Increased Expression of Anaphylatoxin C5a-Receptor-1 in Neutrophils and Natural Killer Cells of Preterm Infants
Abstract
1. Introduction
2. Results
2.1. Cohort Characteristics
2.2. Flow Cytometry
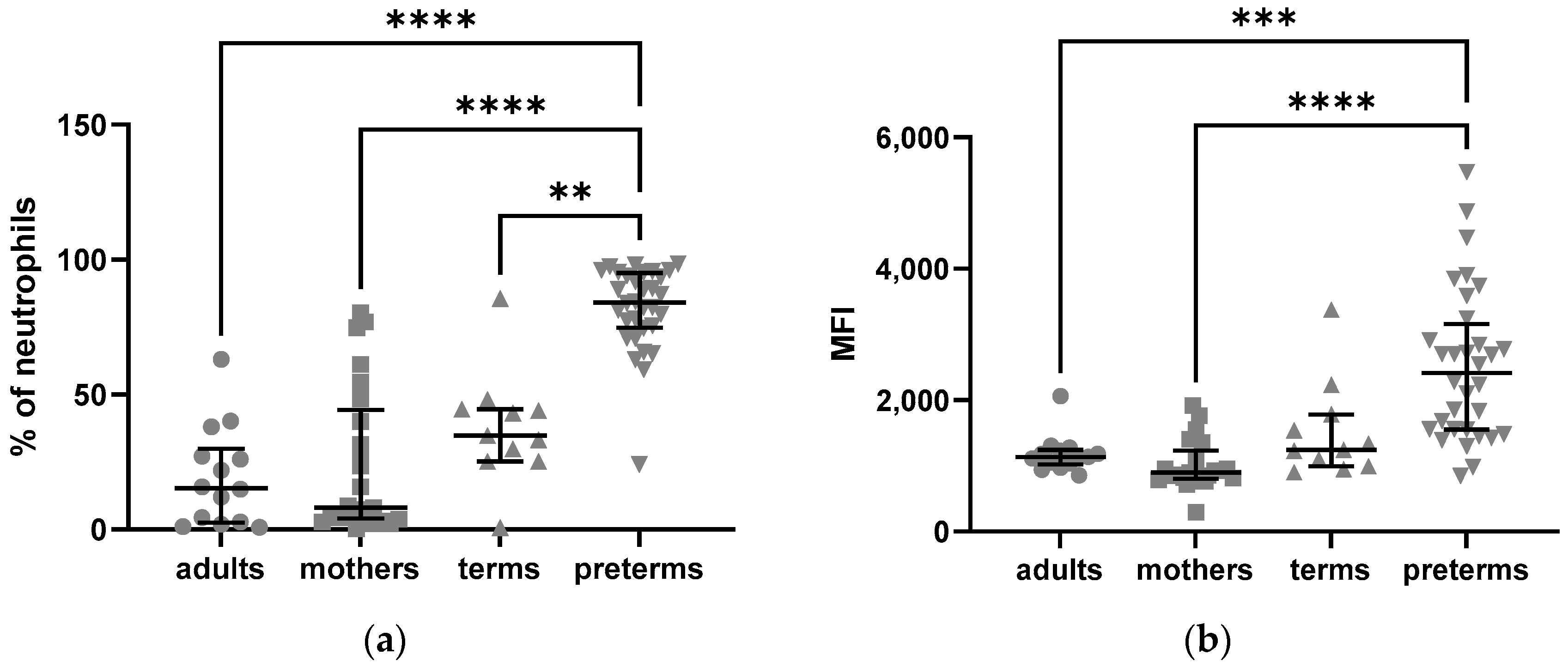
2.2.1. Elevated Intracellular Expression of C5aR1 in Preterm Neutrophils
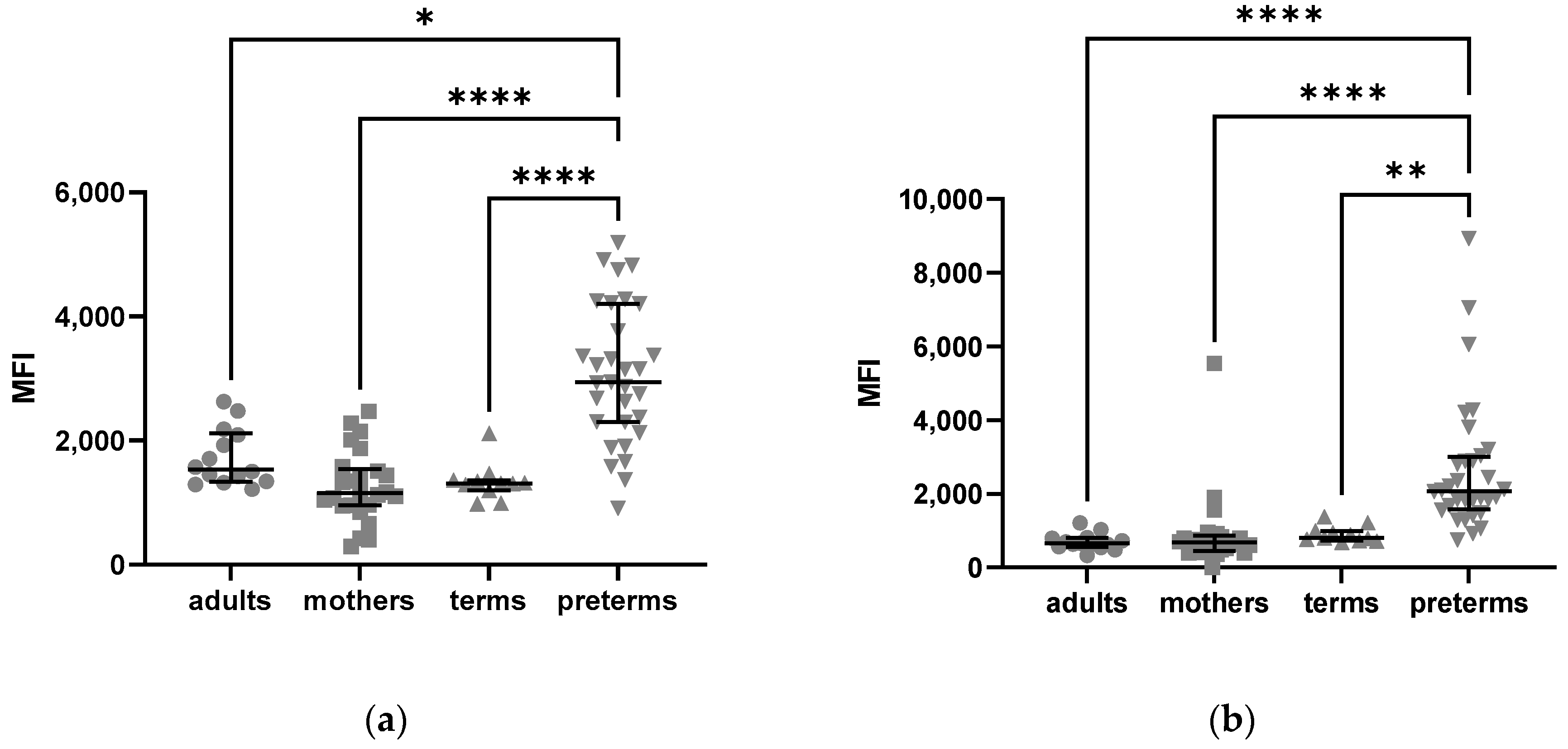
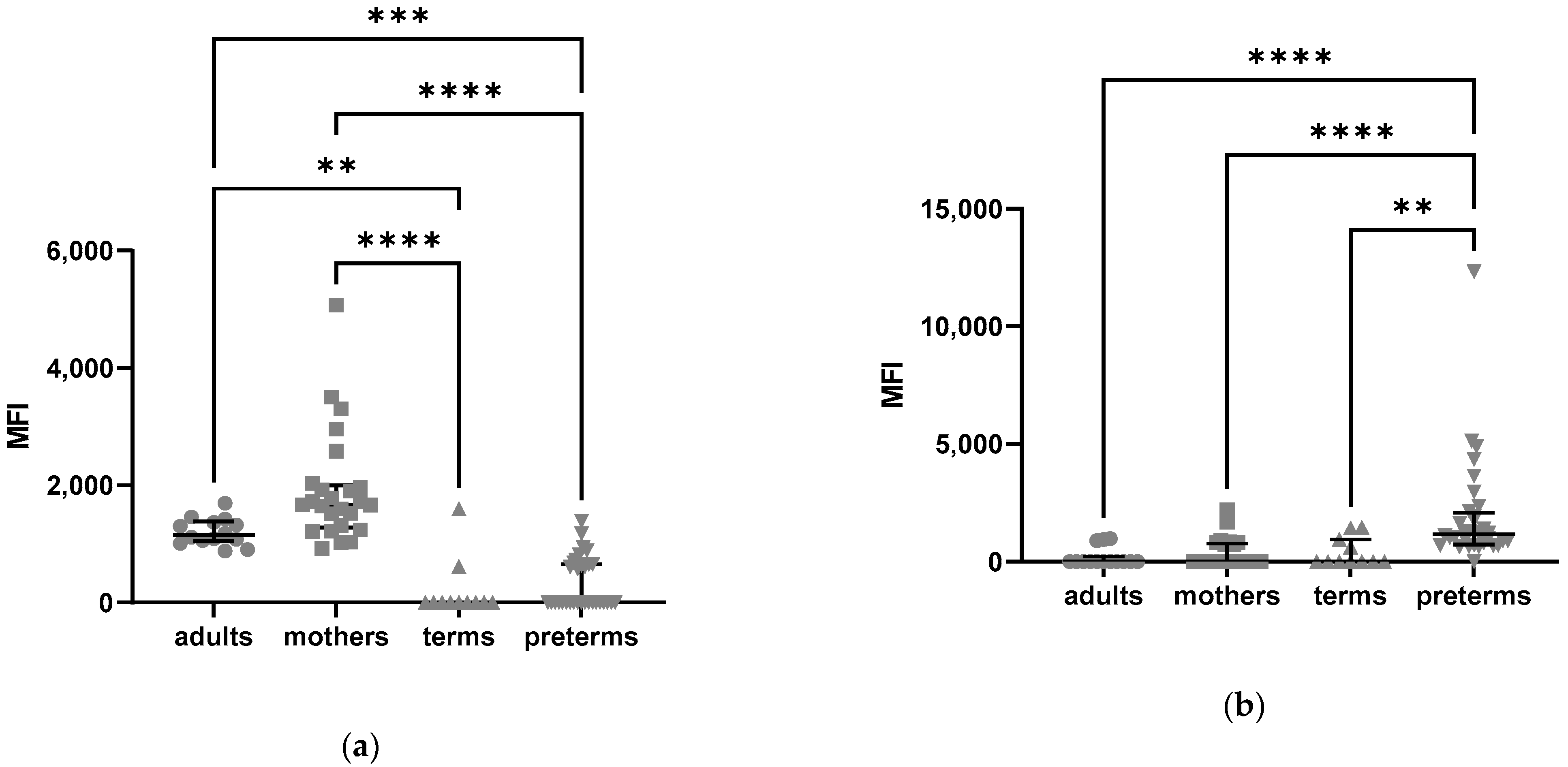
2.2.2. Density of C5aR1 Expression in NK Cells of Preterm Infants and on Their Surface Is Elevated
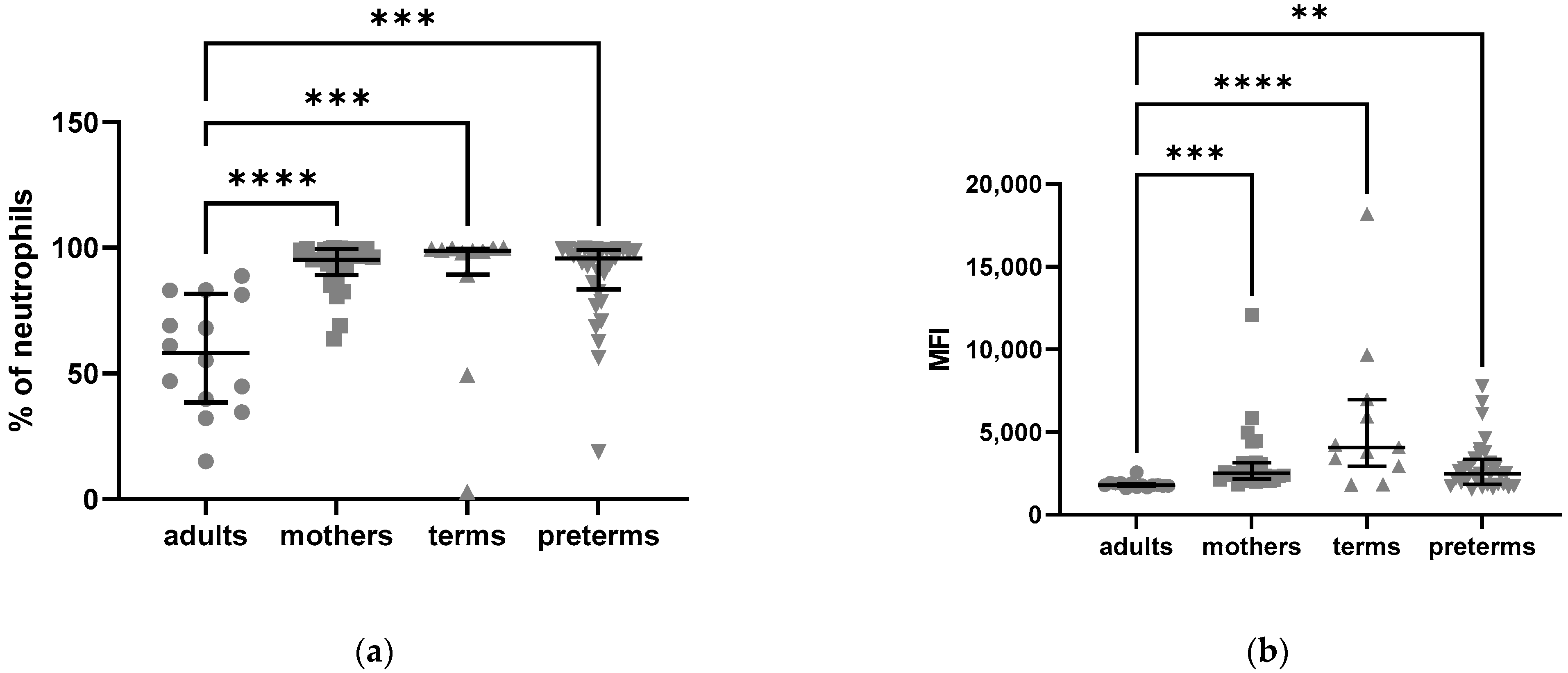
2.2.3. Higher C5aR2 Expression Neutrophils of Preterm and Term Infants as Compared to Healthy Adults
3. Discussion
4. Materials and Methods
4.1. Study Cohort
4.2. Ethics
4.3. Flow Cytometry
4.4. Clinical Data
4.5. Statistical Analysis
Data Analysis Was Performed Using GraphPad Prism®®
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Dele Davies, H. Early-Onset Neonatal Sepsis. Clin. Microbiol. Rev. 2014, 27, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.G.; Kandefer, S.; Do, B.T.; Smith, P.B.; Stoll, B.J.; Bell, E.; Carlo, W.A.; Laptook, A.R.; Sánchez, P.J.; Shankaran, S.; et al. Late-onset Sepsis in Extremely Premature Infants: 2000–2011. Pediatr. Infect. Dis. J. 2017, 36, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Humberg, A.; Härtel, C.; Rausch, T.K.; Stichtenoth, G.; Jung, P.; Wieg, C.; Kribs, A.; Von Der Wense, A.; Weller, U.; Höhn, T.; et al. Active perinatal care of preterm infants in the German Neonatal Network. Arch. Dis. Child.-Fetal Neonatal Ed. 2019, 105, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Humberg, A.; Fortmann, I.; Siller, B.; Kopp, M.V.; Herting, E.; Gopel, W.; Hartel, C. German Neonatal Network; German Center for Lung Research and Priming Immunity at the beginning of life (PRIMAL) Consortium. Preterm birth and sustained inflammation: Consequences for the neonate. Semin. Immunopathol. 2020, 42, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Eichberger, J.; Resch, E.; Resch, B. Diagnosis of Neonatal Sepsis: The Role of Inflammatory Markers. Front. Pediatr. 2022, 10, 840288. [Google Scholar] [CrossRef]
- Olin, A.; Henckel, E.; Chen, Y.; Lakshmikanth, T.; Pou, C.; Mikes, J.; Gustafsson, A.; Bernhardsson, A.K.; Zhang, C.; Bohlin, K.; et al. Stereotypic Immune System Development in Newborn Children. Cell 2018, 174, 1277–1292.e14. [Google Scholar] [CrossRef]
- Strunk, T.; Temming, P.; Gembruch, U.; Reiss, I.; Bucsky, P.; Schultz, C. Differential Maturation of the Innate Immune Response in Human Fetuses. Pediatr. Res. 2004, 56, 219–226. [Google Scholar] [CrossRef]
- Källman, J.; Schollin, J.; Schalèn, C.; Erlandsson, A.; Kihlström, E. Impaired phagocytosis and opsonisation towards group B streptococci in preterm neonates. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 78, F46–F50. [Google Scholar] [CrossRef]
- Yu, J.C.; Khodadadi, H.; Malik, A.; Davidson, B.; da silva Lopes Salles, E.; Bhatia, J.; Hale, V.L.; Baban, B. Innate Immunity of Neonates and Infants. Front. Immunol. 2018, 9, 1759. [Google Scholar] [CrossRef]
- Drossou, V.; Kanakoudi, F.; Tzimouli, V.; Sarafidis, K.; Taparkou, A.; Bougiouklis, D.; Petropoulou, T.; Kremenopoulos, G. Impact of Prematurity, Stress and Sepsis on the Neutrophil Respiratory Burst Activity of Neonates. Neonatology 1997, 72, 201–209. [Google Scholar] [CrossRef]
- Nupponen, I.; Turunen, R.; Nevalainen, T.; Peuravuori, H.; Pohjavuori, M.; Repo, H.; Andersson, S. ARTICLES Extracellular Release of Bactericidal/Permeability-Increasing Protein in Newborn Infants. Pediatr. Res. 2002, 51, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, C.; Gloning, A.; Pruenster, M.; Frommhold, D.; Bierschenk, S.; Genzel-Boroviczény, O.; von Andrian, U.H.; Quackenbush, E.; Sperandio, M. Neutrophil and endothelial adhesive function during human fetal ontogeny. J. Leukoc. Biol. 2013, 93, 175–184. [Google Scholar] [CrossRef]
- Raymond, S.L.; Mathias, B.J.; Murphy, T.J.; Rincon, J.C.; López, M.C.; Ungaro, R.; Ellett, F.; Jorgensen, J.; Wynn, J.L.; Baker, H.V.; et al. Neutrophil chemotaxis and transcriptomics in term and preterm neonates. Transl. Res. 2017, 190, 4–15. [Google Scholar] [CrossRef]
- Sampah, M.E.S.; Hackam, D.J. Dysregulated Mucosal Immunity and Associated Pathogeneses in Preterm Neonates. Front. Immunol. 2020, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Luevano, M.; Daryouzeh, M.; Alnabhan, R.; Querol, S.; Khakoo, S.; Madrigal, A.; Saudemont, A. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum. Immunol. 2012, 73, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Qazi, K.R.; Jensen, G.B.; Heiden, M.; Björkander, S.; Marchini, G.; Jenmalm, M.C.; Abrahamsson, T.; Sverremark-Ekström, E. Extreme prematurity and sepsis strongly influence frequencies and functional characteristics of circulating γδ T and natural killer cells. Clin. Transl. Immunol. 2021, 10, e1294. [Google Scholar] [CrossRef]
- Gaddy, J.; Broxmeyer, H.E. Cord Blood CD16 56 0 Cells with Low Lytic Activity Are Possible Precursors of Mature Natural Killer Cells. Cell. Immunol. 1997, 180, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Björkström, N.K.; Ljunggren, H.-G.; Sandberg, J.K. CD56 negative NK cells: Origin, function, and role in chronic viral disease. Trends Immunol. 2010, 31, 401–406. [Google Scholar] [CrossRef]
- Klos, A.; Tenner, A.J.; Johswich, K.-O.; Ager, R.R.; Reis, E.S.; Köhl, J. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef]
- Unnewehr, H.; Rittirsch, D.; Sarma, J.V.; Zetoune, F.; Flierl, M.A.; Perl, M.; Denk, S.; Weiss, M.; Schneider, M.E.; Monk, P.N.; et al. Changes and Regulation of the C5a Receptor on Neutrophils during Septic Shock in Humans. J. Immunol. 2013, 190, 4215–4225. [Google Scholar] [CrossRef]
- Gressner, O.A.; Koch, A.; Sanson, E.; Trautwein, C.; Tacke, F. High C5a levels are associated with increased mortality in sepsis patients—No enhancing effect by actin-free Gc-globulin. Clin. Biochem. 2008, 41, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.; Sarma, V.J.; Lu, K.T.; McGuire, S.R.; Padgaonkar, V.A.; Guo, R.-F.; Younkin, E.M.; Kunkel, R.G.; Ding, J.; Erickson, R.; et al. Role of C5a in Multiorgan Failure During Sepsis. J. Immunol. 2001, 166, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.A. The Harmful Role of C5a on Innate Immunity in Sepsis. J. Innate Immun. 2010, 2, 439–445. [Google Scholar] [CrossRef]
- Merle, N.S.; Noé, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.A.; Monk, P.N. The Orphan Receptor C5L2 Has High Affinity Binding Sites for Complement Fragments C5a and C5a des-Arg74. J. Biol. Chem. 2002, 277, 7165–7169. [Google Scholar] [CrossRef]
- Bamberg, C.E.; Mackay, C.R.; Lee, H.; Zahra, D.; Jackson, J.; Lim, Y.S.; Whitfeld, P.L.; Craig, S.; Corsini, E.; Lu, B.; et al. The C5a Receptor (C5aR) C5L2 Is a Modulator of C5aR-mediated Signal Transduction. J. Biol. Chem. 2010, 285, 7633–7644. [Google Scholar] [CrossRef]
- Zhang, T.; Garstka, M.A.; Li, K. The Controversial C5a Receptor C5aR2: Its Role in Health and Disease. J. Immunol. Res. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Karsten, C.M.; Wiese, A.V.; Mey, F.; Figge, J.; Woodruff, T.M.; Reuter, T.; Scurtu, O.; Kordowski, A.; Almeida, L.N.; Briukhovetska, D.; et al. Monitoring C5aR2 Expression Using a Floxed tdTomato-C5aR2 Knock-In Mouse. J. Immunol. 2017, 199, 3234–3248. [Google Scholar] [CrossRef]
- Okinaga, S.; Slattery, D.; Humbles, A.; Zsengeller, Z.; Morteau, O.; Kinrade, M.B.; Brodbeck, R.M.; Krause, J.E.; Choe, H.-R.; Gerard, N.P.; et al. C5L2, a Nonsignaling C5A Binding Protein. Biochemistry 2003, 42, 9406–9415. [Google Scholar] [CrossRef]
- Rittirsch, D.; A Flierl, M.; A Nadeau, B.; E Day, D.; Huber-Lang, M.; Mackay, C.R.; Zetoune, F.S.; Gerard, N.P.; Cianflone, K.; Köhl, J.; et al. Functional roles for C5a receptors in sepsis. Nat. Med. 2008, 14, 551–557. [Google Scholar] [CrossRef]
- McGreal, E.P.; Hearne, K.; Spiller, O.B. Off to a slow start: Under-development of the complement system in term newborns is more substantial following premature birth. Immunobiology 2012, 217, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Girardi, G.; Lingo, J.J.; Fleming, S.D.; Regal, J.F. Essential Role of Complement in Pregnancy: From Implantation to Parturition and Beyond. Front. Immunol. 2020, 11, 1681. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.A. Role of C5 Activation Products in Sepsis. Sci. World J. 2010, 10, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.S.; Younkin, E.M.; Sarma, J.V.; McGuire, S.R.; Lu, K.T.; Guo, R.F.; Padgaonkar, V.A.; Curnutte, J.T.; Erickson, R.; Ward, P.A. Complement-Induced Impairment of Innate Immunity During Sepsis. J. Immunol. 2002, 169, 3223–3231. [Google Scholar] [CrossRef]
- Morris, A.C.; Brittan, M.; Wilkinson, T.S.; McAuley, D.F.; Antonelli, J.; McCulloch, C.; Barr, L.C.; McDonald, N.A.; Dhaliwal, K.; Jones, R.O.; et al. C5a-mediated neutrophil dysfunction is RhoA-dependent and predicts infection in critically ill patients. Blood 2011, 117, 5178–5188. [Google Scholar] [CrossRef]
- Solomkin, J.; Jenkins, M.K.; Nelson, R.D.; Chenoweth, D.; Simmons, R.L. Neutrophil dysfunction in sepsis. II. Evidence for the role of complement activation products in cellular deactivation. Surgery 1981, 90, 319–327. [Google Scholar]
- Braun, L.; Christophe, T.; Boulay, F. Phosphorylation of Key Serine Residues Is Required for Internalization of the Complement 5a (C5a) Anaphylatoxin Receptor via a β-Arrestin, Dynamin, and Clathrin-dependent Pathway. J. Biol. Chem. 2003, 278, 4277–4285. [Google Scholar] [CrossRef]
- Sacks, T.; Moldow, C.F.; Craddock, P.R.; Bowers, T.K.; Jacob, H.S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J. Clin. Investig. 1978, 61, 1161–1167. [Google Scholar] [CrossRef]
- Mollnes, T.E.; Brekke, O.-L.; Fung, M.; Fure, H.; Christiansen, R.; Bergseth, G.; Videm, V.; Lappegård, K.T.; Koehl, J.; Lambris, J.D. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 2002, 100, 1869–1877. [Google Scholar]
- Karsten, C.M.; Pandey, M.K.; Figge, J.; Kilchenstein, R.; Taylor, P.R.; Rosas, M.; McDonald, J.U.; Orr, S.J.; Berger, M.; Petzold, D.; et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat. Med. 2012, 18, 1401–1406. [Google Scholar] [CrossRef]
- Riedemann, N.C.; Guo, R.-F.; Ward, P.A. A key role of C5a/C5aR activation for the development of sepsis. J. Leukoc. Biol. 2003, 74, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Lin, F.; Bao, C.; Huang, H.; Ji, C.; Wang, S.; Jin, L.; Sun, L.; Li, K.; Zhang, Z.; et al. Complement 5a receptor-mediated neutrophil dysfunction is associated with a poor outcome in sepsis. Cell. Mol. Immunol. 2016, 13, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Muenstermann, M.; Strobel, L.; Klos, A.; Wetsel, R.A.; Woodruff, T.M.; Köhl, J.; Johswich, K.O. Distinct roles of the anaphylatoxin receptors C3aR, C5aR1 and C5aR2 in experimental meningococcal infections. Virulence 2019, 10, 677–694. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Sarma, J.V.; Rittirsch, D.; Schreiber, H.; Weiss, M.; Flierl, M.; Younkin, E.; Schneider, M.; Suger-Wiedeck, H.; Gebhard, F.; et al. Changes in the Novel Orphan, C5a Receptor (C5L2), during Experimental Sepsis and Sepsis in Humans. J. Immunol. 2005, 174, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Pataky, R.; Howie, F.A.; Girardi, G.; Boardman, J.P. Complement C5a is present in CSF of human newborns and is elevated in association with preterm birth. J. Matern. Neonatal Med. 2017, 30, 2413–2416. [Google Scholar] [CrossRef]
- Schultz, C.; Temming, P.; Bucsky, P.; Göpel, W.; Strunk, T.; Härtel, C. Immature anti-inflammatory response in neonates. Clin. Exp. Immunol. 2004, 135, 130–136. [Google Scholar] [CrossRef]
- Morris, A.C.; Kefala, K.; Wilkinson, T.S.; Dhaliwal, K.; Farrell, L.; Walsh, T.; Mackenzie, S.J.; Reid, H.; Davidson, D.J.; Haslett, C.; et al. C5a Mediates Peripheral Blood Neutrophil Dysfunction in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2009, 180, 19–28. [Google Scholar] [CrossRef]
- Melvan, J.N.; Bagby, G.J.; Welsh, D.A.; Nelson, S.; Zhang, P. Neonatal Sepsis and Neutrophil Insufficiencies. Int. Rev. Immunol. 2010, 29, 315–348. [Google Scholar] [CrossRef]
- Peterson, L.S.; Hedou, J.; Ganio, E.A.; Stelzer, I.A.; Feyaerts, D.; Harbert, E.; Adusumelli, Y.; Ando, K.; Tsai, E.S.; Tsai, A.S.; et al. Single-Cell Analysis of the Neonatal Immune System Across the Gestational Age Continuum. Front. Immunol. 2021, 12, 714090. [Google Scholar] [CrossRef]
- Faix, J.D. Biomarkers of sepsis. Crit. Rev. Clin. Lab. Sci. 2013, 50, 23–36. [Google Scholar] [CrossRef]
- Fusakio, M.E.; Mohammed, J.P.; Laumonnier, Y.; Hoebe, K.; Köhl, J.; Mattner, J. C5a Regulates NKT and NK Cell Functions in Sepsis. J. Immunol. 2011, 187, 5805–5812. [Google Scholar] [CrossRef]
- Karsten, C.M.; Laumonnier, Y.; Eurich, B.; Ender, F.; Bröker, K.; Roy, S.; Czabanska, A.; Vollbrandt, T.; Figge, J.; Köhl, J. Monitoring and Cell-Specific Deletion of C5aR1 Using a Novel Floxed GFP-C5aR1 Reporter Knock-in Mouse. J. Immunol. 2015, 194, 1841–1855. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Liu, C.; Wei, Y.; Wang, N.; Yuan, G.; Liu, D.; Li, Z.; Zhou, W.; Li, K. Expression and regulation of complement receptors by human natural killer cells. Immunobiology 2014, 219, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Fehniger, T.A.; Caligiuri, M. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Bochennek, K.; Fryns, E.; Wittekindt, B.; Buxmann, H.; Quaiser, A.; Fischer, D.; Klingebiel, T.; Koehl, U.; Schloesser, R.; Huenecke, S. Immune cell subsets at birth may help to predict risk of late-onset sepsis and necrotizing enterocolitis in preterm infants. Early Hum. Dev. 2016, 93, 9–16. [Google Scholar] [CrossRef]
- Anderson, J.; Thang, C.M.; Thanh, L.Q.; Dai, V.T.T.; Phan, V.T.; Nhu, B.T.H.; Trang, D.N.X.; Trinh, P.T.P.; Nguyen, T.V.; Toan, N.T.; et al. Immune Profiling of Cord Blood From Preterm and Term Infants Reveals Distinct Differences in Pro-Inflammatory Responses. Front. Immunol. 2021, 12, 777927. [Google Scholar] [CrossRef]
- Orange, J.S.; Ballas, Z.K. Natural killer cells in human health and disease. Clin. Immunol. 2006, 118, 1–10. [Google Scholar] [CrossRef]
- Fauriat, C.; Long, E.O.; Ljunggren, H.-G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef]
- Guilmot, A.; Hermann, E.; Braud, V.M.; Carlier, Y.; Truyens, C. Natural Killer Cell Responses to Infections in Early Life. J. Innate Immun. 2011, 3, 280–288. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56brightnatural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Lusyati, S.; Hulzebos, C.V.; Zandvoort, J.; Sauer, P.J. Levels of 25 cytokines in the first seven days of life in newborn infants. BMC Res. Notes 2013, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- Furebring, M.; Håkansson, L.D.; Venge, P.; Nilsson, B.; Sjölin, J. Expression of the C5a receptor (CD88) on granulocytes and monocytes in patients with severe sepsis. Crit. Care 2002, 6, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.; Romero, R.; Richani, K.; Yoon, B.H.; Chaiworapongsa, T.; Vaisbuch, E.; Mittal, P.; Erez, O.; Gotsch, F.; Mazor, M.; et al. Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. J. Matern. Neonatal Med. 2009, 22, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Viemann, D. S100-Alarmins Are Essential Pilots of Postnatal Innate Immune Adaptation. Front. Immunol. 2020, 11, 688. [Google Scholar] [CrossRef]
- Ulas, T.; Pirr, S.; Fehlhaber, B.; Bickes, M.S.; Loof, T.G.; Vogl, T.; Mellinger, L.; Heinemann, A.S.; Burgmann, J.; Schöning, J.; et al. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat. Immunol. 2017, 18, 622–632. [Google Scholar] [CrossRef]
- Garred, P.; Tenner, A.J.; Mollnes, T.E. Therapeutic Targeting of the Complement System: From Rare Diseases to Pandemics. Pharmacol. Rev. 2021, 73, 792–827. [Google Scholar] [CrossRef]
- Biolegend.com. Available online: https://www.biolegend.com/en-us/protocols/cell-surface-flow-cytometry-staining-protocol (accessed on 29 September 2022).
- Biolegend.com. Available online: https://www.biolegend.com/en-us/protocols/intracellular-flow-cytometry-staining-protocol (accessed on 29 September 2022).


| Preterm infants (n) | 32 |
|---|---|
| Gestational age, weeks of gestation (mean/median/SD) | 29.5/29.9/2.69 |
| Birth weight, g (mean/median/SD) | 1331.6/1300.0/464.13 |
| Gender male (n/%) | 14/43.8 |
| Multiples (n/%) | 10/31.3 |
| AIS (n/%) | 8/25.0 |
| EOS (n/%) | 1/3.1 |
| Mothers (n) | 25 |
| Age, years (mean/median/SD) | 29.0/29.0/6.09 |
| Delivery at gestational age, weeks of gestation (mean/median/SD) | 29.7/29.9/2.68 |
| AIS yes (n/%) | 6/24.0 |
| Term infants (n) | 11 |
| Sex, male (n/%) | 4/36.4 |
| Adults (n) | 14 |
| Sex, male (n/%) | 6/42.9 |
| Age, years (mean/median/SD) | 28.2/25.0/5.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boeckel, H.; Karsten, C.M.; Göpel, W.; Herting, E.; Rupp, J.; Härtel, C.; Hartz, A. Increased Expression of Anaphylatoxin C5a-Receptor-1 in Neutrophils and Natural Killer Cells of Preterm Infants. Int. J. Mol. Sci. 2023, 24, 10321. https://doi.org/10.3390/ijms241210321
Boeckel H, Karsten CM, Göpel W, Herting E, Rupp J, Härtel C, Hartz A. Increased Expression of Anaphylatoxin C5a-Receptor-1 in Neutrophils and Natural Killer Cells of Preterm Infants. International Journal of Molecular Sciences. 2023; 24(12):10321. https://doi.org/10.3390/ijms241210321
Chicago/Turabian StyleBoeckel, Hannah, Christian M. Karsten, Wolfgang Göpel, Egbert Herting, Jan Rupp, Christoph Härtel, and Annika Hartz. 2023. "Increased Expression of Anaphylatoxin C5a-Receptor-1 in Neutrophils and Natural Killer Cells of Preterm Infants" International Journal of Molecular Sciences 24, no. 12: 10321. https://doi.org/10.3390/ijms241210321
APA StyleBoeckel, H., Karsten, C. M., Göpel, W., Herting, E., Rupp, J., Härtel, C., & Hartz, A. (2023). Increased Expression of Anaphylatoxin C5a-Receptor-1 in Neutrophils and Natural Killer Cells of Preterm Infants. International Journal of Molecular Sciences, 24(12), 10321. https://doi.org/10.3390/ijms241210321





