd | 0.35 | nd |
| 47 | 22.191 | Pentadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | nd | nd | 5.74 | nd |
| 48 | 22.195 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 2.82 | 2.06 | 9.88 | 1.88 |
| 49 | 22.496 | Phthalic acid, di(6-methylhept-2-yl) ester | nd | nd | 0.39 | nd |
| 50 | 22.508 | Diisooctyl phthalate | 0.33 | nd | nd | nd |
| 51 | 22.680 | Decanoic acid, 2-hydroxy-3-[(1-oxooctyl)oxy]propyl ester | nd | 0.28 | nd | nd |
| 52 | 23.687 | 2-Methyltetracosane | nd | nd | 2.26 | nd |
| 53 | 23.711 | Cinnamyl linolenate | nd | 0.20 | nd | nd |
| 54 | 23.849 | Octadecanoic acid, 2,3-dihydroxypropyl ester | 10.16 | 9.85 | 1.09 | 7.13 |
| 55 | 26.042 | Cholesta-4,6-dien-3-ol, (3β)- | nd | 0.24 | 0.14 | nd |
| 56 | 26.127 | Octacosane, 2-methyl- | nd | nd | 0.14 | nd |
| 57 | 26.513 | cis-Valerenyl acetate | nd | nd | 0.26 | 0.07 |
| 58 | 26.587 | Tetracyclo [5.4.3.0(7,11)]tetradeca-2,5,10-trione, 1,4,6,14-tetramethyl-4-vinyl- | nd | nd | nd | 0.04 |
| 59 | 26.772 | Stigmasta-5,22-dien-3-ol, acetate, (3β)- | nd | nd | 1.24 | nd |
| 60 | 27.13 | 9,19-Cycloergost-24(28)-en-3-ol, 4,14-dimethyl-, acetate, (3β,4α,5α)- | nd | nd | 0.41 | nd |
| 61 | 27.266 | 2-(Decanoyloxy)propane-1,3-diyl dioctanoate | 1.45 | 0.72 | 1.36 | nd |
| 62 | 28.011 | Lupa-13(18),22-dien-3-ol, acetate | nd | nd | 0.99 | nd |
| 63 | 28.602 | 1-Heptacosanol | nd | nd | 0.43 | nd |
| 64 | 28.739 | Campesterol | 12.98 | 6.94 | 8.77 | 0.33 |
| 65 | 29.111 | Acetyl betulinaldehyde | 0.51 | 0.36 | 2.19 | 0.95 |
| 66 | 29.226 | Tricyclo [5.4.3.0(1,8)]tetradecan-3-ol-9-one, 4-ethenyl-6-(2-hydroxyacetoxy)-2,4,7,14-tetramethyl- | nd | 0.22 | nd | 0.82 |
| 67 | 29.368 | 24-Norursa-3,12-diene | nd | 0.20 | 0.37 | nd |
| 68 | 29.514 | 7-Oxo-5-cholesten-3β-yl benzoate | nd | 0.49 | 0.74 | nd |
| 69 | 29.611 | Khusimyl methyl ether | nd | nd | nd | 1.06 |
| 70 | 29.619 | 3β,21α-diacetoxy-18,22,22-trimethyl-17,27,29,30-tetranor-c-homoolean-14-ene | nd | nd | 4.76 | nd |
| 71 | 29.857 | 4-Campestene-3-one | nd | nd | 0.56 | nd |
| 72 | 29.869 | Stigmast-4-en-3-one | 0.39 | nd | nd | nd |
| 73 | 30.898 | Methyl ursa-2,12-dien-28-oate | nd | 0.28 | nd | 0.48 |
| 74 | 30.899 | Olean-12-en-28-oic acid, 2β,3β,23-trihydroxy-, methyl ester | 1.13 | nd | 1.14 | nd |
| 75 | 31.526 | Urs-12-en-28-oic acid, 3-hydroxy-, methyl ester, (3β)- | nd | nd | 0.88 | nd |
| 76 | 31.529 | Methyl 3-hydroxyurs-12-en-28-oate | nd | nd | nd | 0.20 |
| 77 | 34.288 | Urs-12-en-28-oic acid, 2,3,19-trihydroxy-, methyl ester, (2α,3β)- | nd | nd | 0.36 | nd |
| 78 | 34.863 | Betulinaldehyde | nd | nd | 0.22 | nd |
| 79 | 35.743 | 10,12,14-Nonacosatriynoic acid | nd | nd | 0.42 | nd |
| 80 | 36.746 | Phenol, 4-[4,5-bis[4-(dimethylamino)phenyl]-4H-imidazol-2-yl]- | nd | nd | 1.08 | nd |
| Sample | IC50 | ||
|---|---|---|---|
| DPPH● [mg/mL ± SD] | ABTS●+ [mg/mL ± SD] | CHEL [mg/mL ± SD] | |
| KDE | 1.36 ± 0.01 | 0.46 ± 0.07 | 0.15 ± 0.01 |
| KDM | 0.50 ± 0.01 | 0.41 ± 0.01 | 0.06 ± 0.01 |
| KDM-O | 2.30 ± 0.01 | 0.69 ± 0.02 | 0.26 ± 0.04 |
| KDM-B | 1.67 ± 0.02 | 0.50 ± 0.03 | 0.18 ± 0.02 |
| KME | 4.04 ± 0.02 | 1.25 ± 0.02 | 0.51 ± 0.03 |
| KMM | 3.18 ± 0.02 | 1.07 ± 0.05 | 0.40 ± 0.01 |
| KMM-O | 2.63 ± 0.02 | 0.85 ± 0.01 | 0.33 ± 0.02 |
| KMM-B | 2.20 ± 0.01 | 0.70 ± 0.01 | 0.25 ± 0.01 |
| AA | 0.48 ± 0.30 | nt | nt |
| Trolox | nt | 0.09 ± 0.10 | nt |
| Na2EDTA*2H2O | nt | nt | 0.04 ± 0.02 |
| Sample | IC50 [µg/mL] | ||
|---|---|---|---|
| Lipoxygenase Inhibition | COX-1 Inhibition | COX-2 Inhibition | |
| KDE | 14.46 ± 0.92 | 7.63 ± 0.15 | 10.68 ± 0.36 |
| KDM | 27.32 ± 1.17 | 14.99 ± 0.21 | 11.65 ± 0.47 |
| KDM-O | 60.46 ± 2.01 | 31.97 ± 0.47 | 25.69 ± 0.11 |
| KDM-B | 55.86 ± 2.37 | 29.19 ± 0.09 | 24.53 ± 0.43 |
| KME | 101.19 ± 2.85 | 53.76 ± 1.19 | 76.30 ± 0.13 |
| KMM | 65.82 ± 1.79 | 35.02 ± 1.35 | 30.49 ± 0.47 |
| KMM-O | 94.23 ± 2.18 | 48.95 ± 1.05 | 44.17 ± 0.22 |
| KMM-B | 55.58 ± 1.19 | 30.18 ± 0.78 | 31.61 ± 0.35 |
| IND | nt | 4.82 ± 0.09 | 3.90 ± 0.02 |
| NDGA | 6.05 ± 0.07 | nt | nt |
| S. aureus ATCC 25923 | S. epidermidis ATCC 12228 | P. granulosum PCM 2462 | C. acnes PCM 2400 | |||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC/MIC | MIC | MBC/MIC | MIC | MBC/MIC | MIC | MBC/MIC | |
| KMM | nt | nt | nt | nt | >6000 | nt | >6000 | nt |
| KME | nt | nt | nt | nt | >6000 | nt | >6000 | nt |
| KDM | >6000 | nt | 6000 | >4 | 1500 | >4 | 1500 | >4 |
| KDE | >6000 | nt | >6000 | nt | >6000 | nt | 6000 | nt |
| KMM-O | 3000 | >4 | 1500 | >4 | 1500 | >4 | 1500 | >4 |
| KMM-B | >6000 | nt | >6000 | nt | 6000 | nt | 3000 | >4 |
| KDM-O | 3000 | >4 | 1500 | >4 | 750 | >4 | 750 | 4 |
| KDM-B | 3000 | >4 | 3000 | >4 | 1500 | >4 | 750 | >4 |
| Caffeic acid | nt | nt | nt | nt | >6000 | nt | >6000 | nt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrząszcz, M.; Miazga-Karska, M.; Klimek, K.; Dybowski, M.P.; Typek, R.; Tchórzewska, D.; Dos Santos Szewczyk, K. The Anti-Acne Potential and Chemical Composition of Knautia drymeia Heuff. and Knautia macedonica Griseb Extracts. Int. J. Mol. Sci. 2023, 24, 9188. https://doi.org/10.3390/ijms24119188
Chrząszcz M, Miazga-Karska M, Klimek K, Dybowski MP, Typek R, Tchórzewska D, Dos Santos Szewczyk K. The Anti-Acne Potential and Chemical Composition of Knautia drymeia Heuff. and Knautia macedonica Griseb Extracts. International Journal of Molecular Sciences. 2023; 24(11):9188. https://doi.org/10.3390/ijms24119188
Chicago/Turabian StyleChrząszcz, Małgorzata, Małgorzata Miazga-Karska, Katarzyna Klimek, Michał P. Dybowski, Rafał Typek, Dorota Tchórzewska, and Katarzyna Dos Santos Szewczyk. 2023. "The Anti-Acne Potential and Chemical Composition of Knautia drymeia Heuff. and Knautia macedonica Griseb Extracts" International Journal of Molecular Sciences 24, no. 11: 9188. https://doi.org/10.3390/ijms24119188
APA StyleChrząszcz, M., Miazga-Karska, M., Klimek, K., Dybowski, M. P., Typek, R., Tchórzewska, D., & Dos Santos Szewczyk, K. (2023). The Anti-Acne Potential and Chemical Composition of Knautia drymeia Heuff. and Knautia macedonica Griseb Extracts. International Journal of Molecular Sciences, 24(11), 9188. https://doi.org/10.3390/ijms24119188







