Optogenetic and Chemogenic Control of Pain Signaling: Molecular Markers
Abstract
1. Introduction
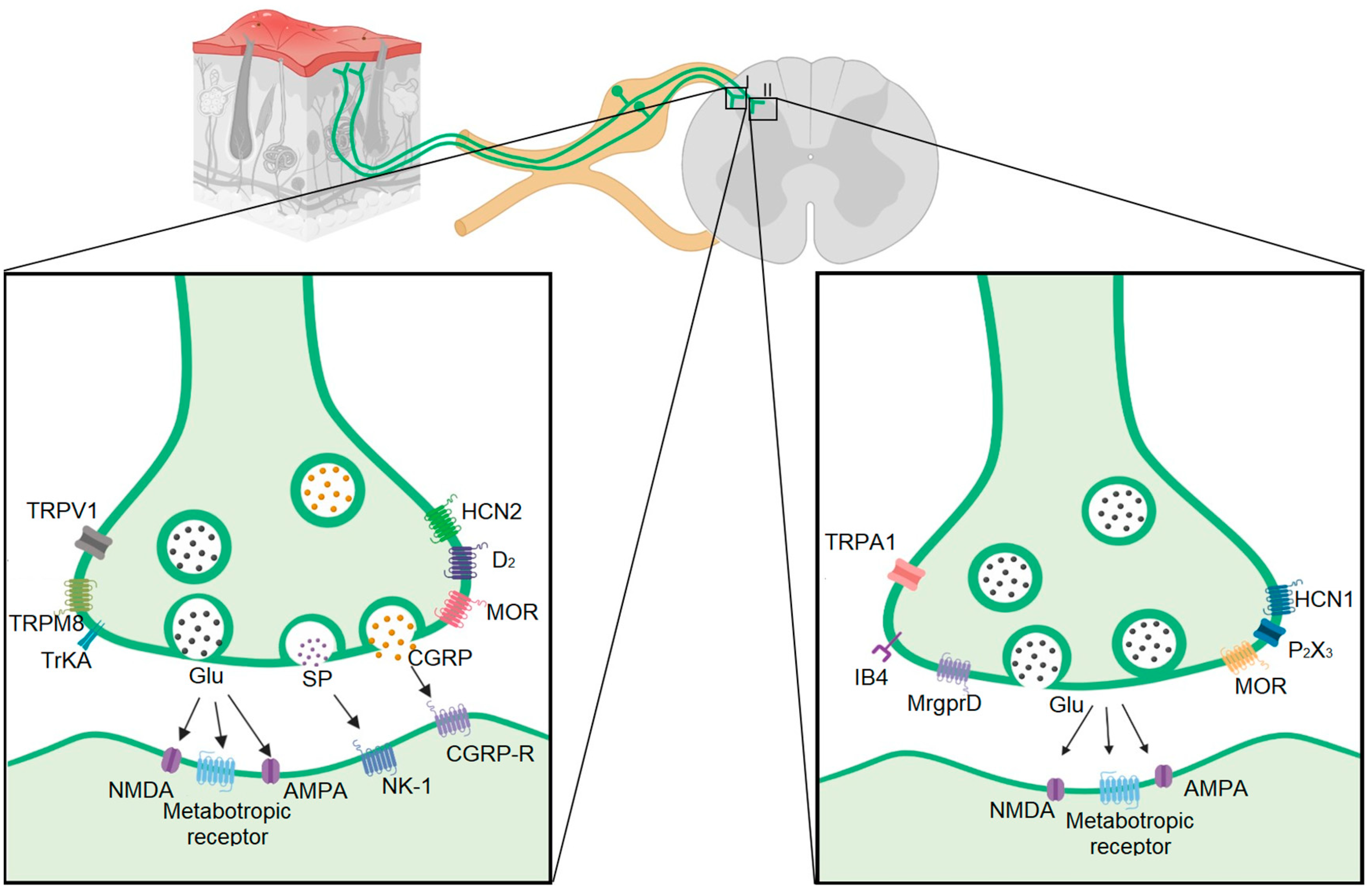
2. Neural Types in the Dorsal Ganglia Root and Their Colocalization with the Vesicular Transporter of Glutamate
| Fiber Type | Conduction Velocity | Vesiculator Type | Protein Markers | Channels | Membrane Receptors | Dorsal Laminar Distribution | Transcription Factor | References | |
|---|---|---|---|---|---|---|---|---|---|
| Aβ | 30–80 m/s | VGluT 1 | NF200 Thy-1 parvalbumin Caderina SPP1 NEFH | Nav1.1 Nav1.6 HCN3 ASIC1 Nav1.8 | DOR TrkC | III, IV, V | RunX3 ETV1 Etv4 Cdh12 | [7,8,9,10,11,13,14,27,28,29,30,31,32,33,34,35] | |
| Aδ | 5–25 m/s | VGluT 2 | NF200 NEFH CGRP Substance P | TRPM8 Nav1.8 HCN1 HCN3 Cav 3.2 | TrkA TRKB MOR DOR | I, II | CNTNAP2 FAM19A RET | [7,13,14,29,31,32,33,36,37,38] | |
| C | Peptidergic | 0.5–2 m/s | VGluT 2 | Substance P CGRP Cdk5 mTOR | TRPV1 TRPA1 TRPM8 Nav1.7 Nav1.8 HCN2 HCN3 | D2 MOR MrgprA3 mGlu2 Ntrk1 PAC1 TrkA Y1R Y2R | I | cMAF GDNF HOB8 LMBX1 RET TAC1 | [7,9,24,25,26,29,31,33,34,35,39,40,41,42,43,44,45,46] |
| Non-peptidergic | 0.5–2 m/s | VGluT 2 | IB4 Cdk5 mTOR | TRPA1 TRPM3 TRPC3 Nav1.7 Nav1.8 Nav1.9 HCN1 HCN3 P2X3 | DOR MrgprA3 MrgprB4 MrgprD Y1R Y2R | II | GfrA1 LMBX1 NGF PLXNC1 RET RunX | [7,9,13,24,26,28,29,31,33,34,36,41,44,45,46,47,48] | |
| LTMR | <0.5 m/s | VGluT 3 | TH | TRPM8 Nav1.8 Nav1.9 | TrkB | I, II, III | GfrA2 PIEZO2 RET | [14,28,29,34,36,48] | |
3. Pain Pathway Alteration
4. Optomodulation of Peripheral Nerve Activity
Optomodulation of Primary Afferent Fibers with Viral Transfections and Constitutive Expression
| Specificity Strategy | Construct | Fiber and Cell Type | Injection Site | Place of Stimulation | Behavioral Phenotype | Painful Condition | Reference |
|---|---|---|---|---|---|---|---|
| No Cre dependent | AAV6-hSyn-ChR2-eYFP Product: Chr2-eYFP | C-fiber: -Unmyelinated primary afferent | intrasciatic | The plantar surface Blue light (473 nm) | Decreases in paw withdrawal Place aversion | No sensitized | [89] |
| AAV6-hSyn-SwiChR-eYFP Product: SwiChR-eYFP | C-fiber: -Unmyelinated primary afferent | intraneural | The plantar surface of the hindpaw Blue light (473 nm) | Increases in mechanical withdrawal threshold and thermal withdrawal latency | [88] | ||
| AAV6-hSyn-biC1C2-TS-eYFP Product: iC1C2-eYFP | C-fiber: -Unmyelinated primary afferent | intraneural | The plantar surface of the hindpaw Blue light (473 nm) | Increases in mechanical withdrawal threshold and thermal withdrawal latency | [88] | ||
| AAV6-hSyn-eNpHR3.0-eYFP | C-fiber: -Unmyelinated primary afferent | Intrasciatic | The plantar surface Yellow light (590 nm) | Increases in mechanical withdrawal threshold and thermal withdrawal latency | [89] | ||
| AAV6-hSyn-HA-HM4D(Gi)-IRES-mCitrine Product:hM4D-Gi | C-fiber: -Unmyelinated primary afferent | Intraneural | Clozapine-N-oxide intraperitoneally | Increases in mechanical withdrawal threshold and thermal withdrawal latency | [88] |
| Specificity Strategy | Construct | Fiber and Cell Type | Injection Site | Place of Stimulation | Behavioral Phenotype | Painful Condition | Reference | |
|---|---|---|---|---|---|---|---|---|
| No Cre-dependent | AAV6-hSyn-SwiChR-eYFP Product: SwiChR-eYFP | C-fiber: Unmyelinated primary afferent | intrasciatic | The plantar surface of the hindpaw Blue light (473 nm) | Reduced pain behavior in phase I | Sensitized | Formalin test | [88] |
| AAV6-hSyn-eNpHR3.0-eYFP Product: eNpHR-eYFP | C-fiber unmyelinated primary afferent | intraneural | The plantar surface of the hindpaw Yellow light (490 nm) | Reduction of mechanical allodynia and thermal hyperalgesia | CCI | [89] | ||
| AAV8/CAG-ArchT-GFP In myelinated neurons. Product: ArchT-GFP | AHTMR-fiber myelinated primary afferent | intrathecal | The plantar surface of the hindpaw Green light | Increase in paw withdrawal threshold | SNL | [90] | ||
5. Long-Term Chemogenic Modulation of Pain
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mischkowski, D.; Palacios-Barrios, E.E.; Banker, L.; Dildine, T.C.; Atlas, L.Y. Pain or Nociception? Subjective Experience Mediates the Effects of Acute Noxious Heat on Autonomic Responses. Pain 2018, 159, 699. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yao, X.; Jiang, Y.; He, X.; Shao, X.; Du, J.; Shen, Z.; He, Q.; Fang, J. Pain Aversion and Anxiety-like Behavior Occur at Different Times during the Course of Chronic Inflammatory Pain in Rats. J. Pain Res. 2017, 10, 2585–2593. [Google Scholar] [CrossRef]
- Campbell, J.N.; Meyer, R.A. Review Mechanisms of Neuropathic Pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef]
- Chung, J.W.Y.; Wong, T.K.S.; Martinez, V.; Fletcher, D.; Edwards, R.R.; Sarlani, E.; Wesselmann, U.; Fillingim, R.B.; Tchicaya, A.; Lorentz, N.; et al. Neuropathic Pain: Redefinition and a Grading System for Clinical and Research Purposes. Pain 2015, 70, 1630–1635. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Ruscheweyh, R.; -Forsthuber, L.; Schoffnegger, D.; Sandkühler, J. Modification of Classical Neurochemical Markers in Identified Primary Afferent Neurons with Aβ-, Aδ-, and C-Fibers after Chronic Constriction Injury in Mice. J. Comp. Neurol. 2007, 502, 325–336. [Google Scholar] [CrossRef]
- Zhang, J.M.; Song, X.J.; LaMotte, R.H. An in Vitro Study of Ectopic Discharge Generation and Adrenergic Sensitivity in the Intact, Nerve-Injured Rat Dorsal Root Ganglion. Pain 1997, 72, 51–57. [Google Scholar] [CrossRef]
- Chiu, I.M.; Barrett, L.B.; Williams, E.K.; Strochlic, D.E.; Lee, S.; Weyer, A.D.; Lou, S.; Bryman, G.S.; Roberson, D.P.; Ghasemlou, N.; et al. Transcriptional Profiling at Whole Population and Single Cell Levels Reveals Somatosensory Neuron Molecular Diversity. Elife 2014, 3, e04660. [Google Scholar] [CrossRef] [PubMed]
- Medici, T.; Shortland, P.J. Effects of Peripheral Nerve Injury on Parvalbumin Expression in Adult Rat Dorsal Root Ganglion Neurons. BMC Neurosci. 2015, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Clark, T.E.; Wu, K.Y.; Thompson, J.A.; Yang, K.; Bahia, P.K.; Ajmo, J.M. Thy1.2 YFP-16 Transgenic Mouse Labels a Subset of Large-Diameter Sensory Neurons That Lack TRPV1 Expression. PLoS ONE 2015, 10, e0119538. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.; Decosterd, I.; Samad, T.A.; Plumpton, C.; Tate, S.; Mannion, R.J.; Costigan, M.; Woolf, C.J. Diversity of Expression of the Sensory Neuron-Specific TTX-Resistant Voltage-Gated Sodium Ion Channels SNS and SNS2. Mol. Cell. Neurosci. 2000, 15, 331–342. [Google Scholar] [CrossRef]
- Smith, T.; Al Otaibi, M.; Sathish, J.; Djouhri, L. Increased Expression of HCN2 Channel Protein in L4 Dorsal Root Ganglion Neurons Following Axotomy of L5- and Inflammation of L4-Spinal Nerves in Rats. Neuroscience 2015, 295, 90–102. [Google Scholar] [CrossRef]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lönnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggström, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased Classification of Sensory Neuron Types by Large-Scale Single-Cell RNA Sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef]
- Acosta, C.; Djouhri, L.; Watkins, R.; Berry, C.; Bromage, K.; Lawson, S.N. TREK2 Expressed Selectively in IB4-Binding C-Fiber Nociceptors Hyperpolarizes Their Membrane Potentials and Limits Spontaneous Pain. J. Neurosci. 2014, 34, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.; McMullan, S.; Djouhri, L.; Gao, L.; Watkins, R.; Berry, C.; Dempsey, K.; Lawson, S.N. HCN1 and HCN2 in Rat DRG Neurons: Levels in Nociceptors and Non-Nociceptors, NT3-Dependence and Influence of CFA-Induced Skin Inflammation on HCN2 and NT3 Expression. PLoS ONE 2012, 7, e50442. [Google Scholar] [CrossRef] [PubMed]
- Levanon, D.; Bettoun, D.; Harris-Cerruti, C.; Woolf, E.; Negreanu, V.; Eilam, R.; Bernstein, Y.; Goldenberg, D.; Xiao, C.; Fliegauf, M.; et al. The Runx3 Transcription Factor Regulates Development and Survival of TrkC Dorsal Root Ganglia Neurons. EMBO J. 2002, 21, 3454–3463. [Google Scholar] [CrossRef]
- de Nooij, J.C.; Doobar, S.; Jessell, T.M. Etv1 Inactivation Reveals Proprioceptor Subclasses That Reflect the Level of NT3 Expression in Muscle Targets. Neuron 2013, 77, 1055–1068. [Google Scholar] [CrossRef]
- Nakamura, S.; Senzaki, K.; Yoshikawa, M.; Nishimura, M.; Inoue, K.I.; Ito, Y.; Ozaki, S.; Shiga, T. Dynamic Regulation of the Expression of Neurotrophin Receptors by Runx3. Development 2008, 135, 1703–1711. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Senzaki, K.; Yokomizo, T.; Takahashi, S.; Ozaki, S.; Shiga, T. Runx1 Selectively Regulates Cell Fate Specification and Axonal Projections of Dorsal Root Ganglion Neurons. Dev. Biol. 2007, 303, 663–674. [Google Scholar] [CrossRef]
- Hu, J.; Huang, T.; Li, T.; Guo, Z.; Cheng, L. C-Maf Is Required for the Development of Dorsal Horn Laminae III/IV Neurons and Mechanoreceptive DRG Axon Projections. J. Neurosci. 2012, 32, 5362–5373. [Google Scholar] [CrossRef]
- Li, B.; Yang, X.Y.; Qian, F.P.; Tang, M.; Ma, C.; Chiang, L.Y. A Novel Analgesic Approach to Optogenetically and Specifically Inhibit Pain Transmission Using TRPV1 Promoter. Brain Res. 2015, 1609, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Costigan, M.; Woolf, C.J. Pain: Molecular Mechanisms. J. Pain 2000, 1, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Brumovsky, P.; Watanabe, M.; Hökfelt, T. Expression of the Vesicular Glutamate Transporters-1 and -2 in Adult Mouse Dorsal Root Ganglia and Spinal Cord and Their Regulation by Nerve Injury. Neuroscience 2007, 147, 469–490. [Google Scholar] [CrossRef]
- Lagerström, M.C.; Rogoz, K.; Abrahamsen, B.; Persson, E.; Reinius, B.; Nordenankar, K.; Ölund, C.; Smith, C.; Mendez, J.A.; Chen, Z.F.; et al. VGLUT2-Dependent Sensory Neurons in the TRPV1 Population Regulate Pain and Itch. Neuron 2010, 68, 529–542. [Google Scholar] [CrossRef]
- Beaudry, H.; Daou, I.; Ase, A.R.; Ribeiro-Da-Silva, A.; Séguela, P. Distinct Behavioral Responses Evoked by Selective Optogenetic Stimulation of the Major TRPV1+ and MrgD+ Subsets of C-Fibers. Pain 2017, 158, 2329–2339. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct Expression of TRPM8, TRPA1, and TRPV1 MRNAs in Rat Primary Afferent Neurons with Aδ/C-Fibers and Colocalization with Trk Receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.J.; Hovhannisyan, A.H.; Akopian, A.N. Characteristics of Sensory Neuronal Groups in CGRP-Cre-ER Reporter Mice: Comparison to Nav1.8-Cre, TRPV1-Cre and TRPV1-GFP Mouse Lines. PLoS ONE 2018, 13, e0198601. [Google Scholar] [CrossRef]
- Peirs, C.; Williams, S.P.G.; Zhao, X.; Walsh, C.E.; Gedeon, J.Y.; Cagle, N.E.; Goldring, A.C.; Hioki, H.; Liu, Z.; Marell, P.S.; et al. Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron 2015, 87, 797–812. [Google Scholar] [CrossRef]
- Scherrer, G.; Low, S.A.; Wang, X.; Zhang, J.; Yamanaka, H.; Urban, R.; Solorzano, C.; Harper, B.; Hnaskod, T.S.; Edwards, R.H.; et al. VGLUT2 Expression in Primary Afferent Neurons Is Essential for Normal Acute Pain and Injury-Induced Heat Hypersensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 22296–22301. [Google Scholar] [CrossRef]
- Scherrer, G.; Imamachi, N.; Cao, Y.Q.; Contet, C.; Mennicken, F.; O’Donnell, D.; Kieffer, B.L.; Basbaum, A.I. Dissociation of the Opioid Receptor Mechanisms That Control Mechanical and Heat Pain. Cell 2009, 137, 1148–1159. [Google Scholar] [CrossRef]
- Wright, D.E.; Snider, W.D. Neurotrophin Receptor MRNA Expression Defines Distinct Populations of Neurons in Rat Dorsal Root Ganglia. J. Comp. Neurol. 1995, 351, 329–338. [Google Scholar] [CrossRef]
- Duan, B.; Cheng, L.; Bourane, S.; Britz, O.; Padilla, C.; Garcia-Campmany, L.; Krashes, M.; Knowlton, W.; Velasquez, T.; Ren, X.; et al. Identification of Spinal Circuits Transmitting and Gating Mechanical Pain. Cell 2014, 159, 1417–1432. [Google Scholar] [CrossRef] [PubMed]
- Häring, M.; Zeisel, A.; Hochgerner, H.; Rinwa, P.; Jakobsson, J.E.T.; Lönnerberg, P.; La Manno, G.; Sharma, N.; Borgius, L.; Kiehn, O.; et al. Neuronal Atlas of the Dorsal Horn Defines Its Architecture and Links Sensory Input to Transcriptional Cell Types. Nat. Neurosci. 2018, 21, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Q.; Sun, Q.; Tu, H.-Y.; Wan, Y. Characteristics of HCN Channels and Their Participation in Neuropathic Pain. Neurochem. Res. 2008, 33, 1979–1989. [Google Scholar] [CrossRef]
- Li, L.; Rutlin, M.; Abraira, V.E.; Cassidy, C.; Kus, L.; Gong, S.; Jankowski, M.P.; Luo, W.; Heintz, N.; Koerber, H.R.; et al. The Functional Organization of Cutaneous Low-Threshold Mechanosensory Neurons. Cell 2011, 147, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Bardoni, R.; Tawfik, V.L.; Wang, D.; François, A.; Solorzano, C.; Shuster, S.A.; Choudhury, P.; Betelli, C.; Cassidy, C.; Smith, K.; et al. Delta Opioid Receptors Presynaptically Regulate Cutaneous Mechanosensory Neuron Input to the Spinal Cord Dorsal Horn. Neuron 2014, 81, 1443. [Google Scholar] [CrossRef]
- Heinke, B.; Gingl, E.; Sandkühler, J. Multiple Targets of μ-Opioid Receptor-Mediated Presynaptic Inhibition at Primary Afferent Aδ- and C-Fibers. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 1313–1322. [Google Scholar] [CrossRef]
- Daou, I.; Beaudry, H.; Ase, A.R.; Wieskopf, J.S.; Ribeiro-da-Silva, A.; Mogil, J.S.; Séguéla, P. Optogenetic Silencing of Nav1.8-Positive Afferents Alleviates Inflammatory and Neuropathic Pain. eNeuro 2016, 3, 702–705. [Google Scholar] [CrossRef]
- Takasaki, I.; Watanabe, A.; Yokai, M.; Watanabe, Y.; Hayakawa, D.; Nagashima, R.; Fukuchi, M.; Okada, T.; Toyooka, N.; Miyata, A.; et al. In Silico Screening Identified Novel Small-Molecule Antagonists of PAC1 Receptors. J. Pharmacol. Exp. Ther. 2018, 365, 1–8. [Google Scholar] [CrossRef]
- Abrahamsen, B.; Zhao, J.; Asante, C.O.; Cendan, C.M.; Marsh, S.; Martinez-Barbera, J.P.; Nassar, M.A.; Dickenson, A.H.; Wood, J.N. The Cell and Molecular Basis of Mechanical, Cold, and Inflammatory Pain. Science 2008, 321, 702–705. [Google Scholar] [CrossRef]
- Szabo, N.E.; da Silva, R.V.; Sotocinal, S.G.; Zeilhofer, H.U.; Mogil, J.S.; Kania, A. Hoxb8 Intersection Defines a Role for Lmx1b in Excitatory Dorsal Horn Neuron Development, Spinofugal Connectivity, and Nociception. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 5233–5246. [Google Scholar] [CrossRef] [PubMed]
- Wende, H.; Lechner, S.G.; Cheret, C.; Bourane, S.; Kolanczyk, M.E.; Pattyn, A.; Reuter, K.; Munier, F.L.; Carroll, P.; Lewin, G.R.; et al. The Transcription Factor C-Maf Controls Touch Receptor Development and Function. Science 2012, 335, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Brumovsky, P.; Stanic, D.; Shuster, S.; Herzog, H.; Villar, M.; Hökfelt, T. Neuropeptide Y2 Receptor Protein Is Present in Peptidergic and Nonpeptidergic Primary Sensory Neurons of the Mouse. J. Comp. Neurol. 2005, 489, 328–348. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.-X.; Zhang, K.-H.; Johnson, R.L.; Jacquin, M.F.; Chen, Z.-F. The Transcription Factor, Lmx1b, Promotes a Neuronal Glutamate Phenotype and Suppresses a GABA One in the Embryonic Trigeminal Brainstem Complex. Somatosens. Mot. Res. 2012, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lopes, C.; Wende, H.; Guo, Z.; Cheng, L.; Birchmeier, C.; Ma, Q. Ontogeny of Excitatory Spinal Neurons Processing Distinct Somatic Sensory Modalities. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 14738–14748. [Google Scholar] [CrossRef]
- Acton, D.; Ren, X.; Di Costanzo, S.; Dalet, A.; Bourane, S.; Bertocchi, I.; Eva, C.; Goulding, M. Spinal Neuropeptide Y1 Receptor-Expressing Neurons Form an Essential Excitatory Pathway for Mechanical Itch. Cell Rep. 2019, 28, 625–639.e6. [Google Scholar] [CrossRef] [PubMed]
- Draxler, P.; Honsek, S.D.; Forsthuber, L.; Hadschieff, V. VGluT3 ϩ Primary Afferents Play Distinct Roles in Mechanical and Cold Hypersensitivity Depending on Pain Etiology. J. Neurosci. 2014, 34, 12015–12028. [Google Scholar] [CrossRef]
- Duitama, M.; Vargas-López, V.; Casas, Z.; Albarracin, S.L.; Sutachan, J.-J.; Torres, Y.P. TRP Channels Role in Pain Associated With Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 782. [Google Scholar] [CrossRef]
- Collo, G.; North, R.A.; Kawashima, E.; Merlo-Pich, E.; Neidhart, S.; Surprenant, A.; Buell, G. Cloning OF P2X5 and P2X6 Receptors and the Distribution and Properties of an Extended Family of ATP-Gated Ion Channels. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 2495–2507. [Google Scholar] [CrossRef]
- Teixeira, J.M.; Bobinski, F.; Parada, C.A.; Sluka, K.A.; Tambeli, C.H. P2X3 and P2X2/3 Receptors Play a Crucial Role in Articular Hyperalgesia Development Through Inflammatory Mechanisms in the Knee Joint Experimental Synovitis. Mol. Neurobiol. 2017, 54, 6174–6186. [Google Scholar] [CrossRef]
- Rau, K.K.; Caudle, R.M.; Cooper, B.Y.; Johnson, R.D. Diverse Immunocytochemical Expression of Opioid Receptors in Electrophysiologically Defined Cells of Rat Dorsal Root Ganglia. J. Chem. Neuroanat. 2005, 29, 255–264. [Google Scholar] [CrossRef]
- Wang, D.; Tawfik, V.L.; Corder, G.; Low, S.A.; François, A.; Basbaum, A.I.; Scherrer, G. Functional Divergence of Delta and Mu Opioid Receptor Organization in CNS Pain Circuits. Neuron 2018, 98, 90–108.e5. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Dou, Y.-N.; Yuan, L.; Li, Q.; Zhu, Y.-J.; Wang, M.; Sun, Y.-G. Different Neuronal Populations Mediate Inflammatory Pain Analgesia by Exogenous and Endogenous Opioids. Elife 2020, 9, e55289. [Google Scholar] [CrossRef]
- Tamae, A.; Nakatsuka, T.; Koga, K.; Kato, G.; Furue, H.; Katafuchi, T.; Yoshimura, M. Direct Inhibition of Substantia Gelatinosa Neurones in the Rat Spinal Cord by Activation of Dopamine D2-like Receptors. J. Physiol. 2005, 568, 243–253. [Google Scholar] [CrossRef]
- Taniguchi, W.; Nakatsuka, T.; Miyazaki, N.; Yamada, H.; Takeda, D.; Fujita, T.; Kumamoto, E.; Yoshida, M. In Vivo Patch-Clamp Analysis of Dopaminergic Antinociceptive Actions on Substantia Gelatinosa Neurons in the Spinal Cord. Pain 2011, 152, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Doroshenko, M.; Lauzadis, J.; Kanjiya, M.P.; Rebecchi, M.J.; Kaczocha, M.; Puopolo, M. Presynaptic Inhibition of Primary Nociceptive Signals to Dorsal Horn Lamina I Neurons by Dopamine. J. Neurosci. 2018, 38, 8809–8821. [Google Scholar] [CrossRef] [PubMed]
- Almanza, A.; Simón-Arceo, K.; Coffeen, U.; Fuentes-García, R.; Contreras, B.; Pellicer, F.; Mercado, F. A D2-like Receptor Family Agonist Produces Analgesia in Mechanonociception but Not in Thermonociception at the Spinal Cord Level in Rats. Pharmacol. Biochem. Behav. 2015, 137, 119–125. [Google Scholar] [CrossRef]
- Mercado-Reyes, J.; Almanza, A.; Segura-Chama, P.; Pellicer, F.; Mercado, F. D2-like Receptor Agonist Synergizes the μ-Opioid Agonist Spinal Antinociception in Nociceptive, Inflammatory and Neuropathic Models of Pain in the Rat. Eur. J. Pharmacol. 2019, 853, 56–64. [Google Scholar] [CrossRef]
- Ohnou, T.; Yokai, M.; Kurihara, T.; Hasegawa-Moriyama, M.; Shimizu, T.; Inoue, K.; Kambe, Y.; Kanmura, Y.; Miyata, A. Pituitary Adenylate Cyclase-Activating Polypeptide Type 1 Receptor Signaling Evokes Long-Lasting Nociceptive Behaviors through the Activation of Spinal Astrocytes in Mice. J. Pharmacol. Sci. 2016, 130, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Yokai, M.; Kurihara, T.; Miyata, A. Spinal Astrocytic Activation Contributes to Both Induction and Maintenance of Pituitary Adenylate Cyclase-Activating Polypeptide Type 1 Receptor-Induced Long-Lasting Mechanical Allodynia in Mice. Mol. Pain 2016, 12, 1744806916646383. [Google Scholar] [CrossRef]
- Crawford, L.T.K.; Caterina, M.J. Functional Anatomy of the Sensory Nervous System: Updates From the Neuroscience Bench. Toxicol. Pathol. 2020, 48, 174–189. [Google Scholar] [CrossRef]
- Brumovsky, P.; Hygge-Blakeman, K.; Villar, M.J.; Watanabe, M.; Wiesenfeld-Hallin, Z.; Hökfelt, T. Phenotyping of Sensory and Sympathetic Ganglion Neurons of a Galanin-Overexpressing Mouse-Possible Implications for Pain Processing. J. Chem. Neuroanat. 2006, 31, 243–262. [Google Scholar] [CrossRef]
- François, A.; Schüetter, N.; Laffray, S.; Sanguesa, J.; Pizzoccaro, A.; Dubel, S.; Mantilleri, A.; Nargeot, J.; Noël, J.; Wood, J.N.; et al. The Low-Threshold Calcium Channel Cav3.2 Determines Low-Threshold Mechanoreceptor Function. Cell Rep. 2015, 10, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Seal, R.P. Do the Distinct Synaptic Properties of VGLUTs Shape Pain? Neurochem. Int. 2016, 98, 82–88. [Google Scholar] [CrossRef]
- Liu, X.J.; Salter, M.W. Glutamate Receptor Phosphorylation and Trafficking in Pain Plasticity in Spinal Cord Dorsal Horn. Eur. J. Neurosci. 2010, 32, 278–289. [Google Scholar] [CrossRef]
- Todd, A.J.; Hughes, D.I.; Polgár, E.; Nagy, G.G.; Mackie, M.; Ottersen, O.P.; Maxwell, D.J. The Expression of Vesicular Glutamate Transporters VGLUT1 and VGLUT2 in Neurochemically Defined Axonal Populations in the Rat Spinal Cord with Emphasis on the Dorsal Horn. Eur. J. Neurosci. 2003, 17, 13–27. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Villalba, R.M.; Zerda, R.; Schneider, S.P. Vesicular Glutamate Transporters in the Spinal Cord, with Special Reference to Sensory Primary Afferent Synapses. J. Comp. Neurol. 2004, 472, 257–280. [Google Scholar] [CrossRef]
- Larsson, M.; Broman, J. Synaptic Organization of VGLUT3 Expressing Low-Threshold Mechanosensitive C Fiber Terminals in the Rodent Spinal Cord. eNeuro 2019, 6, ENEURO.0007-19.2019. [Google Scholar] [CrossRef] [PubMed]
- Deuchars, S.A.; Milligan, C.J.; Stornetta, R.L.; Deuchars, J. GABAergic Neurons in the Central Region of the Spinal Cord: A Novel Substrate for Sympathetic Inhibition. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.A.M.; Boudreau, D.; Bachand, K.; Prescott, S.A.; Nault, F.; Sík, A.; De Koninck, P.; De Koninck, Y. Trans-Synaptic Shift in Anion Gradient in Spinal Lamina I Neurons as a Mechanism of Neuropathic Pain. Nature 2003, 424, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Zeilhofer, H.U.; Fritschy, J.-M. Selective Distribution of GABA(A) Receptor Subtypes in Mouse Spinal Dorsal Horn Neurons and Primary Afferents. J. Comp. Neurol. 2012, 520, 3895–3911. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.T.; Schmouth, J.F.; Lavastre, V.; Latremoliere, A.; Zhang, J.; Andrews, N.; Omura, T.; Laganière, J.; Rochefort, D.; Hince, P.; et al. Inhibition of the Kinase WNK1/HSN2 Ameliorates Neuropathic Pain by Restoring GABA Inhibition. Sci. Signal. 2016, 9, ra32. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Ahmed, M.M.; King, K.C.; Miranpuri, G.S.; Kahle, K.T.; Resnick, D.K.; Sun, D. Persistent Phosphorylation of NKCC1 and WNK1 in the Epicenter of the Spinal Cord Following Contusion Injury. Spine J. 2014, 14, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Tillman, L.; Zhang, J. Crossing the Chloride Channel: The Current and Potential Therapeutic Value of the Neuronal K(+)-Cl(-) Cotransporter KCC2. BioMed Res. Int. 2019, 2019, 8941046. [Google Scholar] [CrossRef]
- Deisseroth, K.; Feng, G.; Majewska, A.K.; Miesenböck, G.; Ting, A.; Schnitzer, M.J. Next-Generation Optical Technologies for Illuminating Genetically Targeted Brain Circuits. J. Neurosci. 2006, 26, 10380–10386. [Google Scholar] [CrossRef]
- Deisseroth, K.; Hegemann, P. The Form and Function of Channelrhodopsin. Science 2017, 357, eaan5544. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-Timescale, Genetically Targeted Optical Control of Neural Activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Chow, B.Y.; Han, X.; Dobry, A.S.; Qian, X.; Chuong, A.S.; Li, M.; Henninger, M.A.; Belfort, G.M.; Lin, Y.; Monahan, P.E.; et al. High-Performance Genetically Targetable Optical Neural Silencing by Light-Driven Proton Pumps. Nature 2010, 463, 98–102. [Google Scholar] [CrossRef]
- Han, X.; Chow, B.Y.; Zhou, H.; Klapoetke, N.C.; Chuong, A.; Rajimehr, R.; Yang, A.; Baratta, M.V.; Winkle, J.; Desimone, R.; et al. A High-Light Sensitivity Optical Neural Silencer: Development and Application to Optogenetic Control of Non-Human Primate Cortex. Front. Syst. Neurosci. 2011, 5, 18. [Google Scholar] [CrossRef]
- Watakabe, A.; Sadakane, O.; Hata, K.; Ohtsuka, M.; Takaji, M.; Yamamori, T. Application of Viral Vectors to the Study of Neural Connectivities and Neural Circuits in the Marmoset Brain. Dev. Neurobiol. 2017, 77, 354–372. [Google Scholar] [CrossRef] [PubMed]
- Anastassiadis, K.; Fu, J.; Patsch, C.; Hu, S.; Weidlich, S.; Duerschke, K.; Buchholz, F.; Edenhofer, F.; Stewart, A.F. Dre Recombinase, like Cre, Is a Highly Efficient Site-Specific Recombinase in E. coli, Mammalian Cells and Mice. Dis. Model. Mech. 2009, 2, 508–515. [Google Scholar] [CrossRef]
- Daou, I.; Tuttle, A.H.; Longo, G.; Wieskopf, J.S.; Bonin, R.P.; Ase, A.R.; Wood, J.N.; De Koninck, Y.; Ribeiro-da-silva, A.; Mogil, J.S.; et al. Remote Optogenetic Activation and Sensitization of Pain Pathways in Freely Moving Mice. J. Neurosci. 2013, 33, 18631–18640. [Google Scholar] [CrossRef] [PubMed]
- Michoud, F.; Seehus, C.; Schönle, P.; Brun, N.; Taub, D.; Zhang, Z.; Jain, A.; Furfaro, I.; Akouissi, O.; Moon, R.; et al. Epineural Optogenetic Activation of Nociceptors Initiates and Amplifies Inflammation. Nat. Biotechnol. 2021, 39, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, S.R.; Ma, H.; Chen, H.; Hittelman, W.N.; Pan, H.L. Regulating Nociceptive Transmission by VGluT2-Expressing Spinal Dorsal Horn Neurons. J. Neurochem. 2018, 147, 526–540. [Google Scholar] [CrossRef]
- Tashima, R.; Koga, K.; Sekine, M.; Kanehisa, K.; Kohro, Y.; Tominaga, K.; Matsushita, K.; Tozaki-Saitoh, H.; Fukazawa, Y.; Inoue, K.; et al. Optogenetic Activation of Non-Nociceptive Aβ Fibers Induces Neuropathic Pain-like Sensory and Emotional Behaviors after Nerve Injury in Rats. eNeuro 2018, 5, ENEURO.0450-17.2018. [Google Scholar] [CrossRef]
- Cavanaugh, D.J.; Lee, H.; Lo, L.; Shields, S.D.; Zylka, M.J.; Basbaum, A.I.; Anderson, D.J. Distinct Subsets of Unmyelinated Primary Sensory Fibers Mediate Behavioral Responses to Noxious Thermal and Mechanical Stimuli. Proc. Natl. Acad. Sci. USA 2009, 106, 9075–9080. [Google Scholar] [CrossRef]
- Iyer, S.M.; Vesuna, S.; Ramakrishnan, C.; Huynh, K.; Young, S.; Berndt, A.; Lee, S.Y.; Gorini, C.J.; Deisseroth, K.; Delp, S.L. Optogenetic and Chemogenetic Strategies for Sustained Inhibition of Pain. Sci. Rep. 2016, 6, 30570. [Google Scholar] [CrossRef]
- Iyer, S.M.; Montgomery, K.L.; Towne, C.; Lee, S.Y.; Ramakrishnan, C.; Deisseroth, K.; Delp, S.L. Virally Mediated Optogenetic Excitation and Inhibition of Pain in Freely Moving Nontransgenic Mice. Nat. Biotechnol. 2014, 32, 274–278. [Google Scholar] [CrossRef]
- Boada, M.D.; Martin, T.J.; Peters, C.M.; Hayashida, K.; Harris, M.H.; Houle, T.T.; Boyden, E.S.; Eisenach, J.C.; Ririe, D.G. Fast-Conducting Mechanoreceptors Contribute to Withdrawal Behavior in Normal and Nerve Injured Rats. Pain 2014, 155, 2646–2655. [Google Scholar] [CrossRef]
- Armbruster, B.N.; Li, X.; Pausch, M.H.; Herlitze, S.; Roth, B.L. Evolving the Lock to Fit the Key to Create a Family of G Protein-Coupled Receptors Potently Activated by an Inert Ligand. Proc. Natl. Acad. Sci. USA 2007, 104, 5163–5168. [Google Scholar] [CrossRef] [PubMed]
- Whissell, P.D.; Tohyama, S.; Martin, L.J. The Use of DREADDs to Deconstruct Behavior. Front. Genet. 2016, 7, 70. [Google Scholar] [CrossRef]
- Hachisuka, J.; Baumbauer, K.M.; Omori, Y.; Snyder, L.M.; Koerber, H.R.; Ross, S.E. Semi-Intact Ex Vivo Approach to Investigate Spinal Somatosensory Circuits. Elife 2016, 5, e22866. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D.; Mata, M.; Fink, D.J. A Human Trial of HSV-Mediated Gene Transfer for the Treatment of Chronic Pain. Gene Ther. 2009, 16, 455–460. [Google Scholar] [CrossRef]
- Fink, D.J.; Wechuck, J.; Mata, M.; Glorioso, J.C.; Goss, J.; Krisky, D.; Wolfe, D. Gene Therapy for Pain: Results of a Phase I Clinical Trial. Ann. Neurol. 2011, 70, 207–212. [Google Scholar] [CrossRef] [PubMed]

| Specificity Strategy | Construct | Fiber and Cell Type | Injection Site | Place of Stimulation | Behavioral Phenotype | Painful Condition | Reference |
|---|---|---|---|---|---|---|---|
| Cre dependent | Nav1.8: Cre/Ai32 (carry the ChR2(H134R)–EYFP in Gt(ROSA)26Sor locus) Product: Nav1.8–ChR2+ | Aβ Fiber Aδ Fiber C-fibers: -Peptidergic and -nonpeptidergic | NA | The plantar surface of the hindpaw blue light (473 nm) | Paw withdrawal and paw licking | Not sensitized | [83] |
| TRPV1: Cre/AAV5-TRPV1-ArchT-eGFP Product: TRPV1-Arch+ | C-fiber Peptidergic | DRG injection | The plantar surface of the hindpaw Green light (532 nm) | Increases in mechanical withdrawal threshold and thermal latency | [22] | ||
| TRPV1: Cre/AAV2/8-CAG-floxed stop-ChR2[H134R]-tdTomato-WPRE Product: TRPV1-ChR2 | C-fiber Peptidergic | Intrathecal | The plantar surface of the hindpaw Blue light (473 nm) | Increases in paw withdrawal, paw lifting and paw licking | [26] | ||
| TRPV1: Cre/Ai27D (carry Rosa-CAG-LSL-hChR2(H134R)-tdTomato-WPRE) Product: TRPV1-ChR2 | C-fiber Peptidergic | NA | Epineural in sciatic nerve Blue light (470 nm) | Thermal and mechanical sensitivity | [84] | ||
| MrgD: CreERT2/Ai32 (carry in the ROSA26 locus the floxed stop-ChR2(H134R)-EYFP) Product: MrgD-ChR2 (Opsin induced by tamoxiofen) | C-fiber Nonpeptidergic | NA | The plantar surface of the hindpaw Blue light (473 nm) | Paw withdrawal and lifting | [26] | ||
| VGluT2: Cre/AAV8-hSyn-DIO-hM3Dq-mCherry Product: VGluT2- hM3D-Gq | C-fiber: -Peptidergic and –nonpeptidergic (VGluT2-dorsal horn) | Dorsal horn of the spinal cord | clozapine N-oxide intraperitoneally | Increases in mechanical and thermal sensitivities | [85] |
| Specificity Strategy | Construct | Fiber and Cell Type | Injection Site | Place of Stimulation | Behavioral Phenotype | Painful Condition | Reference | |
|---|---|---|---|---|---|---|---|---|
| Cre-dependent | VGluT3: Cre/Ai32 carry ChR2 Product: VGluT3-ChR2 | LTMR Fiber: VGluT3+ | NA | The plantar surface of the hindpaw Blue light (470 nm) | Elicit nociceptive behavior | sensitized | Oxaliplatin-induced neuropathy | [48] |
| Nav1.8: Cre/Ai35 (Carry the floxed stop-Arch-EGFP gene in the ROSA26 locus) Product: Nav1.8-Arch+ | C-fiber Peptidergic | NA | The plantar surface of the hindpaw Yellow light | Decrease in mechanical allodynia | Spared nerve injury | [39] | ||
| VGluT2: Cre/AAV8-hSyn-DIO-HA-KORD-IRES-mCitrine Product: VGluT2-KORD-Gi | Peptidergic and nonpeptidergic (VGluT2-dorsal horn) | Dorsal horn of the spinal cord | Salvinorin B intraperitoneal | Decrease in tactile allodynia/ Decrease in pain hypersensibility | SNI/CFA | [85] | ||
| Thy1-COP4/YFP/W-TChR2V4 | Aβ fibers | NA | The plantar surface of the hindpaw Yellow light | Lifting and flinching behaviors | PNI | [86] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Juárez, J.V.; Chiquete, E.; Estañol, B.; Aceves, J.d.J. Optogenetic and Chemogenic Control of Pain Signaling: Molecular Markers. Int. J. Mol. Sci. 2023, 24, 10220. https://doi.org/10.3390/ijms241210220
Espinosa-Juárez JV, Chiquete E, Estañol B, Aceves JdJ. Optogenetic and Chemogenic Control of Pain Signaling: Molecular Markers. International Journal of Molecular Sciences. 2023; 24(12):10220. https://doi.org/10.3390/ijms241210220
Chicago/Turabian StyleEspinosa-Juárez, Josue Vidal, Erwin Chiquete, Bruno Estañol, and José de Jesús Aceves. 2023. "Optogenetic and Chemogenic Control of Pain Signaling: Molecular Markers" International Journal of Molecular Sciences 24, no. 12: 10220. https://doi.org/10.3390/ijms241210220
APA StyleEspinosa-Juárez, J. V., Chiquete, E., Estañol, B., & Aceves, J. d. J. (2023). Optogenetic and Chemogenic Control of Pain Signaling: Molecular Markers. International Journal of Molecular Sciences, 24(12), 10220. https://doi.org/10.3390/ijms241210220






