Characterization of the LysP2110-HolP2110 Lysis System in Ralstonia solanacearum Phage P2110
Abstract
:1. Introduction
2. Results
2.1. Genome Analysis of Bacteriophage P2110
2.2. Bioinformatics Analysis of the Phage P2110 Lysis System
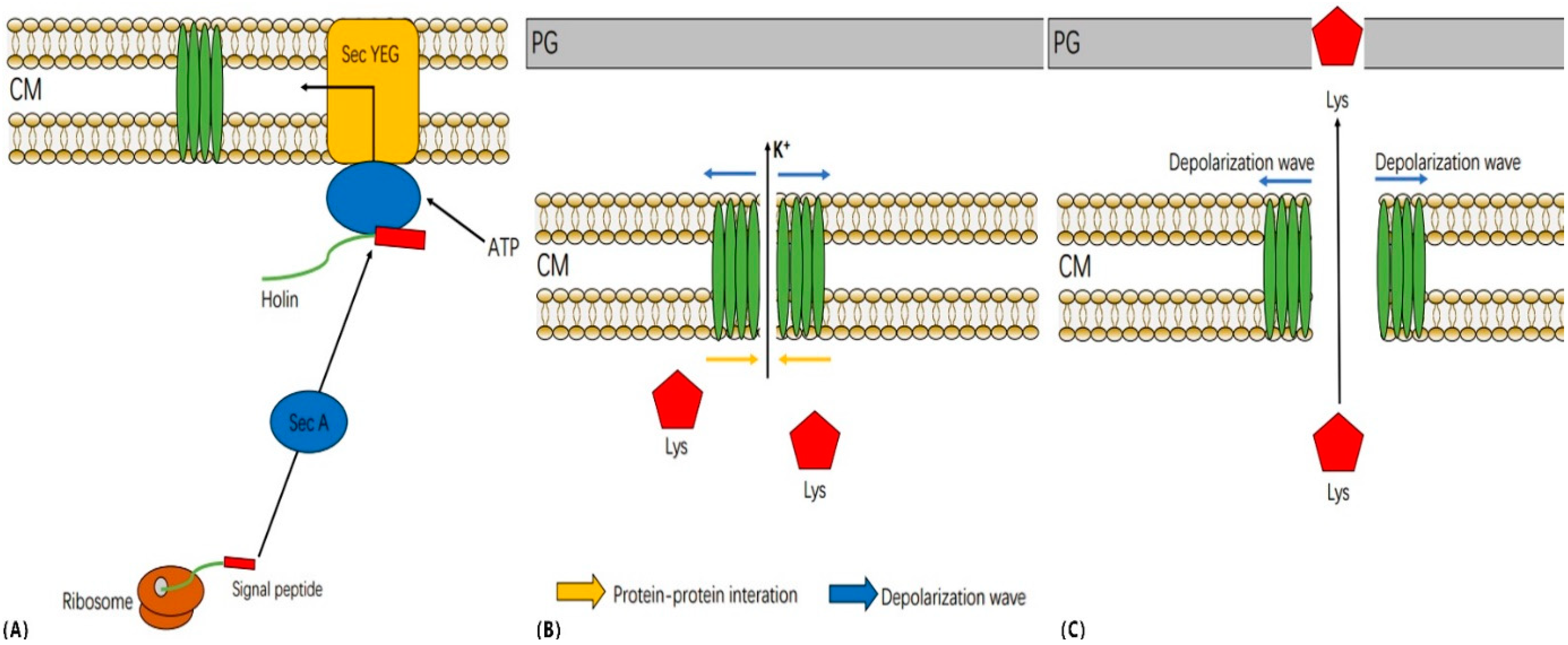
2.3. HolP2110 Is a Specific Holin Structure in the Ralstonia Phage
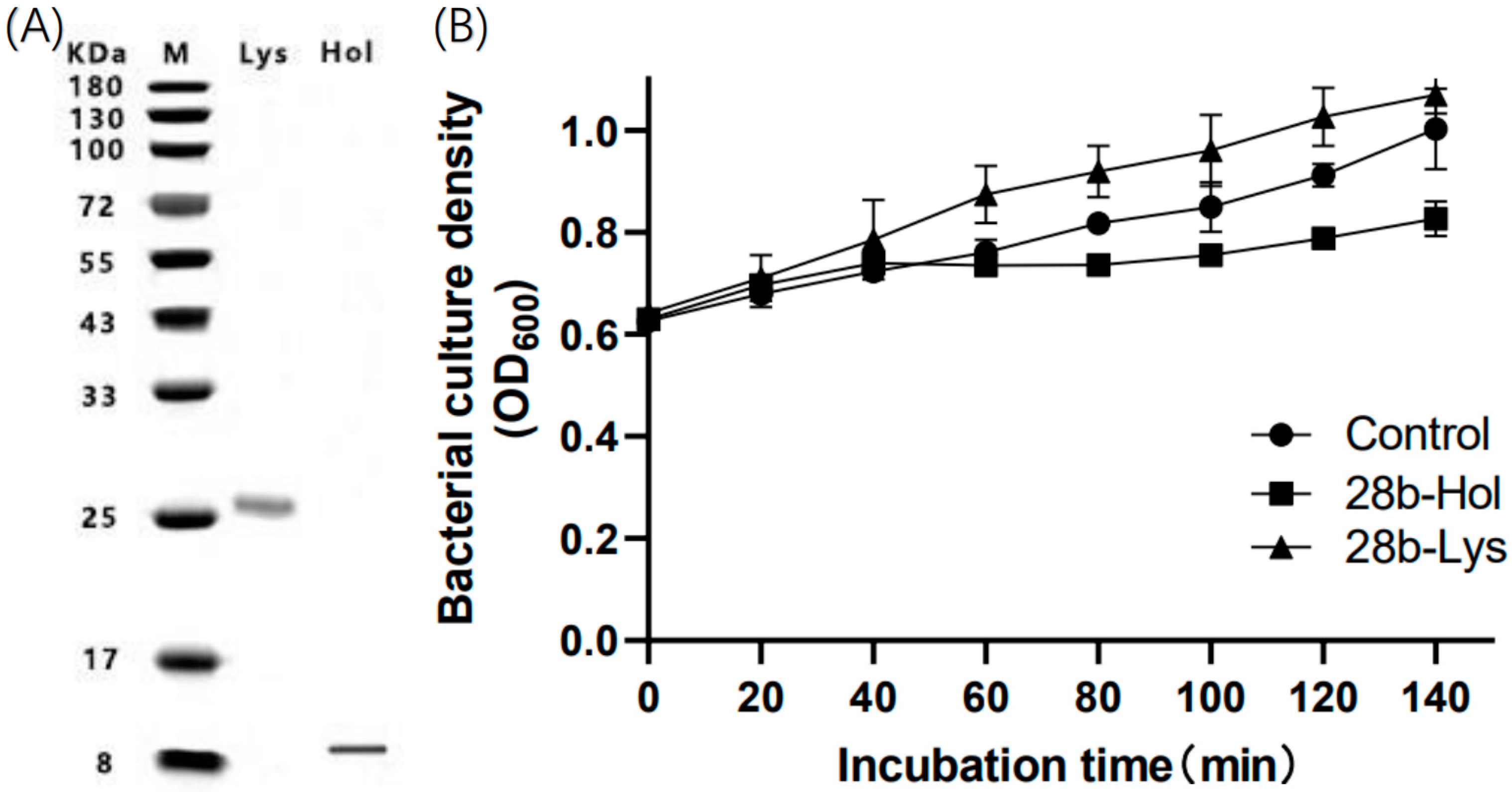
2.4. Impact of LysP2110 and HolP2110 Expression on Bacterial Growth
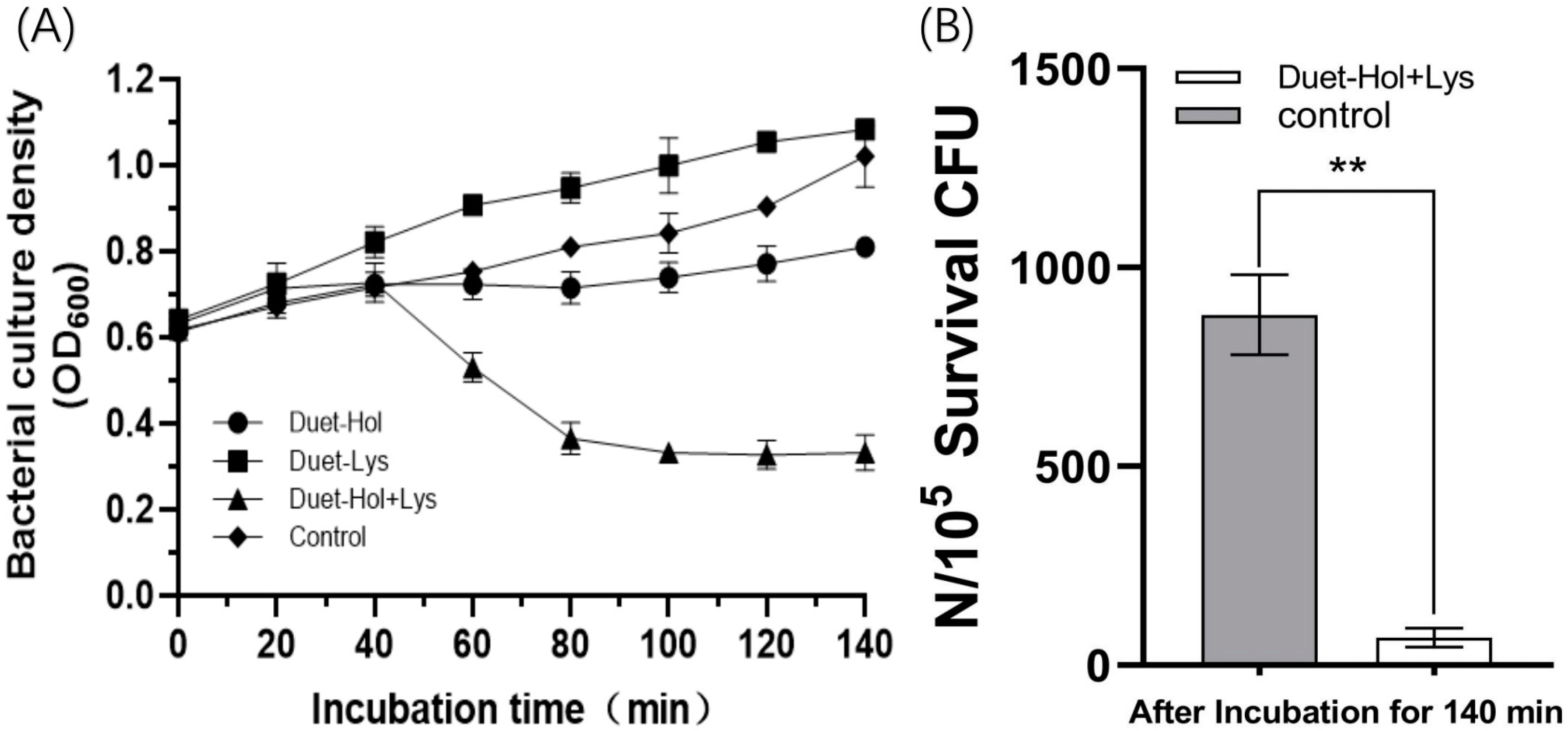
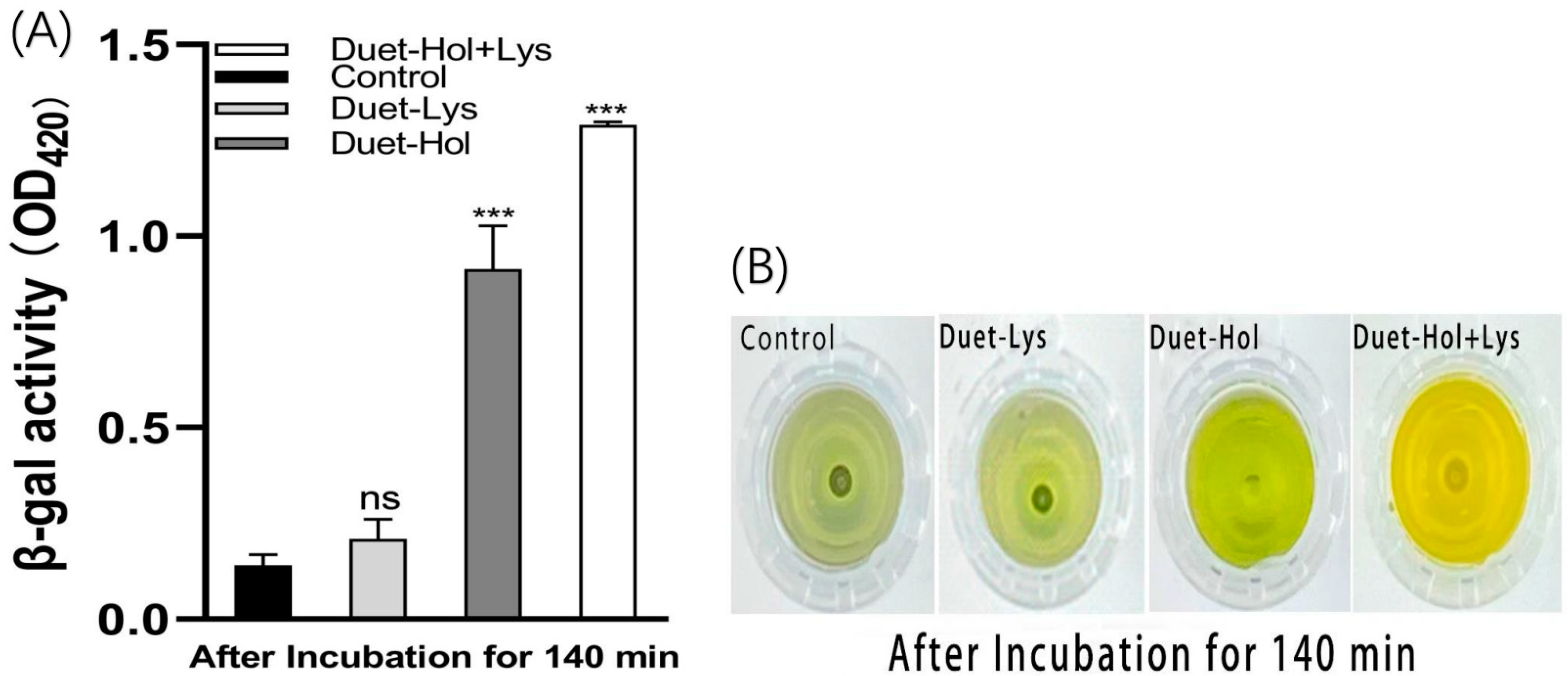
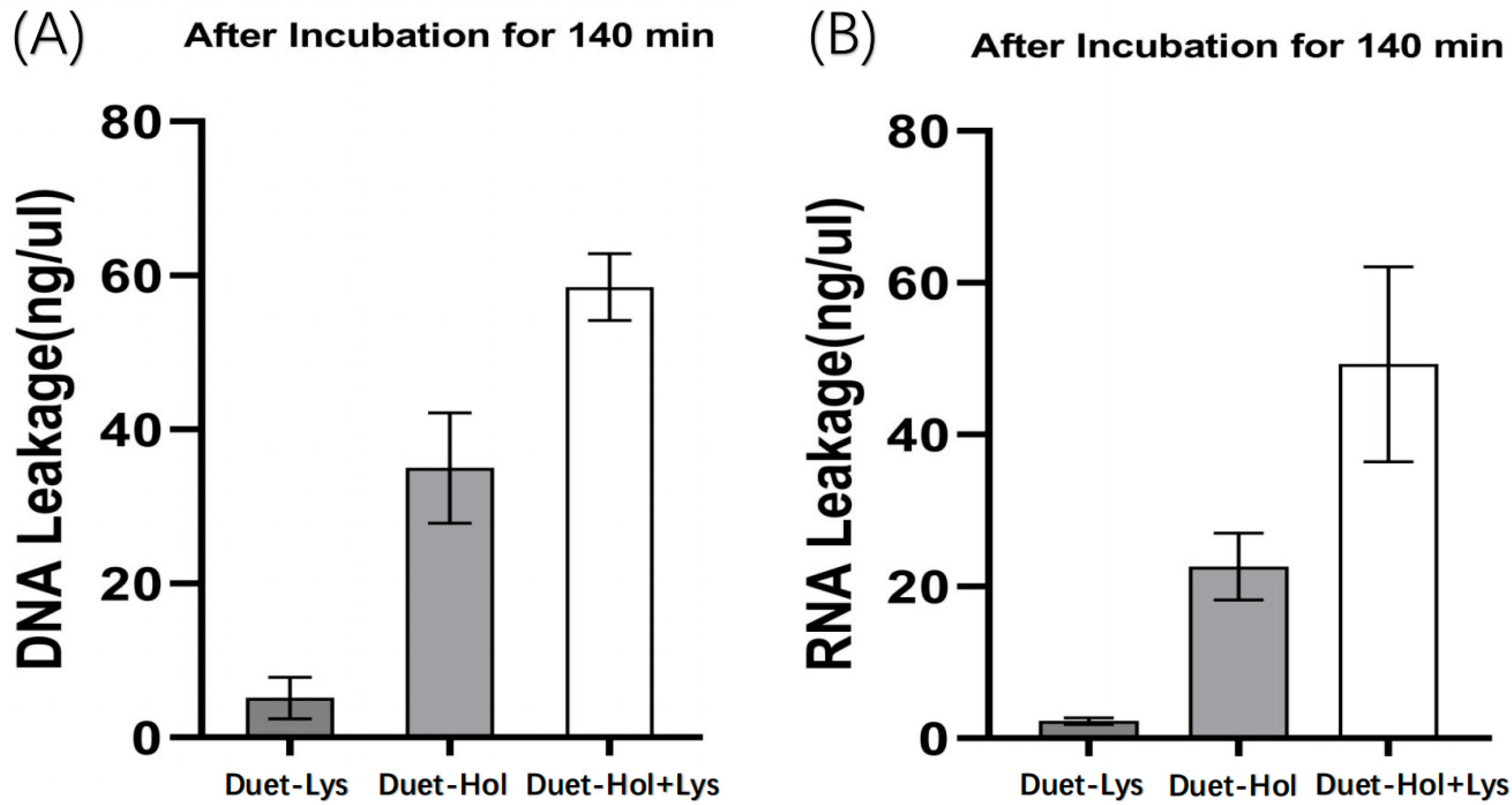
2.5. LysP2110 and HolP2110 Co-Expression and Their Effects on Cells
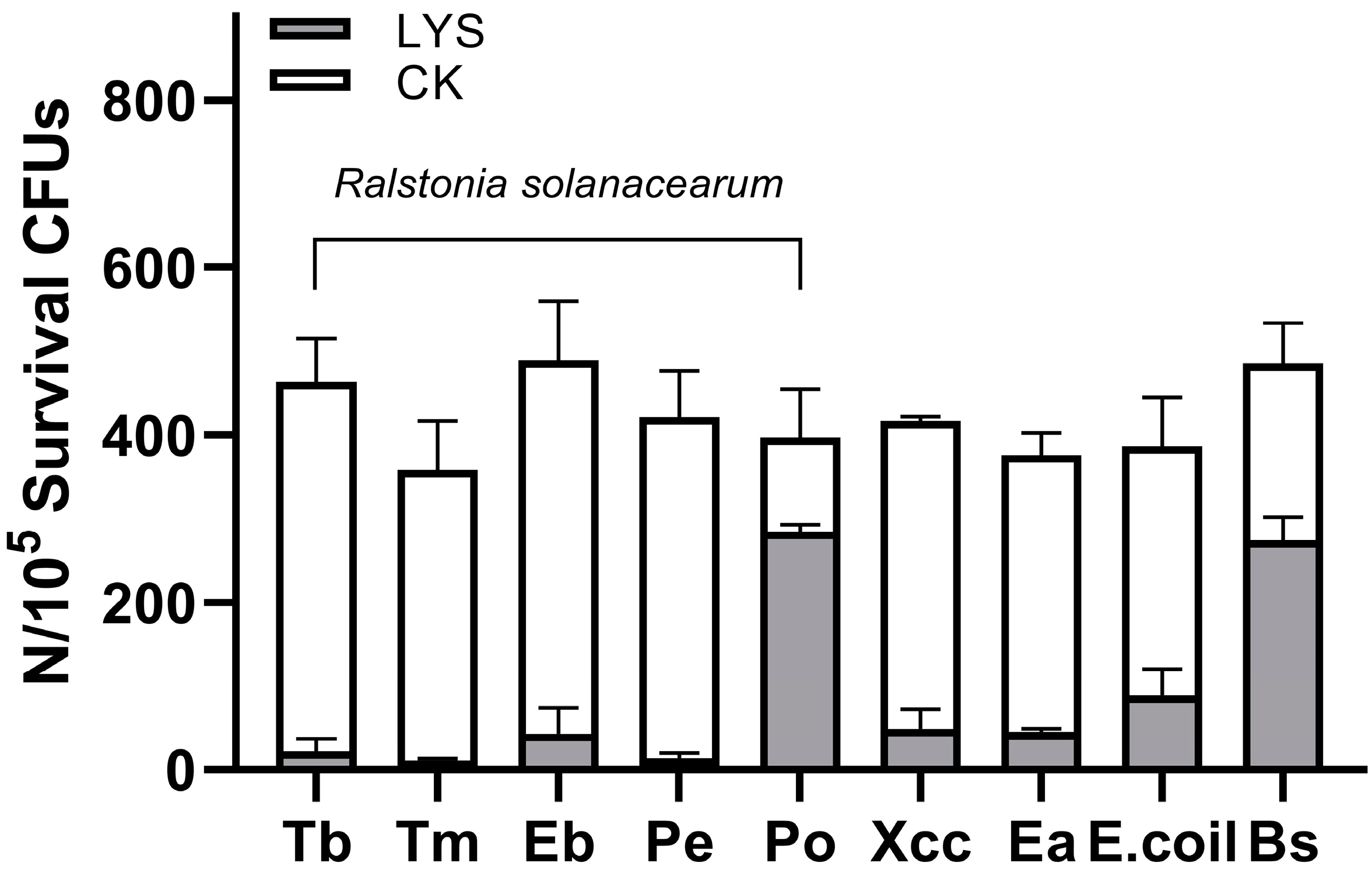
2.6. Purified LysP2110 Has Broad-Spectrum Antibacterial Activity
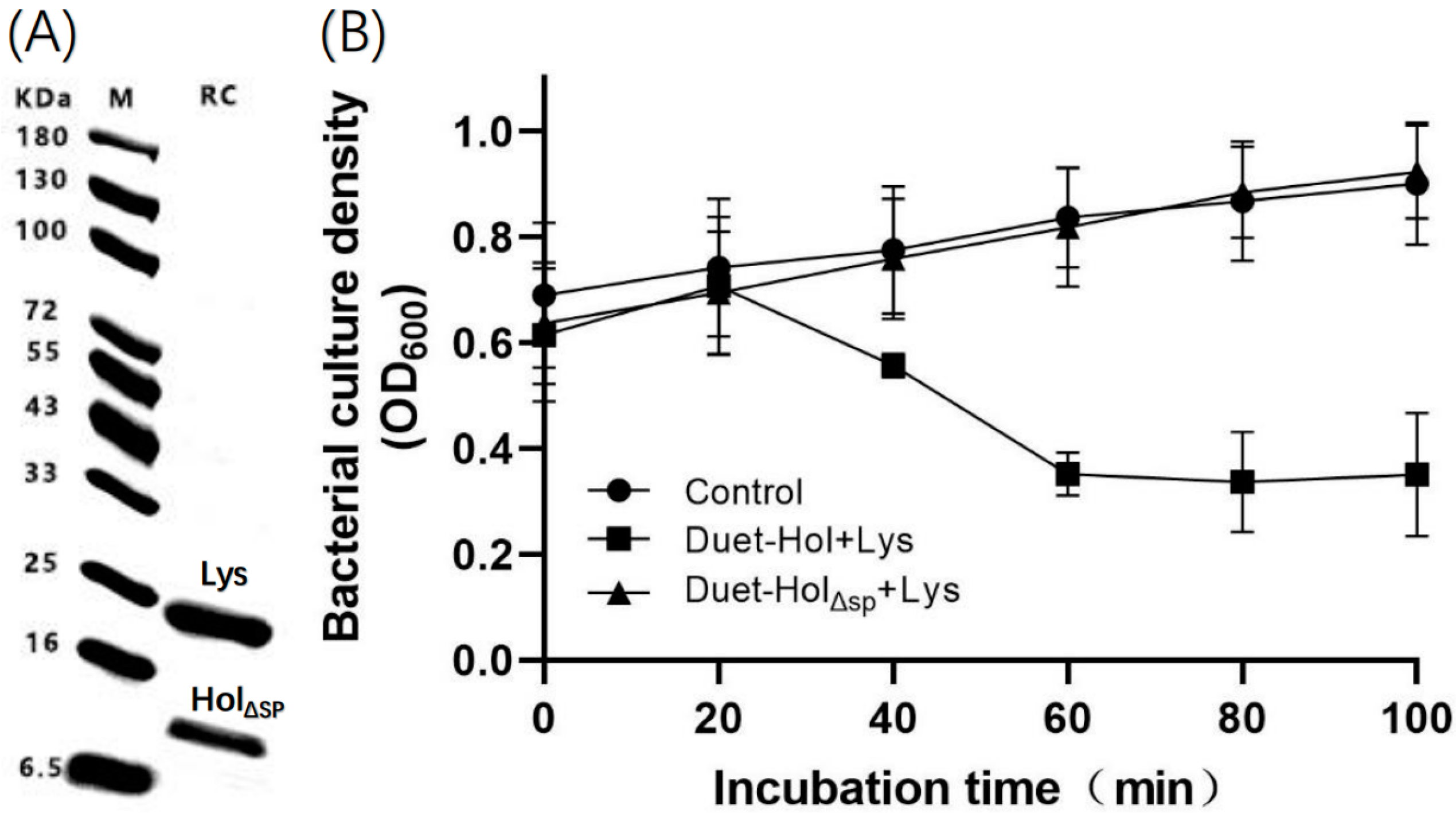
2.7. The N-Terminal Signal Peptide (SP) Plays an Important Role in HolP2110 Lysis
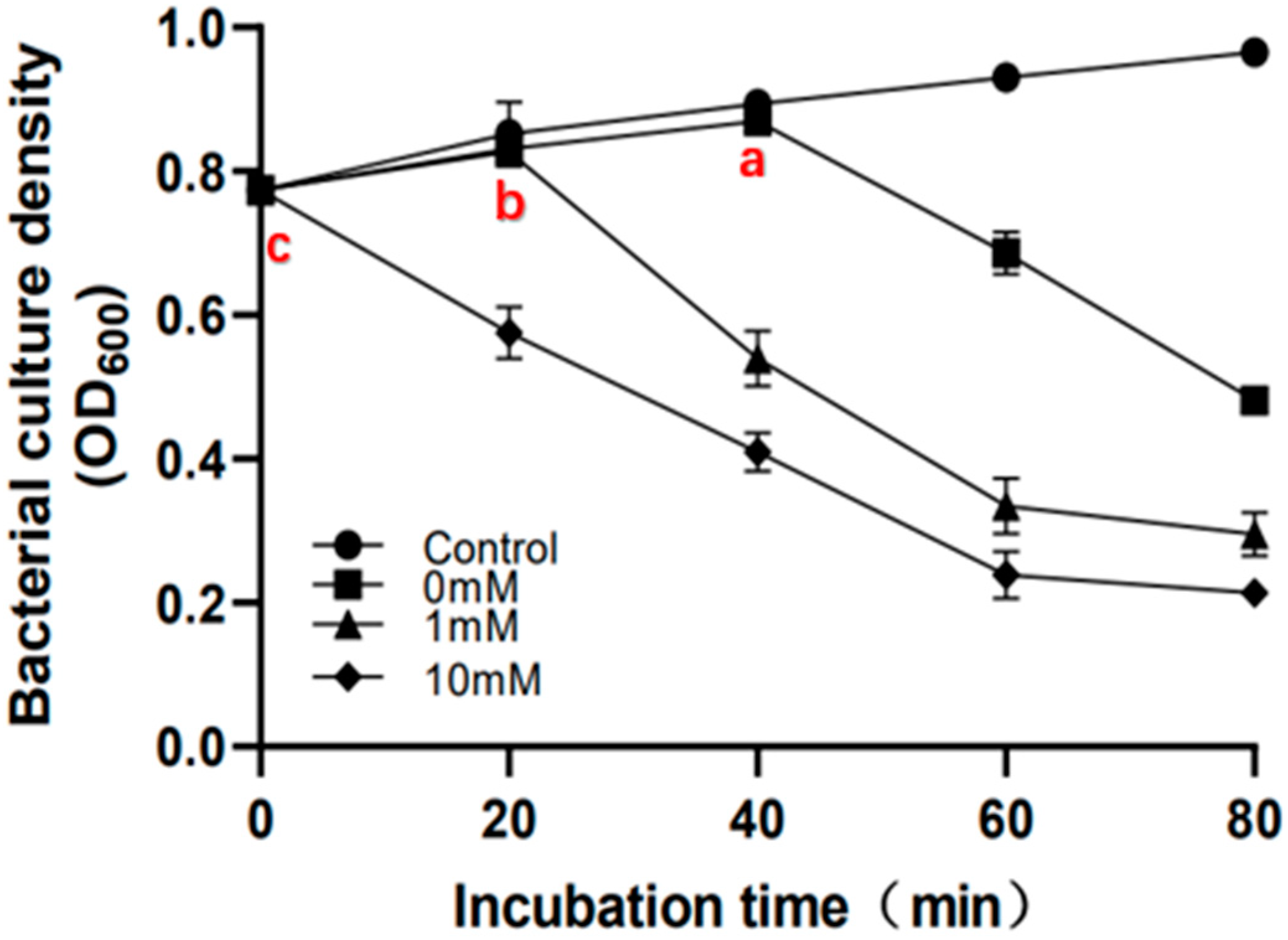
2.8. Sodium Azide Treatment Accelerates Bacterial Lysis Time
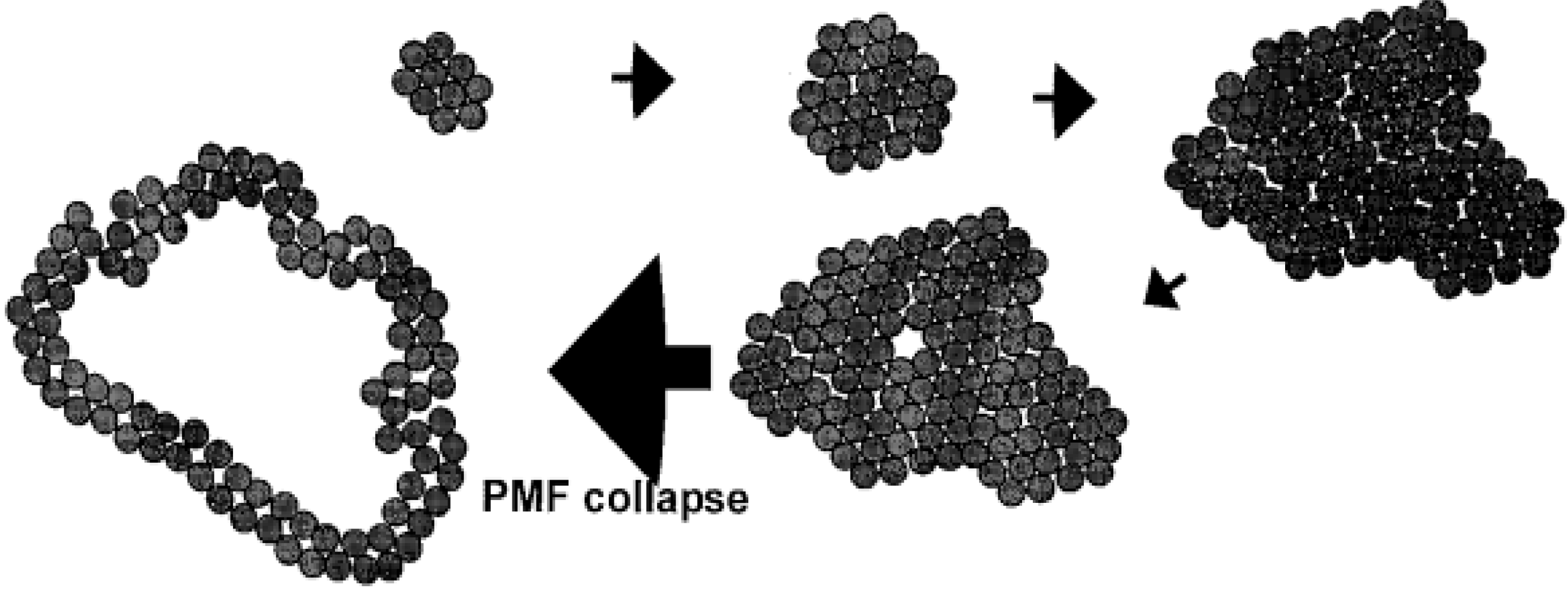
2.9. Early Lysis Leads to the Formation of Smaller Pores by Holin Proteins on the Inner Membrane
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Bacteriophages, Plasmids, and Growth Conditions
4.2. Genome Sequencing and Phylogenetic Analysis of Bacteriophage P2110
4.3. Prediction and Bioinformatics Analysis of the Lysis System in the Complete Genome of Bacteriophage P2110
4.4. Construction of Recombinant Plasmids and Cells
4.5. Growth Measurement
4.6. Detection of Extracellular β-Galactosidase Activity and Nucleic Acid Concentration
4.7. Purification of LysP2110, Antibacterial Activity, Determination of Lysis Spectrum, and Western Blotting
4.8. Effect of Energy Inhibitors on the PhageP2110 Lysis System
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayward, A.C. Biology and epidemiology of bacterial wilt caused by pseudomonas solanacearum. Annu. Rev. Phytopathol. 1991, 29, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wei, Z.; Xu, J.; Chen, H.; Zhang, Y.; She, X.; Macho, A.P.; Ding, W.; Liao, B. Bacterial Wilt in China: History, Current Status, and Future Perspectives. Front. Plant Sci. 2017, 8, 1549. [Google Scholar] [CrossRef] [PubMed]
- Nischal, P.M. First global report on antimicrobial resistance released by the WHO. Natl. Med. J. India 2015, 27, 241. [Google Scholar]
- Gill, J.J.; Hyman, P. Phage choice, isolation, and preparation for phage therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Pirisi, A. Phage therapy—Advantages over antibiotics? Lancet 2000, 356, 1418. [Google Scholar] [CrossRef] [PubMed]
- Leptihn, S.; Loh, B. Complexity, challenges and costs of implementing phage therapy. Futur. Microbiol. 2022, 17, 643–646. [Google Scholar] [CrossRef]
- Manohar, P.; Royam, M.M.; Loh, B.; Bozdogan, B.; Nachimuthu, R.; Leptihn, S. Synergistic Effects of Phage–Antibiotic Combinations against Citrobacter amalonaticus. ACS Infect. Dis. 2022, 8, 59–65. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Vijay, S.G.; Kusum, H.; Sanjay, C. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar]
- Bai, J.; Lee, S.; Ryu, S. Identification and in vitro Characterization of a Novel Phage Endolysin that Targets Gram-Negative Bacteria. Microorganisms 2020, 8, 447. [Google Scholar] [CrossRef] [Green Version]
- Wang, I.N.; Smith, D.L.; Young, R. Holins: The Protein Clocks of Bacteriophage Infections. Annu. Rev. Microbiol. 2000, 54, 799–825. [Google Scholar] [CrossRef]
- Rennell, D.; Poteete, A.R. Phage P22 lysis genes: Nucleotide sequences and functional relationships with T4 and lambda genes. Virology 1985, 143, 280–289. [Google Scholar] [CrossRef]
- Catalao, M.J.; Gil, F.; Moniz-Pereira, J.; São-José, C.; Pimentel, M. Diversity in bacterial lysis systems: Bacteriophages show the way. FEMS Microbiol. Rev. 2013, 37, 554–571. [Google Scholar] [CrossRef] [Green Version]
- Young, R. Phage lysis: Do we have the hole story yet? Curr. Opin. Microbiol. 2013, 16, 790–797. [Google Scholar] [CrossRef] [Green Version]
- Gründling, A.; Bläsi, U.; Young, R. Biochemical and genetic evidence for three transmembrane domains in the class I holin, lambda S. J. Biol. Chem. 2000, 275, 769–776. [Google Scholar] [CrossRef] [Green Version]
- White, R.; Chiba, S.; Pang, T.; Dewey, J.S.; Savva, C.G.; Holzenburg, A.; Pogliano, K.; Young, R. Holin triggering in real time. Proc. Natl. Acad. Sci. USA 2010, 108, 798–803. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Arulandu, A.; Struck, D.K.; Swanson, S.; Sacchettini, J.C.; Young, R. Disulfide isomerization after membrane release of its Sar domain activates P1 lysozyme. Science 2005, 307, 113–117. [Google Scholar] [CrossRef]
- Jan, B.; Beata, W.; Andrzej, G. Bacteriophage Endolysins as a Novel Class of Antibacterial Agents. Exp. Biol. Med. 2006, 231, 366–377. [Google Scholar]
- Ghose, C.; Euler, C.W. Gram-Negative Bacterial Lysins. Antibiotics 2020, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Pastagia, M.; Euler, C.; Chahales, P.; Fuentes-Duculan, J.; Krueger, J.G.; Fischetti, V.A. A Novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus Strains. Antimicrob. Agents Chemother. 2011, 55, 738–744. [Google Scholar] [CrossRef] [Green Version]
- Jutta, M.L.; Daniel, N.; Vincent, A.F. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 2001, 294, 2170–2172. [Google Scholar]
- Cheng, Q.; Nelson, D.; Zhu, S.; Fischetti, V.A. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob. Agents Chemother. 2005, 49, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, J.; Young, R. Phage LYSIS: Multiple Genes for Multiple Barriers. In Advances in Virus Research; Academic Press Inc.: Cambridge, MA, USA, 2019; Volume 103, pp. 33–70. ISBN 9780128177228. [Google Scholar]
- Raab, R.; Neal, G.; Sohaskey, C.; Smith, J.; Young, R. Dominance in lambda S mutations and evidence for translational control. J. Mol. Biol. 1988, 199, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.L.; Saier, M.H. Topological and phylogenetic analyses of bacterial holin families and superfamilies. Biochim. Biophys. Acta (BBA)—Biomembr. 2013, 1828, 2654–2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Thapa Magar, R.; Kim, H.J.; Choi, K.; Lee, S.W. Complete genome sequence of a novel bacteriophage RpY1 infecting Ralstonia solanacearum Strains. Curr. Microbiol. 2021, 78, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, D.; Liu, Q.; Zhu, P.; Sun, M.; Peng, D. Isolation and characterization of novel lytic bacteriophage vB_RsoP_BMB50 infecting Ralstonia solanacearum. Curr. Microbiol. 2022, 79, 1–12. [Google Scholar] [CrossRef]
- da Silva Xavier, A.; da Silva, F.P.; Vidigal, P.M.P.; Lima, T.T.M.; de Souza, F.O.; Alfenas-Zerbini, P. Genomic and biological characterization of a new member of the genus Phikmvvirus infecting phytopathogenic Ralstonia bacteria. Arch. Virol. 2018, 163, 3275–3290. [Google Scholar] [CrossRef]
- São-José, C.; Parreira, R.; Vieira, G.; Santos, M.A. The N-terminal region of the Oenococcus oeni bacteriophage fOg44 lysin behaves as a bona fide signal peptide in Escherichia coli and as a cis-inhibitory element, preventing lytic activity on oenococcal cells. J. Bacteriol. 2000, 182, 5823–5831. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, N.; Manohar, P.; Eniyan, K.; Archana, L.; Athira, S.; Loh, B.; Ma, L.; Leptihn, S. A lysozyme murein hydrolase with broad-spectrum antibacterial activity from enterobacter phage myPSH1140. Antimicrob. Agents Chemother. 2022, 66, e00506-22. [Google Scholar] [CrossRef]
- Briers, Y.; Peeters, L.M.; Volckaert, G.; Lavigne, R. The lysis cassette of bacteriophage ϕKMV encodes a signal-arrest-release endolysin and a pinholin. Bacteriophage 2011, 1, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Krupovič, M.; Daugelavičius, R.; Bamford, D.H. A novel lysis system in PM2, a lipid-containing marine double-stranded DNA bacteriophage. Mol. Microbiol. 2007, 64, 1635–1648. [Google Scholar] [CrossRef]
- Wang, I.N.; Deaton, J.; Youn, R. Sizing the holin lesion with an endolysin-beta-galactosidase fusion. J. Bacteriol. 2003, 185, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Žiedaitė, G.; Daugelavičius, R.; Bamford, J.K.H.; Bamford, D.H. The holin protein of bacteriophage PRD1 forms a pore for small-molecule and endolysin translocation. J. Bacteriol. 2005, 187, 5397–5405. [Google Scholar] [CrossRef] [Green Version]
- Gaidelyte, A.; Jaatinen, S.T.; Daugelavičius, R.; Bamford, J.K.H.; Bamford, D.H. The linear double-stranded dna of phage Bam35 enters lysogenic host cells, but the late phage functions are suppressed. J. Bacteriol. 2005, 187, 3521–3527. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Hong, F.-L.; Wang, H.-H.; Yuan, Q.-H.; Ma, W.-Y.; Gao, X.-N.; Shi, R.; Zhang, R.-J.; Sun, C.-S.; Wang, S.-B. Modified recombinant proteins can be exported via the sec pathway in escherichia coli. PLoS ONE 2012, 7, e42519. [Google Scholar] [CrossRef]
- Tsirigotaki, A.; De Geyter, J.; Šoštaric, N.; Economou, A.; Karamanou, S. Protein export through the bacterial Sec pathway. Nat. Rev. Microbiol. 2017, 15, 21–36. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Grymonprez, B.; Biebl, M.; Pirnay, J.-P.; Defraine, V.; Michiels, J.; Cenens, W.; Aertsen, A.; Miller, S.; et al. Art-175 is a highly efficient antibacterial against multidrug-resistant strains and persisters of pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 3774–3784. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Zhao, S.; Dou, J.; Xu, X.; Zhi, Y.; Wen, L. Engineered endolysin-based “artilysins” for controlling the gram-negative pathogen Helicobacter pylori. AMB Express 2021, 11, 63. [Google Scholar] [CrossRef]
- Ahrenholtz, I.; Harms, K.; De Vries, J.; Wackernagel, W. Increased killing of Bacillus subtilison the hair roots of transgenic T4 lysozyme-producing potatoes. Appl. Environ. Microbiol. 2000, 66, 1862–1865. [Google Scholar] [CrossRef] [Green Version]
- Serrano, C.; Arce-Johnson, P.; Torres, H.; Gebauer, M.; Gutierrez, M.; Moreno, M.; Jordana, X.; Venegas, A.; Kalazich, J.; Holuigue, L. Expression of the chicken lysozyme gene in potato enhances resistance to infection by Erwinia carotovora subsp. atroseptica. Am. J. Potato Res. 2000, 77, 191–199. [Google Scholar] [CrossRef]
- Düring, K. Genetic engineering for resistance to bacteria in transgenic plants by introduction of foreign genes. Mol. Breed. 1996, 2, 297–305. [Google Scholar] [CrossRef]
- Gründling, A.; Manson, M.D.; Young, R. Holins kill without warning. Proc. Natl. Acad. Sci. USA 2001, 98, 9348–9352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakočiūnė, D.; Moodley, A. A rapid bacteriophage DNA extraction method. Methods Protoc. 2018, 1, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleimani-Delfan, A.; Bouzari, M.; Wang, R. A rapid competitive method for bacteriophage genomic DNA extraction. J. Virol. Methods 2021, 293, 114148. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Chen, J.; Hong, X.; Xu, X.; Wu, Z.; Ahmed, T.; Loh, B.; Leptihn, S.; Hassan, S.; et al. Identification and Characterization of a new Type of holin-endolysin lysis cassette in acidovorax oryzae phage AP1. Viruses 2022, 14, 167. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Lavigne, R. Use of bacteriophage endolysin EL188 and outer membrane permeabilizers against Pseudomonas aeruginosa. J. Appl. Microbiol. 2011, 110, 778–785. [Google Scholar] [CrossRef] [Green Version]
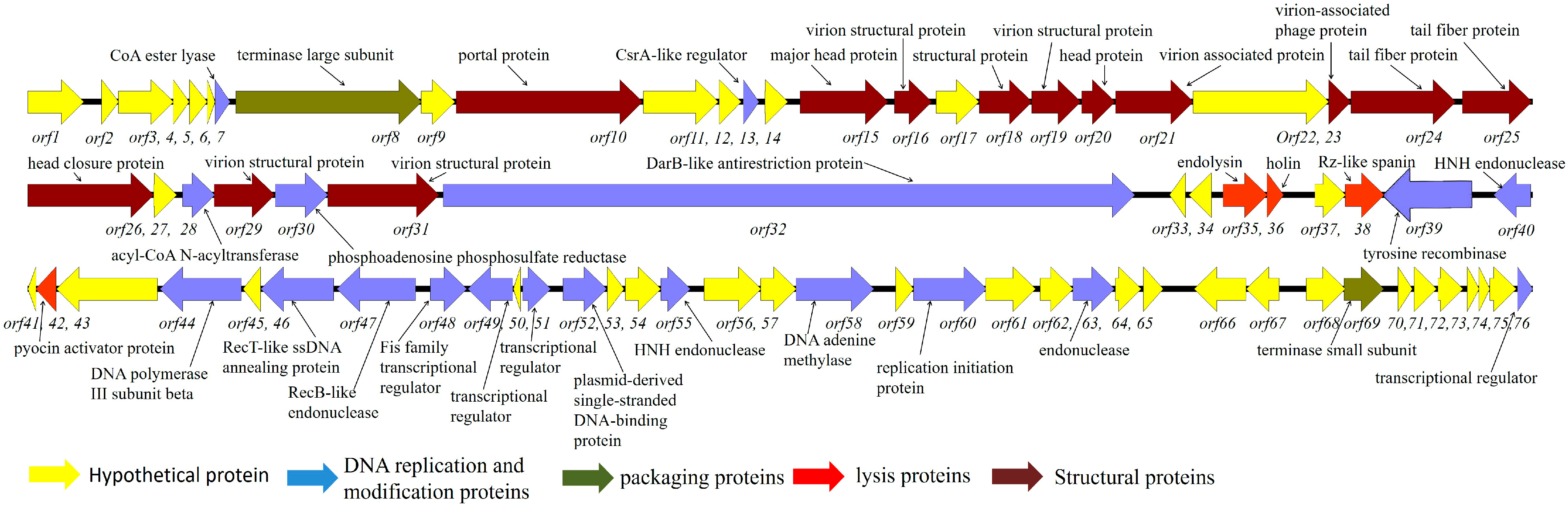
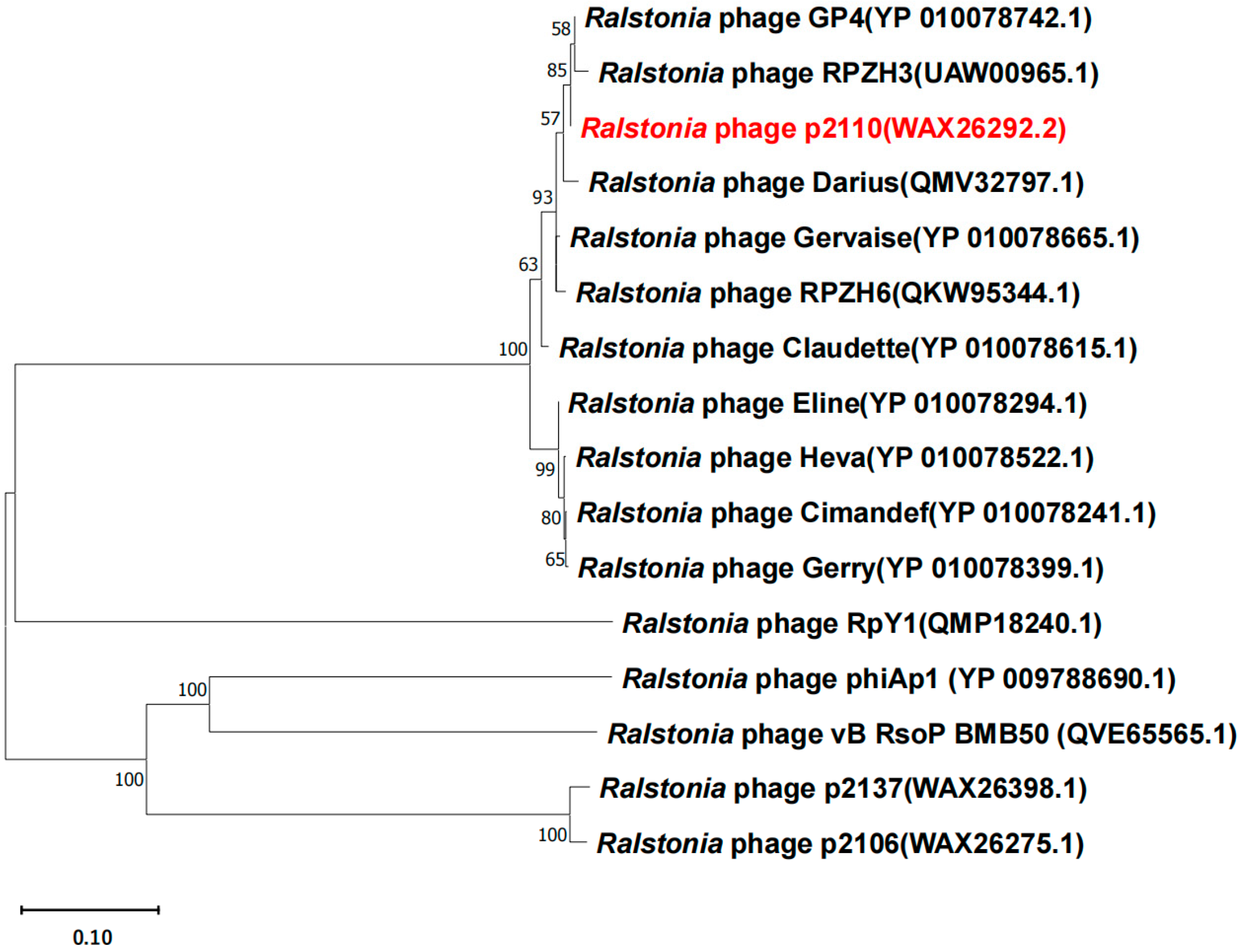
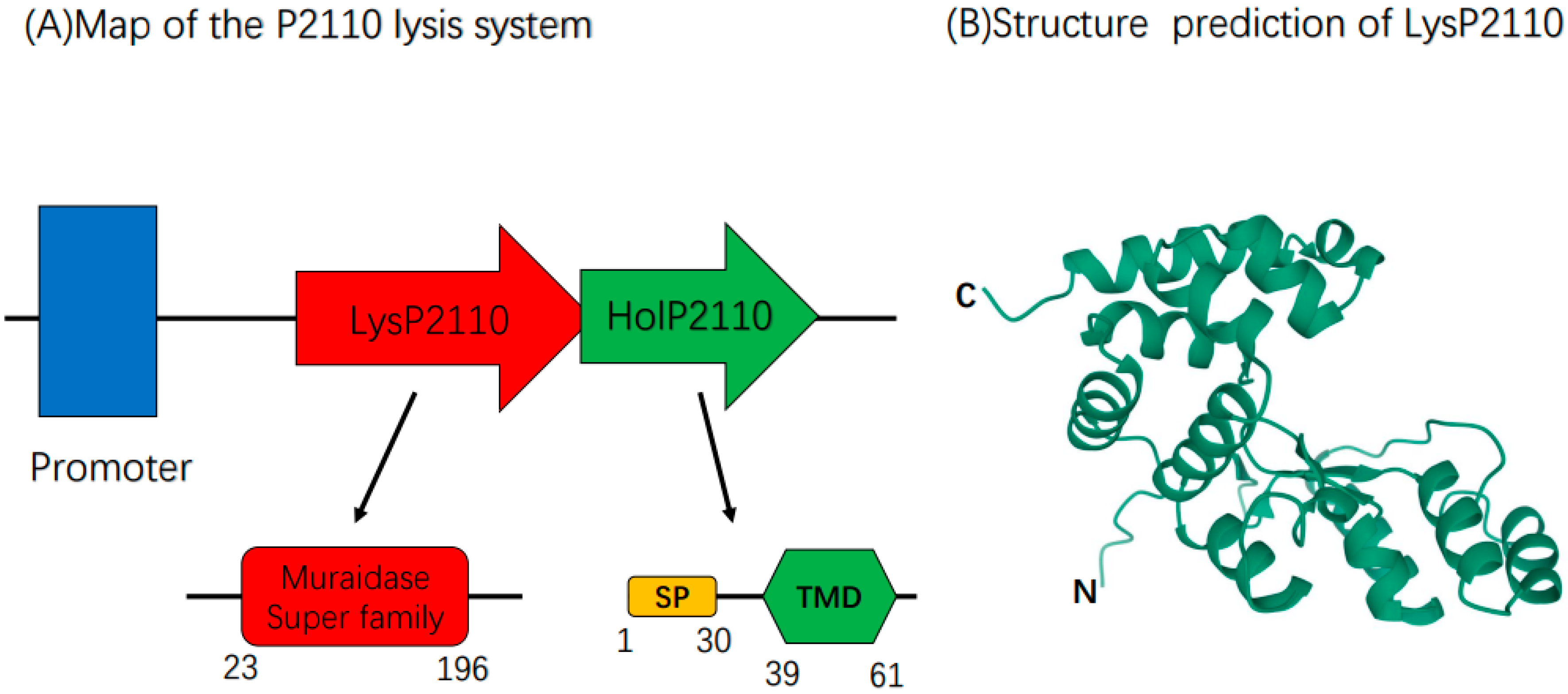

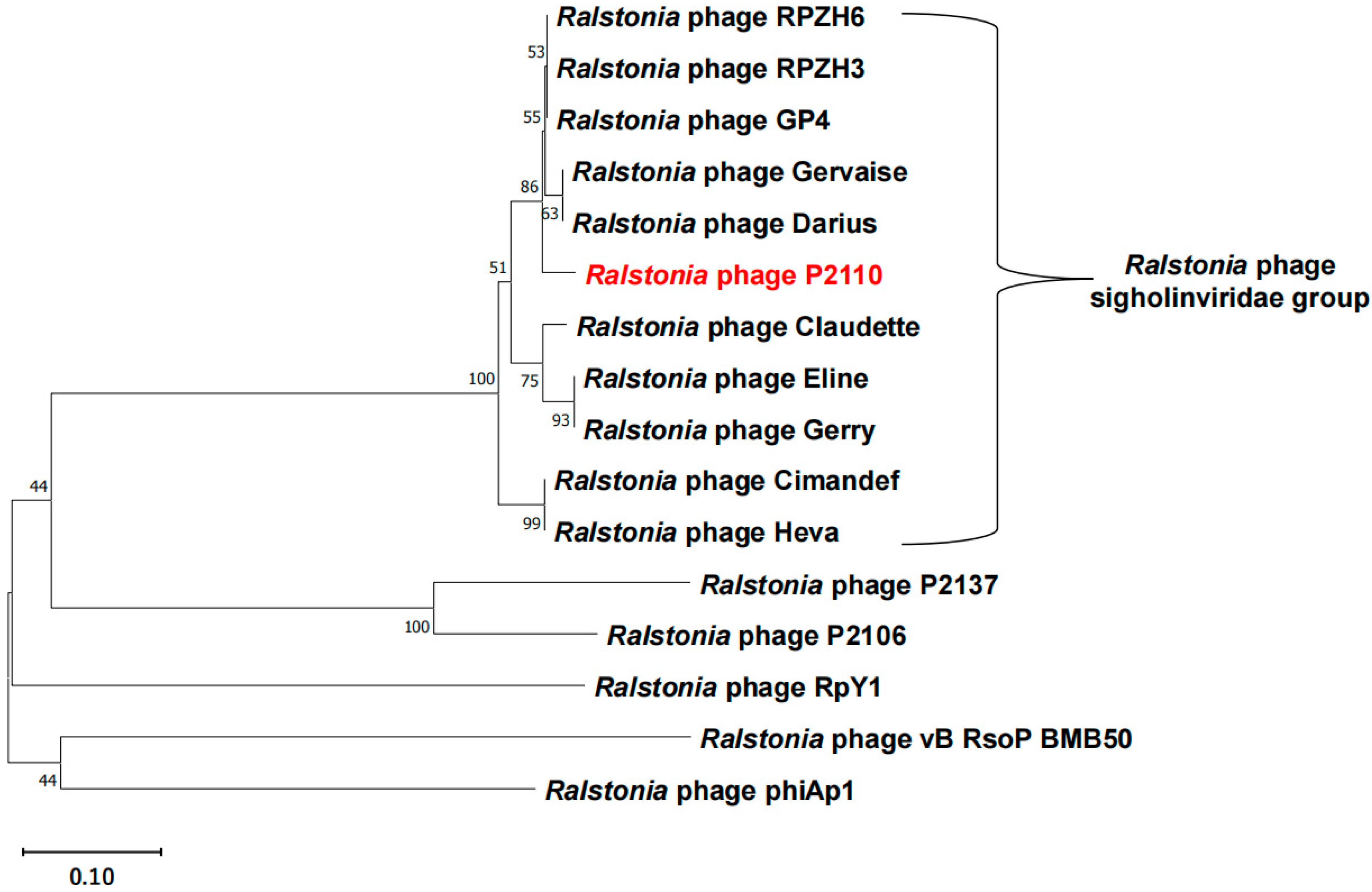



| Name | Length | Accession No. |
|---|---|---|
| Ralstonia phage p2110 | 59,380 bp | OP947226.1 |
| Ralstonia phage GP4 | 61,129 bp | NC_054964.1 |
| Ralstonia phage Claudette | 57,085 bp | NC_054962.1 |
| Ralstonia phage Gervaise | 61,164 bp | NC_054963.1 |
| Ralstonia phage RpY1 | 43,284 bp | QMP18240.1 |
| Ralstonia phage RPZH6 | 64,657 bp | MT361768.1 |
| Ralstonia phage Darius | 60,734 bp | MT740732.1 |
| Ralstonia phage RPZH3 | 65,958 bp | MZ870514.1 |
| Ralstonia phage Cimandef | 55,171 bp | NC_054956.1 |
| Ralstonia phage Heva | 58,352 bp | NC_054960.1 |
| Ralstonia phage P2137 | 37,595 bp | WAX26398.1 |
| Ralstonia phage P2106 | 35,928 bp | WAX26275.1 |
| Ralstonia phage Gerry | 60,898 bp | NC_054959.1 |
| Ralstonia phage Eline | 57,352 bp | NC_054957.1 |
| Ralstonia phage phiAp1 | 44,793 bp | YP 009788690.1 |
| Ralstonia phage vB RsoP BMB50 | 43,665 bp | QVE65565.1 |
| Name | Source | Length | Accession No. |
|---|---|---|---|
| holin | Ralstonia phage Cimandef | 76 | YP_010078203.1 |
| holin | Ralstonia phage p2110 | 75 | WAX26318.1 |
| holin | Ralstonia phage GP4 | 75 | YP_010078796.1 |
| holin | Ralstonia phage Gervaise | 75 | YP_010078692.1 |
| holin | Ralstonia phage Eline | 76 | YP_010078319.1 |
| holin | Ralstonia phage Claudette | 76 | YP_010078659.1 |
| holin | Ralstonia phage Heva | 76 | YP_010078482.1 |
| hypothetical protein | Ralstonia phage Darius | 75 | QMV32770.1 |
| hypothetical protein | Ralstonia phage Alix | 76 | QMV32463.1 |
| hypothetical protein | Ralstonia phage RPZH3 | 75 | UAW01020.1 |
| hypothetical protein | Ralstonia phage RPZH6 | 75 | QKW95398.1 |
| hypothetical protein | Ralstonia phage P2137 | 70 | WAX26396.1 |
| hypothetical protein | Ralstonia phage P2106 | 69 | WAX26273.1 |
| holin | Ralstonia phage BMB50 | 60 | QVE65567.1 |
| holin | Ralstonia phage phiAp1 | 152 | APU03191.1 |
| holin | Ralstonia phage RpY1 | 72 | QMP18260.1 |
| Type | Organism | Name | Source |
|---|---|---|---|
| Bacteria | Ralstonia solanacearum | Tb1546 | SCAUBL |
| TmSZ-1 | SCAUBL | ||
| Eg1906 | SCAUBL | ||
| Po36 | SCAUBL | ||
| Pe1301 | SCAUBL | ||
| Pectobacterium carotovorum subsp. caroto-vorum | Pcc1806 | SCAUBL | |
| Xanthomonas citri subsp. citri | Xcc63 | SCAUBL | |
| Bacillus subtilis | Bs03 | SCAUBL | |
| Escherichia coli | BL21(ED3) | BJTB | |
| Phage | Ralstonia phage | P2110 | SCAUBL |
| Plasmids | ND | Pet28b | BJTB |
| PetDuet1 | BJTB |
| Primer | Sequences (5′-3′) | Size (bp) | Characterization |
|---|---|---|---|
| 28b-Lys-F | CCCCATATGATGCCACAAACTAGCAAGC | 28 | Gene of Lys from P2110 phage |
| 28b-Lys-R | TTCTCGAGTCATGCGGGTCCCGTTTG | 26 | |
| 28b-Hol-F | GAATTCATGAACCTCATCGAAAACGCC | 27 | Gene of Hol from P2110 phage |
| 28b-Hol-R | AAGCTTGGTCTTCACTTCGGGTCGG | 25 | |
| Duet-Lys-F | CCCATATGCCACAAACTAGCAAGC | 24 | Gene of Lys from P2110 phage |
| Duet-Lys-R | TTCTCGAGTCATGCGGGTCCCGTTTG | 26 | |
| Duet-Hol-F | CCGAATTCGATGAACCTCATCGAAAACGCC | 30 | Gene of Hol from P2110 phage |
| Duet-Hol-R | TTAAGCTTGGTCTTCACTTCGGGTCGG | 27 | |
| Duet-HolΔSP | TATGAATTCGCCCTGCCGCTGTGGA | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Guan, Y.; Hu, R.; Cui, X.; Liu, Q. Characterization of the LysP2110-HolP2110 Lysis System in Ralstonia solanacearum Phage P2110. Int. J. Mol. Sci. 2023, 24, 10375. https://doi.org/10.3390/ijms241210375
Chen K, Guan Y, Hu R, Cui X, Liu Q. Characterization of the LysP2110-HolP2110 Lysis System in Ralstonia solanacearum Phage P2110. International Journal of Molecular Sciences. 2023; 24(12):10375. https://doi.org/10.3390/ijms241210375
Chicago/Turabian StyleChen, Kaihong, Yanhui Guan, Ronghua Hu, Xiaodong Cui, and Qiongguang Liu. 2023. "Characterization of the LysP2110-HolP2110 Lysis System in Ralstonia solanacearum Phage P2110" International Journal of Molecular Sciences 24, no. 12: 10375. https://doi.org/10.3390/ijms241210375




