Quantitative Loop-Mediated Isothermal Amplification Detection of Ustilaginoidea virens Causing Rice False Smut
Abstract
1. Introduction
2. Results
2.1. Design of Primers for U. virens Detection
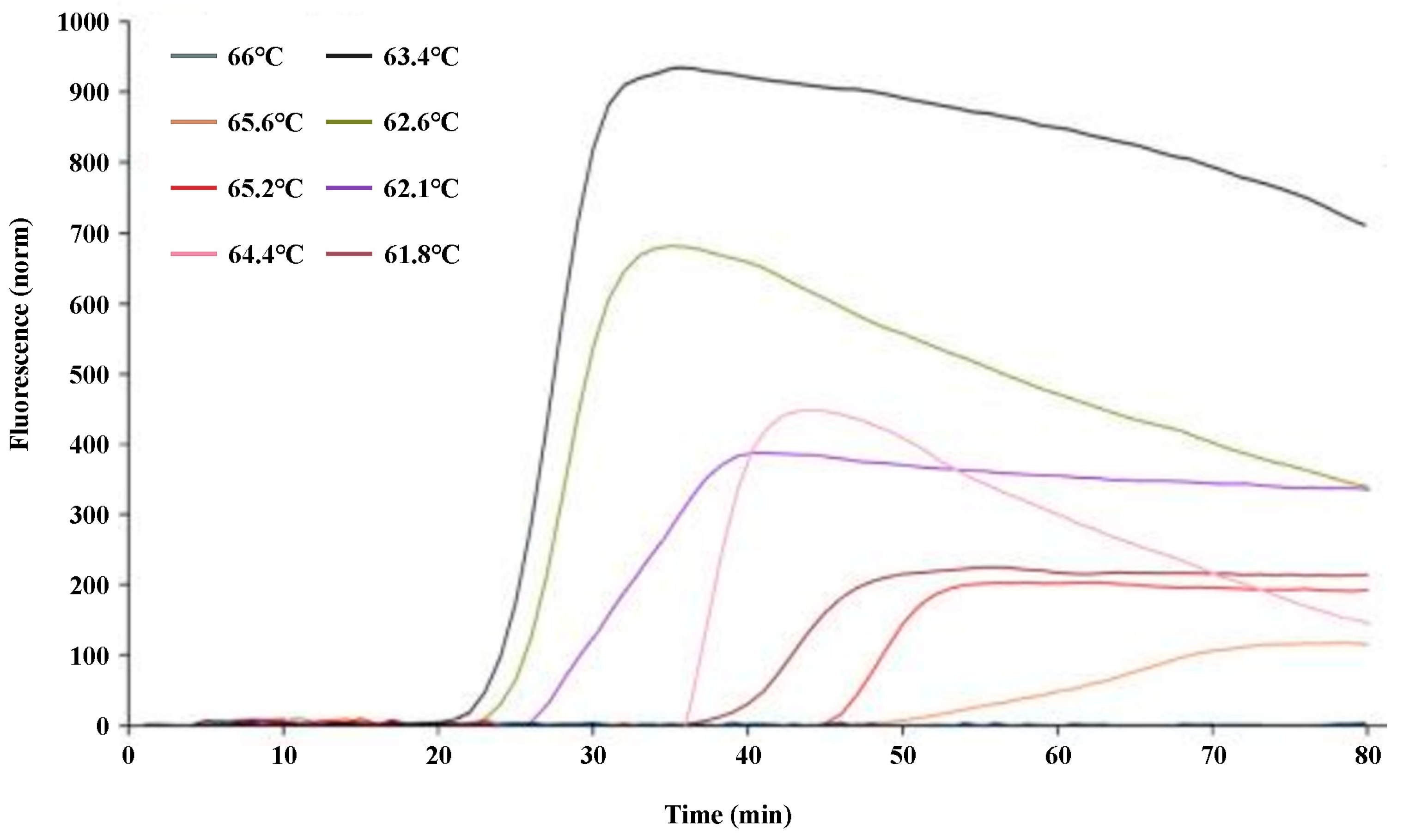
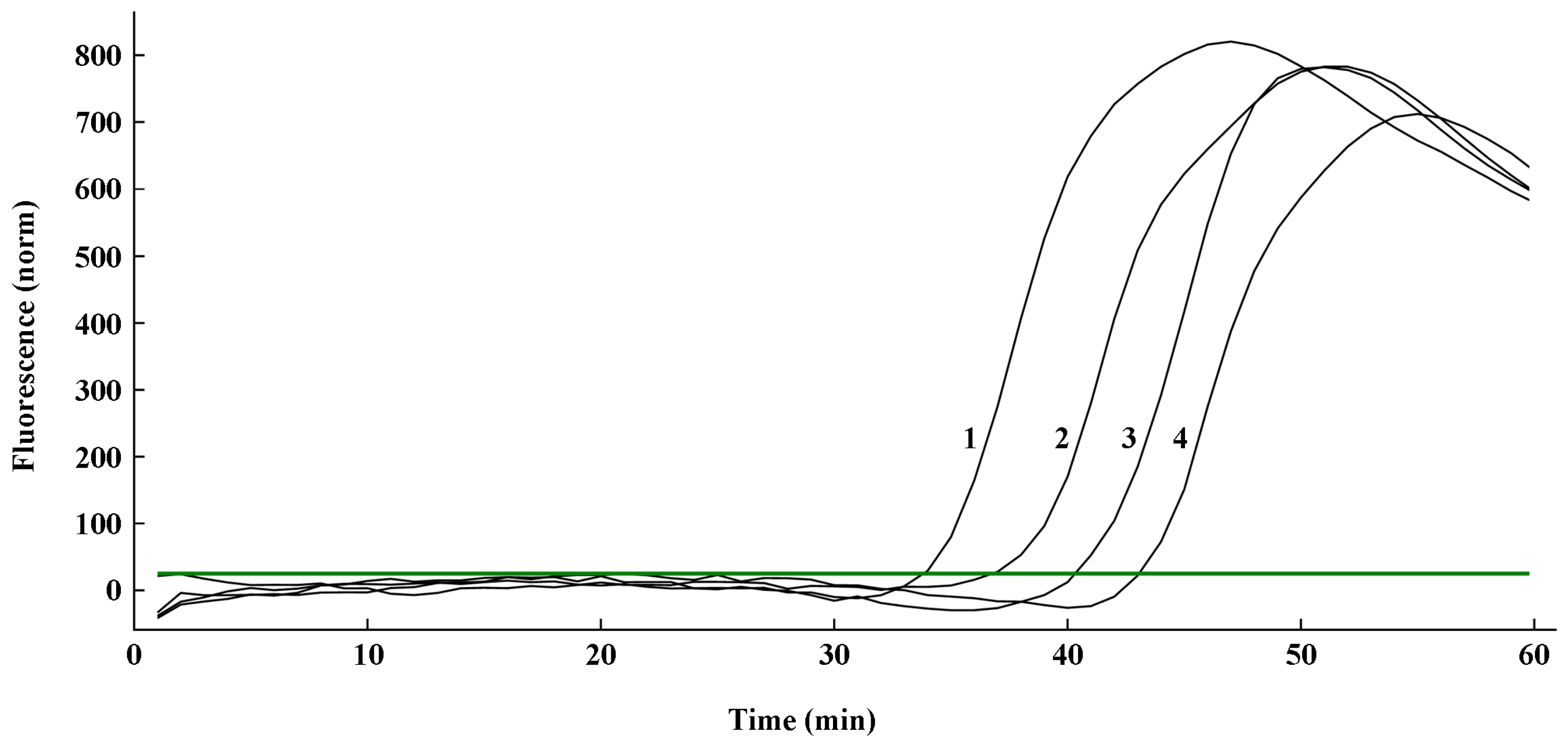
2.2. Optimization of the q-LAMP Assay
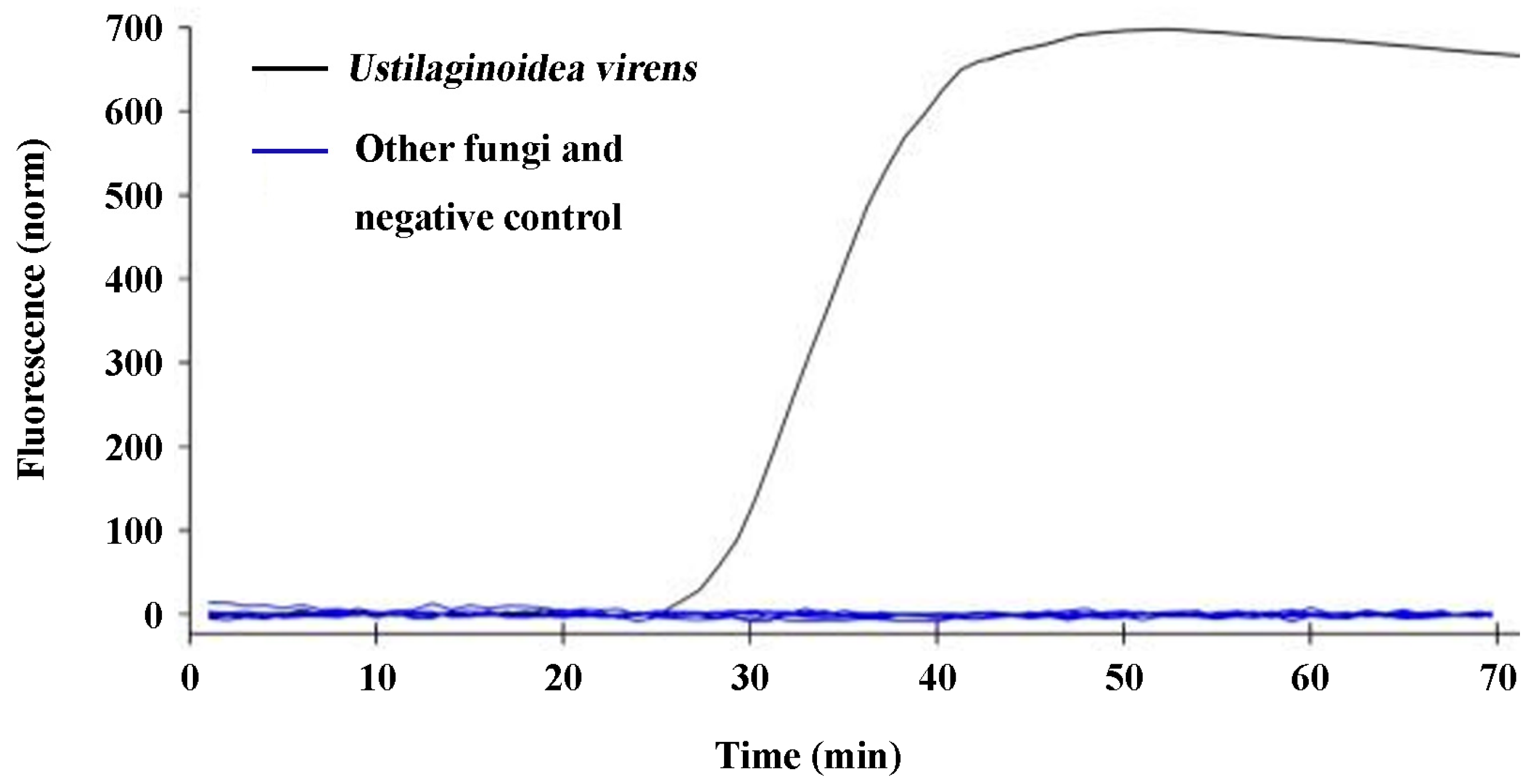
2.3. Specificity Validation of the q-LAMP Assay System
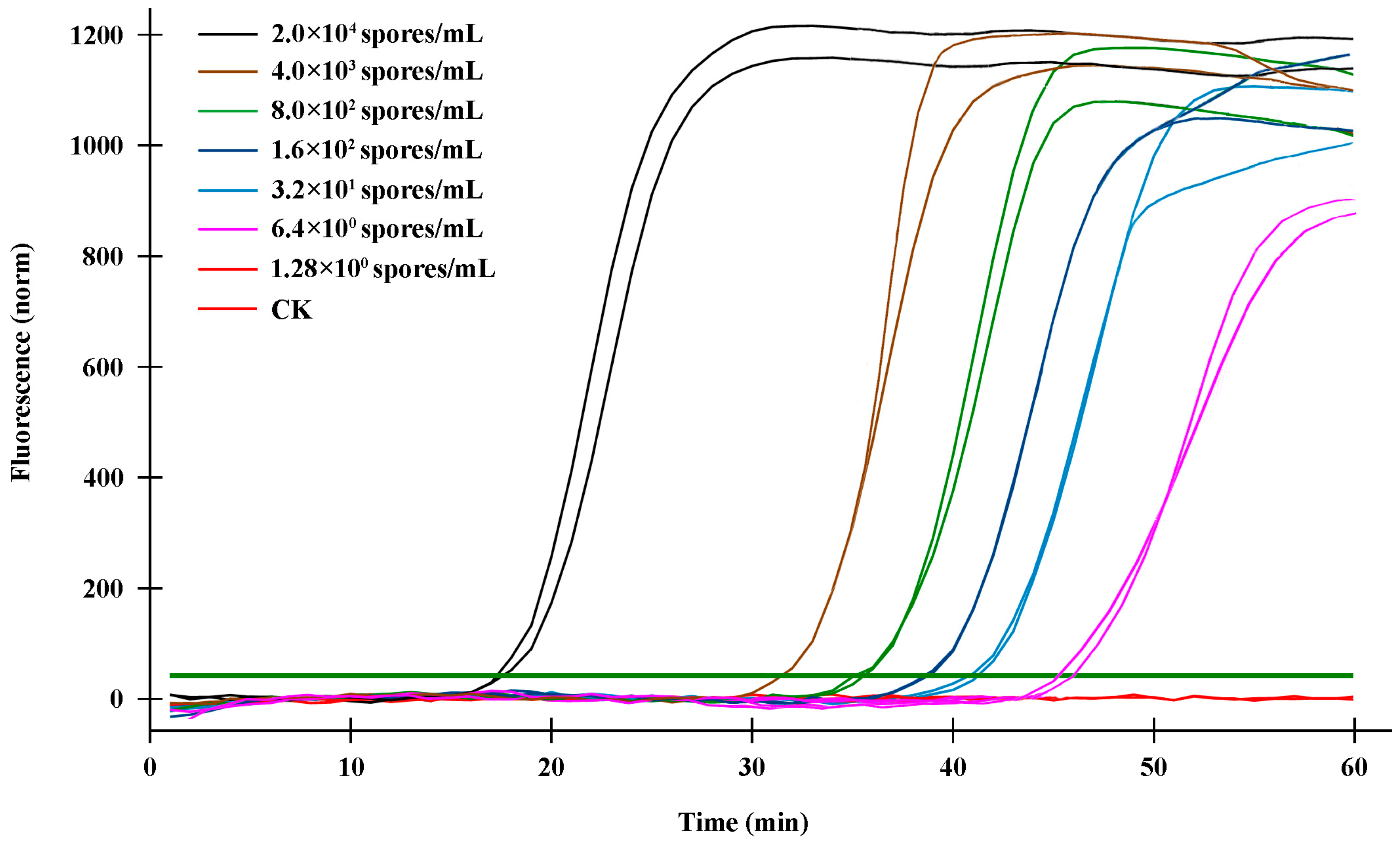
2.4. Sensitivity Validation of the q-LAMP Assay System
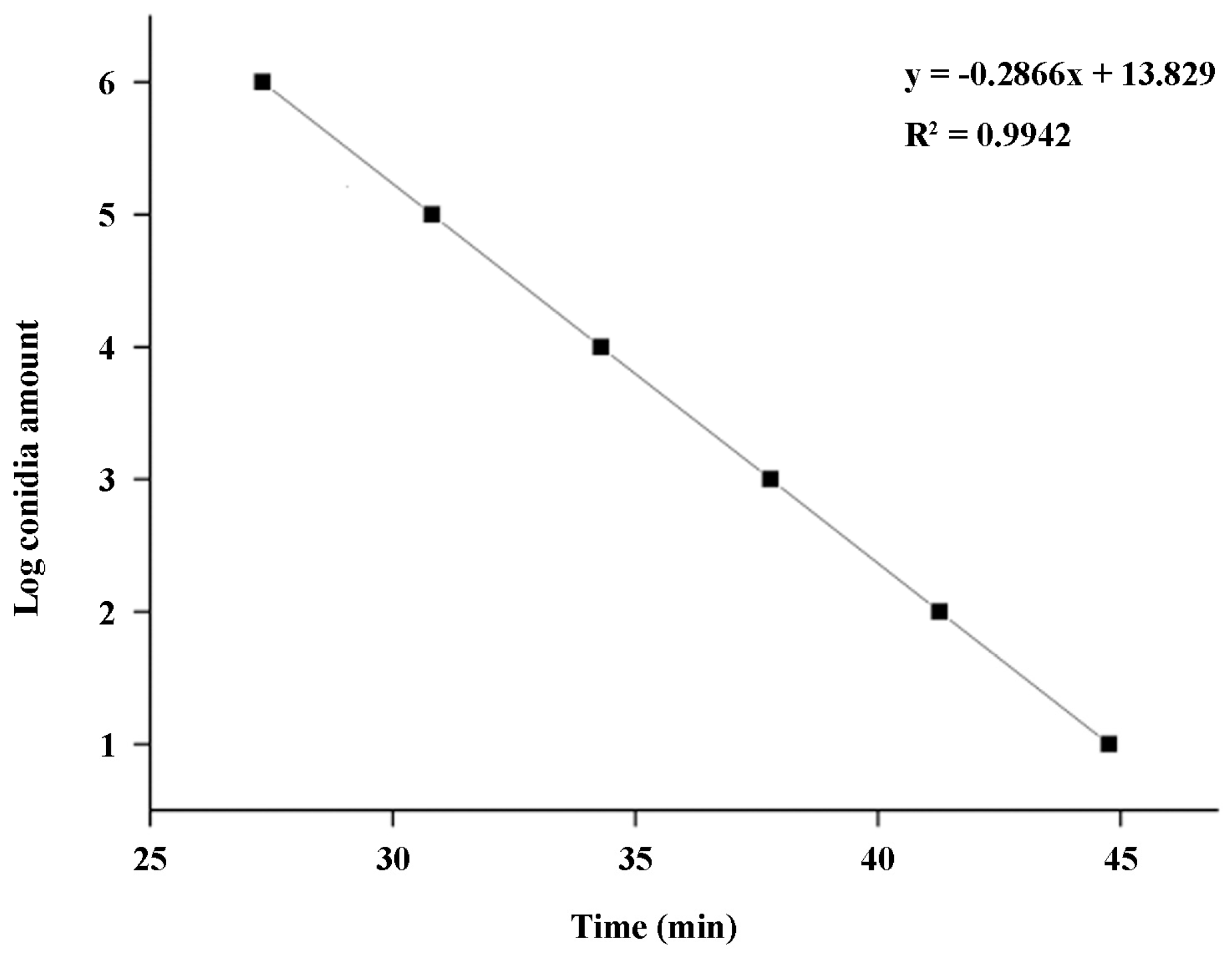
2.5. Establishment of a Standard Curve for q-LAMP Detection of U. virens
2.6. Application of q-LAMP Assay for U. virens Spore Calculation
2.7. Field Application of q-LAMP Assay System
3. Discussion
4. Materials and Methods
4.1. Fungal Isolates
4.2. DNA Template Preparation from Mycelium and Spores for q-PCR and q-LAMP Analysis
4.3. Design of q-LAMP Primers for U. virens Detection
4.4. Determination of Optimum Condition of the q-LAMP Assay
4.5. Validation of the Specificity for q-LAMP Assay Systems
4.6. Sensitivity Validation of q-LAMP and q-PCR Assay Systems
4.7. Establishment of Standard Curves for q-LAMP Assay Systems
4.8. Calculating of U. virens Spore Using q-LAMP System
4.9. Field Application of q-LAMP Assay by U. virens
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, S.; Wang, Y.; Zhou, J.; Xiang, S.; Sun, W.; Peng, X.; Li, J.; Hai, Y.; Wang, Y.; Li, S. The conserved effector UvHrip1 interacts with OsHGW and infection of Ustilaginoidea virens regulates defense- and heading date-related signaling pathway. Int. J. Mol. Sci. 2020, 21, 3376. [Google Scholar] [CrossRef]
- Andargie, M.; Li, J. Arabidopsis thaliana: A model host plant to study plant–pathogen interaction using rice false smut isolates of Ustilaginoidea virens. Front. Plant Sci. 2016, 7, 192. [Google Scholar] [CrossRef]
- Song, J.-H.; Wei, W.; Lv, B.; Lin, Y.; Yin, W.-X.; Peng, Y.-L.; Schnabel, G.; Huang, J.-B.; Jiang, D.-H.; Luo, C.-X. Rice False smut fungus hijacks the rice nutrients supply by blocking and mimicking the fertilization of rice ovary. Environ. Microbiol. 2016, 18, 3840–3849. [Google Scholar] [CrossRef]
- Meng, S.; Xiong, M.; Jagernath, J.S.; Wang, C.; Qiu, J.; Shi, H.; Kou, Y. UvAtg8-mediated autophagy regulates fungal growth, stress responses, conidiation, and pathogenesis in Ustilaginoidea virens. Rice 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Devi, T.K.; Singh, N.I. Aerobiology and epidemiology of false smut disease of rice by Ustilagnoidea virens (Syn. Claviceps oryzae sativae) in Thoubal District. J. Mycopatholog. Res. 2007, 45, 107–108. [Google Scholar]
- Zhou, Y.-L.; Izumitsu, K.; Sonoda, R.; Nakazaki, T.; Tanaka, E.; Tsuda, M.; Tanaka, C. PCR-based specific detection of Ustilaginoidea virens and Ephelis japonica. J. Phytopathol. 2003, 151, 513–518. [Google Scholar] [CrossRef]
- Ashizawa, T.; Takahashi, M.; Moriwaki, J.; Hirayae, K. Quantification of the rice false smut pathogen Ustilaginoidea virens from soil in Japan using real-time PCR. Eur. J. Plant Pathol. 2010, 128, 221–232. [Google Scholar] [CrossRef]
- Tanaka, E.; Ashizawa, T.; Sonoda, R.; Tanaka, C. Villosiclava virens gen. nov., comb. nov., teleomorph of Ustilaginoidea virens, the causal agent of rice false smut. Mycotaxon 2008, 106, 491–501. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Fang, A.; Han, Y.; Yang, J.; Xue, M.; Bao, J.; Hu, D.; Zhou, B.; Sun, X.; et al. Specific adaptation of Ustilaginoidea virens in occupying host florets revealed by comparative and functional genomics. Nat. Commun. 2014, 5, 3849. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-L.; Xie, X.-W.; Zhang, F.; Wang, S.; Liu, X.-Z.; Zhu, L.-H.; Xu, J.-L.; Gao, Y.-M.; Li, Z.-K. Detection of quantitative resistance loci associated with resistance to rice false smut (Ustilaginoidea virens) using introgression lines. Plant Pathol. 2013, 63, 365–372. [Google Scholar] [CrossRef]
- Sun, X.; Kang, S.; Zhang, Y.; Tan, X.; Yu, Y.; He, H.; Zhang, X.; Liu, Y.; Wang, S.; Sun, W.; et al. Genetic diversity and population structure of rice pathogen Ustilaginoidea virens in China. PLoS ONE 2013, 8, e76879. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, S.; Shan, T.; Wang, P.; Wang, S. Chemistry and biology of mycotoxins from rice false smut pathogen. In Mycotoxins: Properties, Applications, and Hazards; Melbor, B., Greene, J., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Fu, X.; Wang, A.; Wang, X.; Lin, F.; He, L.; Lai, D.; Liu, Y.; Li, Q.X.; Zhou, L.; Wang, B. Development of a monoclonal antibody-based icELISA for the detection of Ustiloxin B in rice false smut balls and rice grains. Toxins 2015, 7, 3481–3496. [Google Scholar] [CrossRef]
- Yu, M.; Yu, J.; Cao, H.; Song, T.; Pan, X.; Qi, Z.; Du, Y.; Zhang, R.; Huang, S.; Liu, W.; et al. SUN-family protein UvSUN1 regulates the development and virulence of Ustilaginoidea virens. Front. Microbiol. 2021, 12, 739453. [Google Scholar] [CrossRef]
- Yu, J.; Yu, M.; Song, T.; Cao, H.; Pan, X.; Yong, M.; Qi, Z.; Du, Y.; Zhang, R.; Yin, X.; et al. A homeobox transcription factor UvHOX2 regulates chlamydospore formation, conidiogenesis, and pathogenicity in Ustilaginoidea virens. Front. Microbiol. 2019, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-X.; Jin, J.; Hu, D.-W.; Yong, M.-L.; Xu, Y.; He, L.-P. Elucidation of the infection process of Ustilaginoidea virens (teleomorph: Villosiclava virens) in rice spikelets. Plant Pathol. 2012, 62, 1–8. [Google Scholar] [CrossRef]
- Hu, Y. Infection processes of Ustilaginoidea virens during artificial inoculation of rice panicles. Eur. J. Plant Pathol. 2014, 139, 67–77. [Google Scholar] [CrossRef]
- Yong, M.; Deng, Q.; Fan, L.; Miao, J.; Lai, C.; Chen, H.; Yang, X.; Wang, S.; Chen, F.; Jin, L.; et al. The role of Ustilaginoidea virens sclerotia in increasing incidence of rice false smut disease in the subtropical zone in China. Eur. J. Plant Pathol. 2017, 150, 669–677. [Google Scholar] [CrossRef]
- Tsukui, T.; Nagano, N.; Umemura, M.; Kumagai, T.; Terai, G.; Machida, M.; Asai, K. Ustiloxins, fungal cyclic peptides, are ribosomally synthesized in Ustilaginoidea virens. Bioinformatics 2014, 31, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.W.; Zhang, C.-Q. Q-LAMP assays for the detection of Botryosphaeria dothidea causing Chinese hickory canker in trunk, water, and air samples. Plant Dis. 2019, 103, 3142–3149. [Google Scholar] [CrossRef] [PubMed]
- Harrison, N.A.; Womack, M.; Carpio, M.L. Detection and characterization of a lethal yellowing (16SrIV) group phytoplasma in Canary Island date palms affected by lethal decline in Texas. Plant Dis. 2002, 86, 676–681. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Aryan, E.; Makvandi, M.; Farajzadeh, A.; Huygen, K.; Bifani, P.; Mousavi, S.-L.; Fateh, A.; Jelodar, A.; Gouya, M.-M.; Romano, M. A novel and more sensitive loop-mediated isothermal amplification assay targeting IS6110 for detection of Mycobacterium tuberculosis complex. Microbiol. Res. 2010, 165, 211–220. [Google Scholar] [CrossRef] [PubMed]
- McKenna, J.P.; Fairley, D.J.; Shields, M.D.; Cosby, S.L.; Wyatt, D.E.; McCaughey, C.; Coyle, P.V. Development and clinical validation of a loop-mediated isothermal amplification method for the rapid detection of Neisseria meningitidis. Diagn. Microbiol. Infect. Dis. 2011, 69, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, Z.; Zhao, G.; Liu, J.; Pang, Y.; Deng, X.; Xie, Z.; Fan, Q.; Luo, S. A loop-mediated isothermal amplification assay for the visual detection of duck circovirus. Virol. J. 2014, 11, 76. [Google Scholar] [CrossRef]
- Soleimani, M.; Shams, S.; Majidzadeh, A.K. Developing a real-time quantitative loop-mediated isothermal amplification assay as a rapid and accurate method for detection of Brucellosis. J. Appl. Microbiol. 2013, 115, 828–834. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Yang, X.; Al-Attala, M.N.; Zhang, Y.; Zhang, A.-F.; Zang, H.-Y.; Gu, C.-Y.; Gao, T.-C.; Chen, Y.; Ali, F.; Li, Y.-F.; et al. Rapid detection of Ustilaginoidea virens from rice using Loop-Mediated Isothermal Amplification Assay. Plant Dis. 2018, 102, 1741–1747. [Google Scholar] [CrossRef]
- Stella, S.; Gottardi, E.M.; Favout, V.; Gonzalez, E.B.; Errichiello, S.; Vitale, S.R.; Fava, C.; Luciano, L.; Stagno, F.; Grimaldi, F.; et al. The q-LAMP method represents a valid and rapid alternative for the detection of the BCR-ABL1 rearrangement in Philadelphia-positive leukemias. Int. J. Mol. Sci. 2019, 20, 6106. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Xu, G.; Chen, C.; Song, G.; Dong, Z.; Lin, L.; Wang, Y.; Xu, Z.; Yu, M.; et al. Low-cost and Scalable platform with multiplexed microwell array biochip for rapid diagnosis of COVID-19. Research 2021, 2021, 2813643. [Google Scholar] [CrossRef]
- Ku, J.; Chauhan, K.; Hwang, S.-H.; Jeong, Y.-J.; Kim, D.-E. Enhanced specificity in loop-mediated isothermal amplification with poly(ethylene glycol)-engrafted graphene oxide for detection of viral genes. Biosensors 2022, 12, 661. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, X.; Zheng, L.; Huang, J.; Zhang, Q.; Liu, H. Development of generic immuno-magnetic bead-based enzyme-linked immunoassay for Ustiloxins in rice coupled with enrichment. Toxins 2021, 13, 907. [Google Scholar] [CrossRef]
- Wang, X.; Fu, X.; Lin, F.; Sun, W.; Meng, J.; Wang, A.; Lai, D.; Zhou, L.; Liu, Y. The contents of Ustiloxins A and B along with their distribution in rice false smut balls. Toxins 2016, 8, 262. [Google Scholar] [CrossRef]
- Fu, R.; Chen, C.; Wang, J.; Liu, Y.; Zhao, L.; Lu, D. Transcription profiling of rice panicle in response to crude toxin extract of Ustilaginoidea virens. Front. Microbiol. 2022, 13, 701489. [Google Scholar] [CrossRef]
- Abbas, H.K.; Shier, W.T.; Cartwright, R.D.; Sciumbato, G.L. Ustilaginoidea virens infection of rice in Arkansas: Toxicity of false smut galls, their extracts and the Ustiloxin fraction. Am. J. Plant Sci. 2014, 05, 3166–3176. [Google Scholar] [CrossRef]
- Rovira, A.; Abrahante, J.; Murtaugh, M.; Claudia, M.-Z. Reverse transcription loop-mediated isothermal amplification for the detection of porcine reproductive and respiratory syndrome virus. J. Veter-Diagn. Investig. 2009, 21, 350–354. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.Y.; Zhang, C.-Q. Establishment of a rapid detection method for rice blast fungus based on one-step loop-mediated isothermal amplification (LAMP). Plant Dis. 2019, 103, 1967–1973. [Google Scholar] [CrossRef]
- Li, H.; Ni, D.; Duan, Y.; Chen, Y.; Li, J.; Song, F.; Li, L.; Wei, P.; Yang, J. Quantitative detection of the rice false smut pathogen Ustilaginoidea virens by real-time PCR. Genet. Mol. Res. 2013, 12, 6433–6441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, X.; Lyu, L.; Yuan, B.; Chang, X.; Zhang, S. Progress and prospective of Villosiclava virens infection mechanism. Hubei Agric. Sci. 2019, 58, 5. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Luo, H. Test pesticides against rice false smut and choose optimum application period. J. Huazhong Agric. Univ. 2007, 26, 178. [Google Scholar]
- Fu, X.; Xie, R.; Wang, J.; Chen, X.; Wang, X.; Sun, W.; Meng, J.; Lai, D.; Zhou, L.; Wang, B. Development of colloidal gold-based lateral flow immunoassay for rapid qualitative and semi-quantitative analysis of Ustiloxins A and B in rice samples. Toxins 2017, 9, 79. [Google Scholar] [CrossRef]
- Tomlinson, J.; Boonham, N. Real-time LAMP for Chalara fraxinea diagnosis. Methods Mol. Biol. 2015, 1302, 75–83. [Google Scholar] [CrossRef] [PubMed]


| Serial Number | Sequence (5′–3′) | |
|---|---|---|
| UV-2 | F3 | GGCACAGCATGACAGGATG |
| B3 | TGCTCCCACACTGGTAGT | |
| FIP(F1c-F2) | CCTGACATGGCCGGTTTCCCGACGCATGGCCAATAACTCC | |
| BIP(B1c-B2) | AGCGGGGCACTTAGGTTCTGCCAATCAAGGCAGCTGATCT | |
| Spore Concentration (Spores/mL) | Time a (min) (Mean ± Standard Deviation) | Fluorescence Signals b |
|---|---|---|
| 2 × 104 | 17.90 ± 1.64 | + |
| 4 × 103 | 31.44 ± 0.71 | + |
| 8 × 102 | 34.68 ± 1.26 | + |
| 1.6 × 102 | 37.92 ± 1.53 | + |
| 3.2 × 101 | 41.16 ± 0.98 | + |
| 6.4 | 44.40 ± 2.42 | + |
| 1.28 | − | |
| CK c | − |
| Ct a | Manually Added Spores (Spores/mL) | Predictive Spores (Spores/mL) | R2 | p Value |
|---|---|---|---|---|
| 34.03 | 450 | 446.07 | 0.999 | 0.639 |
| 37.12 | 116 | 118.51 | ||
| 40.46 | 29 | 28.29 | ||
| 43.17 | 9 | 8.85 |
| Species | Isolate NO. | Host | Origin |
|---|---|---|---|
| Fusarium fujikuroi | / | Rice | Zhejiang, China |
| F. oxysporum | ACCC30927 a | Rice | Hainan, China |
| F. proliferatum | CICC2489 b | Rice | Anhui, China |
| F. solani | ACCC37119 | Rice | Hebei, China |
| F. graminearum | ACCC37680 | Wheat | Jiangxi, China |
| Penicillium sp. | ACCC31507 | Soil | Shandong, China |
| Ustilaginoidea virens | ACCC2711 | Rice | Hunan, China |
| Pyricularia oryzae | ACCC37631 | Rice | Fujian, China |
| Alternaria alternata | ACCC36843 | Rice | Hainan, China |
| Rhizoctonia solani | ACCC36246 | Rice | Beijing, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, X.; Zhang, S.; Ma, T.; Mao, C.; Zhang, C. Quantitative Loop-Mediated Isothermal Amplification Detection of Ustilaginoidea virens Causing Rice False Smut. Int. J. Mol. Sci. 2023, 24, 10388. https://doi.org/10.3390/ijms241210388
Zhang Y, Li X, Zhang S, Ma T, Mao C, Zhang C. Quantitative Loop-Mediated Isothermal Amplification Detection of Ustilaginoidea virens Causing Rice False Smut. International Journal of Molecular Sciences. 2023; 24(12):10388. https://doi.org/10.3390/ijms241210388
Chicago/Turabian StyleZhang, Yu, Xinyue Li, Shuya Zhang, Tianling Ma, Chengxin Mao, and Chuanqing Zhang. 2023. "Quantitative Loop-Mediated Isothermal Amplification Detection of Ustilaginoidea virens Causing Rice False Smut" International Journal of Molecular Sciences 24, no. 12: 10388. https://doi.org/10.3390/ijms241210388
APA StyleZhang, Y., Li, X., Zhang, S., Ma, T., Mao, C., & Zhang, C. (2023). Quantitative Loop-Mediated Isothermal Amplification Detection of Ustilaginoidea virens Causing Rice False Smut. International Journal of Molecular Sciences, 24(12), 10388. https://doi.org/10.3390/ijms241210388






