Evidence That DDR1 Promotes Oligodendrocyte Differentiation during Development and Myelin Repair after Injury
Abstract
1. Introduction
2. Results
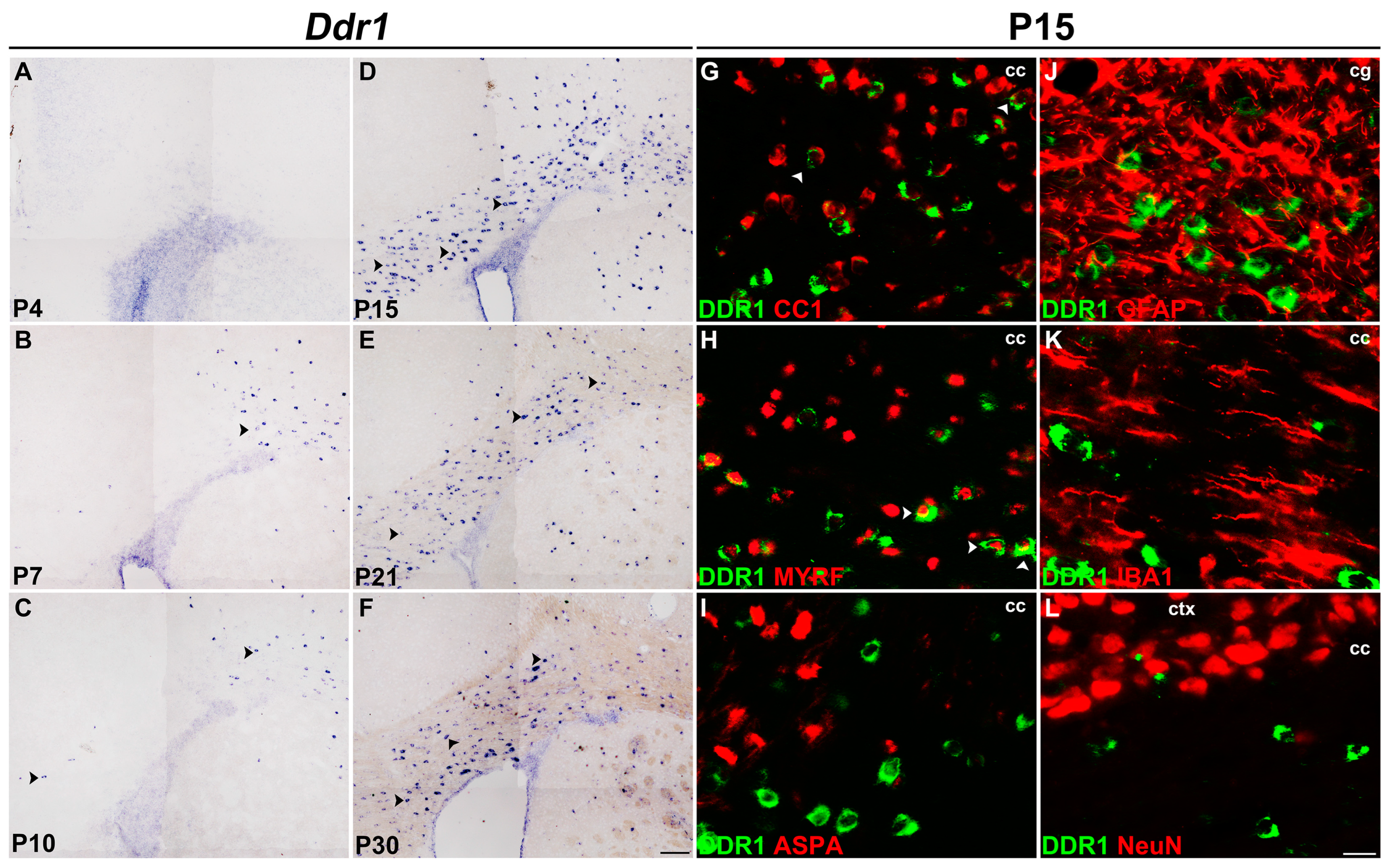
2.1. Ddr1 Is Selectively Up-Regulated in Newly Differentiated Oligodendrocytes
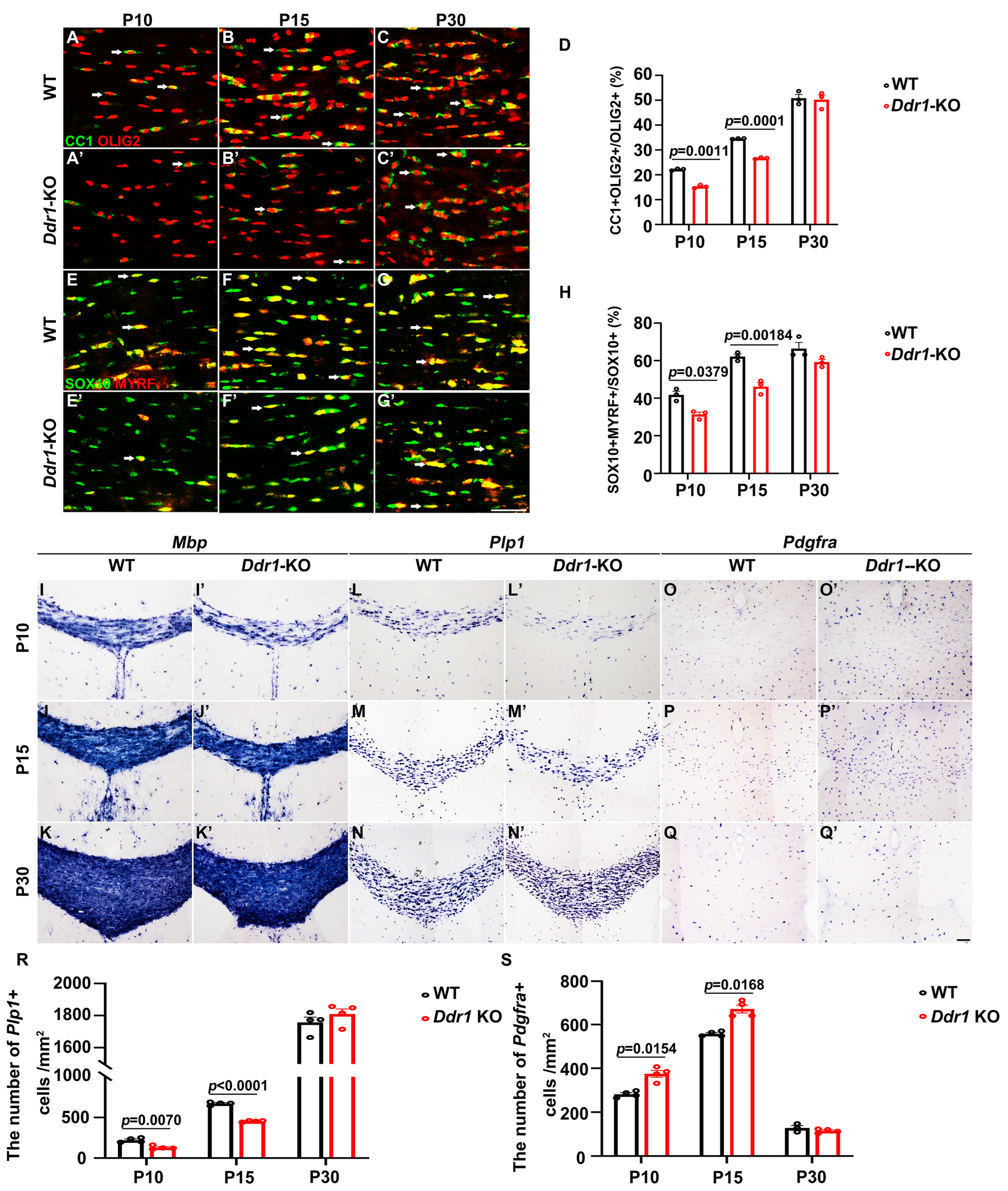
2.2. Ddr1 Mutation Results in a Transient Delay of OL Differentiation
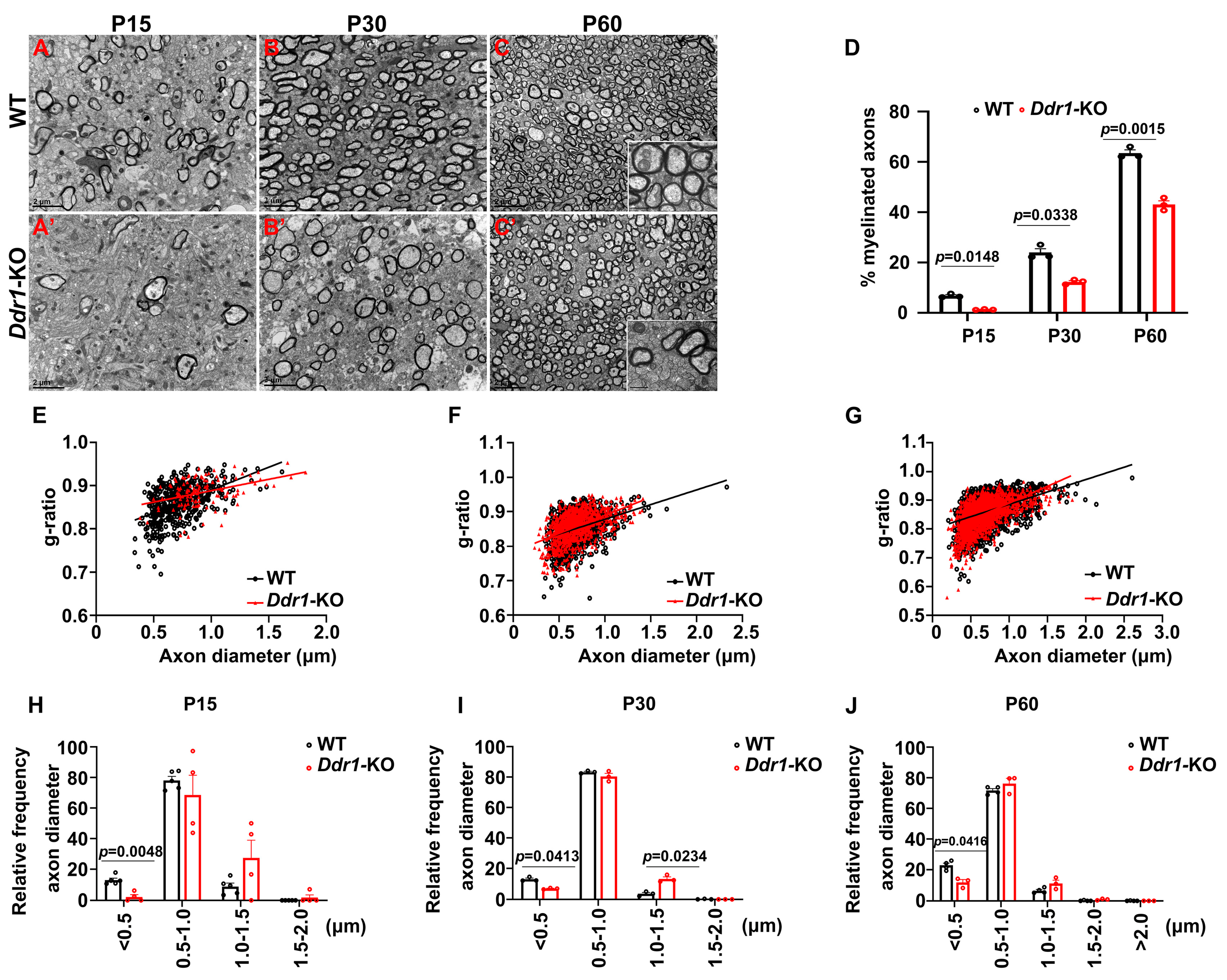
2.3. Deficiency of Ddr1 in OL Impairs Axonal Myelination in the CNS
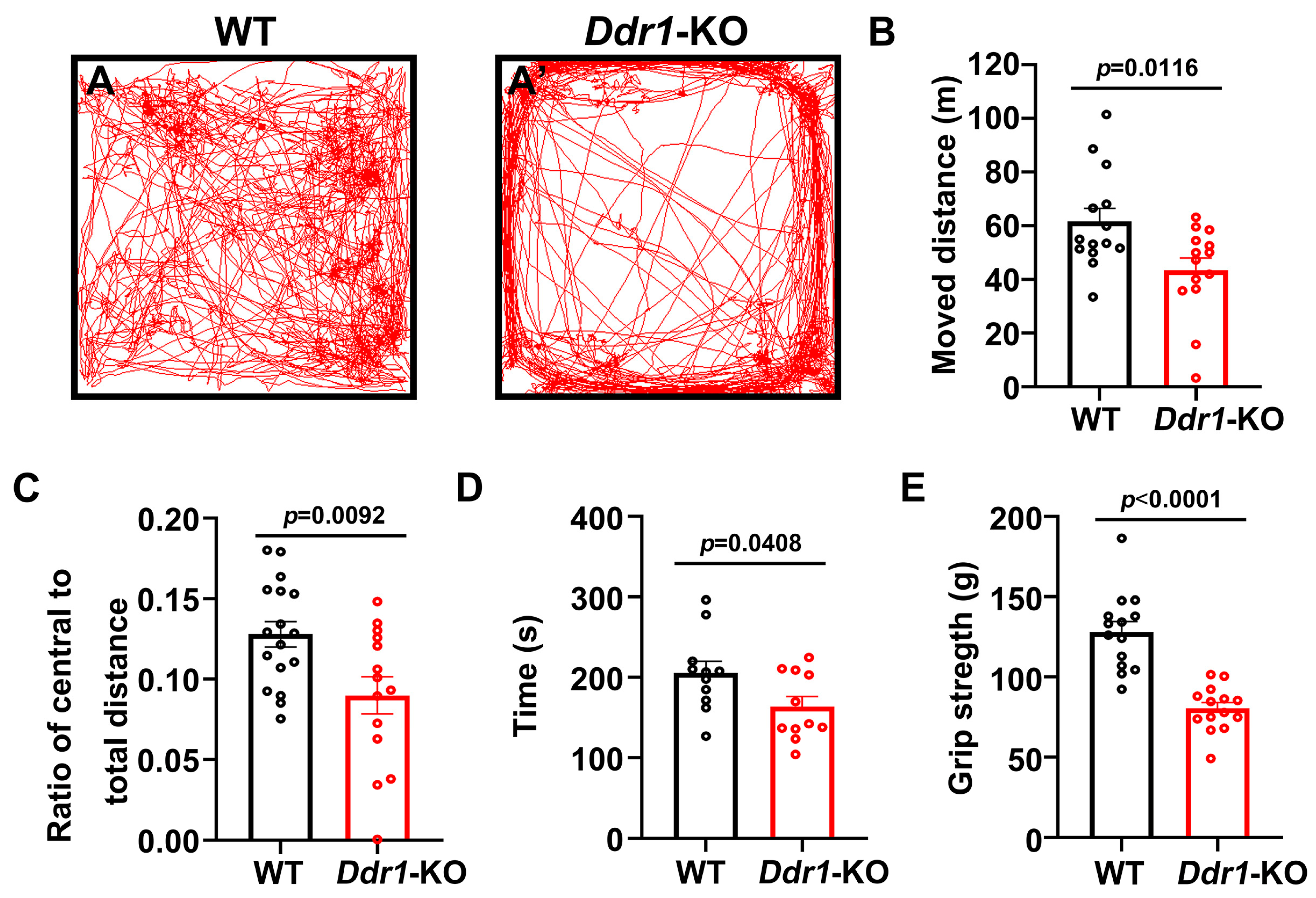
2.4. Ablation Ddr1 Leads to an Abnormal Motor Function
2.5. Ddr1 Is Critical for Remyelination in the CNS
2.6. Ddr1 Might Enhance the Differentiation of Oligodendrocytes by Restraining ERK Signaling
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. In Situ RNA Hybridization (ISH)
4.3. Immunofluorescence Staining
4.4. Transmission Electron Microscopy (TEM)
4.5. Black Gold II Staining
4.6. Induction of White Matter Demyelination
4.7. Western Blot
4.8. Behavior Assays
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.F.; Liu, K.; Hu, B.; Li, R.R.; Xin, W.; Chen, H.; Wang, F.; Chen, L.; Li, R.X.; Ren, S.Y.; et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 2021, 109, 2292–2307.e2295. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, H.; He, W.; Zhou, F.; Yu, X.; Yi, M.; Du, J.; Xie, B.; Qiu, M. Id2 and Id4 are not the major negative regulators of oligodendrocyte differentiation during early central nervous system development. Glia 2022, 70, 590–601. [Google Scholar] [CrossRef]
- Mei, R.; Fu, J.; Jiang, C.; Yang, J.; Zheng, K.; Yang, A.; Qiu, M.; Zhao, X. TAPP1 Represses the Differentiation of Oligodendrocyte and its Deficiency Accelerates Myelin Regeneration after Demyelinating Injuries. Neurosci. Bull. 2021, 37, 385–388. [Google Scholar] [CrossRef]
- Wang, P.S.; Wang, J.; Xiao, Z.C.; Pallen, C.J. Protein-tyrosine phosphatase alpha acts as an upstream regulator of Fyn signaling to promote oligodendrocyte differentiation and myelination. J. Biol. Chem. 2009, 284, 33692–33702. [Google Scholar] [CrossRef] [PubMed]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I. Oligodendrogliopathy in neurodegenerative diseases with abnormal protein aggregates: The forgotten partner. Prog. Neurobiol. 2018, 169, 24–54. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Huang, L.; Wu, M.; Jiang, C.; Yang, A.; Tao, H.; Zheng, K.; Yang, J.; Shen, W.; Chen, X.; et al. Evidence That ITPR2-Mediated Intracellular Calcium Release in Oligodendrocytes Regulates the Development of Carbonic Anhydrase II + Type I/II Oligodendrocytes and the Sizes of Myelin Fibers. Front. Cell. Neurosci. 2021, 15, 751439. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Brooks, J.M.; Chen, J.; Su, J.; Fox, M.A. Cell-specific and developmental expression of lectican-cleaving proteases in mouse hippocampus and neocortex. J. Comp. Neurol. 2015, 523, 629–648. [Google Scholar] [CrossRef]
- Hubert, T.; Grimal, S.; Carroll, P.; Fichard-Carroll, A. Collagens in the developing and diseased nervous system. Cell. Mol. Life Sci. 2009, 66, 1223–1238. [Google Scholar] [CrossRef]
- Chernousov, M.A.; Yu, W.M.; Chen, Z.L.; Carey, D.J.; Strickland, S. Regulation of Schwann cell function by the extracellular matrix. Glia 2008, 56, 1498–1507. [Google Scholar] [CrossRef]
- Mehta, P.; Piao, X. Adhesion G-protein coupled receptors and extracellular matrix proteins: Roles in myelination and glial cell development. Dev. Dyn. 2017, 246, 275–284. [Google Scholar] [CrossRef]
- Silva, M.E.; Hernández-Andrade, M.; Abasolo, N.; Espinoza-Cruells, C.; Mansilla, J.B.; Reyes, C.R.; Aranda, S.; Esteban, Y.; Rodriguez-Calvo, R.; Martorell, L.; et al. DDR1 and Its Ligand, Collagen IV, Are Involved in In Vitro Oligodendrocyte Maturation. Int. J. Mol. Sci. 2023, 24, 1742. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Roig, B.; Franco-Pons, N.; Martorell, L.; Tomàs, J.; Vogel, W.F.; Vilella, E. Expression of the tyrosine kinase discoidin domain receptor 1 (DDR1) in human central nervous system myelin. Brain Res. 2010, 1336, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Franco-Pons, N.; Virgos, C.; Vogel, W.F.; Ureña, J.M.; Soriano, E.; del Rio, J.A.; Vilella, E. Expression of discoidin domain receptor 1 during mouse brain development follows the progress of myelination. Neuroscience 2006, 140, 463–475. [Google Scholar] [CrossRef]
- Vilella, E.; Gas, C.; Garcia-Ruiz, B.; Rivera, F.J. Expression of DDR1 in the CNS and in myelinating oligodendrocytes. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118483. [Google Scholar] [CrossRef] [PubMed]
- Lappe-Siefke, C.; Goebbels, S.; Gravel, M.; Nicksch, E.; Lee, J.; Braun, P.E.; Griffiths, I.R.; Nave, K.A. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 2003, 33, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Ben Haim, L.; Schirmer, L.; Zulji, A.; Sabeur, K.; Tiret, B.; Ribon, M.; Chang, S.; Lamers, W.H.; Boillée, S.; Chaumeil, M.M.; et al. Evidence for glutamine synthetase function in mouse spinal cord oligodendrocytes. Glia 2021, 69, 2812–2827. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhao, X.; Zheng, K.; Li, H.; Huang, H.; Zhang, Z.; Mastracci, T.; Wegner, M.; Chen, Y.; Sussel, L.; et al. Genetic evidence that Nkx2.2 and Pdgfra are major determinants of the timing of oligodendrocyte differentiation in the developing CNS. Development 2014, 141, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, F.; Zhou, S.; Qiu, M. MYRF: A Mysterious Membrane-Bound Transcription Factor Involved in Myelin Development and Human Diseases. Neurosci. Bull. 2021, 37, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Mattan, N.S.; Ghiani, C.A.; Lloyd, M.; Matalon, R.; Bok, D.; Casaccia, P.; de Vellis, J. Aspartoacylase deficiency affects early postnatal development of oligodendrocytes and myelination. Neurobiol. Dis. 2010, 40, 432–443. [Google Scholar] [CrossRef]
- Koenning, M.; Jackson, S.; Hay, C.M.; Faux, C.; Kilpatrick, T.J.; Willingham, M.; Emery, B. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J. Neurosci. 2012, 32, 12528–12542. [Google Scholar] [CrossRef]
- Poggi, G.; Albiez, J.; Pryce, C.R. Effects of chronic social stress on oligodendrocyte proliferation-maturation and myelin status in prefrontal cortex and amygdala in adult mice. Neurobiol. Stress 2022, 18, 100451. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Thompson, P.M.; Toga, A.W. The development of the corpus callosum in the healthy human brain. J. Neurosci. 2010, 30, 10985–10990. [Google Scholar] [CrossRef]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef]
- Keogh, C.E.; Kim, D.H.J.; Pusceddu, M.M.; Knotts, T.A.; Rabasa, G.; Sladek, J.A.; Hsieh, M.T.; Honeycutt, M.; Brust-Mascher, I.; Barboza, M.; et al. Myelin as a regulator of development of the microbiota-gut-brain axis. Brain Behav. Immun. 2021, 91, 437–450. [Google Scholar] [CrossRef]
- Suo, N.; Guo, Y.E.; He, B.; Gu, H.; Xie, X. Inhibition of MAPK/ERK pathway promotes oligodendrocytes generation and recovery of demyelinating diseases. Glia 2019, 67, 1320–1332. [Google Scholar] [CrossRef]
- Allan, K.C.; Hu, L.R.; Scavuzzo, M.A.; Morton, A.R.; Gevorgyan, A.S.; Cohn, E.F.; Clayton, B.L.L.; Bederman, I.R.; Hung, S.; Bartels, C.F.; et al. Non-canonical Targets of HIF1a Impair Oligodendrocyte Progenitor Cell Function. Cell Stem Cell 2021, 28, 257–272.e211. [Google Scholar] [CrossRef] [PubMed]
- Chappell, W.H.; Candido, S.; Abrams, S.L.; Akula, S.M.; Steelman, L.S.; Martelli, A.M.; Ratti, S.; Cocco, L.; Cervello, M.; Montalto, G.; et al. Influences of TP53 and the anti-aging DDR1 receptor in controlling Raf/MEK/ERK and PI3K/Akt expression and chemotherapeutic drug sensitivity in prostate cancer cell lines. Aging 2020, 12, 10194–10210. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, S.; Luo, L.; Xiang, Q.; Zhu, Z.; Wang, J.; Liu, Y.; Luo, J. miR-199b-5p-DDR1-ERK signalling axis suppresses prostate cancer metastasis via inhibiting epithelial-mesenchymal transition. Br. J. Cancer 2021, 124, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, G.; Alcover-Sanchez, B.; Wandosell, F.; Cubelos, B. Pathways Involved in Remyelination after Cerebral Ischemia. Curr. Neuropharmacol. 2022, 20, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Shafit-Zagardo, B.; Gruber, R.C.; DuBois, J.C. The role of TAM family receptors and ligands in the nervous system: From development to pathobiology. Pharmacol. Ther. 2018, 188, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Krämer-Albers, E.M.; White, R. From axon-glial signalling to myelination: The integrating role of oligodendroglial Fyn kinase. Cell. Mol. Life Sci. 2011, 68, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, M.; Hudson, L.D. Regulation of tyrosine phosphorylation and protein tyrosine phosphatases during oligodendrocyte differentiation. Mol. Cell. Neurosci. 1996, 7, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Miller, R.; Krane, I.; Vartanian, T. The erbB2 gene is required for the development of terminally differentiated spinal cord oligodendrocytes. J. Cell Biol. 2001, 154, 1245–1258. [Google Scholar] [CrossRef]
- Bercury, K.K.; Macklin, W.B. Dynamics and mechanisms of CNS myelination. Dev. Cell 2015, 32, 447–458. [Google Scholar] [CrossRef]
- Choi, C.I.; Yoo, K.H.; Hussaini, S.M.; Jeon, B.T.; Welby, J.; Gan, H.; Scarisbrick, I.A.; Zhang, Z.; Baker, D.J.; van Deursen, J.M.; et al. The progeroid gene BubR1 regulates axon myelination and motor function. Aging 2016, 8, 2667–2688. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Li, T.; Guo, Y.; Tian, Y.; Wang, F.; Liu, S.; Shen, H.Y.; Feng, Y.; Xiao, L. Impairment of Oligodendroglia Maturation Leads to Aberrantly Increased Cortical Glutamate and Anxiety-Like Behaviors in Juvenile Mice. Front. Cell. Neurosci. 2015, 9, 467. [Google Scholar] [CrossRef]
- Franco-Pons, N.; Tomàs, J.; Roig, B.; Auladell, C.; Martorell, L.; Vilella, E. Discoidin domain receptor 1, a tyrosine kinase receptor, is upregulated in an experimental model of remyelination and during oligodendrocyte differentiation in vitro. J. Mol. Neurosci. 2009, 38, 2–11. [Google Scholar] [CrossRef]
- Canoll, P.D.; Kraemer, R.; Teng, K.K.; Marchionni, M.A.; Salzer, J.L. GGF/neuregulin induces a phenotypic reversion of oligodendrocytes. Mol. Cell. Neurosci. 1999, 13, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Fressinaud, C.; Vallat, J.M.; Labourdette, G. Basic fibroblast growth factor down-regulates myelin basic protein gene expression and alters myelin compaction of mature oligodendrocytes in vitro. J. Neurosci. Res. 1995, 40, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Pfeiffer, S.E. FGF-2 converts mature oligodendrocytes to a novel phenotype. J. Neurosci. Res. 1997, 50, 215–228. [Google Scholar] [CrossRef]
- Xiao, J.; Ferner, A.H.; Wong, A.W.; Denham, M.; Kilpatrick, T.J.; Murray, S.S. Extracellular signal-regulated kinase 1/2 signaling promotes oligodendrocyte myelination in vitro. J. Neurochem. 2012, 122, 1167–1180. [Google Scholar] [CrossRef]
- Ishii, A.; Furusho, M.; Dupree, J.L.; Bansal, R. Strength of ERK1/2 MAPK Activation Determines Its Effect on Myelin and Axonal Integrity in the Adult CNS. J. Neurosci. 2016, 36, 6471–6487. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Sloane, J.A.; Li, H.; Aytan, N.; Giannaris, E.L.; Zeldich, E.; Hinman, J.D.; Dedeoglu, A.; Rosene, D.L.; Bansal, R.; et al. The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J. Neurosci. 2013, 33, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xiao, G.; He, L.; Niu, X.; Li, H.; Lou, T.; Hu, Q.; Yang, Y.; Xu, Q.; Wei, Z.; et al. Sustained ErbB Activation Causes Demyelination and Hypomyelination by Driving Necroptosis of Mature Oligodendrocytes and Apoptosis of Oligodendrocyte Precursor Cells. J. Neurosci. 2021, 41, 9872–9890. [Google Scholar] [CrossRef]
- Joerger-Messerli, M.S.; Thomi, G.; Haesler, V.; Keller, I.; Renz, P.; Surbek, D.V.; Schoeberlein, A. Human Wharton’s Jelly Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles Drive Oligodendroglial Maturation by Restraining MAPK/ERK and Notch Signaling Pathways. Front. Cell Dev. Biol. 2021, 9, 622539. [Google Scholar] [CrossRef]
- Nam, H.; Kundu, A.; Brinkley, G.J.; Chandrashekar, D.S.; Kirkman, R.L.; Chakravarthi, B.; Orlandella, R.M.; Norian, L.A.; Sonpavde, G.; Ghatalia, P.; et al. PGC1α suppresses kidney cancer progression by inhibiting collagen-induced SNAIL expression. Matrix Biol. 2020, 89, 43–58. [Google Scholar] [CrossRef]
- Lyon, A.; Tripathi, R.; Meeks, C.; He, D.; Wu, Y.; Liu, J.; Wang, C.; Chen, J.; Zhu, H.; Mukherjee, S.; et al. ABL1/2 and DDR1 Drive MEKi Resistance in NRAS-Mutant Melanomas by Stabilizing RAF/MYC/ETS1 and Promoting RAF Homodimerization. Cancers 2023, 15, 954. [Google Scholar] [CrossRef] [PubMed]
- Vogel, W.F.; Aszódi, A.; Alves, F.; Pawson, T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol. Cell. Biol. 2001, 21, 2906–2917. [Google Scholar] [CrossRef]
- Tonniges, J.R.; Albert, B.; Calomeni, E.P.; Roy, S.; Lee, J.; Mo, X.; Cole, S.E.; Agarwal, G. Collagen Fibril Ultrastructure in Mice Lacking Discoidin Domain Receptor 1. Microsc. Microanal. 2016, 22, 599–611. [Google Scholar] [CrossRef]
- Chavez, M.B.; Kolli, T.N.; Tan, M.H.; Zachariadou, C.; Wang, C.; Embree, M.C.; Lira Dos Santos, E.J.; Nociti, F.H., Jr.; Wang, Y.; Tatakis, D.N.; et al. Loss of Discoidin Domain Receptor 1 Predisposes Mice to Periodontal Breakdown. J. Dent. Res. 2019, 98, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Mastracci, T.L.; Lin, C.S.; Sussel, L. Generation of mice encoding a conditional allele of Nkx2.2. Transgenic Res. 2013, 22, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, Q.; Fang, M.; Yi, M.; Yang, A.; Xie, B.; Yang, J.; Zhang, Z.; Dai, Z.; Qiu, M. Stage-specific regulation of oligodendrocyte development by Hedgehog signaling in the spinal cord. Glia 2020, 68, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Holleran, L.; Kim, J.H.; Gangolli, M.; Stein, T.; Alvarez, V.; McKee, A.; Brody, D.L. Axonal disruption in white matter underlying cortical sulcus tau pathology in chronic traumatic encephalopathy. Acta Neuropathol. 2017, 133, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Sui, R.X.; Miao, Q.; Wang, J.; Wang, Q.; Song, L.J.; Yu, J.W.; Cao, L.; Xiao, W.; Xiao, B.G.; Ma, C.G. Protective and therapeutic role of Bilobalide in cuprizone-induced demyelination. Int. Immunopharmacol. 2019, 66, 69–81. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Gan, J.; Zhang, Z.; Liang, X.; Li, T.; Huang, N.; Zhao, X.; Mei, F.; Xiao, L. Myelin Deficits Caused by Olig2 Deficiency Lead to Cognitive Dysfunction and Increase Vulnerability to Social Withdrawal in Adult Mice. Neurosci. Bull. 2020, 36, 419–426. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, R.; Qiu, W.; Yang, Y.; Xu, S.; Rao, Y.; Li, Q.; Luo, Y.; Huang, H.; Yang, A.; Tao, H.; et al. Evidence That DDR1 Promotes Oligodendrocyte Differentiation during Development and Myelin Repair after Injury. Int. J. Mol. Sci. 2023, 24, 10318. https://doi.org/10.3390/ijms241210318
Mei R, Qiu W, Yang Y, Xu S, Rao Y, Li Q, Luo Y, Huang H, Yang A, Tao H, et al. Evidence That DDR1 Promotes Oligodendrocyte Differentiation during Development and Myelin Repair after Injury. International Journal of Molecular Sciences. 2023; 24(12):10318. https://doi.org/10.3390/ijms241210318
Chicago/Turabian StyleMei, Ruyi, Wanwan Qiu, Yingying Yang, Siyu Xu, Yueyu Rao, Qingxin Li, Yuhao Luo, Hao Huang, Aifen Yang, Huaping Tao, and et al. 2023. "Evidence That DDR1 Promotes Oligodendrocyte Differentiation during Development and Myelin Repair after Injury" International Journal of Molecular Sciences 24, no. 12: 10318. https://doi.org/10.3390/ijms241210318
APA StyleMei, R., Qiu, W., Yang, Y., Xu, S., Rao, Y., Li, Q., Luo, Y., Huang, H., Yang, A., Tao, H., Qiu, M., & Zhao, X. (2023). Evidence That DDR1 Promotes Oligodendrocyte Differentiation during Development and Myelin Repair after Injury. International Journal of Molecular Sciences, 24(12), 10318. https://doi.org/10.3390/ijms241210318





