Spin Crossover and Thermochromism in Iron(II) Complexes with 2,6-Bis(1H-imidazol-2-yl)-4-methoxypyridine
Abstract
1. Introduction
2. Results
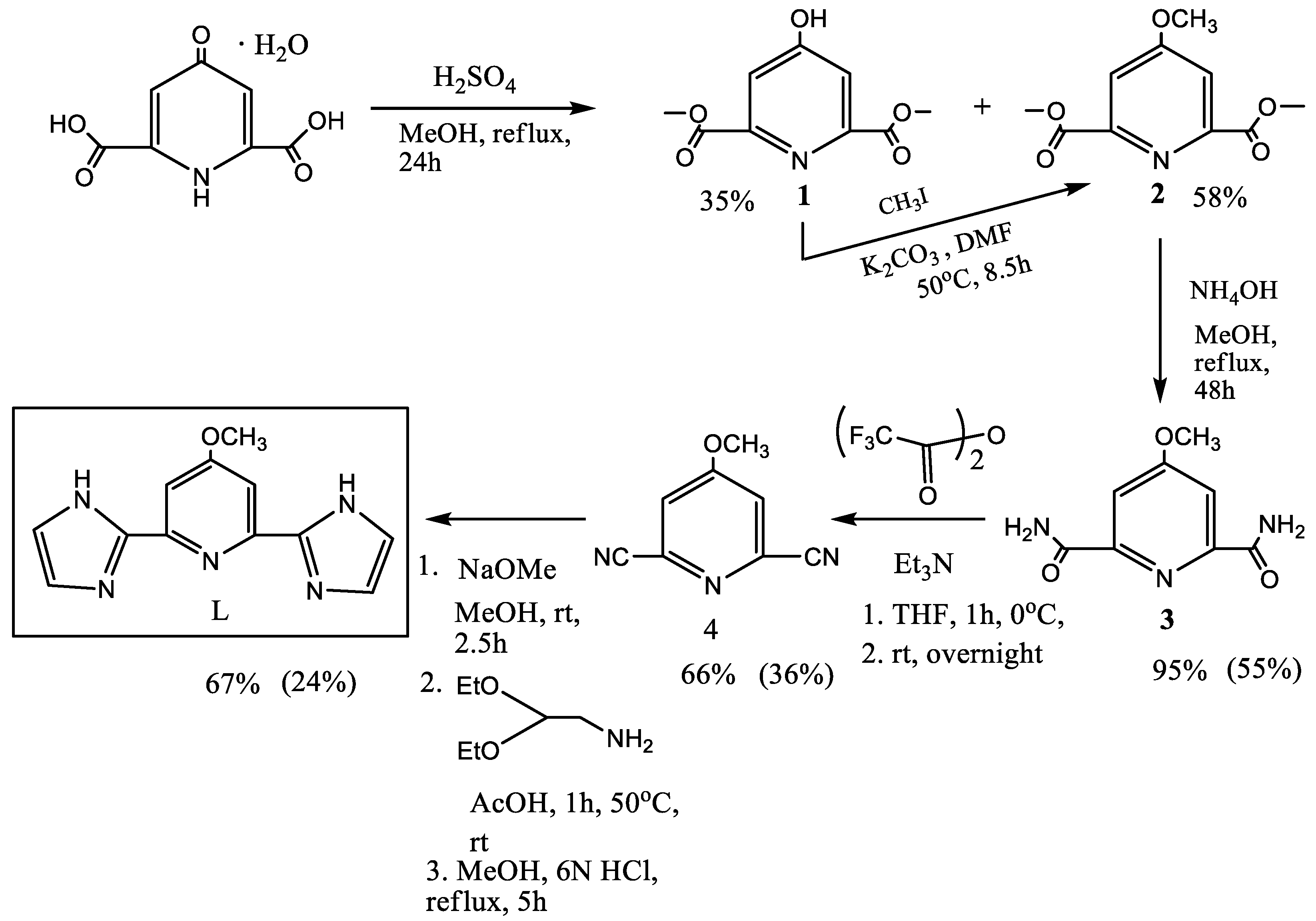
2.1. Synthesis and Characterization
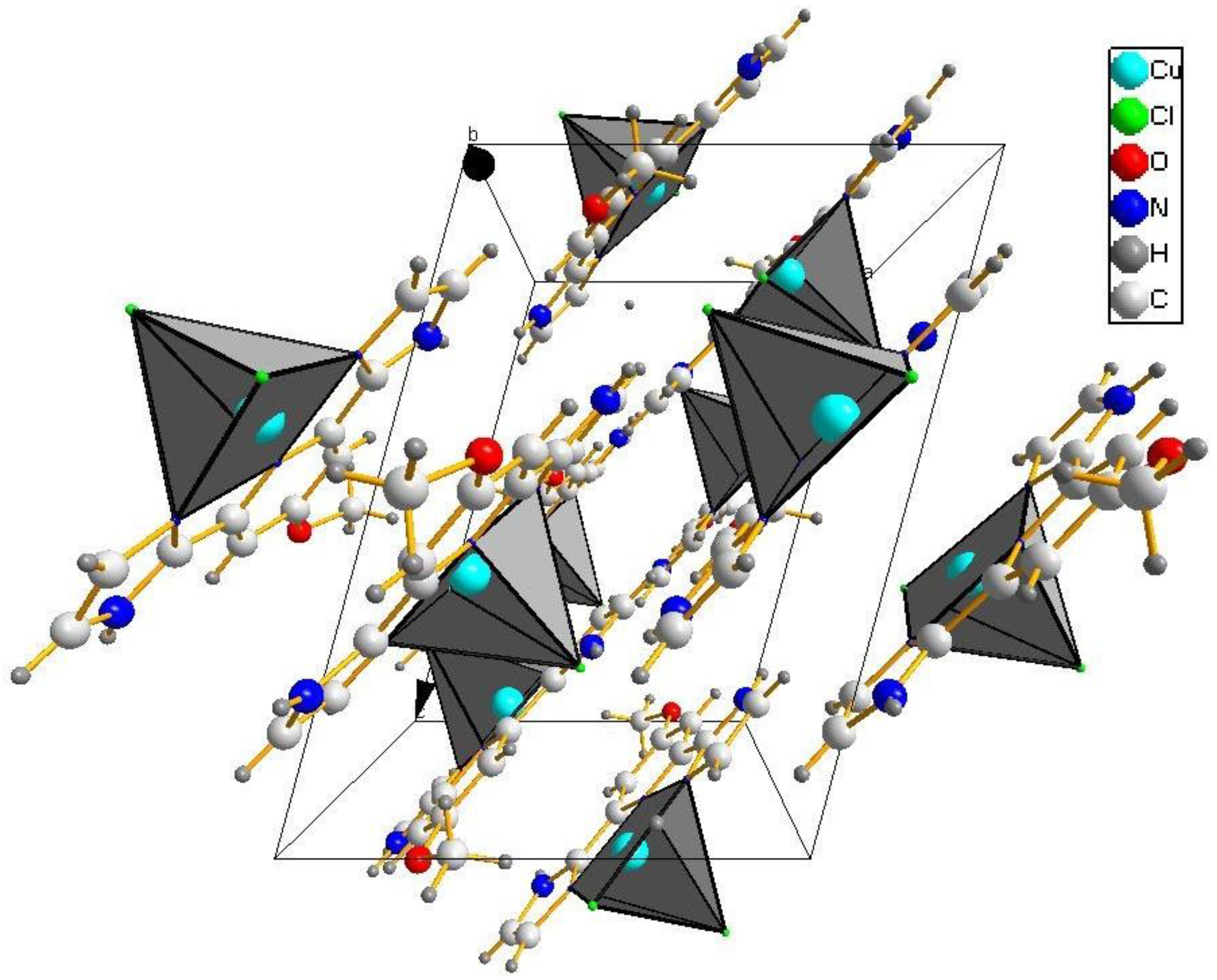
2.2. X-ray Structure Determination
2.3. Infrared Spectroscopy
2.4. Diffuse Reflectance Spectroscopy
2.5. Mössbauer Spectra
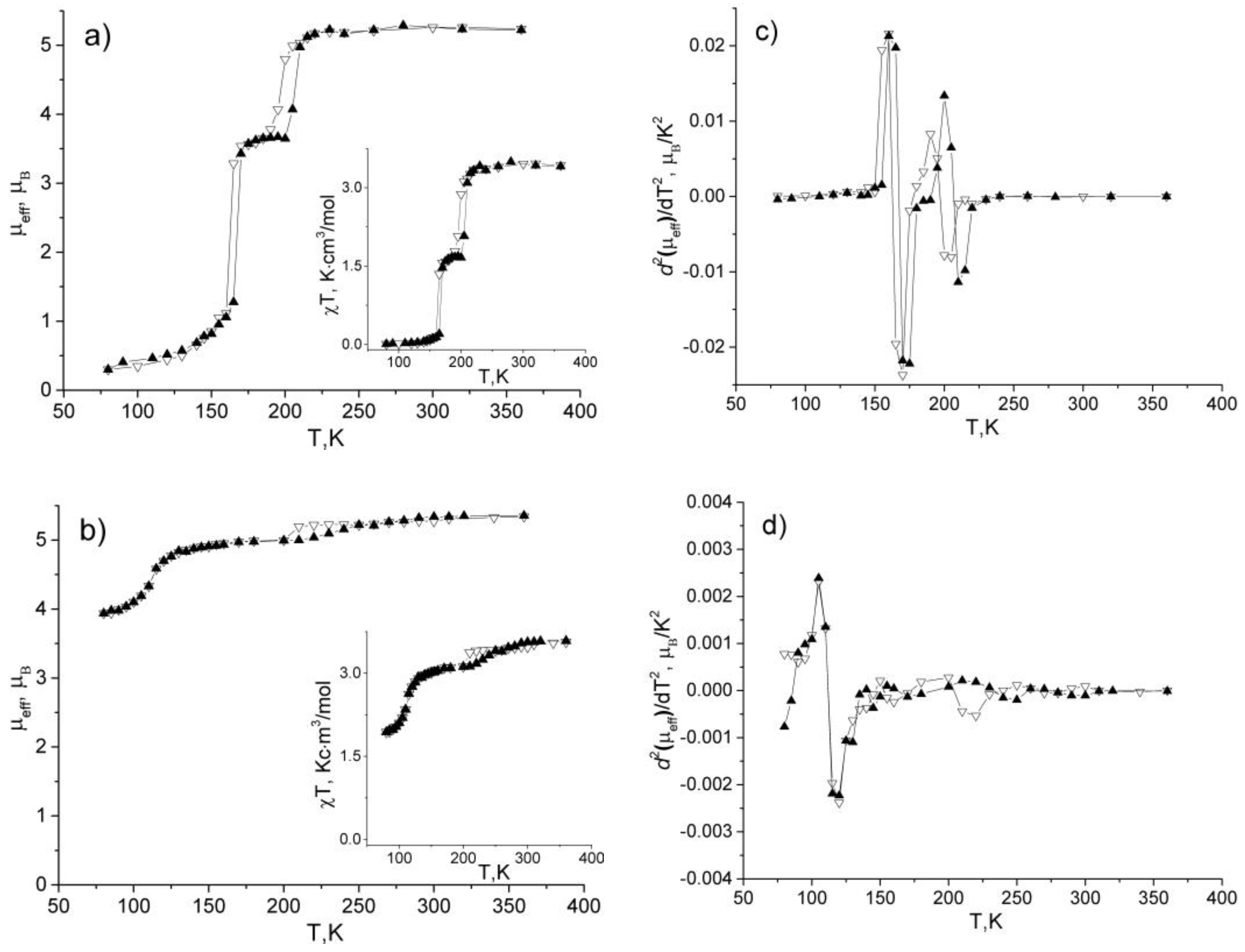
2.6. Study of the Complexes Magnetic Properties
3. Discussion
3.1. Structure of the [CuLCl2]
3.2. IR Spectra of the Complexes I–III
3.3. DRS of the Complexes I–III
3.4. Mössbauer Spectra of the Complexes I–III
3.5. Magnetic Properties of the Complexes
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H. Spin Crossover in Transition Metal Compounds I–III. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; pp. 233–235. [Google Scholar]
- Halcrow, M.A. Spin-Crossover Materials Properties and Applications; John Wiley & Sons Ltd.: London, UK, 2013; 562p. [Google Scholar]
- Halcrow, M.A. The effect of ligand design on metal ion spin state—Lessons from spin crossover complexes. Crystals 2016, 6, 58. [Google Scholar] [CrossRef]
- Boillot, M.-L.; Zarembowitch, J.; Sour, A. Ligand-Driven Light-Induced Spin Change (LD-LISC): A Promising Photomagnetic Effect. In Spin Crossover in Transition Metal Compounds II; Springer: Berlin/Heidelberg, Germany, 2004; pp. 261–276. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Lavrenova, L.G. Spin crossover in new iron(II) coordination compounds with tris(pyrazol-1-yl)methane. Crystals 2020, 10, 843. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Molnár, G.; Rat, S.; Salmon, L.; Nicolazzi, W.; Bousseksou, A. Spin Crossover Nanomaterials: From Fundamental Concepts to Devices. Adv. Mater. 2018, 30, 1703862. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.M.J.N.; Said, S.M.; Mainal, A.; Sabri, M.F.M.; Abdullah, N.; Hasnan, M.M.I.; Hassan, H.C.; Salleh, M.F.M.; Mahiyiddin, W.W.M. Molecular design strategies for spin crossover (SCO) metal complexes (Fe(II) and Co(II)) for thermoelectricity. Mater. Res. Bull. 2020, 126, 110828. [Google Scholar] [CrossRef]
- Boča, M.; Jameson, R.F.; Linert, W. Fascinating variability in the chemistry and properties of 2,6-bis-(benzimidazol-2-yl)-pyridine and 2,6-bis-(benzthiazol-2-yl)-pyridine and their complexes. Coord. Chem. Rev. 2011, 255, 290–317. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Dyukova, I.I.; Korotaev, E.V.; Sheludyakova, L.A.; Varnek, V.A. Spin Crossover in New Iron(II) Complexes with 2,6-bis(1H-benzimidazol-2-yl)pyridine. Rus. J. Inorg. Chem. 2020, 65, 30–35. [Google Scholar] [CrossRef]
- Ivanova, A.D.; Korotaev, E.V.; Komarov, V.Y.; Sheludyakova, L.A.; Varnek, V.A.; Lavrenova, L.G. Spin crossover in iron(II) coordination compounds with 2,6-bis(1H-benzimidazol-2-yl)pyridine. New J. Chem. 2020, 44, 5834–5840. [Google Scholar] [CrossRef]
- Ivanova, A.D.; Lavrenova, L.G.; Korotaev, E.V.; Trubina, S.V.; Sheludyakova, L.A.; Petrov, S.A.; Zhizhin, K.Y.; Kuznetsov, N.T. High-Temperature Spin Crossover in Complexes of Iron(II) closo-Borates with 2,6-bis(1H-benzimidazol-2-yl)pyridine. Russ. J. Inorg. Chem. 2020, 65, 1687–1694. [Google Scholar] [CrossRef]
- Ivanova, A.D.; Korotaev, E.V.; Komarov, V.Y.; Sukhikh, T.S.; Trubina, S.V.; Sheludyakova, L.A.; Petrov, S.A.; Tikhonov, A.Y.; Lavrenova, L.G. Spin Crossover in iron(II) complexes with new ligand 2,6-bis(4,5-dimethyl-1H-imidazol-2-yl)pyridine. Inorg. Chim. Acta 2022, 532, 120746. [Google Scholar] [CrossRef]
- Ivanova, A.D.; Lavrenova, L.G.; Korotaev, E.V.; Trubina, S.V.; Tikhonov, A.Y.; Kriventsov, V.V.; Petrov, S.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Study Spin Crossover in Iron(II) Complexes with 2,6-Bis(4,5-Dimethyl-1H-Imidazol-2-yl)Pyridine and closo-Borate Anions. Russ. J. Inorg. Chem. 2022, 67, 1158–1168. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Shakirova, O.G.; Korotaev, E.V.; Trubina, S.V.; Tikhonov, A.Y.; Os’kina, I.N.; Petrov, S.A.; Zhizhin, K.Y.; Kuznetsov, N.T. High-temperature spin crossover in iron(II) complexes with 2,6-bis(1H-imidazol-2-yl)pyridine. Molecules 2022, 27, 5093. [Google Scholar] [CrossRef] [PubMed]
- Lavrenova, L.G.; Shakirova, O.G. Spin Crossover and Thermochromism of Iron(II) Coordination Compounds with 1,2,4-Triazoles and Tris(pyrazol-1-yl)methanes. Eur. J. Inorg. Chem. 2013, 5–6, 670–682. [Google Scholar] [CrossRef]
- Lavrenova, L.G. Spin crossover in homo-and heteroligand iron (II) complexes with tris(pyrazol-1-yl)methane derivatives. Russ. Chem. Bull. 2018, 67, 1142–1152. [Google Scholar] [CrossRef]
- Sanning, J.; Ewen, P.R.; Stegemann, L.; Schmidt, J.; Daniliuc, C.G.; Koch, T.; Doltsinis, N.L.; Wegner, D.; Strassert, C.A. Scanning-Tunneling-Spectroscopy-Directed Design of Tailored Deep-Blue Emitters. Angew. Chem. Int. Ed. 2015, 54, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Wagenen, B.V.; Stormann, T.M.; Moe, S.T.; Sheehan, S.M.; McLeod, D.A.; Smith, D.L.; Isaac, M.B.; Slassi, A. Heteropolycyclic Compounds and Their Use As Metabotropic Glutamate Receptor Antagonists. U.S. Patent 20030055085A1, 9 December 2003. [Google Scholar]
- Voss, M.E.; Beer, C.M.; Mitchell, S.A.; Blomgren, P.A.; Zhichkin, P.E. A simple and convenient one-pot method for the preparation of heteroaryl-2-imidazoles from nitriles. Tetrahedron 2008, 64, 645–651. [Google Scholar] [CrossRef]
- Carlin, R.L. Magnetochemistry; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Rakitin, Y.V.; Kalinnikov, V.T. Modern Magnetochemistry; Nauka: St. Petersburg, Russia, 1994. [Google Scholar]
- Crowley, J.D.; Bandeen, P.H.; Hanton, L.R. A one pot multi-component CuAAC “click” approach to bidentate and tridentate pyridyl-1,2,3-triazole ligands: Synthesis, X-ray structures and copper(II) and silver(I) complexes. Polyhedron 2010, 29, 70–83. [Google Scholar] [CrossRef]
- Schwartz, T.M.; Burnett, M.E.; Green, K.N. Electronic influence of substitution on the pyridine ring within NNN pincer-type molecules. Dalton Trans. 2020, 49, 2356–2363. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 6th ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Sugano, S.; Tanabe, Y.; Kamimura, H. Multiplets of Transition—Metal ions in Crystals; Academic Press Pure and Applied Physics: London, UK, 1970. [Google Scholar]
- Figgis, B.N.; Hitchman, M.A. Ligand Field Theory and Its Application; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Hauser, A. Ligand field theoretical considerations. Top. Curr. Chem. 2004, 233, 49–58. [Google Scholar] [CrossRef]
- Bruker AXS Inc. APEX2 (version 2012.2-0), SAINT (version 8.18c), and SADABS (version 2008/1). In Bruker Advanced X-ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2000–2012. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]




| Empirical Formula | C12H11Cl2CuN5O |
|---|---|
| Formula weight (g/mol) | 375.70 |
| Space group | P 21/n |
| Cell Parameters | a = 9.2008(7) Å = 90° b = 12.3775(7) Å = 105.219(2)° c = 12.7209(9) Å = 90° |
| V (Å3) | 1397.89(17) |
| Z | 4 |
| (g/cm−3) | 1.785 |
| μ (mm−1) | 1.949 |
| F(000) | 756 |
| Crystal size, mm | 0.22 × 0.10 × 0.06 |
| Scan range, θ, degree | 2.34–26.38 |
| Range h, k, l | −11 ≤ h ≤ 11, −10 ≤ k ≤ 15, −15 ≤ l ≤ 15 |
| Number of measured reflections | 9505 |
| Number of independent reflections | 2860 [R(int) = 0.0688] |
| Number of reflexes/constraints/parameters | 2860/0/191 |
| Goodness-of-fit on F2 | 1.050 |
| R-factor, [I > 2σ(I)] R1/wR2 | R1 = 0.0472, wR2 = 0.0988 |
| R-factor (for all Ihkl) R1/wR2 | R1 = 0.0682, wR2 = 0.1062 |
| Residual electron density (max/min) e/Å3 | 0.520/−0.720 |
| Bond | Distance (Å) | Angle | Degree (°) | Angle | Degree (°) |
|---|---|---|---|---|---|
| Cu1-N1 | 1.983 (3) | N1-Cu1-N2 | 78.78 (13) | N3-Cu1-Cl1 | 97.49 (9) |
| Cu1-N2 | 2.054 (3) | N1-Cu1-N3 | 78.94 (13) | N3-Cu1-Cl2 | 98.67 (9) |
| Cu1-N3 | 2.032 (3) | N3-Cu1-N2 | 155.84 (13) | N1-Cu1-Cl2 | 168.16 (10) |
| Cu1-Cl1 | 2.6761 (11) | N1-Cu1-Cl1 | 94.93 (10) | N2-Cu1-Cl2 | 101.21 (9) |
| Cu1-Cl2 | 2.2137 (11) | N2-Cu1-Cl1 | 93.61 (9) | Cl2-Cu1-Cl1 | 96.89 (4) |
| L | [FeL2]SO4∙2H2O | [FeL2](ReO4)2∙H2O | [FeL2]Br2∙2H2O | Assignments |
|---|---|---|---|---|
| 3600–3300 | 3415–3403 | 3450–3400 | 3500–3365 | ν (O–H) |
| 3138, 3113 | 3156, 3118 | 3156, 3124 | 3146, 3116 | ν (N–H) associated |
| 3074 | 3069 | 3089 | 3076 | ν (Cring–H) |
| 2981, 2939 | 2937 | 2985, 2948 | 2979, 2937 | νas (CH3) |
| 2850 | 2870 | 2879, 2833 | 2879, 2833 | νs (CH3) |
| 2815 | 2808 | 2802 | 2810 | ν (O–CH3) |
| 1606, 1570, 1551 | 1676, 1625, 1596, 1564 | 1660, 1622, 1558 | 1660, 1624, 1600, 1563 | R(py) |
| 1477, 1437 | 1504, 1475, 1460, 1412 | 1489, 1480, 1405 | 1482, 1435, 1400 | R(im) |
| 1356, 1315, 1286, 1260 | 1340, 1320, 1295 | 1370, 1340, 1300 | 1370, 1340, 1300 | δ (C–H) scissoring |
| 1240 | 1253 | 1256 | 1258 | ν (Cring–O) |
| 1130, 1103 | 1137, 1120, 1109 | 1133, 1115 | δ (C–H) twisting | |
| 1115 | ν3 (SO4) | |||
| 1043 | 1043 | 1053 | 1053 | δ (O–CH3) |
| 990, 960, 924 | 987, 959, 906 | 990, 960 | 990, 960, 910 | δ (C–H) out-of-plane |
| 926, 912, 895 | ν3 (ReO4) | |||
| 877, 864 | 862 | 855 | 880, 860 | δ (C–N) |
| 766, 746, 714 | 767, 745, 703 | 786, 770, 751, 705 | 795, 750, 703 | δ (C–H) rocking |
| 615 | 618 | 618 | 615 | δ (N–H) |
| 332 | 334 | 335 | ν (FeHS–N) |
| Complex | δ, mm/s | ΔEQ, mm/s | Г, mm/s |
|---|---|---|---|
| I | 0.950 (1) | 1.881 (2) | 0.29 (1) |
| II | 0.971 (4) | 2.378 (7) | 0.29 (2) |
| III | 0.970 (3) | 1.956 (5) | 0.28 (1) |
| Compound | Tc↑, K | Tc↓, K | ΔTc, K |
|---|---|---|---|
| I | 167 | 163 | 4 |
| 207 | 197 | 10 | |
| II | 112 | 112 | 0 |
| 234 | 204 | 30 | |
| Ia | 217 | 213 | 4 |
| IIa | 112 | 112 | 0 |
| 222 | 201 | 21 | |
| IIIa | 121 | 121 | 0 |
| Complex | B | C | P | |||
|---|---|---|---|---|---|---|
| I | 23,095 | 15,650 | 10,685 | 562 | 2478 | 18,130 |
| II | 24,570 | 15,650 | 10,835 | 570 | 2515 | 19,755 |
| III | 24,570 | 15,650 | 10,685 | 562 | 2478 | 19,605 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakirova, O.G.; Os’kina, I.A.; Korotaev, E.V.; Petrov, S.A.; Kuratieva, N.V.; Tikhonov, A.Y.; Lavrenova, L.G. Spin Crossover and Thermochromism in Iron(II) Complexes with 2,6-Bis(1H-imidazol-2-yl)-4-methoxypyridine. Int. J. Mol. Sci. 2023, 24, 9853. https://doi.org/10.3390/ijms24129853
Shakirova OG, Os’kina IA, Korotaev EV, Petrov SA, Kuratieva NV, Tikhonov AY, Lavrenova LG. Spin Crossover and Thermochromism in Iron(II) Complexes with 2,6-Bis(1H-imidazol-2-yl)-4-methoxypyridine. International Journal of Molecular Sciences. 2023; 24(12):9853. https://doi.org/10.3390/ijms24129853
Chicago/Turabian StyleShakirova, Olga G., Irina A. Os’kina, Evgeniy V. Korotaev, Sergey A. Petrov, Natalia V. Kuratieva, Alexsei Ya. Tikhonov, and Lyudmila G. Lavrenova. 2023. "Spin Crossover and Thermochromism in Iron(II) Complexes with 2,6-Bis(1H-imidazol-2-yl)-4-methoxypyridine" International Journal of Molecular Sciences 24, no. 12: 9853. https://doi.org/10.3390/ijms24129853
APA StyleShakirova, O. G., Os’kina, I. A., Korotaev, E. V., Petrov, S. A., Kuratieva, N. V., Tikhonov, A. Y., & Lavrenova, L. G. (2023). Spin Crossover and Thermochromism in Iron(II) Complexes with 2,6-Bis(1H-imidazol-2-yl)-4-methoxypyridine. International Journal of Molecular Sciences, 24(12), 9853. https://doi.org/10.3390/ijms24129853








