In Silico and In Vitro Identification of P-Glycoprotein Inhibitors from a Library of 375 Phytochemicals
Abstract
:1. Introduction
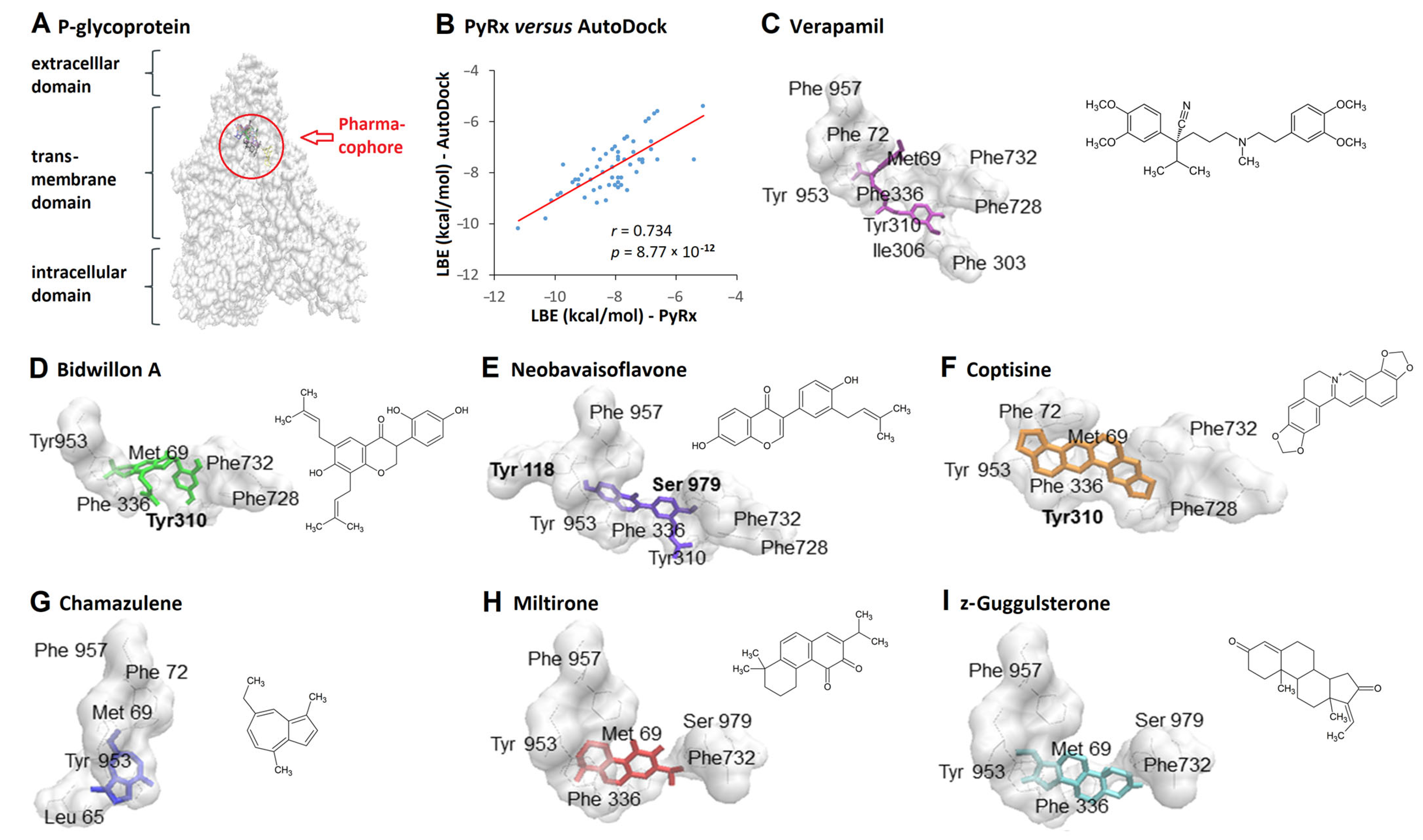
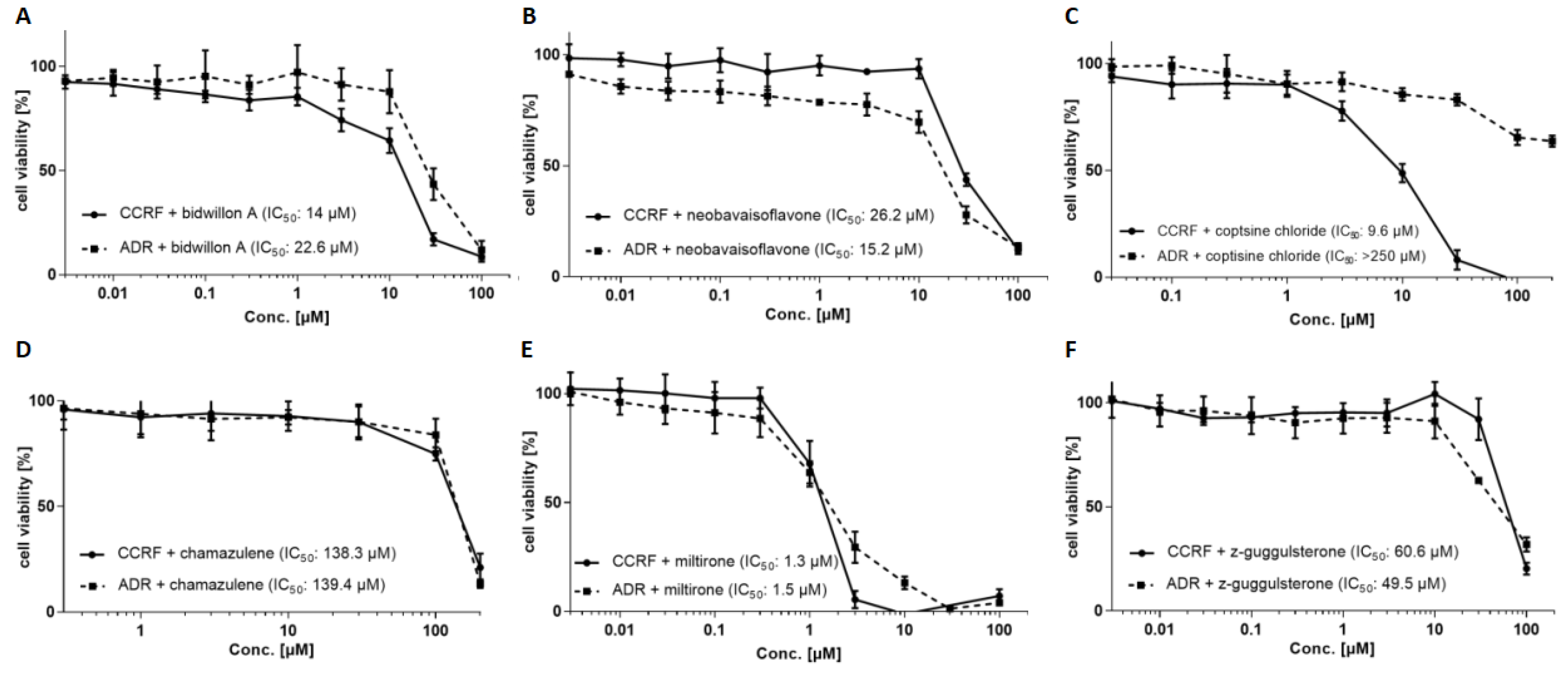
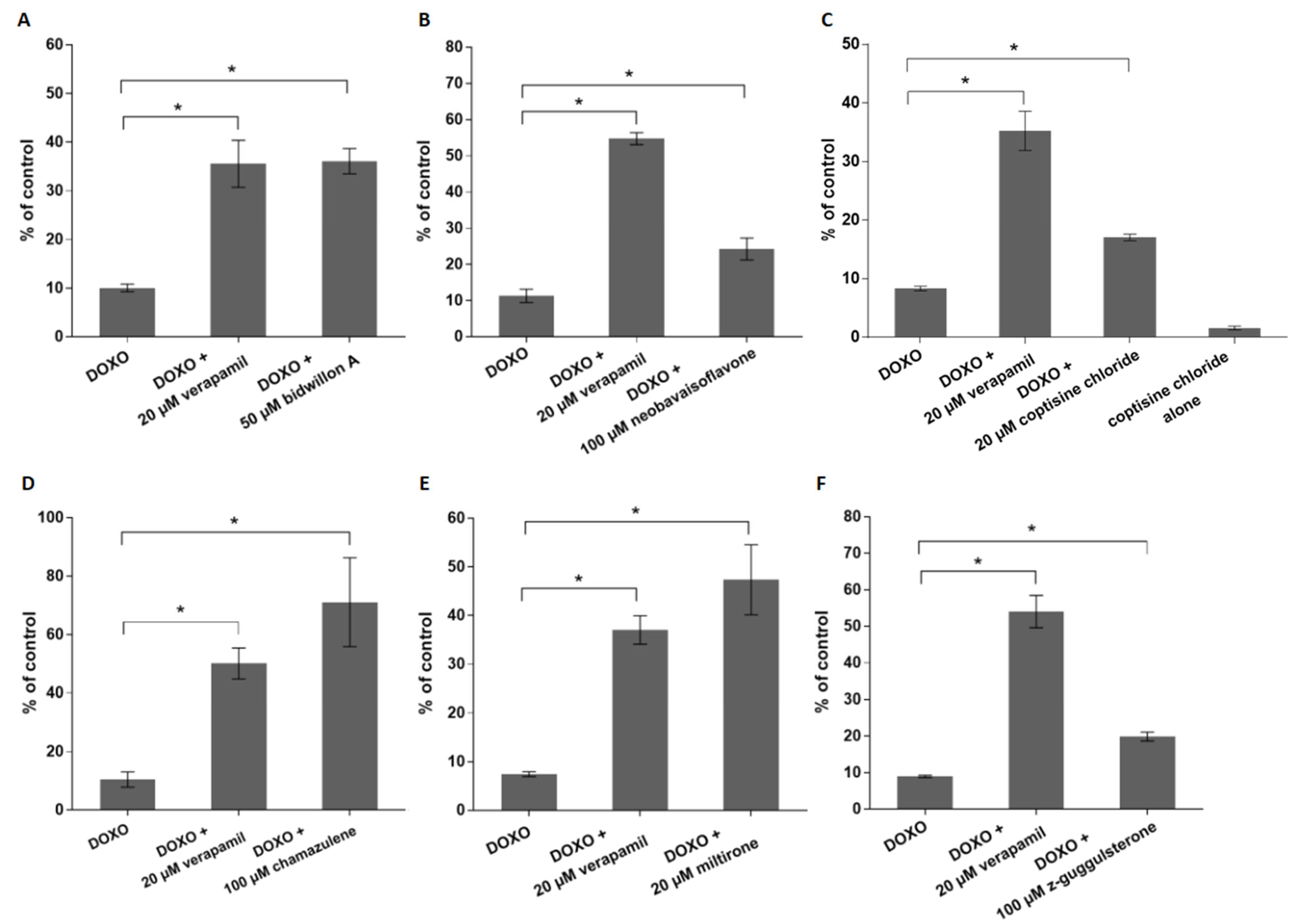
2. Results
3. Discussion
4. Material and Methods
4.1. Virtual Screening and Molecular Docking
4.2. Torch3D Screening
4.3. Toxicity Prediction
4.4. Cell Culture
4.5. Resazurin Cell Viability Assay
4.6. Flow Cytometry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, X.; Yu, H. Global burden of cancer. Yale J. Biol. Med. 2006, 79, 85–94. [Google Scholar] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volm, M.; Efferth, T. Prediction of cancer drug resistance and implications for personalized medicine. Front. Oncol. 2015, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Vagner, S.; Robert, C. Persistent cancer cells: The deadly survivors. Cell 2020, 183, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Pich, O.; Bailey, C.; Watkins, T.B.K.; Zaccaria, S.; Jamal-Hanjani, M.; Swanton, C. The translational challenges of precision oncology. Cancer Cell 2022, 40, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, W.K.; Trumpp, A.; Müller-Tidow, C. Therapy resistance mechanisms in hematological malignancies. Int. J. Cancer 2023, 152, 340–347. [Google Scholar] [CrossRef]
- Efferth, T.; Grassmann, R. Impact of viral oncogenesis on responses to anti-cancer drugs and irradiation. Crit. Rev. Oncog. 2000, 11, 165–187. [Google Scholar]
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.P. Toward a better understanding of the complexity of cancer drug resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Ling, V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett. 2006, 580, 998–1009. [Google Scholar] [CrossRef] [Green Version]
- Efferth, T.; Volm, M. Multiple resistance to carcinogens and xenobiotics: P-glycoproteins as universal detoxifiers. Arch. Toxicol. 2017, 91, 2515–2538. [Google Scholar] [CrossRef]
- Engle, K.; Kumar, G. Cancer multidrug-resistance reversal by ABCB1 inhibition: A recent update. Eur. J. Med. Chem. 2022, 239, 114542. [Google Scholar] [CrossRef]
- Tamaki, A.; Ierano, C.; Szakacs, G.; Robey, R.W.; Bates, S.E. The controversial role of ABC transporters in clinical oncology. Essays Biochem. 2011, 50, 209–232. [Google Scholar] [CrossRef]
- Amiri-Kordestani, L.; Basseville, A.; Kurdziel, K.; Fojo, A.T.; Bates, S.E. Targeting MDR in breast and lung cancer: Discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist. Updat. 2012, 15, 50–61. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023. Available online: https://www.who.int/publications/i/item/9789241506096 (accessed on 13 May 2023).
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl. 1), 69–75. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Gullett, N.P.; Ruhul Amin, A.R.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.J.; Kucuk, O. Cancer prevention with natural compounds. Semin. Oncol. 2010, 37, 258–281. [Google Scholar] [CrossRef]
- Poswal, F.S.; Russell, G.; Mackonochie, M.; MacLennan, E.; Adukwu, E.C.; Rolfe, V. Herbal teas and their health benefits: A scoping review. Plant Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef]
- Yuan, R.; Hou, Y.; Sun, W.; Yu, J.; Liu, X.; Niu, Y.; Lu, J.J.; Chen, X. Natural products to prevent drug resistance in cancer chemotherapy: A review. Ann. N. Y. Acad. Sci. 2017, 1401, 19–27. [Google Scholar] [CrossRef]
- Efferth, T.; Kadioglu, O.; Saeed, M.E.M.; Seo, E.-J.; Mbaveng, A.T.; Kuete, V. Medicinal plants and phytochemicals against multidrug-resistant tumor cells expressing ABCB1, ABCG2, or ABCB5: A synopsis of 2 decades. Phytochem. Rev. 2021, 20, 7–53. [Google Scholar] [CrossRef]
- Ramirez, D. Computational methods applied to rational drug design. Open Med. Chem. J. 2016, 10, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Dar, K.B.; Bhat, A.H.; Amin, S.; Hamid, R.; Anees, S.; Anjum, S.; Reshi, B.A.; Zargar, M.A.; Masood, A.; Ganie, S.A. Modern computational strategies for designing drugs to curb human diseases: A prospect. Curr. Top. Med. Chem. 2018, 18, 2702–2719. [Google Scholar] [CrossRef] [PubMed]
- Nayarisseri, A. Experimental and computational approaches to improve binding affinity in chemical biology and drug discovery. Curr. Top. Med. Chem. 2020, 20, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Macalino, S.J.; Gosu, V.; Hong, S.; Choi, S. Role of computer-aided drug design in modern drug discovery. Arch. Pharm. Res. 2015, 38, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Malathi, K.; Ramaiah, S. Bioinformatics approaches for new drug discovery: A review. Biotechnol. Genet. Eng. Rev. 2018, 34, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Saeed, M.E.M.; Munder, M.; Spuller, A.; Greten, H.J.; Efferth, T. Effect of ABC transporter expression and mutational status on survival rates of cancer patients. Biomed. Pharmacother. 2020, 131, 110718. [Google Scholar] [CrossRef]
- Pluchino, K.M.; Hall, M.D.; Goldsborough, A.S.; Callaghan, R.; Gottesman, M.M. Collateral sensitivity as a strategy against cancer multidrug resistance. Drug Resist. Updat. 2012, 15, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Joshi, P.; Vishwakarma, R.A.; Bharate, S.B. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur. J. Med. Chem. 2017, 138, 273–292. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef]
- Efferth, T.; Saeed, M.E.M.; Kadioglu, O.; Seo, E.J.; Shirooie, S.; Mbaveng, A.T.; Nabavi, S.M.; Kuete, V. Collateral sensitivity of natural products in drug-resistant cancer cells. Biotechnol. Adv. 2020, 38, 107342. [Google Scholar] [CrossRef]
- Zhu, X.; Sui, M.; Fan, W. In vitro and in vivo characterizations of tetrandrine on the reversal of P-glycoprotein-mediated drug resistance to paclitaxel. Anticancer Res. 2005, 25, 1953–1962. [Google Scholar]
- Huang, M.; Jin, J.; Sun, H.; Liu, G.T. Reversal of P-glycoprotein-mediated multidrug resistance of cancer cells by five schizandrins isolated from the Chinese herb Fructus Schizandrae. Cancer Chemother. Pharmacol. 2008, 62, 1015–1026. [Google Scholar] [CrossRef]
- Sun, W.; Wong, I.L.K.; Law, H.K.; Su, X.; Chan, T.C.F.; Sun, G.; Yang, X.; Wang, X.; Chan, T.H.; Wan, S.; et al. In vivo reversal of P-glycoprotein-mediated drug resistance in a breast cancer xenograft and in leukemia models using a novel, potent, and nontoxic epicatechin EC31. Int. J. Mol. Sci. 2023, 24, 4377. [Google Scholar] [CrossRef]
- Zeino, M.; Saeed, M.E.; Kadioglu, O.; Efferth, T. The ability of molecular docking to unravel the controversy and challenges related to P-glycoprotein—A well-known, yet poorly understood drug transporter. Investig. New Drugs 2014, 32, 618–625. [Google Scholar] [CrossRef]
- López-Vallejo, F.; Caulfield, T.; Martínez-Mayorga, K.; Giulianotti, M.A.; Nefzi, A.; Houghten, R.A.; Medina-Franco, J.L. Integrating virtual screening and combinatorial chemistry for accelerated drug discovery. Comb. Chem. High Throughput Screen 2011, 14, 475–487. [Google Scholar] [CrossRef]
- Kadioglu, O.; Cao, J.; Kosyakova, N.; Mrasek, K.; Liehr, T.; Efferth, T. Genomic and transcriptomic profiling of resistant CEM/ADR-5000 and sensitive CCRF-CEM leukaemia cells for unravelling the full complexity of multi-factorial multidrug resistance. Sci. Rep. 2016, 6, 36754. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Tanaka, H.; Yamaguchi, R.; Kato, K.; Etoh, H. Synergistic effects of mupirocin and an isoflavanone isolated from Erythrina variegata on growth and recovery of methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2004, 24, 241–246. [Google Scholar] [CrossRef]
- Djeussi, D.E.; Sandjo, L.P.; Noumedem, J.A.; Omosa, L.K.; Ngadjui, B.T.; Kuete, V. Antibacterial activities of the methanol extracts and compounds from Erythrina sigmoidea against Gram-negative multi-drug resistant phenotypes. BMC Complement. Altern. Med. 2015, 15, 453. [Google Scholar] [CrossRef] [Green Version]
- Cottiglia, F.; Casu, L.; Bonsignore, L.; Casu, M.; Floris, C.; Leonti, M.; Gertsch, J.; Heilmann, J. New cytotoxic prenylated isoflavonoids from Bituminaria morisiana. Planta Med. 2005, 71, 254–260. [Google Scholar] [CrossRef]
- Kuete, V.; Sandjo, L.P.; Djeussi, D.E.; Zeino, M.; Kwamou, G.M.; Ngadjui, B.; Efferth, T. Cytotoxic flavonoids and isoflavonoids from Erythrina sigmoidea towards multi-factorial drug resistant cancer cells. Invest. New Drugs 2014, 32, 1053–1062. [Google Scholar] [CrossRef]
- Zhou, Y.T.; Zhu, L.; Yuan, Y.; Ling, S.; Xu, J.W. Effects and mechanisms of five Psoralea prenylflavonoids on aging-related diseases. Oxid. Med. Cell Longev. 2020, 2020, 2128513. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Skaba, D.; Czuba, Z.P.; Krol, W. Inhibition of inflammatory mediators by neobavaisoflavone in activated RAW264.7 macrophages. Molecules 2011, 16, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, T.; Ren, M.; Wang, X. Neobavaisoflavone improves medial collateral ligament-induced osteoarthritis through repressing the nuclear factor-κB/hypoxia-inducible factor-2α axis. J. Physiol. Pharmacol. 2022, 73, 645–657. [Google Scholar] [CrossRef]
- Liang, R.; Yuan, Y.; Bai, Y.; Liu, X.; Chen, J.; Jiang, D.; Meng, D.; Chen, G.; Li, B.; Zhou, L.; et al. Neobavaisoflavone inhibits allergic inflammatory responses by suppressing mast cell activation. Int. Immunopharmacol. 2022, 110, 108953. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, W.I.; Ko, H.; So, Y.; Kang, K.S.; Kim, I.; Kim, K.; Yoon, H.G.; Kim, T.J.; Choi, K.C. Neobavaisoflavone sensitizes apoptosis via the inhibition of metastasis in TRAIL-resistant human glioma U373MG cells. Life Sci. 2014, 95, 101–107. [Google Scholar] [CrossRef]
- Ye, H.; He, X.; Feng, X. Developing neobavaisoflavone nanoemulsion suppresses lung cancer progression by regulating tumor microenvironment. Biomed. Pharmacother. 2020, 129, 110369. [Google Scholar] [CrossRef]
- Guo, J.; Shen, Y.; Hu, S.; Rui, T.; Liu, J.; Yuan, Y. Neobavaisoflavone inhibits antitumor immunosuppression via myeloid-derived suppressor cells. Int. Immunopharmacol. 2022, 111, 109103. [Google Scholar] [CrossRef]
- Li, L.; Dong, F.; Wang, B.; Song, J.; Zhang, H.; Wang, P.; Wang, F.; Yan, Y.; Zhang, X. Metabolites identification and mechanism prediction of neobavaisoflavone in vitro and in vivo of rats through UHPLC-Q-exactive plus orbitrap MS integrated network pharmacology. Molecules 2022, 27, 8413. [Google Scholar] [CrossRef]
- Wu, J.; Luo, Y.; Deng, D.; Su, S.; Li, S.; Xiang, L.; Hu, Y.; Wang, P.; Meng, X. Coptisine from Coptis chinensis exerts diverse beneficial properties: A concise review. J. Cell Mol. Med. 2019, 23, 7946–7960. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Hu, L.; Liao, W.; Yin, D.; Rui, F. Coptisine prevented IL-β-induced expression of inflammatory mediators in chondrocytes. Inflammation 2016, 39, 1558–1565. [Google Scholar] [CrossRef]
- Huang, T.; Xiao, Y.; Yi, L.; Li, L.; Wang, M.; Tian, C.; Ma, H.; He, K.; Wang, Y.; Han, B.; et al. Coptisine from Rhizoma Coptidis suppresses HCT-116 cells-related tumor growth in vitro and in vivo. Sci. Rep. 2017, 7, 38524. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Jiang, P.; Li, Z.; Yu, Y.; Huang, T.; Ye, X.; Li, X. Coptisine-induced apoptosis in human colon cancer cells (HCT-116) is mediated by PI3K/Akt and mitochondrial-associated apoptotic pathway. Phytomedicine 2018, 48, 152–160. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A review of ethnomedicinal use, phytochemistry and pharmacological uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Safayhi, H.; Sabieraj, J.; Sailer, E.R.; Ammon, H.P. Chamazulene: An antioxidant-type inhibitor of leukotriene B4 formation. Planta Med. 1994, 60, 410–413. [Google Scholar] [CrossRef]
- Russo, A.; Bruno, M.; Avola, R.; Cardile, V.; Rigano, D. Chamazulene-rich Artemisia arborescens essential oils affect the cell growth of human melanoma cells. Plants 2020, 9, 1000. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, L.; Xu, M.; Liu, Q.; Gao, N.; Li, P.; Liu, E.H. Miltirone exhibits antileukemic activity by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction pathways. Sci. Rep. 2016, 6, 20585. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wang, Y.; Lee, W.Y.; Or, P.M.; Wan, D.C.; Kwan, Y.W.; Yeung, J.H. Miltirone is a dual inhibitor of P-glycoprotein and cell growth in doxorubicin-resistant HepG2 cells. J. Nat. Prod. 2015, 78, 2266–2275. [Google Scholar] [CrossRef]
- Yamada, T.; Sugimoto, K. Guggulsterone and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 329–361. [Google Scholar] [CrossRef]
- Urizar, N.L.; Liverman, A.B.; Dodds, D.T.; Silva, F.V.; Ordentlich, P.; Yan, Y.; Gonzalez, F.J.; Heyman, R.A.; Mangelsdorf, D.J.; Moore, D.D. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 2002, 296, 1703–1706. [Google Scholar] [CrossRef]
- Xiao, D.; Singh, S.V. z-Guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, inhibits angiogenesis in vitro and in vivo. Mol. Cancer Ther. 2008, 7, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.B.; Chen, X.Z.; Yu, Z.L.; Xue, F. Guggulsterone from Commiphora mukul potentiates anti-glioblastoma efficacy of temozolomide in vitro and in vivo via down-regulating EGFR/PI3K/Akt signaling and NF-κB activation. J. Ethnopharmacol. 2023, 301, 115855. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.B.; Shen, Z.L.; Fu, J.; Xu, L.Z. Reversal of doxorubicin resistance by guggulsterone of Commiphora mukul in vivo. Phytomedicine 2014, 21, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- cBioportal for Cancer Genomics. Available online: https://www.cbioportal.org (accessed on 17 May 2023).
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. Suppressor mutations in the transmembrane segments of P-glycoprotein promote maturation of processing mutants and disrupt a subset of drug-binding sites. J. Biol. Chem. 2007, 282, 32043–32052. [Google Scholar] [CrossRef] [Green Version]
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. Arginines in the first transmembrane segment promote maturation of a P-glycoprotein processing mutant by hydrogen bond interactions with tyrosines in transmembrane segment 11. J. Biol. Chem. 2008, 283, 24860–24870. [Google Scholar] [CrossRef] [Green Version]
- Chufan, E.E.; Kapoor, K.; Ambudkar, S.V. Drug-protein hydrogen bonds govern the inhibition of the ATP hydrolysis of the multidrug transporter P-glycoprotein. Biochem. Pharmacol. 2016, 101, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Chufan, E.E.; Kapoor, K.; Sim, H.M.; Singh, S.; Talele, T.T.; Durell, S.R.; Ambudkar, S.V. Multiple transport-active binding sites are available for a single substrate on human P-glycoprotein (ABCB1). PLoS ONE 2013, 8, e82463. [Google Scholar] [CrossRef] [Green Version]
- Cosconati, S.; Forli, S.; Perryman, A.L.; Harris, R.; Goodsell, D.S.; Olson, A.J. Virtual screening with AutoDock: Theory and practice. Expert Opin. Drug Discov. 2010, 5, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Robert, J.; Jarry, C. Multidrug resistance reversal agents. J. Med. Chem. 2003, 46, 4805–4817. [Google Scholar] [CrossRef]
- Wen, P.C.; Verhalen, B.; Wilkens, S.; Mchaourab, H.S.; Tajkhorshid, E. On the origin of large flexibility of P-glycoprotein in the inward-facing state. J. Biol. Chem. 2013, 288, 19211–19220. [Google Scholar] [CrossRef] [Green Version]
- Ho, B.K.; Brasseur, R. The Ramachandran plots of glycine and pre-proline. BMC Struct. Biol. 2005, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- PyRx–Virtual Screening Tool. Available online: https://sourceforge.net/projects/pyrx/ (accessed on 17 May 2023).
- Ma, Z.; Zhang, M.; Song, Z. Characterization of tanshinones with quinone reductase induction activity from Radix Salvia miltiorrhiza by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2857–2866. [Google Scholar] [CrossRef]
- Burmaoğlu, S.; Seçinti, H.; Mozioğlu, E.; Gören, A.C.; Altundaş, R.; Seçen, H. Syntheses and evaluation of multicaulin and miltirone-like compounds as antituberculosis agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Binder, R.J.; Hatfield, M.J.; Chi, L.; Potter, P.M. Facile synthesis of 1,2-dione-containing abietane analogues for the generation of human carboxylesterase inhibitors. Eur. J. Med. Chem. 2018, 149, 79–89. [Google Scholar] [CrossRef]
- Li, J.; Jaimes, K.F.; Aller, S.G. Refined structures of mouse P-glycoprotein. Protein Sci. 2014, 23, 34–46. [Google Scholar] [CrossRef]
- Tajima, Y.; Nakagawa, H.; Tamura, A.; Kadioglu, O.; Satake, K.; Mitani, Y.; Murase, H.; Regasini, L.O.; Bolzani, V.d.S.; Ishikawa, T.; et al. Nitensidine A, a guanidine alkaloid from Pterogyne nitens, is a novel substrate for human ABC transporter ABCB1. Phytomedicine 2014, 21, 323–332. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.W.; Lindstrom, W.; Olson, A.J.; Belew, R.K. Analysis of HIV wild-type and mutant structures via in silico docking against diverse ligand libraries. J. Chem. Inform. Model. 2007, 47, 1258–1262. [Google Scholar] [CrossRef] [Green Version]
- Segall, M.D.; Barber, C. Addressing toxicity risk when designing and selecting compounds in early drug discovery. Drug Discov. Today 2014, 19, 688–993. [Google Scholar] [CrossRef]
- Kimmig, A.; Gekeler, V.; Neumann, M.; Frese, G.; Handgretinger, R.; Kardos, G.; Diddens, H.; Niethammer, D. Susceptibility of multidrug-resistant human leukemia cell lines to human interleukin 2-activated killer cells. Cancer Res. 1990, 50, 6793–6799. [Google Scholar]
- Efferth, T.; Sauerbrey, A.; Olbrich, A.; Gebhart, E.; Rauch, P.; Weber, H.O.; Hengstler, J.G.; Halatsch, M.E.; Volm, M.; Tew, K.D.; et al. Molecular modes of action of artesunate in tumor cell lines. Mol. Pharmacol. 2003, 64, 382–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efferth, T.; Konkimalla, V.B.; Wang, Y.F.; Sauerbrey, A.; Meinhardt, S.; Zintl, F.; Mattern, J.; Volm, M. Prediction of broad-spectrum resistance of tumors towards anticancer drugs. Clin. Cancer Res. 2008, 14, 2405–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adham, A.N.; Abdelfatah, S.; Naqishbandi, A.M.; Mahmoud, N.; Efferth, T. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine 2021, 80, 153371. [Google Scholar] [CrossRef] [PubMed]
- Ooko, E.; Alsalim, T.; Saeed, B.; Saeed, M.E.M.; Kadioglu, O.; Abbo, H.S.; Titinchi, S.J.J.; Efferth, T. Modulation of P-glycoprotein activity by novel synthetic curcumin derivatives in sensitive and multidrug-resistant T-cell acute lymphoblastic leukemia cell lines. Toxicol. Appl. Pharmacol. 2016, 305, 216–233. [Google Scholar] [CrossRef]
- Yusa, K.; Tsuruo, T. Reversal mechanism of multidrug resistance by verapamil: Direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989, 49, 5002–5006. [Google Scholar]

| Compound | P-gp Interaction | Torch3D Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Defined mode (AutoDock) | Blind Mode (PyRx) | Clomipramine | Verapamil | Lidocain | Trifluoperazine | Valspodar | Tamoxifen | Propanalol | Reserpine | Tariquidar | |
| Flavonoids: | |||||||||||
| Bidwillon A | −9.0 | −9.0 | 0.6311 | 0.5551 | 0.6711 | 0.6435 | 0.3237 | 0.6063 | 0.6763 | 0.5544 | 0.5170 |
| Neobavaisoflavone | −9.2 | −7.9 | 0.5918 | 0.5228 | 0.5579 | 0.6147 | 0.3632 | 0.5825 | 0.5996 | 0.5390 | 0.4852 |
| Alkaloids: | |||||||||||
| Coptisine | −9.0 | −9.0 | 0.6164 | 0.5666 | 0.5851 | 0.6185 | 0.6148 | 0.5381 | 0.2949 | 0.4526 | 0.6024 |
| Terpenes: | |||||||||||
| Chamazulene | −8.3 | −6.3 | 0.5150 | 0.2559 | 0.6204 | 0.2657 | 0.6476 | 0.5223 | 0.6289 | 0.4540 | 0.6225 |
| Miltirone | −10.0 | −7.1 | 0.5046 | 0.3069 | 0.5965 | 0.2952 | 0.6160 | 0.5874 | 0.6280 | 0.4940 | 0.6674 |
| z-Guggulsterone | −9.2 | −7.8 | 0.4904 | 0.2942 | 0.5516 | 0.2720 | 0.5176 | 0.5645 | 0.5116 | 0.4808 | 0.5038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, J.; Klösgen, V.J.; Omer, E.A.; Kadioglu, O.; Mbaveng, A.T.; Kuete, V.; Hildebrandt, A.; Efferth, T. In Silico and In Vitro Identification of P-Glycoprotein Inhibitors from a Library of 375 Phytochemicals. Int. J. Mol. Sci. 2023, 24, 10240. https://doi.org/10.3390/ijms241210240
Schäfer J, Klösgen VJ, Omer EA, Kadioglu O, Mbaveng AT, Kuete V, Hildebrandt A, Efferth T. In Silico and In Vitro Identification of P-Glycoprotein Inhibitors from a Library of 375 Phytochemicals. International Journal of Molecular Sciences. 2023; 24(12):10240. https://doi.org/10.3390/ijms241210240
Chicago/Turabian StyleSchäfer, Julia, Vincent Julius Klösgen, Ejlal A. Omer, Onat Kadioglu, Armelle T. Mbaveng, Victor Kuete, Andreas Hildebrandt, and Thomas Efferth. 2023. "In Silico and In Vitro Identification of P-Glycoprotein Inhibitors from a Library of 375 Phytochemicals" International Journal of Molecular Sciences 24, no. 12: 10240. https://doi.org/10.3390/ijms241210240
APA StyleSchäfer, J., Klösgen, V. J., Omer, E. A., Kadioglu, O., Mbaveng, A. T., Kuete, V., Hildebrandt, A., & Efferth, T. (2023). In Silico and In Vitro Identification of P-Glycoprotein Inhibitors from a Library of 375 Phytochemicals. International Journal of Molecular Sciences, 24(12), 10240. https://doi.org/10.3390/ijms241210240








