The Role of Fractalkine in the Regulation of Endometrial Iron Metabolism in Iron Deficiency
Abstract
:1. Introduction
2. Results
2.1. Iron Deficiency Affects the Expression of the Iron-Related Genes in HEC-1A Endometrium Cells
2.2. Fractalkine Alters the mRNA Expression Levels of the Iron Transporters Transferrin Receptor 1, Divalent Metal Transporter-1 and Ferroportin
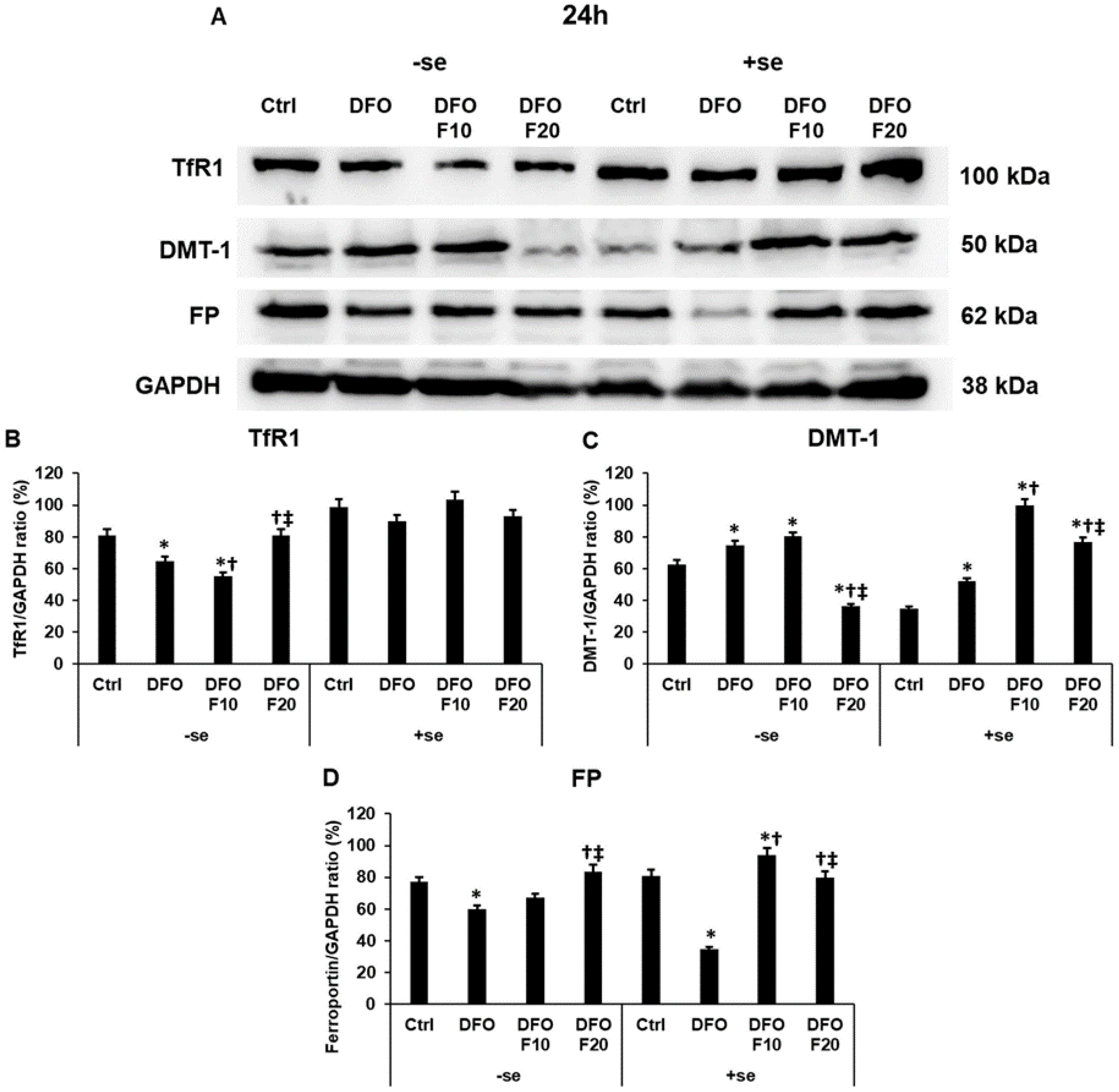
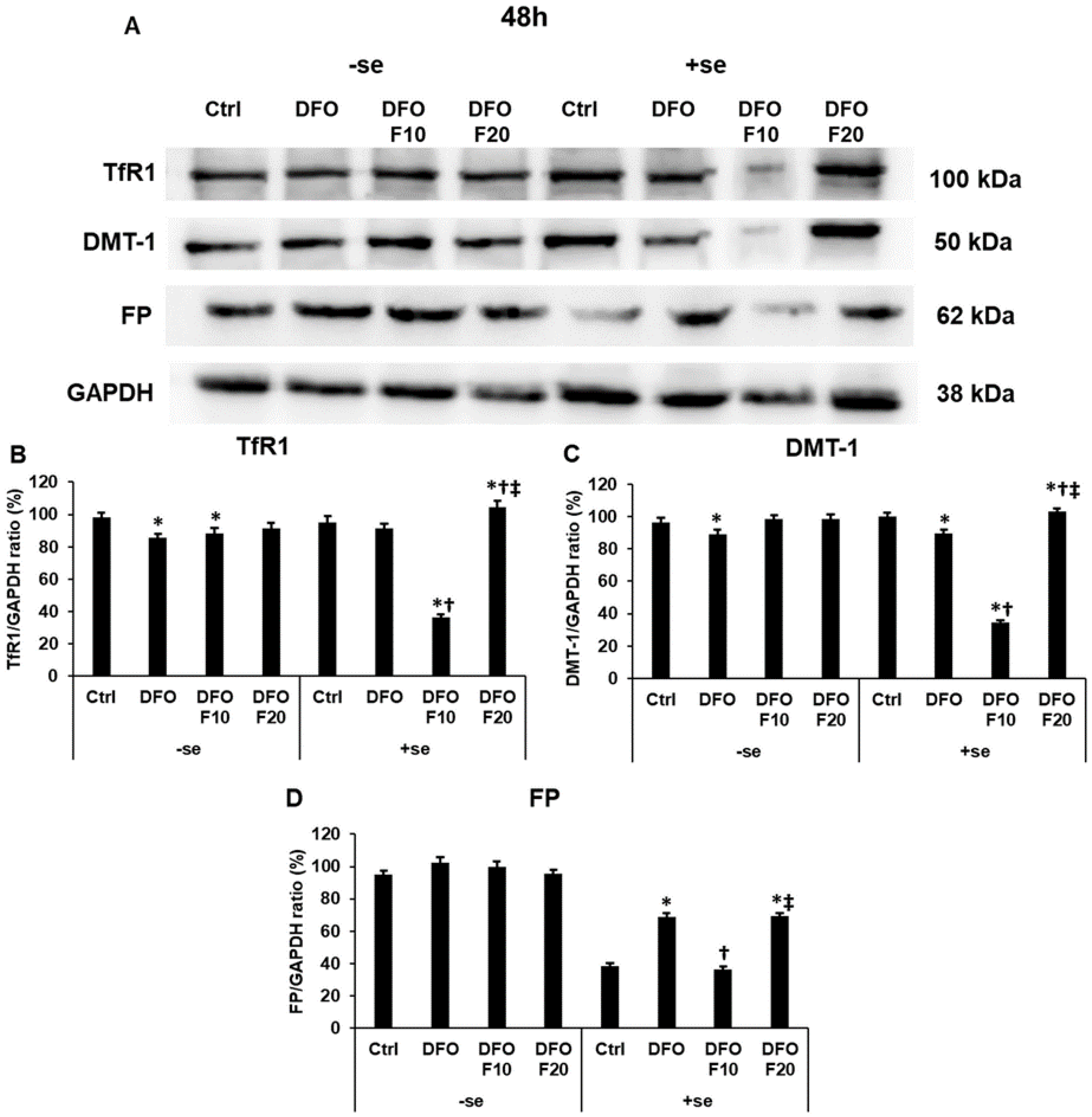
2.3. Fractalkine Alters the Protein Levels of the Iron Transporters Transferrin Receptor 1, Divalent Metal Transporter-1 and Ferroportin in Iron Deficiency
2.4. Fractalkine Acts Differently on the Hepcidin Gene (HAMP) and Ferritin Heavy Chain mRNA Expression Levels with or without Serum Supplementation
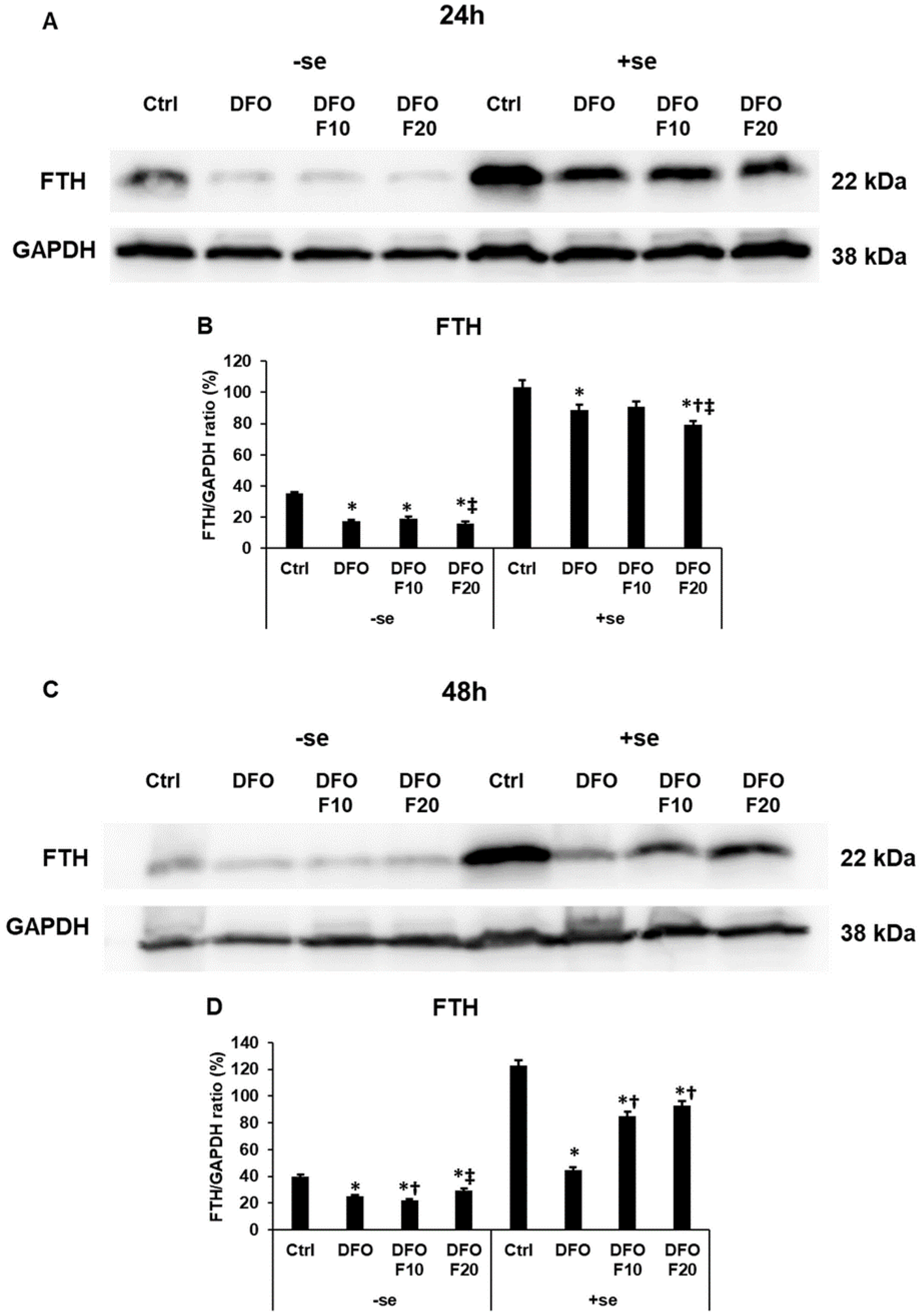
2.5. Fractalkine Affects the Level of the Iron Storage Protein Ferritin Heavy Chain
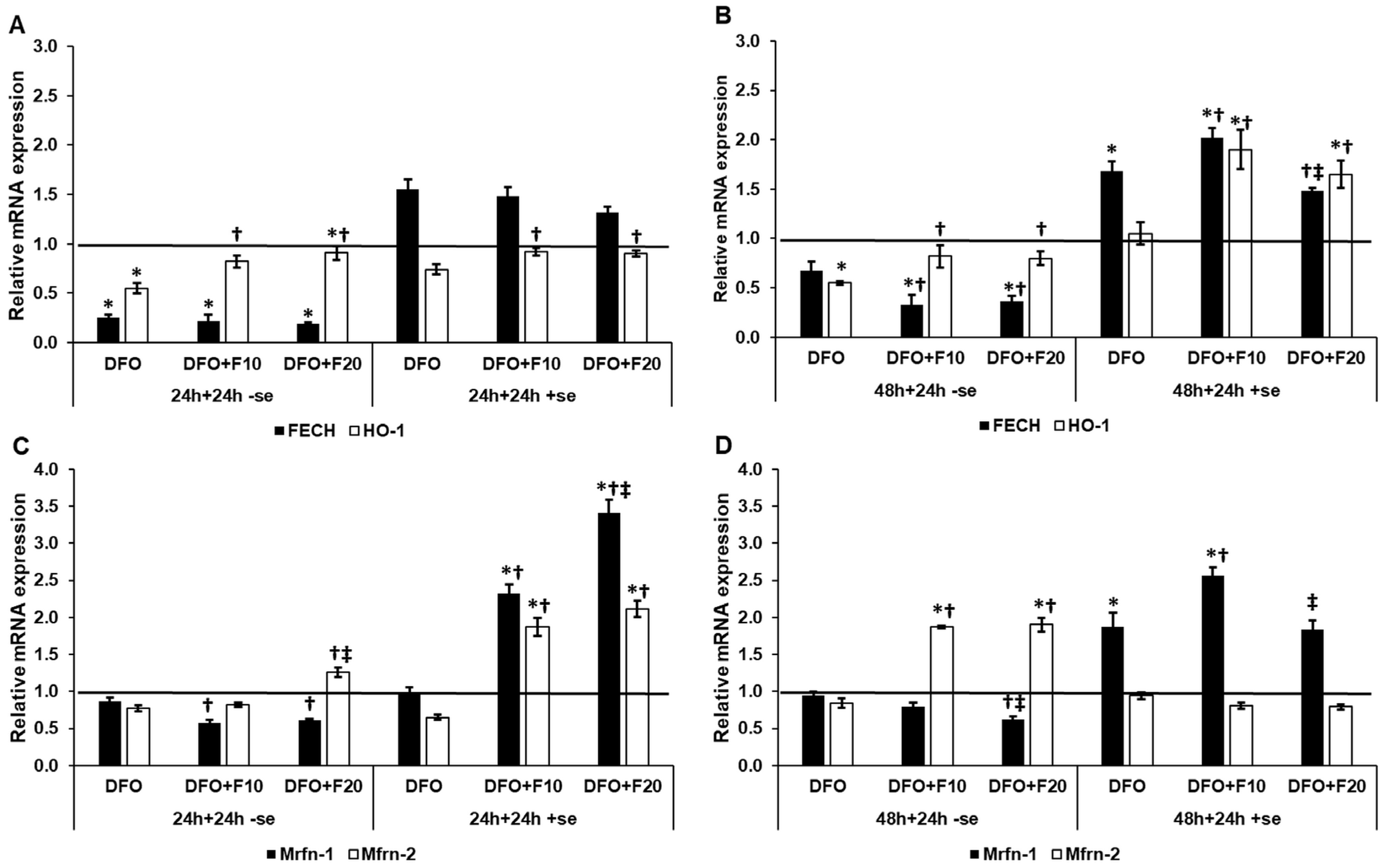
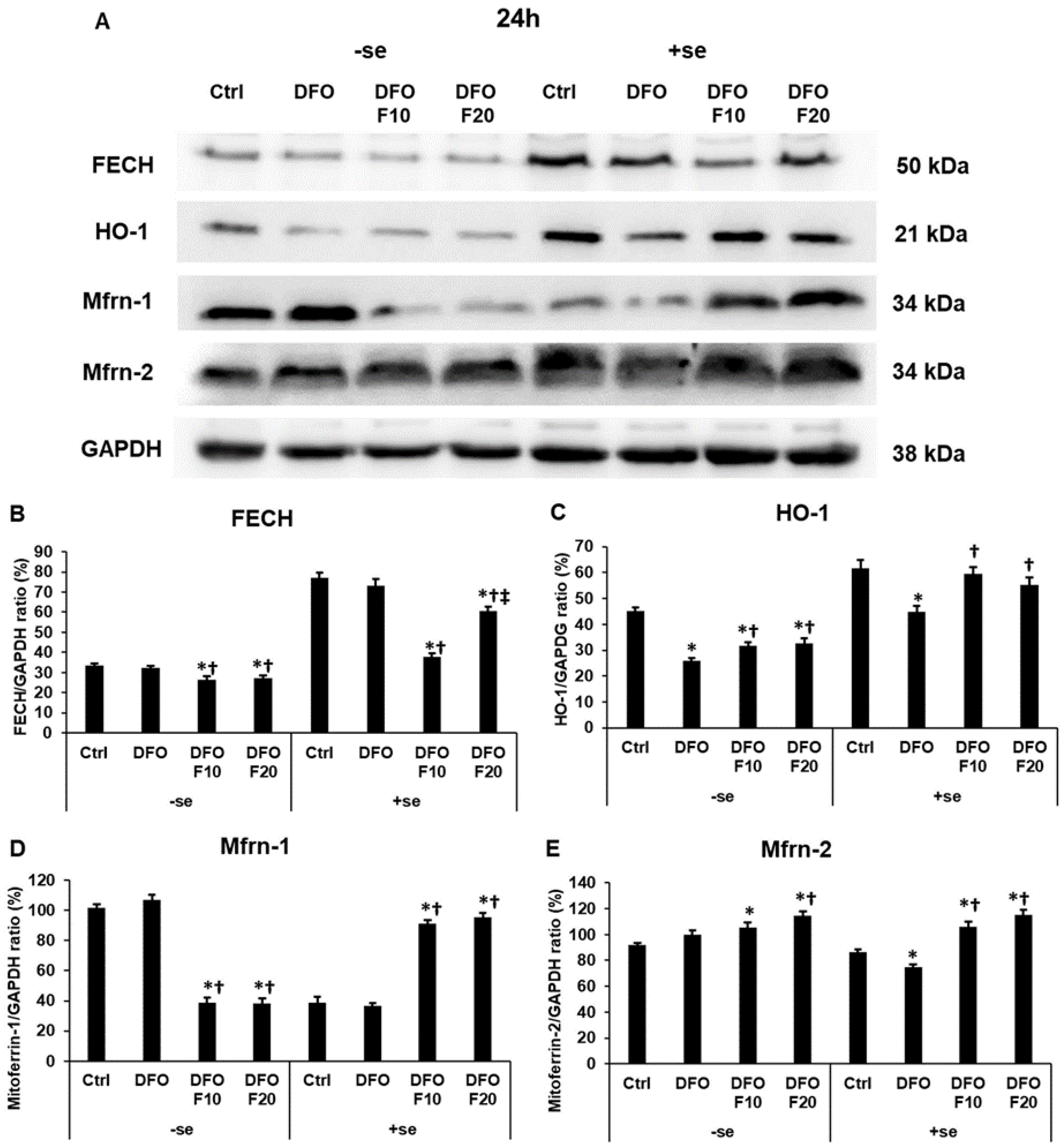
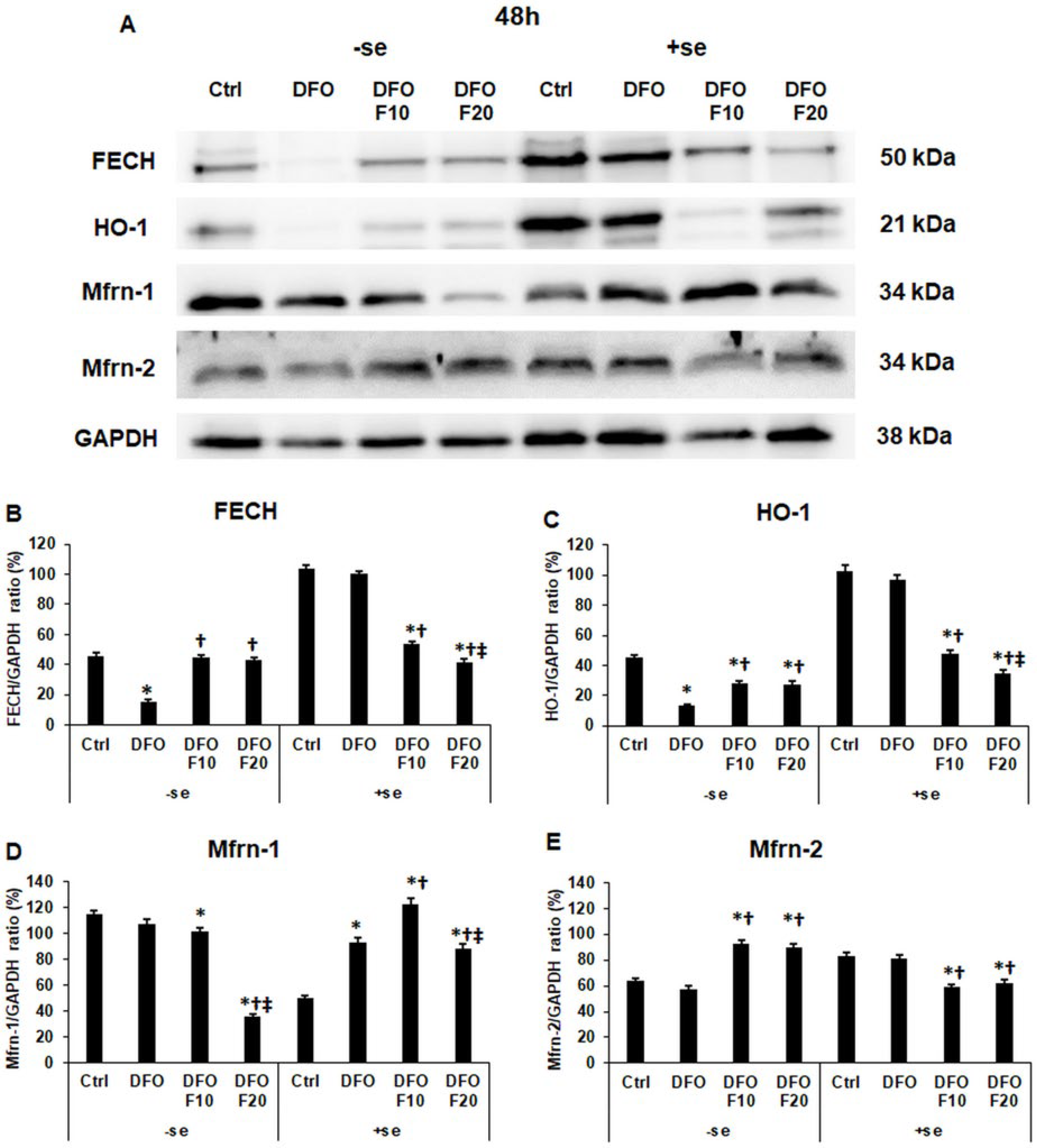
2.6. Fractalkine Alters the mRNA Expression of the Genes Related to Mitochondrial Iron Utilization
2.7. Fractalkine Alters the Protein Levels of the Genes Related to Mitochondrial Iron Utilization after DFO Treatment
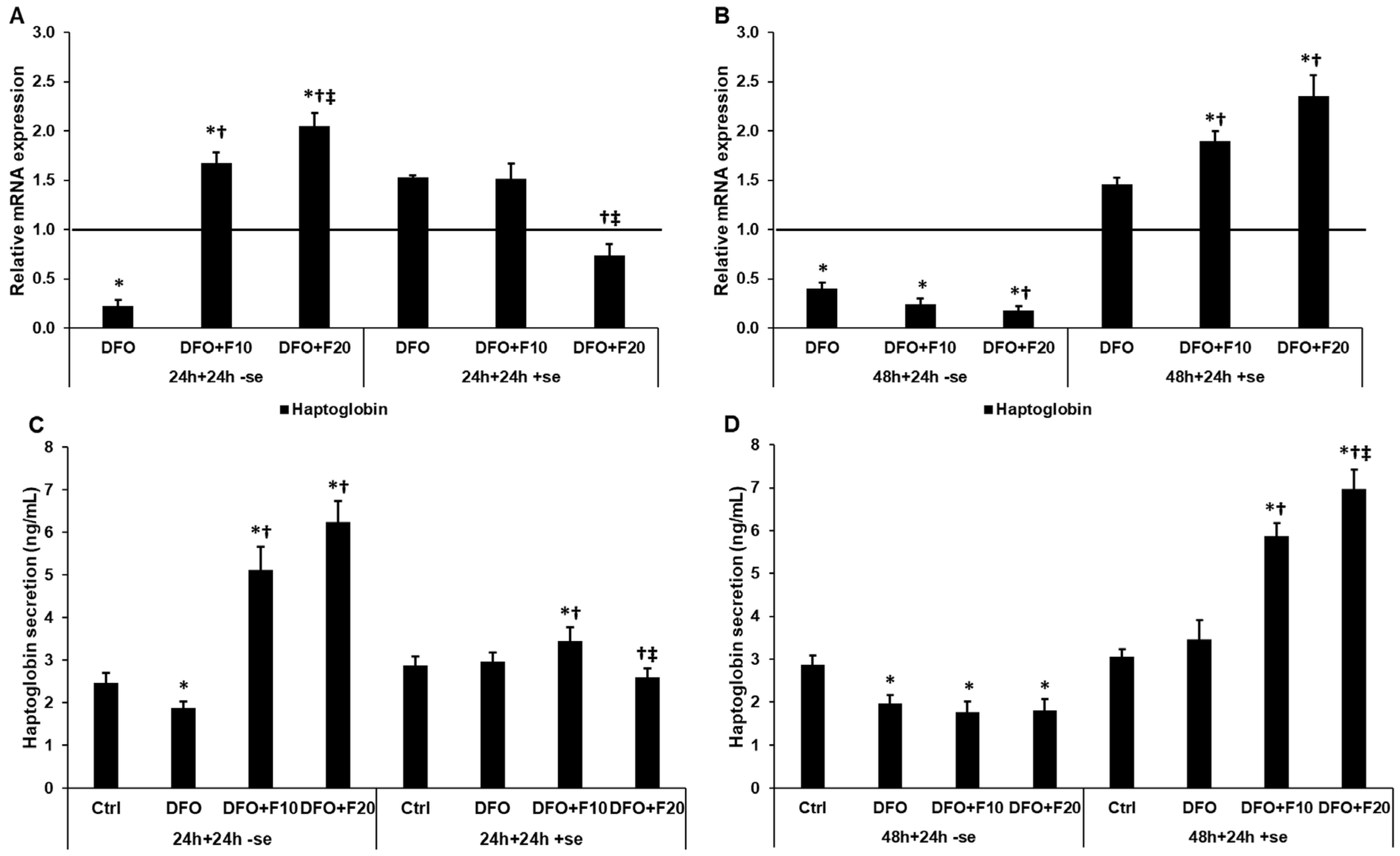
2.8. Fractalkine Changes the mRNA and Protein Levels of Haptoglobin
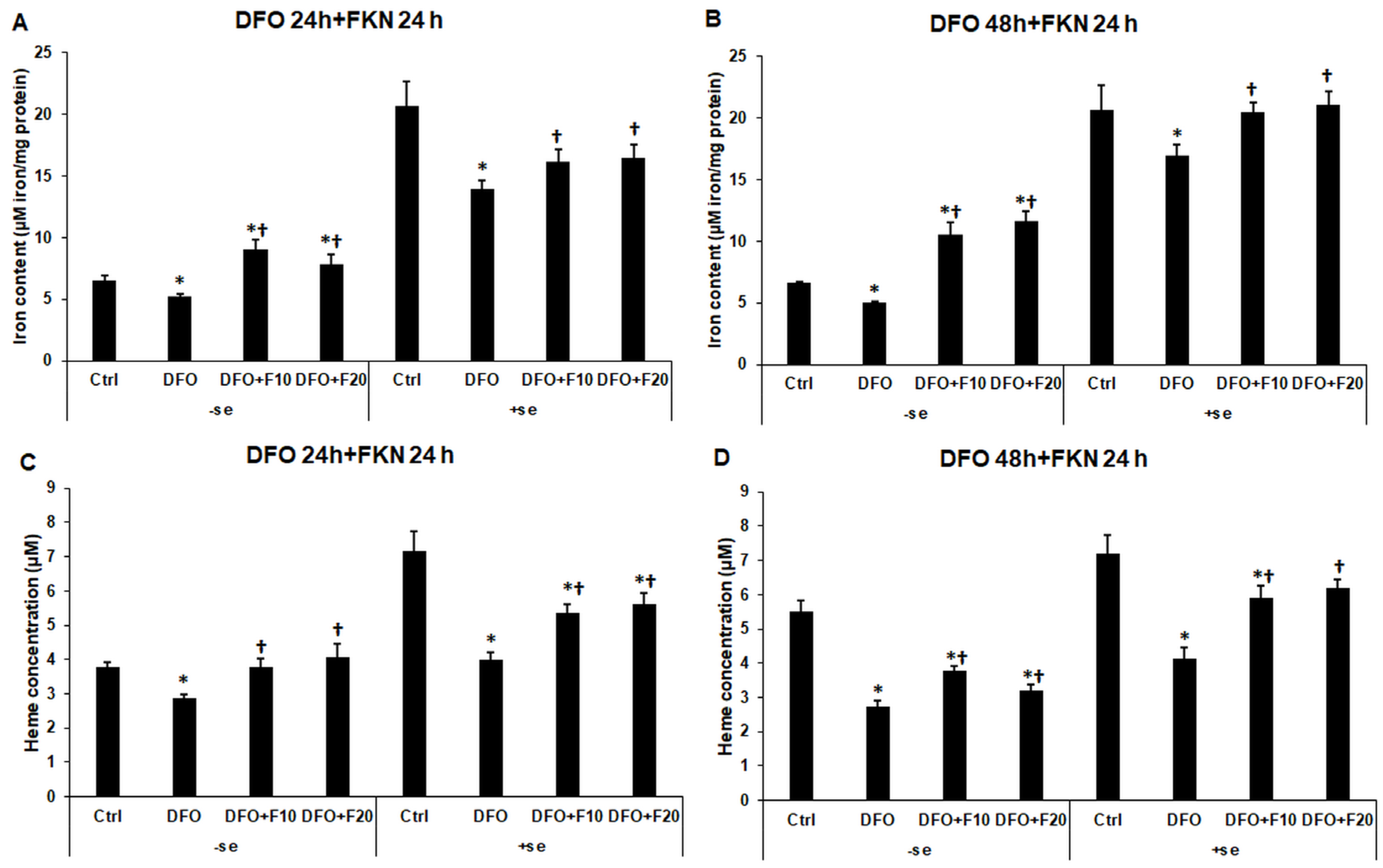
2.9. Alterations in the Total Iron Content and Heme Concentration of the Iron-Deficient HEC-1A Cells after Fractalkine Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Treatments
4.2. Real-Time PCR
4.3. Western Blotting
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Total Iron Measurements
4.6. Determination of the Heme Concentration
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogt, A.C.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V. Iron Homeostasis and Metabolism: Two Sides of a Coin. In Ferroptosis: Mechanism and Diseases; Advances in Experimental Medicine and Biology; Florez, A.F., Alborzinia, H., Eds.; Springer: Cham, Switzerland, 2021; Volume 1301, pp. 25–40. [Google Scholar]
- Nemeth, E.; Ganz, T. Hepcidin and Iron in Health and Disease. Annu. Rev. Med. 2023, 74, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.L.; Babitt, J.L. Coordination of Iron Homeostasis by Bone Morphogenetic Proteins: Current Understanding and Unanswered Questions. Dev. Dyn. 2022, 251, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.E.; Eddowes, L.A.; Gileadi, U.; Cole, S.; Spottiswoode, N.; Selvakumar, T.A.; Ho, L.P.; Townsend, A.R.M.; Drakesmith, H. Hepcidin Regulation by Innate Immune and Infectious Stimuli. Blood 2011, 118, 4129–4139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of Erythroferrone as an Erythroid Regulator of Iron Metabolism. Nat. Genet. 2014, 46, 678–684, Correction in Nat. Genet. 2020, 52, 463. [Google Scholar] [CrossRef] [Green Version]
- Cerami, C. Iron Nutriture of the Fetus, Neonate, Infant, and Child. Ann. Nutr. Metab. 2017, 71, 8–14. [Google Scholar] [CrossRef]
- Pandur, E.; Pap, R.; Montskó, G.; Jánosa, G.; Sipos, K.; Kovács, G.L. Fractalkine Enhances Endometrial Receptivity and Activates Iron Transport towards Trophoblast Cells in an in Vitro Co-Culture System of HEC-1A and JEG-3 Cells. Exp. Cell Res. 2021, 403, 112583. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Y. Iron-Loaded Extracellular Vesicles: Angel or Demon? Free Radic. Res. 2023, 57, 2191813. [Google Scholar] [CrossRef]
- Sangkhae, V.; Fisher, A.L.; Wong, S.; Koenig, M.D.; Tussing-Humphreys, L.; Chu, A.; Lelić, M.; Ganz, T.; Nemeth, E. Effects of Maternal Iron Status on Placental and Fetal Iron Homeostasis. J. Clin. Investig. 2020, 130, 625–640. [Google Scholar] [CrossRef]
- Li, Y.Q.; Cao, X.X.; Bai, B.; Zhang, J.N.; Wang, M.Q.; Zhang, Y.H. Severe Iron Deficiency Is Associated with a Reduced Conception Rate in Female Rats. Gynecol. Obstet. Investig. 2014, 77, 19–23. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Iron Intake and Risk of Ovulatory Infertility. Obstet. Gynecol. 2006, 108, 1145–1152. [Google Scholar] [CrossRef]
- Kopeć, Z.; Starzyński, R.R.; Jończy, A.; Mazgaj, R.; Lipiński, P. Role of Iron Metabolism-Related Genes in Prenatal Development: Insights from Mouse Transgenic Models. Genes 2021, 12, 1382. [Google Scholar] [CrossRef]
- Gambling, L.; Danzeisen, R.; Gair, S.; Lea, R.G.; Charania, Z.; Solanky, N.; Joory, K.D.; Srai, S.K.S.; McArdle, H.J. Effect of Iron Deficiency on Placental Transfer of Iron and Expression of Iron Transport Proteins in Vivo and in Vitro. Biochem. J. 2001, 356, 883–889. [Google Scholar] [CrossRef]
- Georgieff, M.K. Iron Deficiency in Pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef]
- Du, M.R.; Wang, S.C.; Li, D.J. The Integrative Roles of Chemokines at the Maternal-Fetal Interface in Early Pregnancy. Cell. Mol. Immunol. 2014, 11, 438–448. [Google Scholar] [CrossRef] [Green Version]
- Kervancioglu Demirci, E.; Salamonsen, L.A.; Gauster, M. The Role of CX3CL1 in Fetal-Maternal Interaction during Human Gestation. Cell Adhes. Migr. 2016, 10, 189–196. [Google Scholar] [CrossRef]
- Jones, B.A.; Beamer, M.; Ahmed, S. Fractalkine/CX3CL1: A Potential New Target for Inflammatory Diseases. Mol. Interv. 2010, 10, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Garton, K.J.; Gough, P.J.; Blobel, C.P.; Murphy, G.; Greaves, D.R.; Dempsey, P.J.; Raines, E.W. Tumor Necrosis Factor-α-Converting Enzyme (ADAM17) Mediates the Cleavage and Shedding of Fractalkine (CX3CL1). J. Biol. Chem. 2001, 276, 37993–38001. [Google Scholar] [CrossRef]
- Pandur, E.; Tamási, K.; Pap, R.; Varga, E.; Miseta, A.; Sipos, K. Fractalkine Induces Hepcidin Expression of BV-2 Microglia and Causes Iron Accumulation in SH-SY5Y Cells. Cell. Mol. Neurobiol. 2019, 39, 985–1001. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Morimoto, H.; Ohgidani, M.; Ueki, T. Modulation of Inflammatory Responses by Fractalkine Signaling in Microglia. PLoS ONE 2021, 16, e0252118. [Google Scholar] [CrossRef]
- Subbarayan, M.S.; Joly-Amado, A.; Bickford, P.C.; Nash, K.R. CX3CL1/CX3CR1 Signaling Targets for the Treatment of Neurodegenerative Diseases. Pharmacol. Ther. 2022, 231, 107989. [Google Scholar] [CrossRef] [PubMed]
- Pap, R.; Montskó, G.; Jánosa, G.; Sipos, K.; Kovács, G.L.; Pandur, E. Fractalkine Regulates Hec-1a/Jeg-3 Interaction by Influencing the Expression of Implantation-Related Genes in an in Vitro Co-Culture Model. Int. J. Mol. Sci. 2020, 21, 3175. [Google Scholar] [CrossRef] [PubMed]
- Machairiotis, N.; Vasilakaki, S.; Thomakos, N. Inflammatory Mediators and Pain in Endometriosis: A Systematic Review. Biomedicines 2021, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Toprak Sager, E.B.; Kale, I.; Sager, H.; Ozel, A.; Muhcu, M. Maternal Serum Fractalkine Concentrations in Pregnancies Complicated by Fetal Growth Restriction. Gynecol. Obstet. Reprod. Med. 2022, 9, 1342. [Google Scholar] [CrossRef]
- Huang, S.J.; Chen, C.P.; Buchwalder, L.; Yu, Y.C.; Piao, L.; Huang, C.Y.; Schatz, F.; Lockwood, C.J. Regulation of CX3CL1 Expression in Human First-Trimester Decidual Cells: Implications for Preeclampsia. Reprod. Sci. 2019, 26, 1256–1265. [Google Scholar] [CrossRef]
- Pandur, E.; Tamási, K.; Pap, R.; Jánosa, G.; Sipos, K. Modulatory Effects of Fractalkine on Inflammatory Response and Iron Metabolism of Lipopolysaccharide and Lipoteichoic Acid-Activated THP-1 Macrophages. Int. J. Mol. Sci. 2022, 23, 2629. [Google Scholar] [CrossRef]
- Pandur, E.; Pap, R.; Jánosa, G.; Horváth, A.; Sipos, K. Fractalkine Improves the Expression of Endometrium Receptivity-Related Genes and Proteins at Desferrioxamine-Induced Iron Deficiency in HEC-1A Cells. Int. J. Mol. Sci. 2023, 24, 7924. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Makieva, S.; Giacomini, E.; Ottolina, J.; Sanchez, A.M.; Papaleo, E.; Viganò, P. Inside the Endometrial Cell Signaling Subway: Mind the Gap(S). Int. J. Mol. Sci. 2018, 19, 2477. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Yachida, N.; Ueda, H.; Sugino, K.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. Three-Dimensional Understanding of the Morphological Complexity of the Human Uterine Endometrium. iScience 2021, 24, 102258. [Google Scholar] [CrossRef]
- Di Nezza, L.A.; Jobling, T.; Salamonsen, L.A. Progestin Suppresses Matrix Metalloproteinase Production in Endometrial Cancer. Gynecol. Oncol. 2003, 89, 325–333. [Google Scholar] [CrossRef]
- Horne, A.W.; Lalani, E.N.; Margara, R.A.; White, J.O. The Effects of Sex Steroid Hormones and Interleukin-1-Beta on MUC1 Expression in Endometrial Epithelial Cell Lines. Reproduction 2006, 131, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Chervenak, J.L.; Illsley, N.P. Episialin Acts as an Antiadhesive Factor in an in Vitro Model of Human Endometrial-Blastocyst Attachment. Biol. Reprod. 2000, 63, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for Study of Human Embryo Implantation: Choice of Cell Lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Herbison, C.E.; Thorstensen, K.; Chua, A.C.G.; Graham, R.M.; Leedman, P.; Olynyk, J.K.; Trinder, D. The Role of Transferrin Receptor 1 and 2 in Transferrin-Bound Iron Uptake in Human Hepatoma Cells. Am. J. Physiol.-Cell Physiol. 2009, 297, C1567–C1575. [Google Scholar] [CrossRef] [Green Version]
- Glickstein, H.; Ben El, R.; Shvartsman, M.; Cabantchik, Z.I. Intracellular Labile Iron Pools as Direct Targets of Iron Chelators: A Fluorescence Study of Chelator Action in Living Cells. Blood 2005, 106, 3242–3250. [Google Scholar] [CrossRef]
- Tabuchi, M.; Yoshimori, T.; Yamaguchi, K.; Yoshida, T.; Kishi, F. Human NRAMP2/DMT1, Which Mediates Iron Transport across Endosomal Membranes, Is Localized to Late Endosomes and Lysosomes in HEp-2 Cells. J. Biol. Chem. 2000, 275, 22220–22228. [Google Scholar] [CrossRef] [Green Version]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron Deficiency Impairs Contractility of Human Cardiomyocytes through Decreased Mitochondrial Function. Eur. J. Heart Fail. 2018, 20, 910–919. [Google Scholar] [CrossRef] [Green Version]
- Przybyszewska, J.; Zekanowska, E. The Role of Hepcidin, Ferroportin, HCP1, and DMT1 Protein in Iron Absorption in the Human Digestive Tract. Prz. Gastroenterol. 2014, 9, 208–213. [Google Scholar] [CrossRef]
- Allison, S.J. Iron Deficiency of Kidney Macrophages in CKD. Nat. Rev. Nephrol. 2023, 19, 73. [Google Scholar] [CrossRef]
- Anderson, E.R.; Shah, Y.M. Iron Homeostasis in the Liver. In Comprehensive Physiology; Prakash, M.S., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 3, pp. 315–330. [Google Scholar]
- Tacchini, L.; Gammella, E.; De Ponti, C.; Recalcati, S.; Cairo, G. Role of HIF-1 and NF-ΚB Transcription Factors in the Modulation of Transferrin Receptor by Inflammatory and Anti-Inflammatory Signals. J. Biol. Chem. 2008, 283, 20674–20686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volz, K. The Functional Duality of Iron Regulatory Protein 1. Curr. Opin. Struct. Biol. 2008, 18, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahoum, L.; Belisowski, S.; Ghatpande, N.; Guttmann-Raviv, N.; Zhang, W.; Li, K.; Tong, W.-H.; Nyska, A.; Waterman, M.; Weisshof, R.; et al. Iron Regulatory Protein 1 Is Required for the Propagation of Inflammation in Inflammatory Bowel Disease. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nakayama, T.; Watanabe, Y.; Oiso, N.; Higuchi, T.; Shigeta, A.; Mizuguchi, N.; Katou, F.; Hashimoto, K.; Kawada, A.; Yoshie, O. Eotaxin-3/CC Chemokine Ligand 26 Is a Functional Ligand for CX3CR1. J. Immunol. 2010, 185, 6472–6479. [Google Scholar] [CrossRef] [Green Version]
- Ward, D.M.; Kaplan, J. Ferroportin-Mediated Iron Transport: Expression and Regulation. Biochim. Biophys. Acta-Mol. Cell Res. 2012, 1823, 1426–1433. [Google Scholar] [CrossRef] [Green Version]
- Imaizumi, T.; Yoshida, H.; Satoh, K. Regulation of CX3CL1/Fractalkine Expression in Endothelial Cells. J. Atheroscler. Thromb. 2004, 11, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Pagani, A.; Nai, A.; Silvestri, L.; Camaschella, C. Hepcidin and Anemia: A Tight Relationship. Front. Physiol. 2019, 10, e01294. [Google Scholar] [CrossRef]
- Entezari, S.; Haghi, S.M.; Norouzkhani, N.; Sahebnazar, B.; Vosoughian, F.; Akbarzadeh, D.; Islampanah, M.; Naghsh, N.; Abbasalizadeh, M.; Deravi, N. Iron Chelators in Treatment of Iron Overload. J. Toxicol. 2022, 2022, 4911205. [Google Scholar] [CrossRef]
- Brasil, F.B.; Bertolini Gobbo, R.C.; Souza de Almeida, F.J.; Luckachaki, M.D.; Dall’Oglio, E.L.; de Oliveira, M.R. The Signaling Pathway PI3K/Akt/Nrf2/HO-1 Plays a Role in the Mitochondrial Protection Promoted by Astaxanthin in the SH-SY5Y Cells Exposed to Hydrogen Peroxide. Neurochem. Int. 2021, 146, 105024. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Ali, M.Y.; Oliva, C.R.; Flor, S.; Griguer, C.E. Mitoferrin, Cellular and Mitochondrial Iron Homeostasis. Cells 2022, 11, 3464. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.nih.gov/ij/index.html (accessed on 12 May 2023).




| Primer | Sequence 5′ → 3′ |
|---|---|
| HAMP forward | CAGCTGGATGCCCATGTT |
| HAMP reverse | TGCAGCACATCCCACATC |
| GAPDH forward | TGTTCCAATATGATTCCACCC |
| GAPDH reverse | CCACTTGATTTTGGAGGGAT |
| TfR1 forward | CATGTGGAGATGAAACTTGC |
| TfR1 reverse | TCCCATAGCAGATACTTCCA |
| DMT-1 forward | GTGGTTACTGGGCTGCATCT |
| DMT-1 reverse | CCCACAGAGGAATTCTTCCT |
| FP forward | AAAGGAGGCTGTTTCCATAG |
| FP reverse | TTCCTTCTCTACCTTGGTCA |
| FTH forward | GAGGTGGCCGAATCTTCCTTC |
| FTH reverse | TCAGTGGCCAGTTTGTGCAG |
| FECH forward | GATGAATTGTCCCCCAACAC |
| FECH reverse | AGCCCTTTCTAGGCCATCTC |
| HO-1 forward | ACCCATGACACCAAGGACCA |
| HO-1 reverse | ATGCCTGCATTCACATGGCA |
| Haptoglobin forward | CAAGAAGACACCTGCTATGG |
| Haptoglobin reverse | GTCACCTTCACATACACACC |
| Mfrn-1 forward | TTAAATGACGTTTTCCACCAC |
| Mfrn-1 reverse | TTCTGCTGGATTCATTACCG |
| Mfrn-2 forward | ATAGCCATATTGCCAATGGTG |
| Mfrn-2 reverse | CCGGTGGTATGGTGAGTTGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandur, E.; Pap, R.; Jánosa, G.; Horváth, A.; Sipos, K. The Role of Fractalkine in the Regulation of Endometrial Iron Metabolism in Iron Deficiency. Int. J. Mol. Sci. 2023, 24, 9917. https://doi.org/10.3390/ijms24129917
Pandur E, Pap R, Jánosa G, Horváth A, Sipos K. The Role of Fractalkine in the Regulation of Endometrial Iron Metabolism in Iron Deficiency. International Journal of Molecular Sciences. 2023; 24(12):9917. https://doi.org/10.3390/ijms24129917
Chicago/Turabian StylePandur, Edina, Ramóna Pap, Gergely Jánosa, Adrienn Horváth, and Katalin Sipos. 2023. "The Role of Fractalkine in the Regulation of Endometrial Iron Metabolism in Iron Deficiency" International Journal of Molecular Sciences 24, no. 12: 9917. https://doi.org/10.3390/ijms24129917






