Doubtful Clinical Value of Subtyping Anti-U1-RNP Antibodies Regarding the RNP-70 kDa Antigen in Sera of Patients with Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Results
2.1. Differences within the SLE Group Based on Clinical Routine Analyses of Anti-U1-RNP Antibodies
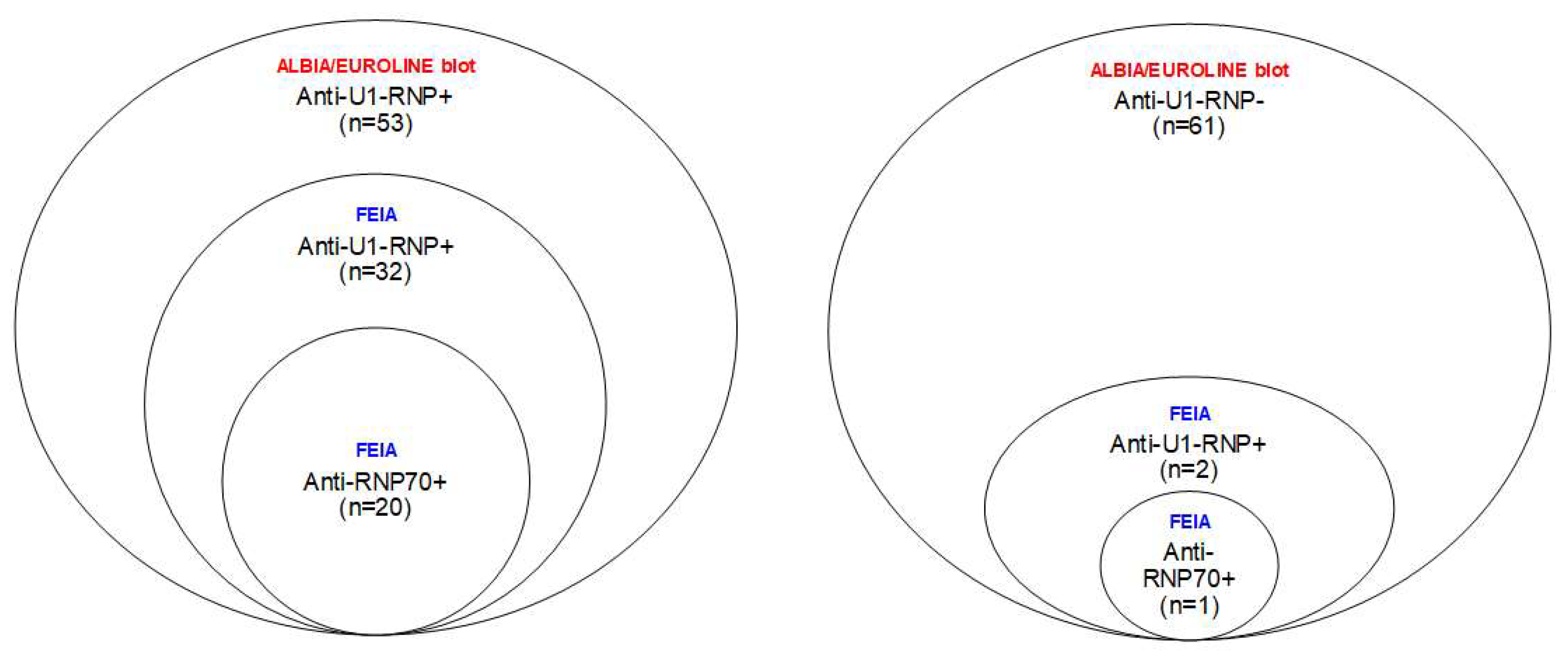
2.2. Anti-U1-RNP and Anti-RNP70 Antibodies (FEIA) in SLE, MCTD, pSS and HBDs
2.3. The Added Value of Analyzing Anti-RNP70 Antibodies in Cases of SLE
2.4. Importance of Anti-U1-RNP and Anti-RNP70 Antibody Levels (FEIA)
3. Discussion
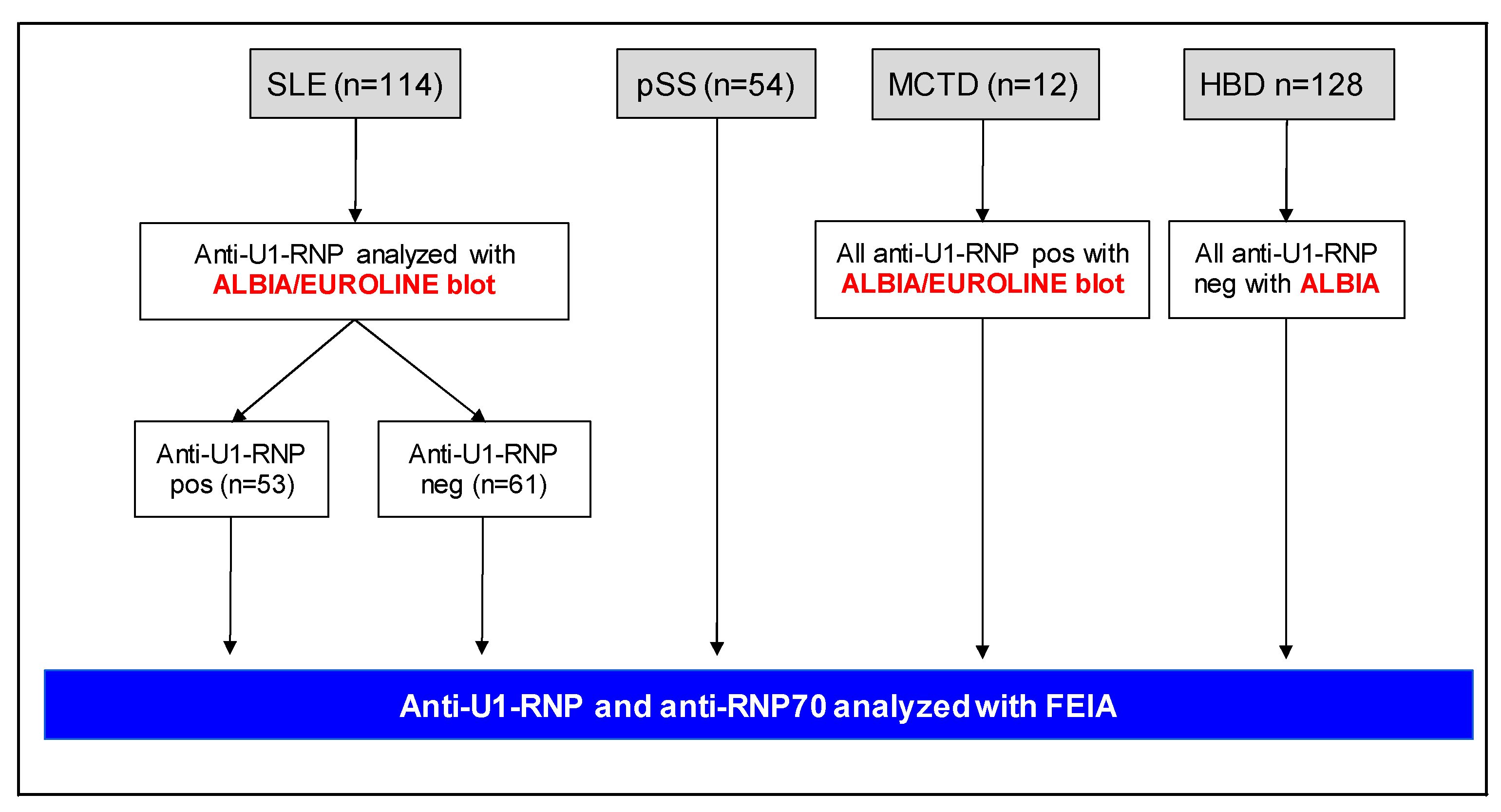
4. Patients and Methods
4.1. Study Population and Data Collection
4.2. Immunoassays
4.3. Statistics
4.4. Ethical Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damoiseaux, J.; Andrade, L.E.C.; Carballo, O.G.; Conrad, K.; Francescantonio, P.L.C.; Fritzler, M.J.; Garcia de la Torre, I.; Herold, M.; Klotz, W.; Cruvinel, W.M.; et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: The International Consensus on ANA patterns (ICAP) perspective. Ann. Rheum. Dis. 2019, 78, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.J.; Karp, D.R. Autoantibodies and SLE: The threshold for disease. Nat. Rev. Rheumatol. 2014, 10, 181–186. [Google Scholar] [CrossRef]
- Didier, K.; Bolko, L.; Giusti, D.; Toquet, S.; Robbins, A.; Antonicelli, F.; Servettaz, A. Autoantibodies Associated with Connective Tissue Diseases: What Meaning for Clinicians? Front. Immunol. 2018, 9, 541. [Google Scholar] [CrossRef]
- Frodlund, M.; Wetterö, J.; Dahle, C.; Dahlström, Ö.; Skogh, T.; Rönnelid, J.; Sjöwall, C. Longitudinal anti-nuclear antibody (ANA) seroconversion in systemic lupus erythematosus: A prospective study of Swedish cases with recent-onset disease. Clin. Exp. Immunol. 2020, 199, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, P.; Baldini, C.; Rocchi, V.; Bombardieri, S. Anti-Sm and anti-RNP antibodies. Autoimmunity 2005, 38, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kattah, N.H.; Kattah, M.G.; Utz, P.J. The U1-snRNP complex: Structural properties relating to autoimmune pathogenesis in rheumatic diseases. Immunol. Rev. 2010, 233, 126–145. [Google Scholar] [CrossRef]
- Riemekasten, G.; Hahn, B.H. Key autoantigens in SLE. Rheumatology (Oxford) 2005, 44, 975–982. [Google Scholar] [CrossRef]
- De Keyser, F.; Hoch, S.O.; Takei, M.; Dang, H.; De Keyser, H.; Rokeach, L.A.; Talal, N. Cross-reactivity of the B/B′ subunit of the Sm ribonucleoprotein autoantigen with proline-rich polypeptides. Clin. Immunol. Immunopathol. 1992, 62, 285–290. [Google Scholar] [CrossRef]
- Lundberg, I.; Nyman, U.; Pettersson, I.; Hedfors, E. Clinical manifestations and anti-(U1)snRNP antibodies: A prospective study of 29 anti-RNP antibody positive patients. Br. J. Rheumatol. 1992, 31, 811–817. [Google Scholar] [CrossRef]
- Salmhofer, W.; Hermann, J.; Joch, M.; Kerl, H.; Graninger, W. High serum levels of antibodies against the recombinant 70 kDa ribonucleoprotein are useful for diagnosing mixed connective tissue disease. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1047–1053. [Google Scholar] [CrossRef]
- Montecucco, C.; Caporali, R.; Ravelli, A.; Ronchetti, A.; Rossi, S.; Martini, A.; Notario, A. Frequency and clinical significance of anti-RNP antibodies in Italian SLE patients. Ann. Ital. Med. Int. 1994, 9, 12–15. [Google Scholar] [PubMed]
- Alarcón-Segovia, D.; Cardiel, M.H. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J. Rheumatol. 1989, 16, 328–334. [Google Scholar] [PubMed]
- Tani, C.; Carli, L.; Vagnani, S.; Talarico, R.; Baldini, C.; Mosca, M.; Bombardieri, S. The diagnosis and classification of mixed connective tissue disease. J. Autoimmun. 2014, 48–49, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.R.; Isenberg, D.A. “Mixed connective tissue disease”: A condition in search of an identity. Clin. Exp. Med. 2020, 20, 159–166. [Google Scholar] [CrossRef]
- Peter, J.B.; Shoenfeld, Y. (Eds.) Spliceosomal snRNPs Autoantibodies. In Autoantibodies, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1996; pp. 774–782. [Google Scholar]
- Tan, E.M. Autoantibodies in Diagnosis and in Identifying Autoantigens. Immunologist 1999, 7, 85–92. [Google Scholar]
- Venrooij, W.J.V.; Maini, R.N. (Eds.) Anti-U1snRNP antibodies and clinical associations. In Manual of Biological Markers of Disease; Kluwer Academic Publishers: Dordecht, The Netherlands, 1996; pp. 1–8. [Google Scholar]
- Nawata, M.; Matsushita, M.; Matsudaira, R.A.N.; Yamada, H.; Kaneda, K.; Asano, M.; Yamanaka, K.; Murakami, A.; Takasaki, Y. Clinical Significance of Anti-U1 RNP Antibodies Recognizing the Conformation Structure on U1 RNA/70-kd Protein Complex in Patients with Mixed Connective Tissue Disease. Juntendo Med. J. 2011, 57, 477–487. [Google Scholar] [CrossRef]
- Irure-Ventura, J.; López-Hoyos, M. The Past, Present, and Future in Antinuclear Antibodies (ANA). Diagnostics 2022, 12, 647. [Google Scholar] [CrossRef]
- Crow, M.K.; Rönnblom, L. Type I interferons in host defence and inflammatory diseases. Lupus Sci. Med. 2019, 6, e000336. [Google Scholar] [CrossRef]
- Abbara, S.; Seror, R.; Henry, J.; Chretien, P.; Gleizes, A.; Hacein-Bey-Abina, S.; Mariette, X.; Nocturne, G. Anti-RNP positivity in primary Sjögren’s syndrome is associated with a more active disease and a more frequent muscular and pulmonary involvement. RMD Open 2019, 5, e001033. [Google Scholar] [CrossRef]
- Matsuda, K.M.; Yoshizaki, A.; Yamaguchi, K.; Fukuda, E.; Okumura, T.; Ogawa, K.; Ono, C.; Norimatsu, Y.; Kotani, H.; Hisamoto, T.; et al. Autoantibody Landscape Revealed by Wet Protein Array: Sum of Autoantibody Levels Reflects Disease Status. Front. Immunol. 2022, 13, 893086. [Google Scholar] [CrossRef]
- Hubbard, E.L.; Pisetsky, D.S.; Lipsky, P.E. Anti-RNP antibodies are associated with the interferon gene signature but not decreased complement levels in SLE. Ann. Rheum. Dis. 2022, 81, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Dima, A.; Jurcut, C.; Baicus, C. The impact of anti-U1-RNP positivity: Systemic lupus erythematosus versus mixed connective tissue disease. Rheumatol. Int. 2018, 38, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Ighe, A.; Dahlström, Ö.; Skogh, T.; Sjöwall, C. Application of the 2012 Systemic Lupus International Collaborating Clinics classification criteria to patients in a regional Swedish systemic lupus erythematosus register. Arthritis Res. Ther. 2015, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.; Mosca, M.; Gordon, C. Assessment of patients with systemic lupus erythematosus and the use of lupus disease activity indices. Best Pract. Res. Clin. Rheumatol. 2005, 19, 685–708. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
| SLE: Anti-U1-RNP Positive (n = 53) | SLE: Anti-U1-RNP Negative (n = 61) | p-Value | |
|---|---|---|---|
| Background variables | |||
| Females, n (%) | 49 (92.5) | 56 (91.8) | n.s. |
| Age in years, mean (range) | 46 (23–79) | 57 (27–87) | <0.0001 |
| SLE duration in years, mean (range) | 10 (0–47) | 23 (9–48) | <0.0001 |
| Caucasian race/ethnicity, n (%) | 46 (86.8) | 56 (91.8) | n.s. |
| cSLEDAI-2K, mean (range) | 1.9 (0–20) | 0.77 (0–12) | n.s. |
| Raynaud’s phenomenon, n (%) | 26 (49.0) | 18 (29.5) | 0.03 |
| SDI, mean (range) | 0.9 (0–7) | 1.7 (0–7) | 0.006 |
| Fulfilled classification criteria (1982 ACR definitions) | |||
| Malar rash, n (%) | 23 (43.4) | 32 (52.5) | n.s. |
| Discoid lupus, n (%) | 9 (17) | 6 (9.8) | n.s. |
| Photosensitivity, n (%) | 30 (56.6) | 30 (49.2) | n.s. |
| Oral ulcers, n (%) | 9 (17) | 6 (9.8) | n.s. |
| Arthritis, n (%) | 36 (67.9) | 46 (75.4) | n.s. |
| Serositis, n (%) | 13 (24.5) | 25 (41) | n.s. |
| Renal disorder, n (%) | 17 (32.1) | 19 (31.1) | n.s. |
| Neurologic disorder, n (%) | 3 (5.7) | 5 (8.2) | n.s. |
| Hematologic disorder, n (%) | 38 (71.7) | 32 (52.5) | 0.04 |
| Immunologic disorder, n (%) | 33 (62.3) | 31 (50.8) | n.s. |
| IIF-ANA, n (%) | 53 (100) | 61 (100) | n.s. |
| Medical treatment (ongoing) | |||
| Hydroxychloroquine, n (%) | 38 (72) | 45 (74) | n.s. |
| 1 Immunosuppressants, n (%) | 23 (43) | 28 (46) | n.s. |
| Prednisolone, daily dose (mg) | 4.5 | 2.6 | 0.03 |
| Laboratory variables at sampling occasion, mean (range) | |||
| Hemoglobin concentration, g/L | 128 (52–177) | 133 (103–171) | n.s. |
| Platelet count, ×109/L | 230 (102–485) | 244 (132–500) | n.s. |
| Leukocyte count, ×109/L | 5.7 (2.9–15.8) | 6.4 (3.0–14.5) | n.s. |
| Basophil count, ×109/L | 0.03 (0.01–0.15) | 0.04 (0.01–0.16) | n.s. |
| Eosinophil count, ×109/L | 0.06 (0.01–0.31) | 0.13 (0.01–0.77) | 0.0005 |
| Lymphocyte count, ×109/L | 1.2 (0.4–3.7) | 1.6 (0.4–4.5) | 0.006 |
| Monocyte count, ×109/L | 0.41 (0.24–0.41) | 0.50 (0.19–1.46) | 0.03 |
| C3, g/L | 0.96 (0.36–1.40) | 1.05 (0.63–1.60) | 0.03 |
| C4, g/L | 0.16 (0.04–0.30) | 0.17 (0.05–0.49) | n.s. |
| SLE: Anti-RNP70 Positive (n = 21) | SLE: Anti-U1-RNP Negative (n = 59) | p-Value | |
|---|---|---|---|
| Background variables | |||
| Females, n (%) | 20 (95.2) | 54 (91.5) | n.s. |
| Age in years, mean (range) | 44 (26–78) | 58 (27–87) | 0.001 |
| SLE duration in years, mean (range) | 11 (1–30) | 23 (9–48) | <0.0001 |
| Caucasian race/ethnicity, n (%) | 17 (81.0) | 54 (91.5) | n.s. |
| cSLEDAI-2K, mean (range) | 1.4 (0–4) | 0.8 (0–12) | n.s. |
| Raynaud’s phenomenon, n (%) | 14 (66.7) | 17 (29.8) | 0.003 |
| SDI, mean (range) | 0.7 (0–4) | 1.8 (0–7) | 0.008 |
| Fulfilled classification criteria (1982 ACR definitions) | |||
| Malar rash, n (%) | 9 (42.9) | 30 (50.8) | n.s. |
| Discoid lupus, n (%) | 2 (9.5) | 6 (10.6) | n.s. |
| Photosensitivity, n (%) | 9 (42.9) | 28 (47.5) | n.s. |
| Oral ulcers, n (%) | 5 (23.8) | 6 (10.2) | n.s. |
| Arthritis, n (%) | 16 (76.2) | 44 (74.6) | n.s. |
| Serositis, n (%) | 5 (23.8) | 24 (40.7) | n.s. |
| Renal disorder, n (%) | 7 (33.3) | 19 (32.2) | n.s. |
| Neurologic disorder, n (%) | 1 (4.8) | 5 (8.5) | n.s. |
| Hematologic disorder, n (%) | 18 (85.7) | 31 (52.5) | 0.007 |
| Immunologic disorder, n (%) | 14 (66.7) | 30 (50.8) | n.s. |
| IIF-ANA, n (%) | 21 (100) | 59 (100) | n.s. |
| Laboratory variables at sampling occasion, mean (range) | |||
| Hemoglobin concentration, g/L | 124 (52–164) | 132 (103–171) | 0.04 |
| Platelet count, ×109/L | 230 (102–349) | 243 (132–500) | n.s. |
| Leukocyte count, ×109/L | 5.2 (2.9–15.8) | 6.4 (3.0–14.5) | 0.04 |
| Basophil count, ×109/L | 0.03 (0.01–0.10) | 0.04 (0.01–0.16) | <0.05 |
| Eosinophil count, ×109/L | 0.09 (0.01–0.31) | 0.13 (0.01–0.77) | 0.0005 |
| Lymphocyte count, ×109/L | 1.1 (0.6–2.6) | 1.6 (0.4–4.5) | 0.008 |
| Monocyte count, ×109/L | 0.39 (0.21–1.46) | 0.50 (0.19–1.46) | <0.05 |
| C3, g/L | 0.96 (0.47–1.40) | 1.06 (0.63–1.60) | n.s. |
| C4, g/L | 0.15 (0.08–0.28) | 0.17 (0.05–0.49) | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.; Brylid, A.; Dahle, C.; Saleh, M.; Dahlström, Ö.; Enocsson, H.; Sjöwall, C. Doubtful Clinical Value of Subtyping Anti-U1-RNP Antibodies Regarding the RNP-70 kDa Antigen in Sera of Patients with Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 10398. https://doi.org/10.3390/ijms241210398
Ahmad A, Brylid A, Dahle C, Saleh M, Dahlström Ö, Enocsson H, Sjöwall C. Doubtful Clinical Value of Subtyping Anti-U1-RNP Antibodies Regarding the RNP-70 kDa Antigen in Sera of Patients with Systemic Lupus Erythematosus. International Journal of Molecular Sciences. 2023; 24(12):10398. https://doi.org/10.3390/ijms241210398
Chicago/Turabian StyleAhmad, Awais, André Brylid, Charlotte Dahle, Muna Saleh, Örjan Dahlström, Helena Enocsson, and Christopher Sjöwall. 2023. "Doubtful Clinical Value of Subtyping Anti-U1-RNP Antibodies Regarding the RNP-70 kDa Antigen in Sera of Patients with Systemic Lupus Erythematosus" International Journal of Molecular Sciences 24, no. 12: 10398. https://doi.org/10.3390/ijms241210398
APA StyleAhmad, A., Brylid, A., Dahle, C., Saleh, M., Dahlström, Ö., Enocsson, H., & Sjöwall, C. (2023). Doubtful Clinical Value of Subtyping Anti-U1-RNP Antibodies Regarding the RNP-70 kDa Antigen in Sera of Patients with Systemic Lupus Erythematosus. International Journal of Molecular Sciences, 24(12), 10398. https://doi.org/10.3390/ijms241210398








