An Overview of the Potential Medicinal and Pharmaceutical Properties of Ru(II)/(III) Complexes
Abstract
:1. Introduction
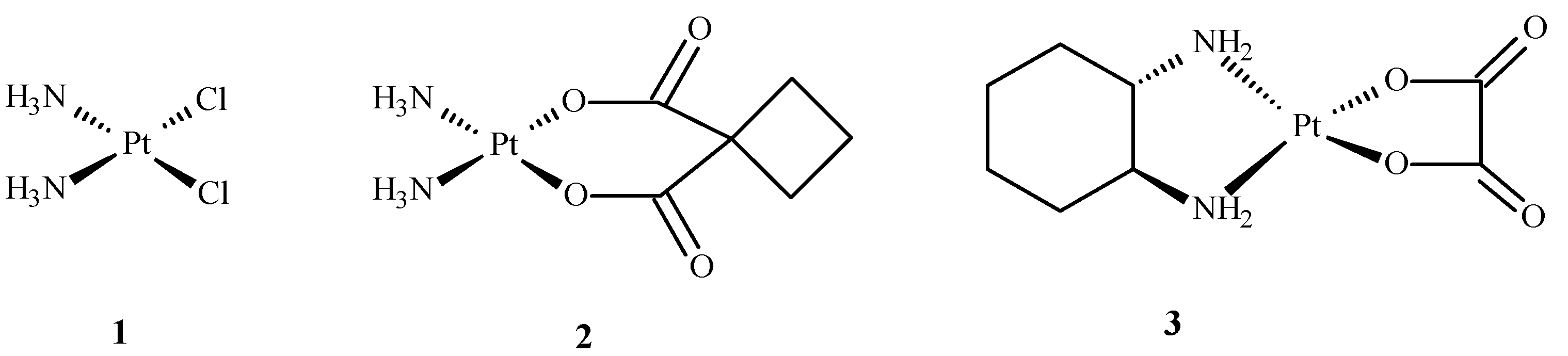
2. Main Mechanisms of Action of Ru(II)/(III) Complexes and Their Therapeutic Targets

3. Medicinal and Pharmaceutical Potential of Ru(II)/(III) Complexes
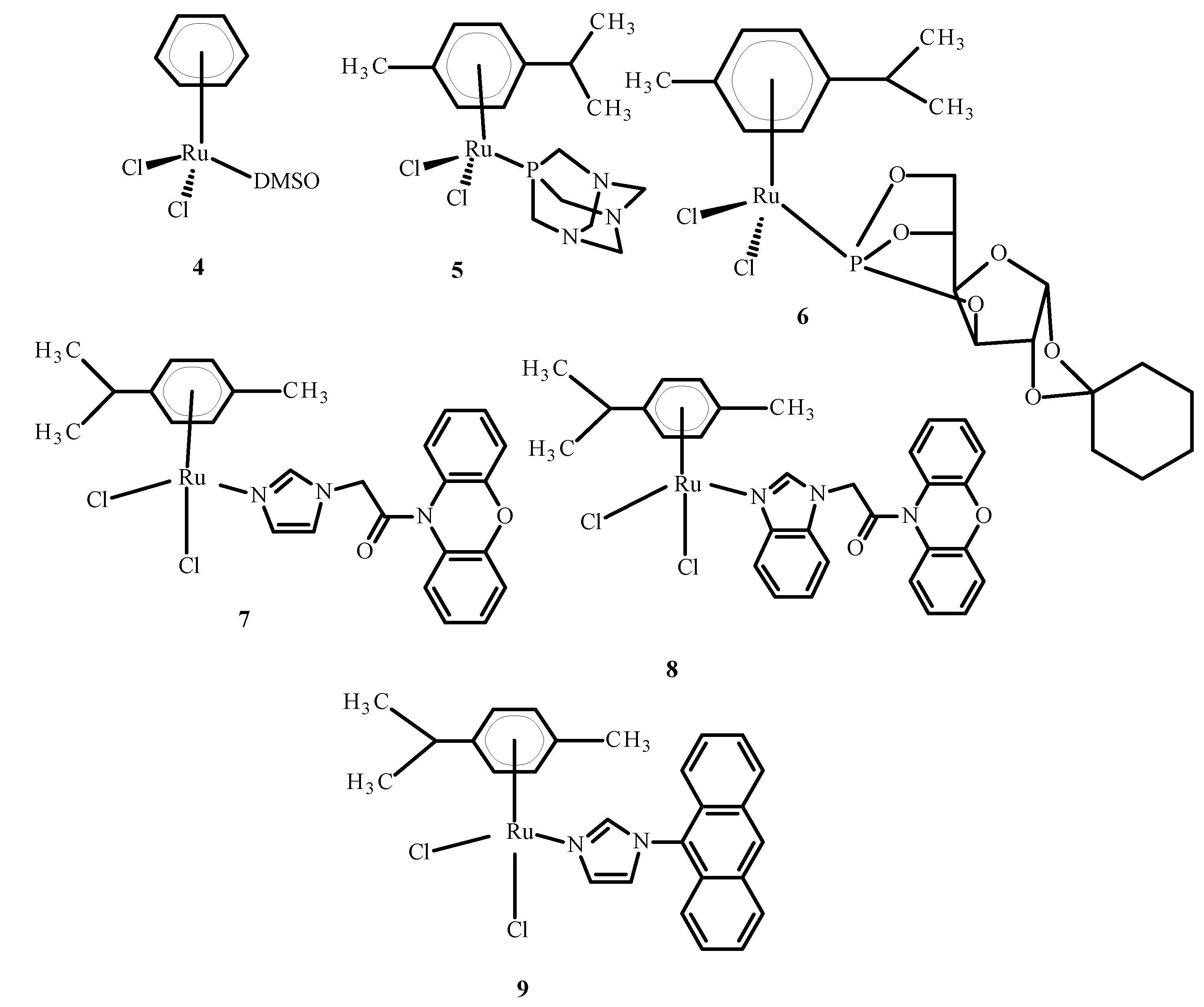
3.1. Application of Complexes of Ru(II)/(III) in Clinical Research
3.2. Antidiabetic Activity of Ru(II)/(III) Complexes
3.3. Anti-HIV Properties of Ru(II)/(III) Complexes
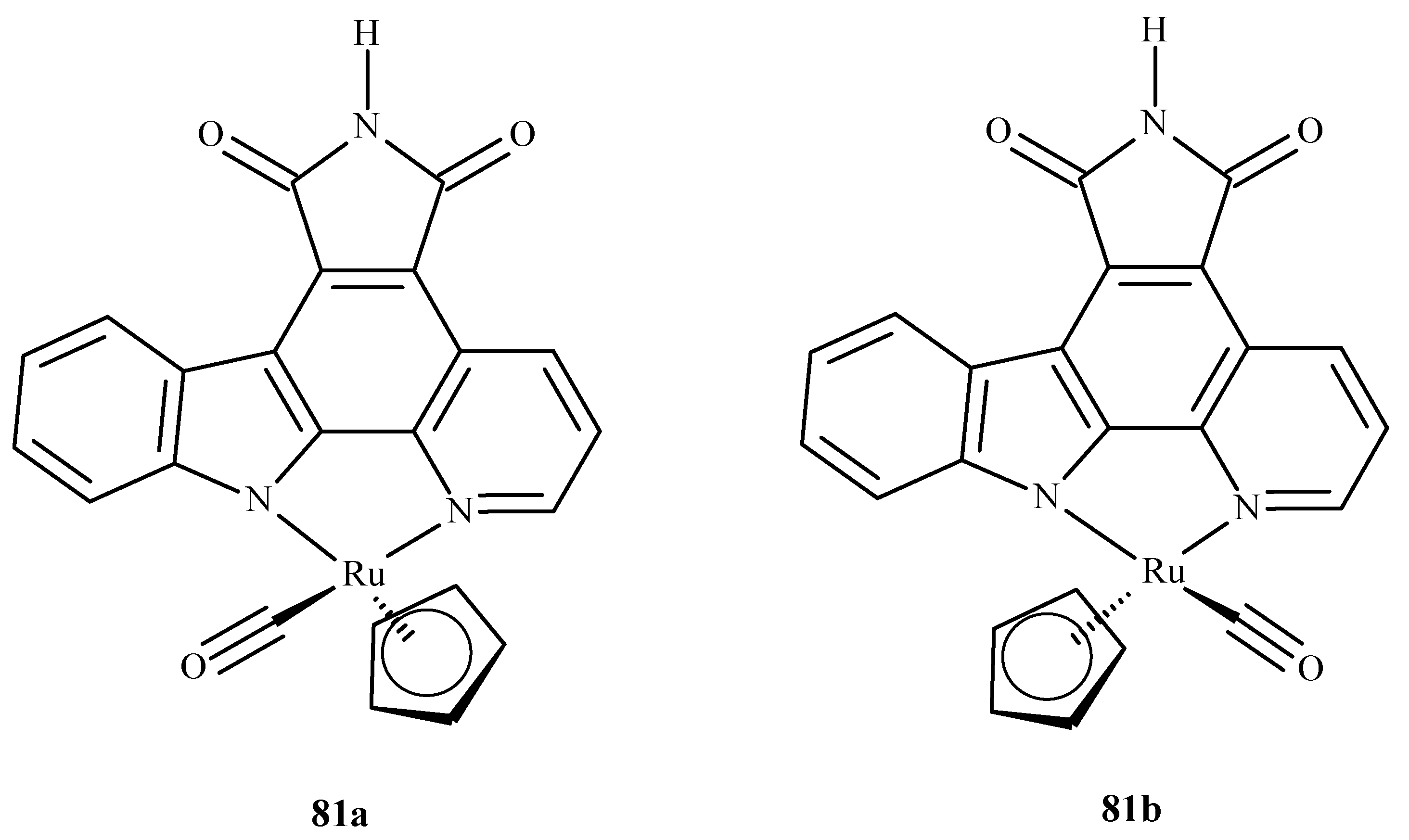
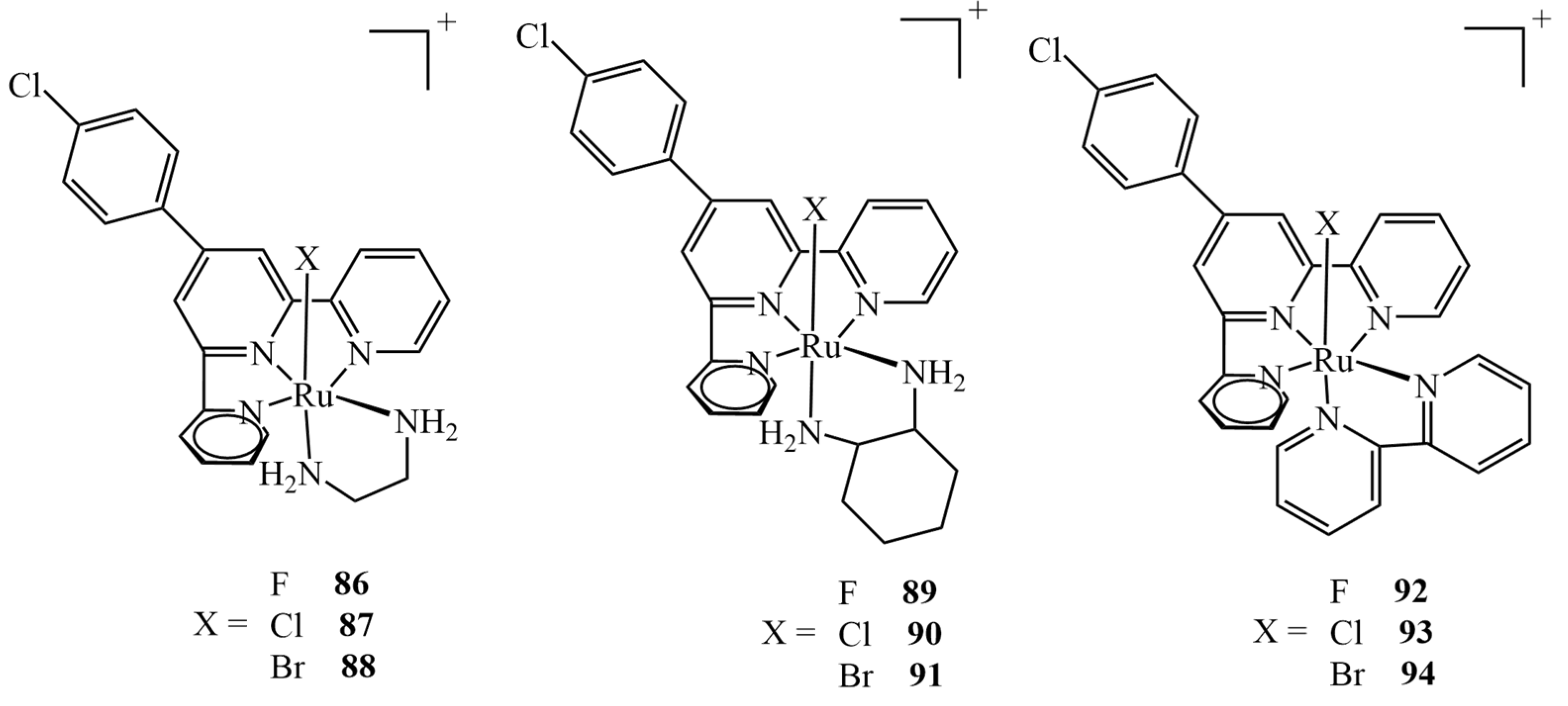
3.4. Cytotoxicity of Ru(II)/(III) Complexes


3.5. Ru(II)/(III) Complexes with Potential Anti Alzheimer’s Disease Properties
3.6. Photosensitizing Complexes of Ru(II)/(III)
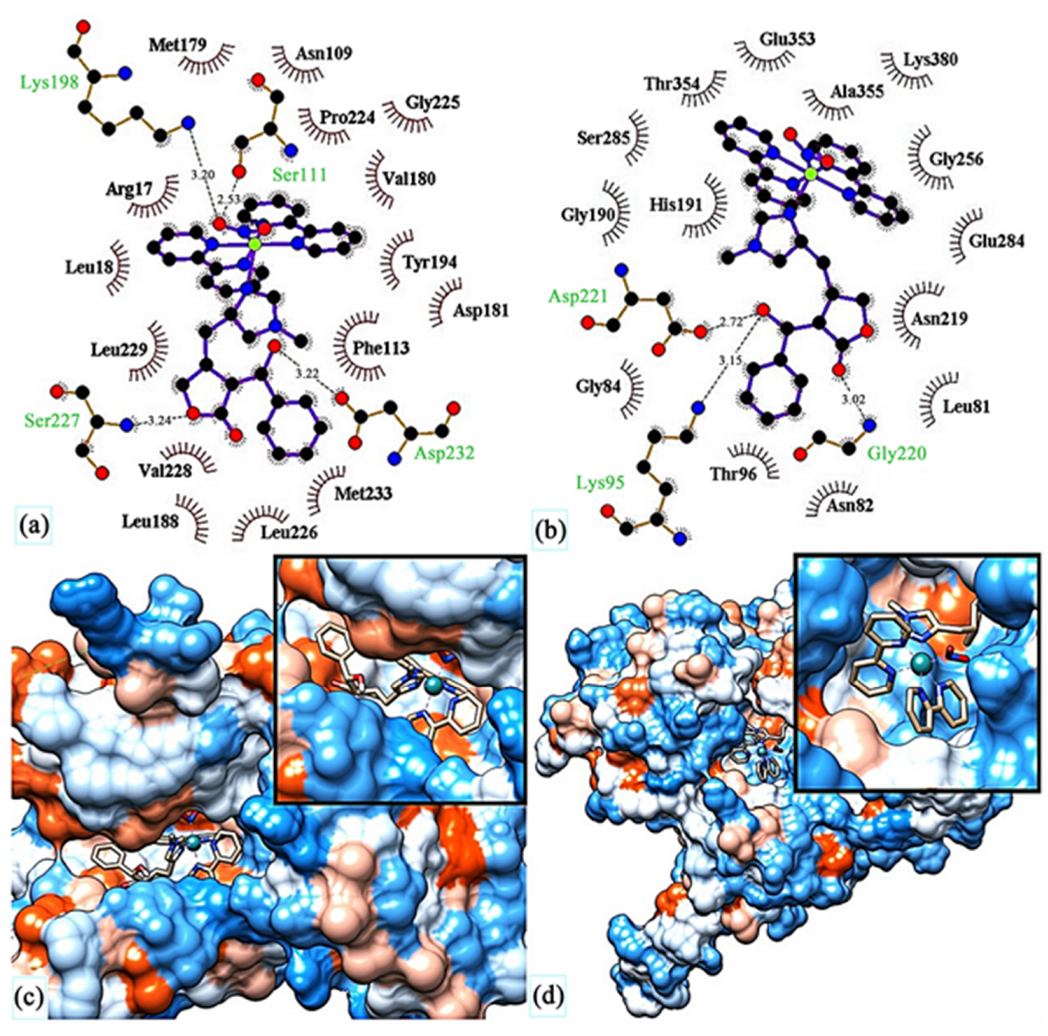
3.7. Computational Approaches to Studying Interactions of Ru(II)/(III) Complexes with Their Biological Targets
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sonkar, C.; Sarkar, S.; Mukhopadhyay, S. Ruthenium(II)–arene complexes as anti-metastatic agents, and related techniques. RSC Med. Chem. 2022, 13, 22–38. [Google Scholar] [CrossRef]
- Boulikas, T.; Vougiouka, M. Cisplatin and platinum drugs at the molecular level. Oncol. Rep. 2003, 10, 1663–1682. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: Current clinical uses, cytotoxicity studies and future perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Metal-based drugs that break the rules. Dalton Trans. 2016, 45, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The development of anticancer ruthenium(ii) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef] [PubMed]
- Strasser, S.; Pump, E.; Fischer, R.C.; Slugovc, C. On the chloride lability in electron-rich second generation ruthenium benzylidene complexes. Monatsh. Chem. Chem. Mon. 2015, 146, 1143–1151. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, C.Y.; Nam, T.-G. Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Dev. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef]
- Muhammad, N.; Guo, Z. Metal-based anticancer chemotherapeutic agents. Curr. Opin. Chem. Biol. 2014, 19, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, G.R.; Sinha, S.; Chhabra, M.; Paira, P. Synthesis of novel anticancer ruthenium-arene pyridinylmethylene scaffolds via three-component reaction. Bioorg. Med. Chem. Lett. 2016, 26, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Gatter, K.C.; Brown, G.; Trowbridge, I.S.; Woolston, R.E.; Mason, D.Y. Transferrin receptors in human tissues: Their distribution and possible clinical relevance. J. Clin. Pathol. 1983, 36, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Kumari, P.; Ryu, J.Y.; Lee, J.; Mobin, S.M.; Lee, C.Y. Mitochondrial Localization of Highly Fluorescent and Photostable BODIPY-Based Ruthenium(II), Rhodium(III), and Iridium(III) Metal Complexes. Inorg. Chem. 2019, 58, 8587–8595. [Google Scholar] [CrossRef]
- Leung, K.-H.; He, H.-Z.; Chan, D.S.-H.; Fu, W.-C.; Leung, C.-H.; Ma, D.-L. An oligonucleotide-based switch-on luminescent probe for the detection of kanamycin in aqueous solution. Sens. Actuators B 2013, 177, 487–492. [Google Scholar] [CrossRef]
- Ma, D.-L.; Kwan, M.H.-T.; Chan, D.S.-H.; Lee, P.; Yang, H.; Ma, V.P.-Y.; Bai, L.-P.; Jiang, Z.-H.; Leung, C.-H. Crystal violet as a fluorescent switch-on probe for i-motif: Label-free DNA-based logic gate. Analyst 2011, 136, 2692–2696. [Google Scholar] [CrossRef]
- Wang, W.; Vellaisamy, K.; Li, G.; Wu, C.; Ko, C.-N.; Leung, C.-H.; Ma, D.-L. Development of a Long-Lived Luminescence Probe for Visualizing β-Galactosidase in Ovarian Carcinoma Cells. Anal. Chem. 2017, 89, 11679–11684. [Google Scholar] [CrossRef]
- Lu, M.; Zhan, X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018, 9, 77–102. [Google Scholar] [CrossRef]
- Gianferrara, T.; Bratsos, I.; Alessio, E. A categorization of metal anticancer compounds based on their mode of action. Dalton Trans. 2009, 37, 7588–7598. [Google Scholar] [CrossRef]
- Duan, L.; Fischer, A.; Xu, Y.; Sun, L. Isolated seven-coordinate Ru(IV) dimer complex with [HOHOH]- bridging ligand as an intermediate for catalytic water oxidation. J. Am. Chem. Soc. 2009, 131, 10397–10399. [Google Scholar] [CrossRef]
- Motswainyana, W.M.; Ajibade, P.A. Anticancer Activities of Mononuclear Ruthenium(II) Coordination Complexes. Adv. Chem. 2015, 2015, 859730. [Google Scholar] [CrossRef]
- Scolaro, C.; Bergamo, A.; Brescacin, L.; Delfino, R.; Cocchietto, M.; Laurenczy, G.; Geldbach, T.J.; Sava, G.; Dyson, P.J. In vitro and in vivo evaluation of ruthenium(II)-arene PTA complexes. Med. Chem. 2005, 48, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.F.; Qian, C.; Wang, J.Q.; Chen, X.; Wang, L.L.; Chao, H.; Ji, L.-N. Chiral ruthenium(II) anthraquinone complexes as dual inhibitors of topoisomerases I and II. J. Biol. Inorg. Chem. 2012, 17, 81–96. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Zhao, Z.-Z.; Bo, H.-B.; Chen, Q.-Z. Synthesis, characterization, and antitumor properties of ruthenium(II) anthraquinone complexes. J. Coord. Chem. 2016, 69, 177–189. [Google Scholar] [CrossRef]
- De Carvalho, N.C.; Neves, S.P.; Dias, R.B.; Valverde, L.F.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; dos Santos, E.R.; Oliveira, R.M.M.; Carlos, R.M.; et al. A novel ruthenium complex with xanthoxylin induces S-phase arrest and causes ERK1/2-mediated apoptosis in HepG2 cells through a p53-independent pathway. Cell Death Dis. 2018, 9, 79. [Google Scholar] [CrossRef]
- Le Gac, S.; Rickling, S.; Gerbaux, P.; Defrancq, E.; Moucheron, C.; Mesmaeker, A.K.-D. A photoreactive ruthenium(II) complex tethered to a guanine-containing oligonucleotide: A biomolecular tool that behaves as a “seppuku molecule”. Angew. Chem. Int. Ed. Engl. 2009, 48, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Rickling, S.; Ghisdavu, L.; Pierard, F.; Gerbaux, P.; Surin, M.; Murat, P.; Defrancq, E.; Moucheron, C.; Mesmaeker, A.K.-D. A rigid dinuclear ruthenium(II) complex as an efficient photoactive agent for bridging two guanine bases of a duplex or quadruplex oligonucleotide. Chem. A Eur. J. 2010, 16, 3951–3961. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, E.; Hiyama, K.; Yokoyama, T.; Matsuura, Y.; Piatyszek, M.A.; Shay, J.W. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat. Med. 1995, 1, 249–255. [Google Scholar] [CrossRef]
- Ambrus, A.; Chen, D.; Dai, J.; Bialis, T.; Jones, R.A.; Yang, D. Human telomeric sequence forms a hybrid-type intramolecular G-quadruplex structure with mixed parallel/antiparallel strands in potassium solution. Nucleic Acids Res. 2006, 34, 2723–2735. [Google Scholar] [CrossRef]
- Rajput, C.; Rutkaite, R.; Swanson, L.; Haq, I.; Thomas, J.A. Dinuclear monointercalating RuII complexes that display high affinity binding to duplex and quadruplex DNA. Chem. A Eur. J. 2006, 12, 4611–4619. [Google Scholar] [CrossRef]
- Shi, S.; Liu, J.; Yao, T.; Geng, X.; Jiang, L.; Yang, Q.; Cheng, L.; Ji, L. Promoting the formation and stabilization of G-quadruplex by dinuclear RuII complex Ru2(obip)L4. Inorg. Chem. 2008, 47, 2910–2912. [Google Scholar] [CrossRef] [PubMed]
- Puckett, C.A.; Barton, J.K. Methods to explore cellular uptake of ruthenium complexes. J. Am. Chem. Soc. 2007, 129, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Groessl, M.; Zava, O.; Dyson, P.J. Cellular uptake and subcellular distribution of ruthenium-based metallodrugs under clinical investigation versus cisplatin. Metallomics 2011, 3, 591–599. [Google Scholar] [CrossRef]
- Wang, J.Q.; Zhang, P.Y.; Qian, C.; Hou, X.J.; Ji, L.N.; Chao, H. Mitochondria are the primary target in the induction of apoptosis by chiral ruthenium(II) polypyridyl complexes in cancer cells. J. Biol. Inorg. Chem. 2014, 19, 335–348. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Li, G.; Zhang, P.; Jin, C.; Zeng, L.; Ji, L.; Chao, H. Ruthenium(II) polypyridyl complexes as mitochondria-targeted two-photon photodynamic anticancer agents. Biomaterials 2015, 56, 140–153. [Google Scholar] [CrossRef]
- Wan, D.; Tang, B.; Wang, Y.J.; Guo, B.H.; Yin, H.; Yi, Q.Y.; Liu, Y.-J. Synthesis and anticancer properties of ruthenium (II) complexes as potent apoptosis inducers through mitochondrial disruption. Eur. J. Med. Chem. 2017, 139, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.R.; Cecchin, D.; Walker, M.G.; Mulla, R.S.; Battaglia, G.; Smythe, C.; Thomas, J.A. Targeting the endoplasmic reticulum with a membrane-interactive luminescent ruthenium(ii) polypyridyl complex. Chem. Sci. 2013, 4, 4512–4519. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Tan, C.; Lai, S.; Wu, S.; Hu, S.; Zhou, L.; Chen, Y.; Wang, M.; Zhy, Y.; Lian, W.; Peng, W.; et al. Nuclear permeable ruthenium(II) beta-carboline complexes induce autophagy to antagonize mitochondrial-mediated apoptosis. J. Med. Chem. 2010, 53, 7613–7624. [Google Scholar] [CrossRef]
- Castonguay, A.; Doucet, C.; Juhas, M.; Maysinger, D. New ruthenium(II)–letrozole complexes as anticancer therapeutics. J. Med. Chem. 2012, 55, 8799–8806. [Google Scholar] [CrossRef]
- Chen, L.; Li, G.; Peng, F.; Jie, X.; Dongye, G.; Cai, K.; Feng, R.; Li, B.; Zeng, Q.; Lun, K.; et al. The induction of autophagy against mitochondria-mediated apoptosis in lung cancer cells by a ruthenium (II) imidazole complex. Oncotarget 2016, 7, 80716–80734. [Google Scholar] [CrossRef]
- Yuan, J.; Lei, Z.; Wang, X.; Zhu, F.; Chen, D. Ruthenium complex Lambda-WH0402 induces hepatocellular carcinoma LM6 (HCCLM6) cel death by triggering the Beclin-1-dependent autophagy pathway. Metallomics 2015, 7, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Nishimura, T.; Chen, Y.; Azeloglu, E.U.; Gottesman, O.; Giannarelli, C.; Zafar, M.U.; Benard, L.; Badimon, J.J.; Hajjar, R.J.; et al. Systems pharmacology of adverse event mitigation by drug combinations. Sci. Transl. Med. 2013, 5, 206ra140. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wong, Y.S.; Chen, T.; Fan, C.; Zheng, W. Ruthenium complexes containing bis-benzimidazole derivatives as a new class of apoptosis inducers. Dalton Trans. 2012, 41, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, Y.; Xu, L.; Zheng, C.; Le, F.; Qin, X.; Liu, Y.; Liu, J. Ruthenium(II) polypyridyl complexes: Cellular uptake, cell image and apoptosis of HeLa cancer cells induced by double targets. Eur. J. Med. Chem. 2014, 82, 82–95. [Google Scholar] [CrossRef]
- Leung, C.-H.; Liu, L.-J.; Leung, K.-H.; Ma, D.-L. Epigenetic modulation by inorganic metal complexes. Coord. Chem. Rev. 2016, 319, 25–34. [Google Scholar] [CrossRef]
- Lehar, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Richard, J.; Rickles, R.J.; Short III, G.F.; Staunton, J.E.; et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Bozic, I.; Reiter, J.G.; Allen, B.; Antal, T.; Chatterjee, K.; Shah, P.; Moon, Y.S.; Yaqubie, A.; Kelly, N.; Le, D.T.; et al. Evolutionary dynamics of cancer in response to targeted combination therapy. eLife 2013, 2, e00747. [Google Scholar] [CrossRef]
- Gelfo, V.; Rodia, M.T.; Pucci, M.; Dall’Ora, M.; Santi, S.; Solmi, R.; Roth, L.; Lindzen, M.; Bonafè, M.; Bertotti, A.; et al. A module of inflammatory cytokines defines resistance of colorectal cancer to EGFR inhibitors. Oncotarget 2016, 7, 72167–72183. [Google Scholar] [CrossRef]
- Robles-Escajeda, E.; Martínez, A.; Varela-Ramirez, A.; Sánchez-Delgado, R.A.; Aguilera, R.J. Analysis of the cytotoxic effects of ruthenium–ketoconazole and ruthenium–clotrimazole complexes on cancer cells. Cell Biol. Toxicol. 2013, 29, 431–443. [Google Scholar] [CrossRef]
- Berger, I.; Hanif, M.; Nazarov, A.A.; Hartinger, C.G.; John, R.O.; Kuznetsov, M.L.; Groessl, M.; Schmitt, F.; Zava, O.; Biba, F.; et al. In vitro anticancer activity and biologically relevant metabolization of organometallic ruthenium complexes with carbohydrate-based ligands. Chem. A Eur. J. 2008, 14, 9046–9057. [Google Scholar] [CrossRef]
- Leijen, S.; Burgers, S.A.; Baas, P.; Pluim, D.; Tibben, M.; van Werkhoven, E.; Enzo Alessio, E.; Sava, G.; Beijnen, J.H.; Schellens, J.H.M. Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy. Investig. New Drugs 2015, 33, 201–214. [Google Scholar] [CrossRef]
- Bergamo, A.; Riedel, T.; Dyson, P.J.; Sava, G. Preclinical combination therapy of the investigational drug NAMI-A+ with doxorubicin for mammary cancer. Investig. New Drugs 2015, 33, 53–63. [Google Scholar] [CrossRef]
- Pries, A.R.; Hopfner, M.; le Noble, F.; Dewhirst, M.W.; Secomb, T.W. The shunt problem: Control of functional shunting in normal and tumour vasculature. Nat. Rev. Cancer 2010, 10, 587–593. [Google Scholar] [CrossRef]
- Siemann, D.W.; Horsman, M.R. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol. Ther. 2015, 153, 107–124. [Google Scholar] [PubMed]
- Weiss, A.; Bonvin, D.; Berndsen, R.H.; Scherrer, E.; Wong, T.J.; Dyson, P.J.; Griffioen, A.W.; Nowak-Sliwinska, P. Angiostatic treatment prior to chemo- or photodynamic therapy improves anti-tumor efficacy. Sci. Rep. 2015, 5, 8990. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics. J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef] [PubMed]
- Smithen, D.A.; Yin, H.; Beh, M.H.R.; Hetu, M.; Cameron, T.S.; McFarland, S.A.; Thompson, A. Synthesis and Photobiological Activity of Ru(II) Dyads Derived from Pyrrole-2-carboxylate Thionoesters. Inorg. Chem. 2017, 56, 4121–4132. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Zorbas-Seifried, S.; Jakupec, M.A.; Kynast, B.; Zorbas, H.; Keppler, B.K.J. From bench to bedside—Preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A). Inorg. Biochem. 2006, 100, 891–904. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Lam, N.Y.S.; Truong, D.H.; Burmeister, M.; Babak, V.; Holtkamp, H.U.; Movassaghi, S.; Ayine-Tora, D.M.; Zafar, A.; Kubanik, M.; Oehninger, L.; et al. From Catalysis to Cancer: Toward Structure–Activity Relationships for Benzimidazol-2-ylidene-Derived N-Heterocyclic-Carbene Complexes as Anticancer Agents. Inorg. Chem. 2018, 57, 14427–14434. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S. Recent advances in anticancer ruthenium Schiff base complexes. Appl. Organomet. Chem. 2020, 34, e5687. [Google Scholar] [CrossRef]
- Moharana, P.; Ghosh, D.; Paira, P. Drive to Organoruthenium and Organoiridium complexes from Organoplatinum: Next-Generation Anticancer Metallotherapeutics. Inorg. Chem. Commun. 2021, 124, 108364. [Google Scholar] [CrossRef]
- Babak, M.V.; Ang, W.H. Multinuclear Organometallic Ruthenium-Arene complexes for Cancer Therapy, in Metallo-Drugs: Development and Action of Anticancer Agents. In Metal Ions in Life Sciences; Sigel, A., Sigel, H., Freisinger, E., Sigel, R.K.O., Eds.; Walter de Gruyter, GmbH: Berlin, Germany, 2018; Volume 18, pp. 171–198. [Google Scholar]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Ma, L.; Ma, R.; Wang, Z.; Yiu, S.-M.; Zhu, G. Heterodinuclear Pt(iv)–Ru(ii) anticancer prodrugs to combat both drug resistance and tumor metastasis. Chem. Commun. 2016, 52, 10735–10738. [Google Scholar] [CrossRef]
- Maikoo, S.; Makayane, D.; Booysen, I.N.; Ngubane, P.; Khathi, A. Ruthenium compounds as potential therapeutic agents for type 2 diabetes mellitus. Eur. J. Med. Chem. 2021, 213, 113064. [Google Scholar] [CrossRef]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Tran, T.; Owen, A.J.; Smith, B.J. The contribution of physical inactivity and socioeconomic factors to type 2 diabetes in Nepal: A structural equation modelling analysis. Nutr. Metabol. Cardiovasc. Dis. 2020, 30, 1758–1767. [Google Scholar] [CrossRef]
- Bradley, B.H.R. Dietary fat and risk for type 2 diabetes: A review of recent research. Cur. Nut. Rep. 2018, 7, 214–226. [Google Scholar] [CrossRef]
- Luedtke, N.W.; Hwang, J.S.; Glazer, E.C.; Gut, D.; Kol, M.; Tor, Y. Eilatin Ru(II) complexes display anti-HIV activity and enantiomeric diversity in the binding of RNA. ChemBioChem 2002, 3, 766–771. [Google Scholar] [CrossRef]
- Yufanyi, D.M.; Abbo, H.S.; Titinchi, S.J.J.; Neville, T. Platinum(II) and Ruthenium(II) complexes in medicine: Antimycobacterial and Anti-HIV activities. Coord. Chem. Rev. 2020, 414, 213285. [Google Scholar] [CrossRef]
- Al-Masoudi, W.A.; Al-Masoudi, N.A. A ruthenium complexes of monastrol and its pyrimidine analogues: Synthesis and biological properties. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 1020–1027. [Google Scholar] [CrossRef]
- Carcelli, M.; Bacchi, A.; Pelagatti, P.; Rispoli, G.; Rogolino, D.; Sanchez, T.W.; Sechi, M.; Neamati, N. Ruthenium arene complexes as HIV-1 integrase strand transfer inhibitors. J. Inorg. Biochem. 2013, 118, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Cao, K.; Li, J.; Hou, Z.; Yuan, S.; Huang, G.; Liu, H.; Liu, Y. Selective Targeting of the Zinc Finger Domain of HIV Nucleocapsid Protein NCp7 with Ruthenium Complexes. Chem. Eur. J. 2018, 24, 19146–19151. [Google Scholar] [CrossRef]
- Zeng, J.; Yu Zhao, Y.; Li, K.; Long, D.; Li, W.; Liang, L. A coordinated ruthenium-rifampicin complex reprogramming the colon carcinoma micro-environment mediated by modulation of p53/AkT/ mTOR/VEGF pathway. Toxicol. Appl. Pharmacol. 2021, 426, 115618. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, A.K.; Karges, J.; Scopelliti, R.; Bobbink, F.D.; Nowak-Sliwinska, P.; Gasser, G.; Dyson, P.J. Towards Light Activated Ruthenium-Arene (RAPTA-type) Prodrug Candidates. ChemBioChem 2019, 20, 2876–2882. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M.; Benson, A.; Opazo, C.; Freedman, D.; Monti, E.; Gariboldi, M.B.; Shaulky, J.; Marchetti, F.; Pettinari, R.; et al. Ruthenium–Arene Complexes of Curcumin: X-Ray and Density Functional Theory Structure, Synthesis, and Spectroscopic Characterization, in Vitro Antitumor Activity, and DNA Docking Studies of (p-Cymene)Ru(curcuminato)chloro. J. Med. Chem. 2012, 55, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Golbaghi, G.; Castonguay, A. Rationally Designed Ruthenium Complexes for Breast Cancer Therapy. Molecules 2020, 25, 265. [Google Scholar] [CrossRef]
- Garcia-Fernandez, A.; Diez, J.; Manteca, A.; Sanchez, J.; Garcia-Navas, R.; Sierra, B.G.; Mollinedo, F.; Gamasa, M.P.; Lastra, E. Antitumor Activity of New Hydridotris(pyrazolyl)borate Ruthenium(II) Complexes Containing the Phosphanes PTA and 1- CH3-PTA. Dalton Trans. 2010, 39, 10186–10196. [Google Scholar] [CrossRef]
- Skoczynska, A.; Lux, K.; Mayer, P.; Lorenz, I.-P.; Krajewska, U.; Rozalski, M.; Dolega, A.; Budzisz, E. Spectroscopic and cytotoxic characteristics of (p-cymene)Ru(II) complexes with bidentate coumarins and density functional theory comparison with selected Pd(II) complexes. Inorg. Chim. Acta 2017, 456, 105–112. [Google Scholar] [CrossRef]
- Skoczynska, A.; Pasternak, B.; Malecka, M.; Krajewska, U.; Mirowski, M.; Merecz-Sadowska, A.; Karwowski, B.T.; Kusz, J.; Budzisz, E. The cytotoxic effect of Ru(II) complexes with 5-(2-hydroxyphenyl)-3-methyl-1-(2-pyridyl)-1H-pyrazole-4-carboxylic acid methyl ester: Synthesis, X-ray structure and DNA damage potential. Polyhedron 2019, 169, 228–238. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, D.; Zhao, C.; He, L.; Du, W. Inhibitory effects of NAMI-A-like ruthenium complexes on prion neuropeptide fibril formation. Metallomics 2015, 7, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-B.; Tao, C.; Tan, C.-P.; Zhao, P. Inhibition of Aβ peptide aggregation by ruthenium(II) polypyridyl complexes through copper chelation. J. Inorg. Biochem. 2021, 224, 111591. [Google Scholar] [CrossRef] [PubMed]
- Cuccioloni, M.; Cecarini, V.; Bonfili, L.; Pettinari, R.; Tombesi, A.; Pagliaricci, N.; Petetta, L.; Angeletti, M.; Eleuteri, A.M. Enhancing the Amyloid-β Anti-Aggregation Properties of Curcumin via Arene-Ruthenium(II) Derivatization. Int. J. Mol. Sci. 2022, 23, 8710. [Google Scholar] [CrossRef]
- Pobłocki, K.; Drzezdzon, J.; Kostrzewa, T.; Jacewicz, D. Coordination Complexes as a New Generation Photosensitizer for Photodynamic Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 8052. [Google Scholar] [CrossRef]
- Smith, G.S.; Therrien, B. Targeted and multifunctional arene ruthenium chemotherapeutics. Dalton Trans. 2011, 40, 10793–10800. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Rees, T.W.; Ke, L.; Ji, L.; Chao, H. Harnessing ruthenium(II) as photodynamic agents: Encouraging advances in cancer therapy. Coord. Chem. Rev. 2018, 363, 17–28. [Google Scholar] [CrossRef]
- Zeng, L.; Kuang, S.; Li, G.; Jin, C.; Ji, L.; Chao, H. A GSH-activatable ruthenium(ii)-azo photosensitizer for two-photon photodynamic therapy. Chem. Commun. 2017, 53, 1977–1980. [Google Scholar] [CrossRef]
- Huang, H.; Yu, B.; Zhang, P.; Huang, J.; Chen, Y.; Gasser, G.; Ji, L.; Chao, H. Highly charged ruthenium(II) polypyridyl complexes as lysosome-localized photosensitizers for two-photon photodynamic therapy. Angew. Chem. Int. Ed. 2015, 54, 14049–14052. [Google Scholar] [CrossRef]
- Hess, J.; Huang, H.; Kaiser, A.; Pierroz, V.; Blacque, O.; Chao, H.; Gasser, G. Evaluation of the medicinal potential of two ruthenium(II) polypyridine complexes as one- and two-photon photodynamic therapy photosensitizers. Chem. Eur. J. 2017, 23, 9888–9896. [Google Scholar] [CrossRef]
- Mjos, K.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 8, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J., III; Konda, P.; Gujar, S.; McFarland, S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2018, 119, 797–828. [Google Scholar] [CrossRef]
- Dolmans, D.; Fukumura, D.; Jain, R. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lei, W.; Jiang, G.; Hou, Y.; Li, C.; Zhang, B.; Zhou, Q.; Wang, X. Fusion of photodynamic therapy and photoactivated chemotherapy: A novel Ru(II) arene complex with dual activities of photobinding and photocleavage toward DNA. Dalton Trans. 2014, 43, 15375–15384. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, P.; Yu, B.; Jin, C.; Ji, L.; Chao, H. Synthesis, characterization and biological evaluation of mixed-ligand ruthenium(II) complexes for photodynamic therapy. Dalton Trans. 2015, 44, 17335–17345. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Rubbiani, R.; Tubafard, S.; Blacque, O.; Anstaett, P.; Felgenträger, A.; Maisch, T.; Spiccia, L.; Gasser, G. Synthesis, characterization, and biological evaluation of new Ru(II) polypyridyl photosensitizers for photodynamic therapy. J. Med. Chem. 2014, 57, 7280–7292. [Google Scholar] [CrossRef]
- Delaey, E.; van Laar, F.; de Vos, D.; Kamuhabwa, A.; Jacobs, P.; de Witte, P. A comparative study of the photosensitizing characteristics of some cyanine dyes. J. Photochem. Photobiol. B 2000, 55, 27–36. [Google Scholar] [CrossRef]
- Karaoun, N.; Renfrew, A. A luminescent ruthenium(II) complex for light-triggered drug release and live cell imaging. Chem. Commun. 2015, 51, 14038–14041. [Google Scholar] [CrossRef]
- Joshi, T.; Pierroz, V.; Mari, C.; Gemperle, L.; Ferrari, S.; Gasser, G. A bis(dipyridophenazine)(2-(2-pyridyl)pyrimidine-4-carboxylic acid)ruthenium(II) complex with anticancer action upon photodeprotection. Angew. Chem. Int. Ed. 2014, 53, 2960–2963. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.J.; Gahbauer, S.; Luttens, A.; Lyu, J.; Webb, C.M.; Stein, R.M.; Fink, E.A.; Balius, T.E.; Carlsson, J.J.; Irwin, J.; et al. A practical guide to large-scale docking. Nat. Protoc. 2021, 16, 4799–4832. [Google Scholar] [CrossRef] [PubMed]
- Dnyandev, K.M.; Babasaheb, G.V.; Chandrashekhar, K.V.; Chandrakant, M.A.; Vasant, O.K. A Review on Molecular Docking. Int. Res. J. Pure Appl. Chem. 2021, 22, 60–68. [Google Scholar] [CrossRef]
- Dar, A.M.; Mir, S. Molecular Docking: Approaches, Types, Applications and Basic Challenges. J. Anal. Bioanal. Tech. 2017, 8, 356. [Google Scholar] [CrossRef]
- Bajorath, J. Integration of virtual and high-throughput screening. Nat. Rev. Drug Discov. 2002, 1, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, H.; Klebe, G. Approaches to the Description and Prediction of the Binding Affinity of Small-Molecule Ligands to Macromolecular Receptors. Angew. Chem. Int. Ed. 2002, 41, 2644–2676. [Google Scholar] [CrossRef]
- Jorgensen, W.L. The Many Roles of Computation in Drug Discovery. Science 2004, 303, 1813–1818. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Walters, W.P.; Stahl, M.T.; Murcko, M.A. Virtual screening—An overview. Drug Discov. Today 1998, 3, 160–178. [Google Scholar] [CrossRef]
- Rizzuti, B.; Grande, F. Chapter 14—Virtual screening in drug discovery: A precious tool for a still-demanding challenge. In Protein Homeostasis Diseases; Pey, A.L., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 309–327. [Google Scholar]
- Vyas, V.; Jain, A.; Jain, A.; Gupta, A. Virtual Screening: A Fast Tool for Drug Design. Sci. Pharm. 2008, 76, 333–360. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Pattanaik, S.; Pattnaik, G.; Mallick, S.; Mohapatra, R. Review on the use of Molecular Docking as the First Line Tool in Drug Discovery and Development. Indian J. Pharm. Sci. 2022, 84, 1334–1337. [Google Scholar] [CrossRef]
- Neethu, K.S.; Eswaran, J.; Theetharappan, M.; Bhuvanesh Nattamai, S.P.; Neelakantan, M.A.; Velusamy, K.M. Organoruthenium (II) complexes featuring pyrazole-linked Schiff base ligands: Crystal structure, DNA/BSA interactions, cytotoxicity and molecular docking. Appl. Organomet. Chem. 2019, 33, e4751. [Google Scholar]
- De, S.; Chaudhuri, S.R.; Panda, A.; Jadhav, G.R.; Kumar, R.S.; Manohar, P.; Ramesh, N.; Mondal, A.; Moorthy, A.; Banerjee, S.; et al. Synthesis, characterisation, molecular docking, biomolecular interaction and cytotoxicity studies of novel ruthenium(II)–arene-2-heteroarylbenzoxazole complexes. New J. Chem. 2019, 43, 3291–3302. [Google Scholar] [CrossRef]
- Guo, W.; Zheng, W.; Luo, Q.; Li, X.; Zhao, Y.; Xiong, S.; Wang, F. Transferrin Serves As a Mediator to Deliver Organometallic Ruthenium(II) Anticancer Complexes into Cells. Inorg. Chem. 2013, 52, 5328–5338. [Google Scholar] [CrossRef] [PubMed]
- Paitandi, R.P.; Singh, R.S.; Mukhopadhyay, S.; Sharma, G.; Koch, B.; Vishnoi, P.; Pandey, D.S. Synthesis, characterization, DNA binding and cytotoxicity of fluoro-dipyrrin based arene ruthenium(II) complexes. Inorg. Chim. Acta 2017, 454, 117–127. [Google Scholar] [CrossRef]
- Gupta, R.K.; Pandey, R.; Sharma, G.; Prasad, R.; Koch, B.; Srikrishna, S.; Li, P.-Z.; Xu, Q.; Pandey, D.S. DNA Binding and Anti-Cancer Activity of Redox-Active Heteroleptic Piano-Stool Ru(II), Rh(III), and Ir(III) Complexes Containing 4-(2-Methoxypyridyl)phenyldipyrromethene. Inorg. Chem. 2013, 52, 3687–3698. [Google Scholar] [CrossRef]
- Vyas, N.A.; Ramteke, S.N.; Kumbhar, A.S.; Kulkarni, P.P.; Jani, V.; Sonawane, U.B.; Joshi, R.R.; Joshi, B.; Erxleben, A. Ruthenium(II) polypyridyl complexes with hydrophobic ancillary ligand as Aβ aggregation inhibitors. Eur. J. Med. Chem. 2016, 121, 793–802. [Google Scholar] [CrossRef]
- Dastmalchi, S.; Hamzeh-Mivehroud, M.; Sokouti, B. (Eds.) Molecular Docking of Biologically Active Substances to Double Helical Nucleic Acids: Problems and Solutions. In Applied Case Studies and Solutions in Molecular Docking-Based Drug Design; IGI Global: Hershey, PA, USA, 2016; pp. 127–157. [Google Scholar]
- Kasprzak, W.K.; Ahmed, N.A.; Shapiro, B.A. Modeling ligand docking to RNA in the design of RNA-based nanostructures. Curr. Opin. Biotechnol. 2020, 63, 16–25. [Google Scholar] [CrossRef]
- Fandzloch, M.; Jędrzejewski, T.; Dobrzańska, L.; Esteban-Parra, G.M.; Wiśniewska, J.; Paneth, A.; Paneth, P.; Sitkowski, J. New organometallic ruthenium(II) complexes with purine analogs—A wide perspective on their biological application. Dalton Trans. 2021, 50, 5557–5573. [Google Scholar] [CrossRef]
- Paitandi, R.P.; Gupta, R.K.; Singh, R.S.; Sharma, G.; Koch, B.; Pandey, D.S. Interaction of ferrocene appended Ru(II), Rh(III) and Ir(III) dipyrrinato complexes with DNA/protein, molecular docking and antitumor activity. Eur. J. Med. Chem. 2014, 84, 17–29. [Google Scholar] [CrossRef]
- Adeniyi, A.A.; Ajibade, P.A. Comparing the Suitability of Autodock, Gold and Glide for the Docking and Predicting the Possible Targets of Ru(II)-Based Complexes as Anticancer Agents. Molecules 2013, 18, 3760–3778. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Ruthenium Complexes, an Emerging Class of Leishmanicidal Drug Candidates. Appl. Biosci. 2022, 1, 129–142. [Google Scholar] [CrossRef]
- Araújo, J.L.; Santos, G.T.; de Sousa, L.A.; Santos, G.T.; de Freitas Silva, W.; de Oliveira Sousa, A.; Rocha, J.A. Molecular docking of rutenum complex with epiisopyloturin and nitric oxide against nucleoside diphosphate kinase protein Leishmania. Res. Soc. Dev. 2020, 9, e59922121. [Google Scholar] [CrossRef]
- Araújo, J.L.; Bastos, R.S.; Santos, G.T.; Alves, M.M.d.M.; Figueiredo, K.A.; de Sousa, L.A.; Passos, I.N.G.; Carvalho, F.A.d.A.; Lima, F.d.C.A.; Rocha, J.A. Molecular Docking and Evaluation of Antileishmania Activity of a Ruthenium Complex with Epiisopiloturine and Nitric Oxide. J. Biosci. Med. 2020, 8, 42–53. [Google Scholar] [CrossRef]
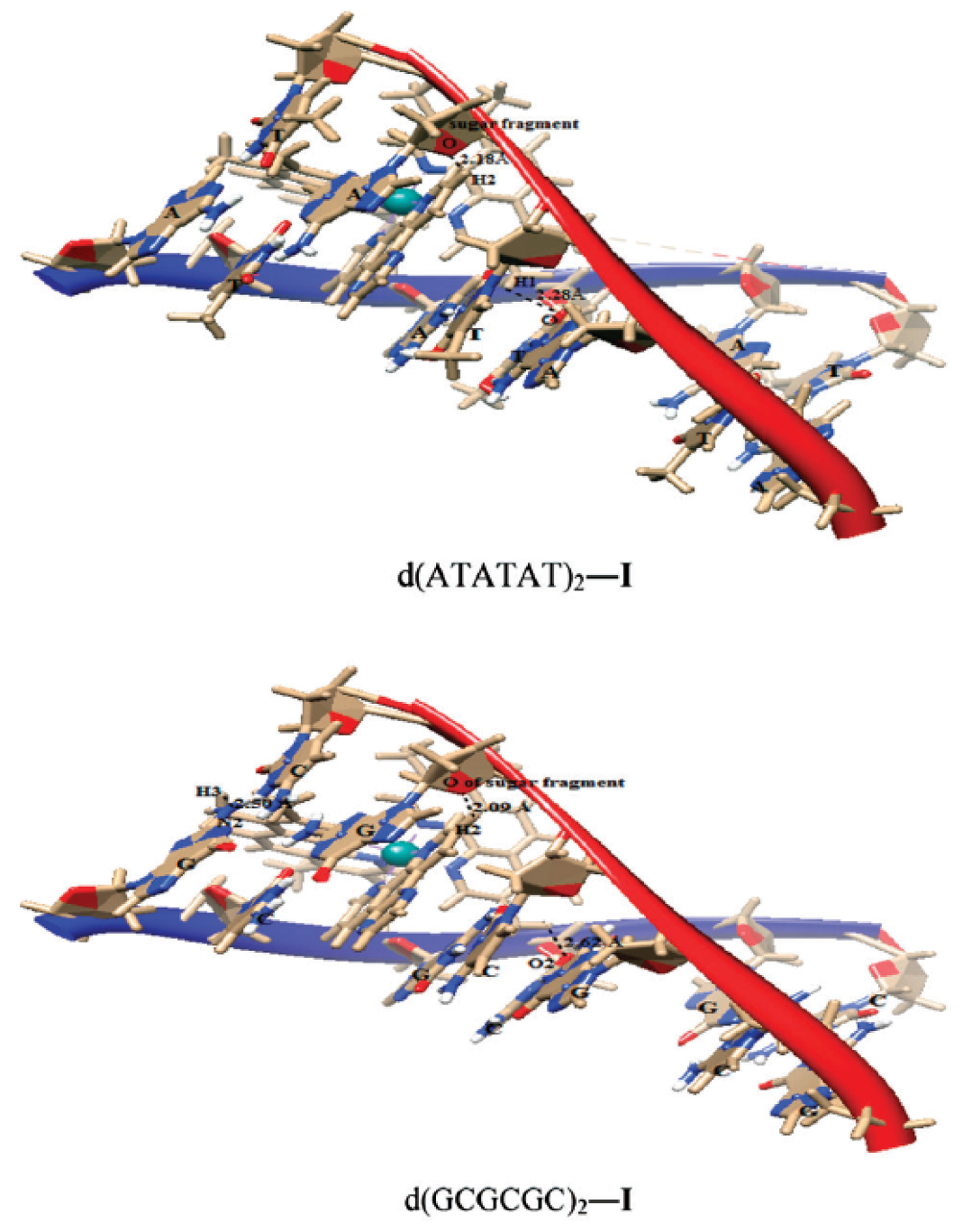
- Das, D.; Mondal, P. Interaction of ruthenium(II) antitumor complexes with d(ATATAT)2 and d(GCGCGC)2: A theoretical study. New J. Chem. 2015, 39, 2515–2522. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.-H.; Lai, Y.-W.; Zhao, R.; Chao, H.; Ji, L.-N. Targeting telomeric G-quadruplexes with the ruthenium(II) complexes [Ru(bpy)2(ptpn)]2+ and [Ru(phen)2(ptpn)]2+. Dalton Trans. 2013, 42, 4386–4397. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Khan, R.A.; AlFawaz, A.; Farshori, N.N.; Paul, A.; Jaafar, M.H.; Alsalme, A. Aminobenzimidazole-based (η6-p-cymene)ruthenium (II) complexes as nascent anticancer chemotherapeutics: Synthesis, crystal structure, DFT studies, HSA interactions, molecular docking, and cytotoxicity. Appl. Organomet. Chem. 2022, 36, e6702. [Google Scholar] [CrossRef]
- Shereef, H.A.; Shaban, S.Y.; Moemen, Y.S.; El-Khouly, M.E.; El-Nahas, A.M. Biophysicochemical studies of a ruthenium (II) nitrosyl thioether-thiolate complex binding to BSA: Mechanistic information, molecular docking, and relationship to antibacterial and cytotoxic activities. Appl. Organomet. Chem. 2022, 36, e6583. [Google Scholar] [CrossRef]
- Tang, B.; Shen, F.; Wan, D.; Guo, B.-H.; Wang, Y.-J.; Yi, Q.-Y.; Liu, Y.-J. DNA-binding, molecular docking studies and biological activity studies of ruthenium(II) polypyridyl complexes. RSC Adv. 2017, 7, 34945–34958. [Google Scholar] [CrossRef]
- Srishailam, A.; Kumar, Y.P.; Venkat Reddy, P.; Nambigari, N.; Vuruputuri, U.; Singh, S.S.; Satyanarayana, S. Cellular uptake, cytotoxicity, apoptosis, DNA-binding, photocleavage and molecular docking studies of ruthenium(II) polypyridyl complexes. J. Photochem. Photobiol. B Biol. 2014, 132, 111–123. [Google Scholar] [CrossRef]
- Golbaghi, G.; Pitard, I.; Lucas, M.; Haghdoost, M.M.; de los Santos, Y.L.; Doucet, N.; Patten, S.A.; Sanderson, J.T.; Castonguay, A. Synthesis and biological assessment of a ruthenium(II) cyclopentadienyl complex in breast cancer cells and on the development of zebrafish embryos. Eur. J. Med. Chem. 2020, 188, 112030. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.A.; Rizk, N.M.H.; Eldourghamy, A.; Farfour, S.; Ismael, M. Molecular docking, theoretical calculations, synthesis of Ru(III), Pd(II) and VO(II) complexes and activity determination as antibacterial and antioxidant. Pol. J. Chem. Technol. 2022, 24, 29–38. [Google Scholar] [CrossRef]
- Gill, M.R.; Jarman, P.J.; Hearnden, V.; Fairbanks, S.D.; Bassetto, M.; Maib, H.; Palmer, J.; Ayscough, K.R.; Thomas, J.A.; Smythe, C. A Ruthenium(II) Polypyridyl Complex Disrupts Actin Cytoskeleton Assembly and Blocks Cytokinesis. Angew. Chem. Int. Ed. 2022, 61, e202117449. [Google Scholar] [CrossRef]
- Spence, P.; Fielden, J.; Waller, Z.A.E. Beyond Solvent Exclusion: I-Motif Detecting Capability and an Alternative DNA Light-Switching Mechanism in a Ruthenium(II) Polypyridyl Complex. J. Am. Chem. Soc. 2020, 142, 13856–13866. [Google Scholar] [CrossRef]
- Liu, X.-W.; Tang, Y.-C.; Liu, N.-Y.; Deng, Y.-Q.; Wang, S.; Liu, T.; Chen, Y.-D.; Lu, J.-L. Topo I inhibition, DNA photocleavage, Molecular docking and cytotoxicities of two new phenanthroline-based ruthenium complexes. Appl. Organomet. Chem. 2020, 34, e5312. [Google Scholar] [CrossRef]
- Liu, X.-W.; Liu, N.-Y.; Deng, Y.-Q.; Wang, S.; Liu, T.; Tang, Y.-C.; Chen, Y.-D.; Lu, J.-L. Anticancer activity, topoisomerase I inhibition, DNA “light switch” behavior and molecular docking of two ruthenium complexes containing phenazine ring. J. Biomol. Struct. Dyn. 2021, 39, 5953–5962. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Kundu, B.K.; Vyas, K.; Sabu, V.; Helen, A.; Dhankhar, S.S.; Nagaraja, C.M.; Bhattacherjee, D.; Bhabak, K.P.; Mukhopadhyay, S. Ruthenium(II) arene NSAID complexes: Inhibition of cyclooxygenase and antiproliferative activity against cancer cell lines. Dalton Trans. 2018, 47, 517–527. [Google Scholar] [CrossRef]
- Tabrizi, L.; Olasunkanmi, L.O.; Fadare, O.A. Experimental and theoretical investigations of cyclometalated ruthenium(II) complex containing CCC-pincer and anti-inflammatory drugs as ligands: Synthesis, characterization, inhibition of cyclooxygenase and in vitro cytotoxicity activities in various cancer cell lines. Dalton Trans. 2019, 48, 728–740. [Google Scholar]
- Sarmento, C.O.; Pinheiro, B.F.A.; Abrahão, J.; Chaves, O.A.; Moreira, M.B.; Nikolaou, S. Interactions of a Ruthenium-Ketoprofen Compound with Human Serum Albumin and DNA: Insights from Spectrophotometric Titrations and Molecular Docking Calculations. ChemistrySelect 2022, 7, e202104020. [Google Scholar] [CrossRef]
- Weynand, J.; Diman, A.; Abraham, M.; Marcélis, L.; Jamet, H.; Decottignies, A.; Dejeu, J.; Defrancq, E.; Elias, B. Towards the Development of Photo-Reactive Ruthenium(II) Complexes Targeting Telomeric G-Quadruplex DNA. Chem. Eur. J. 2018, 24, 19216–19227. [Google Scholar] [CrossRef] [PubMed]
- Noureldeen, A.F.H.; Aziz, S.W.; Shouman, S.A.; Mohamed, M.M.; Attia, Y.M.; Ramadan, R.M.; Elhady, M.M. Molecular Design, Spectroscopic, DFT, Pharmacological, and Molecular Docking Studies of Novel Ruthenium(III)–Schiff Base Complex: An Inhibitor of Progression in HepG2 Cells. Int. J. Environ. Res. Public Health 2022, 19, 13624. [Google Scholar] [CrossRef] [PubMed]
- Mareeswaran, P.M.; Maheshwaran, D.; Babu, E.; Rajagopal, S. Binding and Fluorescence Resonance Energy Transfer (FRET) of Ruthenium(II)-Bipyridine-Calixarene System with Proteins—Experimental and Docking Studies. J. Fluoresc. 2012, 22, 1345–1356. [Google Scholar] [CrossRef]
- Patel, D.; Athar, M.; Jha, P.C. Exploring Ruthenium-Based Organometallic Inhibitors against Plasmodium falciparum Calcium Dependent Kinase 2 (PfCDPK2): A Combined Ensemble Docking, QM/MM and Molecular Dynamics Study. ChemistrySelect 2021, 6, 8189–8199. [Google Scholar] [CrossRef]
- Bansal, A.; Molina-Cruz, A.; Brzostowski, J.; Mu, J.; Miller, L.H. Plasmodium falciparum Calcium-Dependent Protein Kinase 2 Is Critical for Male Gametocyte Exflagellation but Not Essential for Asexual Proliferation. mBio 2017, 8, e01656-17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Agrawal, N.J.; Radhakrishnan, R. A flexible-protein molecular docking study of the binding of ruthenium complex compounds to PIM1, GSK-3β, and CDK2/Cyclin A protein kinases. J. Mol. Model. 2013, 19, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Dutta, A.; Mondal, P. Interactions of the aquated forms of ruthenium(III) anticancer drugs with protein: A detailed molecular docking and QM/MM investigation. RSC Adv. 2014, 4, 60548–60556. [Google Scholar] [CrossRef]
- Erkan, S.; Kaya, S.; Sayin, K.; Karakaş, D. Structural, spectral characterization and molecular docking analyses of mer-ruthenium (II) complexes containing the bidentate chelating ligands. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117399. [Google Scholar] [CrossRef]







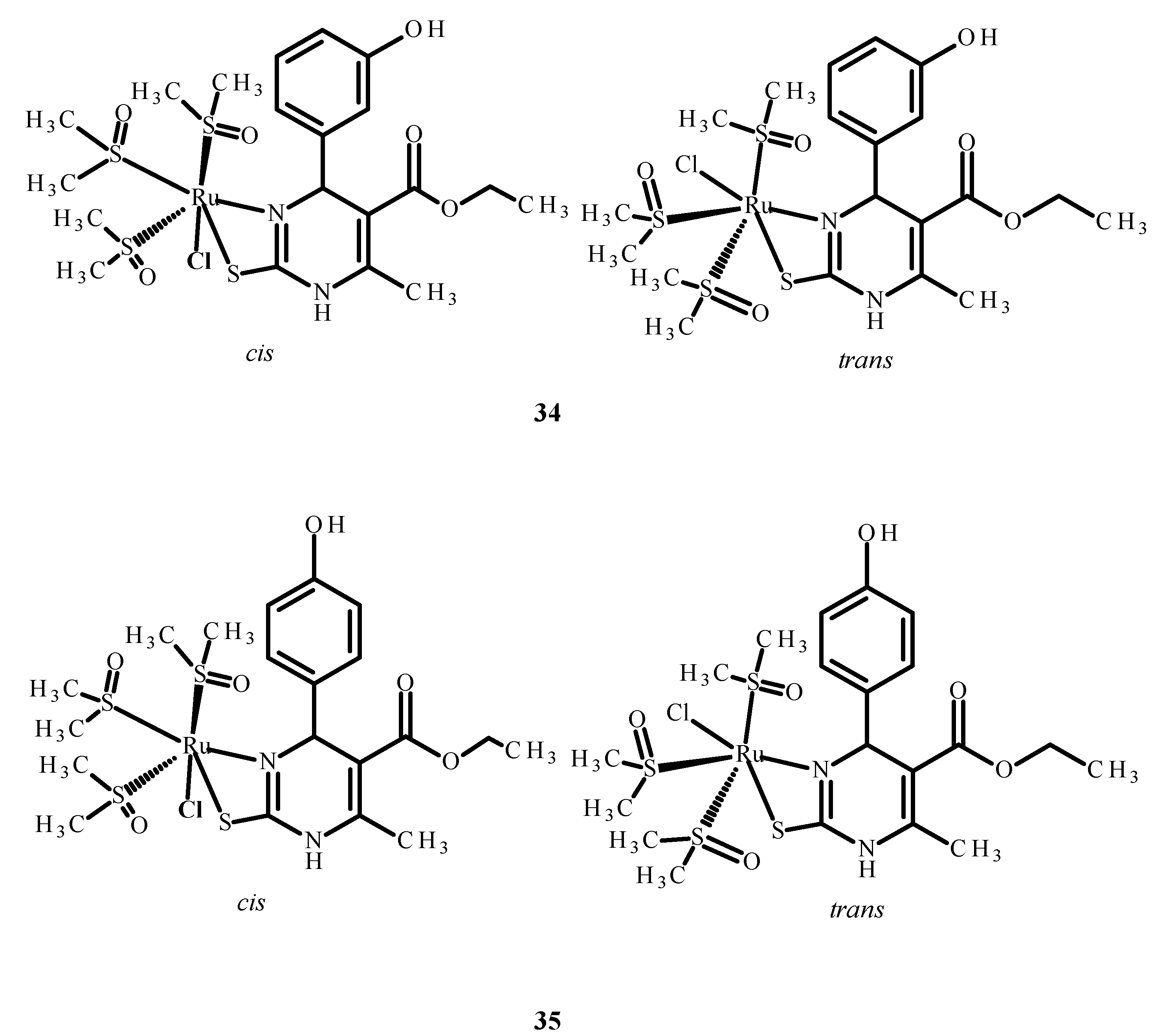
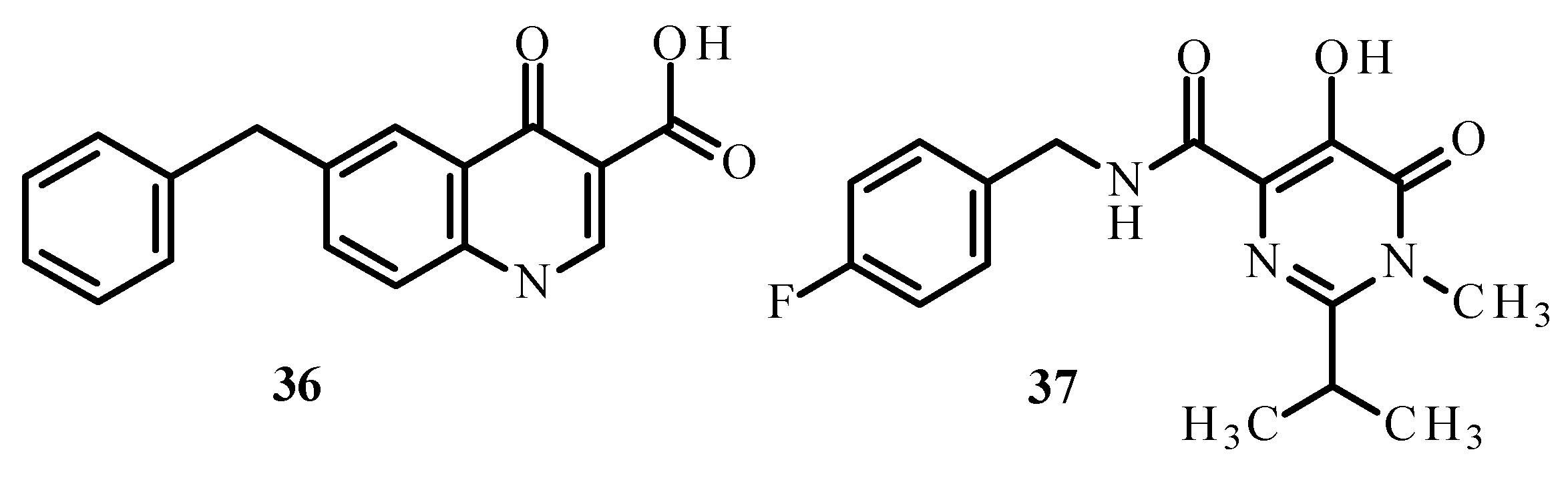

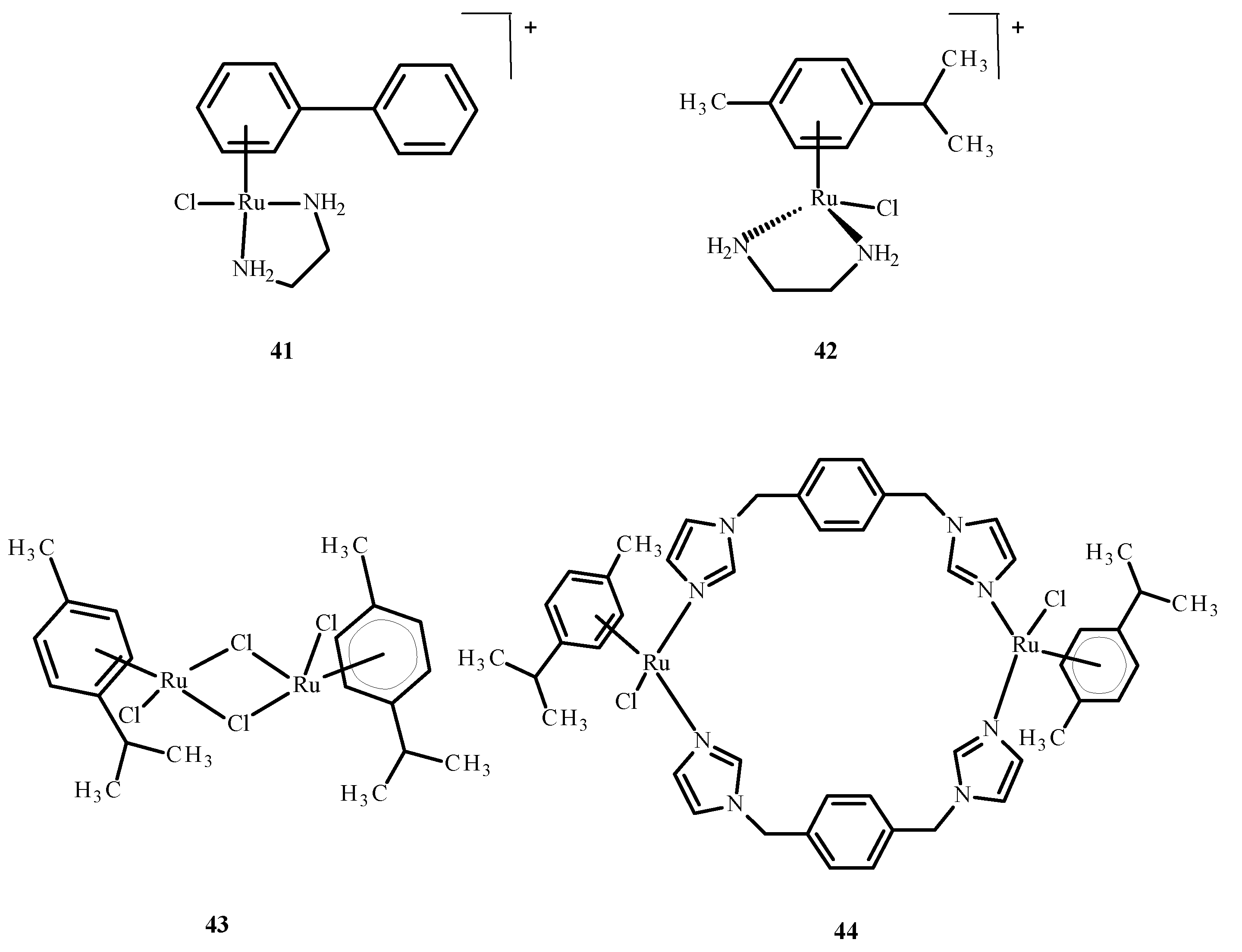


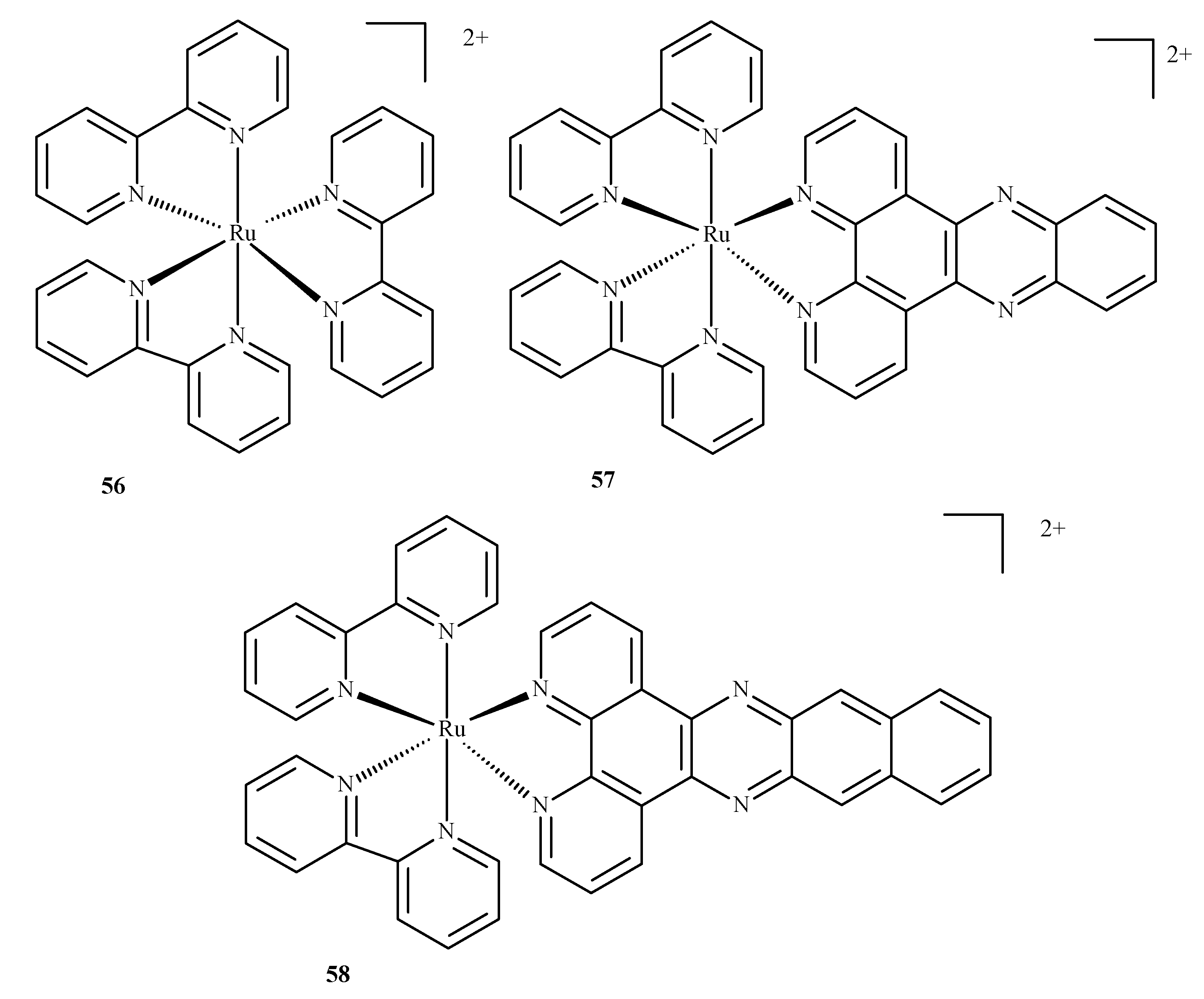


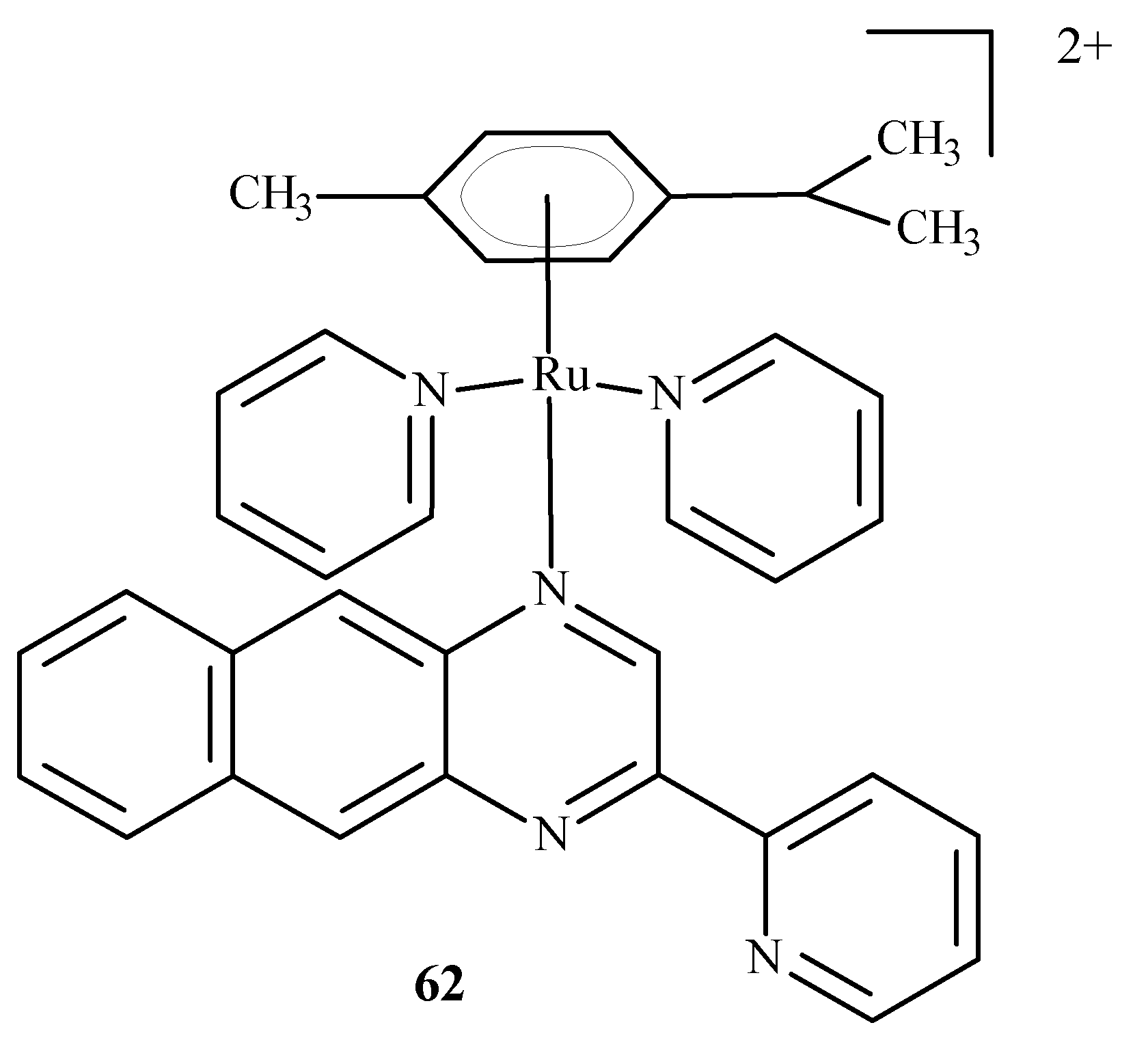
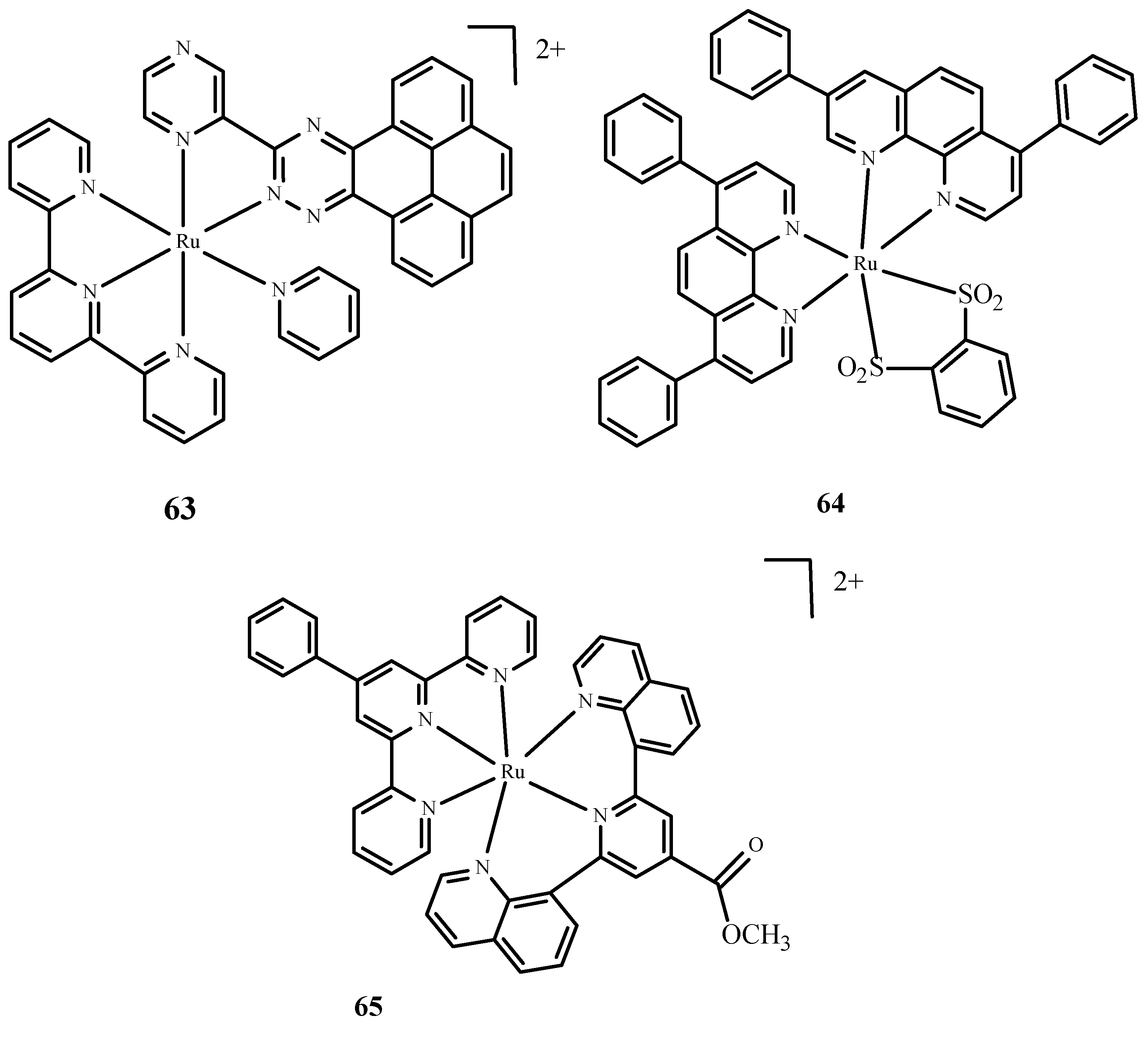
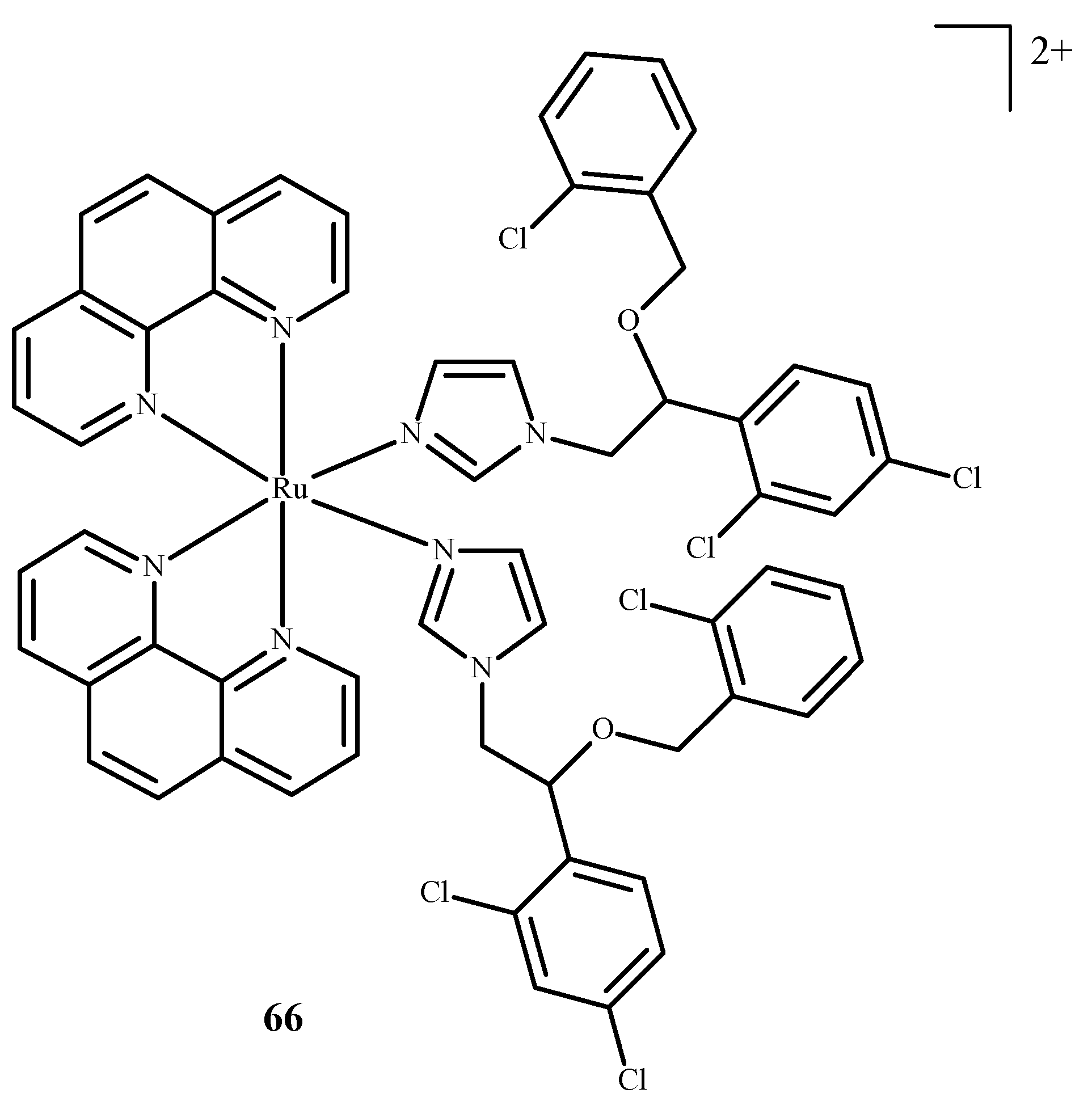
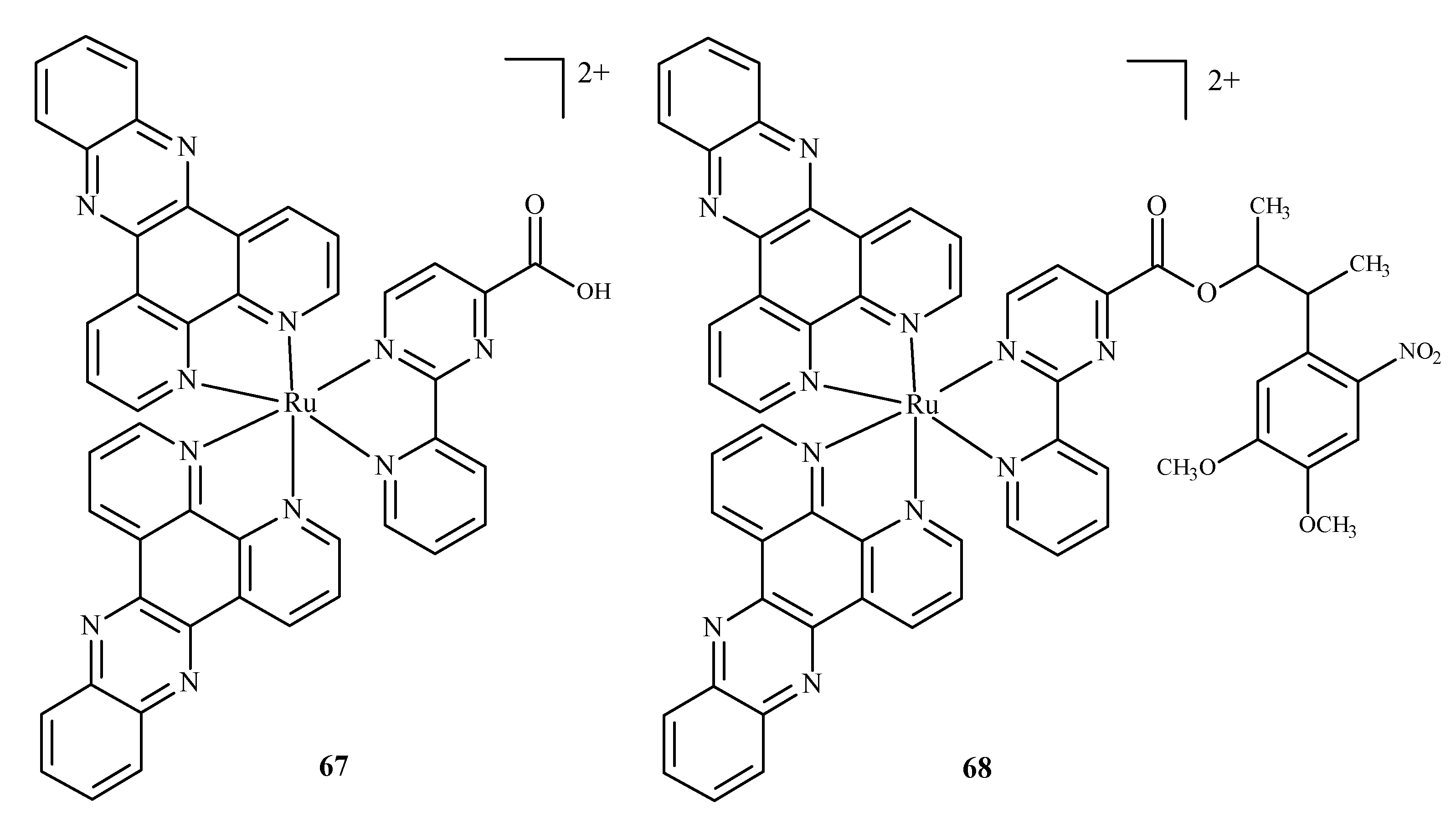




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoczynska, A.; Lewinski, A.; Pokora, M.; Paneth, P.; Budzisz, E. An Overview of the Potential Medicinal and Pharmaceutical Properties of Ru(II)/(III) Complexes. Int. J. Mol. Sci. 2023, 24, 9512. https://doi.org/10.3390/ijms24119512
Skoczynska A, Lewinski A, Pokora M, Paneth P, Budzisz E. An Overview of the Potential Medicinal and Pharmaceutical Properties of Ru(II)/(III) Complexes. International Journal of Molecular Sciences. 2023; 24(11):9512. https://doi.org/10.3390/ijms24119512
Chicago/Turabian StyleSkoczynska, Anna, Andrzej Lewinski, Mateusz Pokora, Piotr Paneth, and Elzbieta Budzisz. 2023. "An Overview of the Potential Medicinal and Pharmaceutical Properties of Ru(II)/(III) Complexes" International Journal of Molecular Sciences 24, no. 11: 9512. https://doi.org/10.3390/ijms24119512
APA StyleSkoczynska, A., Lewinski, A., Pokora, M., Paneth, P., & Budzisz, E. (2023). An Overview of the Potential Medicinal and Pharmaceutical Properties of Ru(II)/(III) Complexes. International Journal of Molecular Sciences, 24(11), 9512. https://doi.org/10.3390/ijms24119512







