End-to-Side vs. Free Graft Nerve Reconstruction—Experimental Study on Rats
Abstract
:1. Introduction
2. Results
2.1. Results of the Analysis the Walking Track
2.2. Results of the Electroneurographic Evaluation
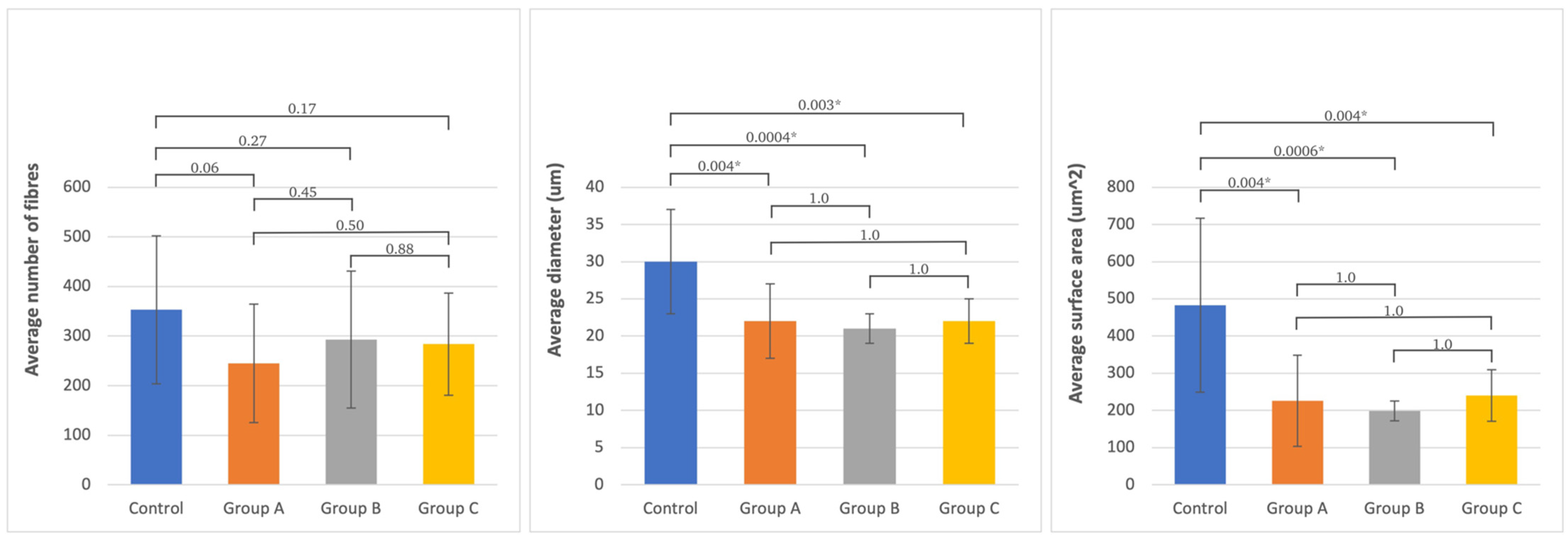
2.3. Histomorphometric Examination Evaluation
3. Discussion
3.1. Analysis of the Walking Track
3.2. Neurophysiological Evaluation
3.3. Histomorphometric Evaluation
3.4. Summary
4. Materials and Methods
4.1. Animals
- A.
- end-to-side suture without an incision of the donor nerve epineurium;
- B.
- end-to-side suture with an incision of the donor nerve epineurium;
- C.
- reconstruction with a free nerve graft.
4.2. Anesthesia and Surgery
- A.
- group A—suturing the stump of the distal peroneal nerve to the side of the tibial nerve;
- B.
- group B—as above, but with a previous creation of an oval window in the epineurium of the tibial nerve;
- C.
- group C—excision of a fragment of the peroneal nerve with a length of about 1 cm, then suturing it to the damaged nerve after inverting it by 180° as a free graft.
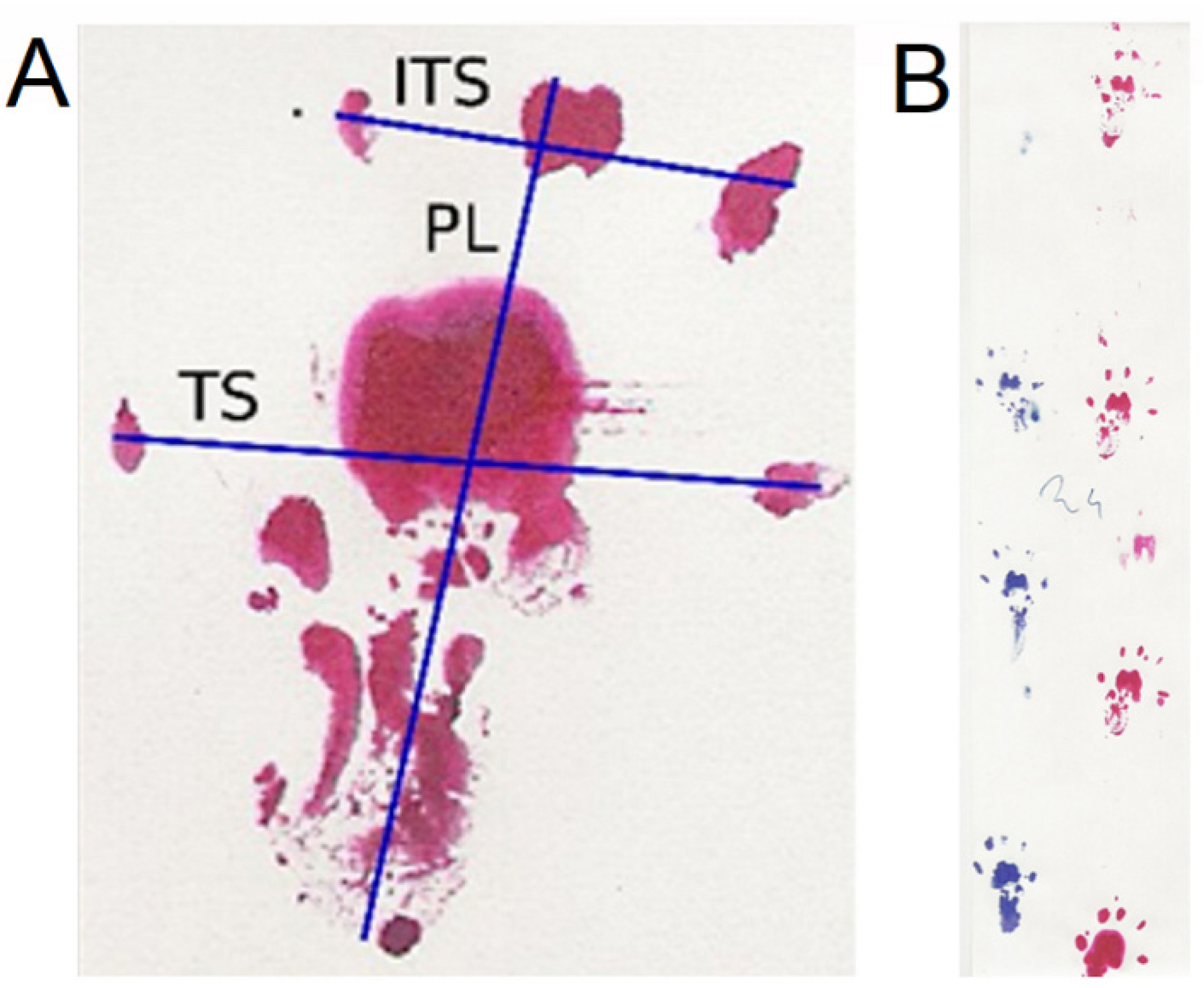
4.3. Walking Track Analysis
- PFI = 174.9 (EPL-NPL)/NPL + 80.3 (ETS-NTS)/NTS-13.4.
- TFI = −37.2 (EPL-NPL)/NPL + 104.4 (ETS-NTS)/NTS + 45.6 (EITS-NITS)/NITS-8.8.
- SFI = −38.8 (EPL-NPL)/NPL + 109.5 (ETS-NTS)/NTS + 13.3 (EITS-NITS)/NITS-8.8.
4.4. Electrophysiological Evaluation
- Motor fibers of the sciatic nerve were electrically stimulated by a pair of silver bipolar hook-shaped electrodes applied to the nerve 1 cm from the fusion site. The cathode was distal, while the anode of the electrode pair was oriented closer to the spinal center. Such an orientation of the simulating poles guarantees the orthodromic excitation of the motor fibers within the nerve. The distance between the recording electrodes poles was 3–4 mm. The ground electrode was placed near the recording electrodes on the muscle. During electroneurographic ENG nerve-to-nerve recordings, special attention was paid not to dry the dissected nerve branches; they were soaked with drops of warm paraffin oil. Electroneurography of the sciatic nerves was applied for bilateral detection of changes in the conduction of neural impulses following surgical nerve grafts. The ENG potentials were recorded from the distal parts of peroneal and tibial nerves with pairs of bipolar silver electrodes, after the application of electrical, rectangular pulses with 0.2 ms duration, at 1 Hz, and intensity from 0 to 20 mA delivered from the other bipolar stimulating silver electrodes in the proximal part of the sciatic nerve. A distance of 3 mm between the recording anode and cathode was maintained. Potentials were recorded in order to verify the conduction of neuronal impulses in the peripheral motor fibers. The recordings were performed at an amplification of 5–5000 µV and a time base of 2–10 ms. The parameters of amplitudes (in µV) and latencies (in ms) in recorded potentials were the outcome measures. Donor tibial and graft peroneal nerve fibers were excited following electrical stimulations of sciatic nerve rectangular pulses with a duration of 0.1 ms at 1 Hz and strength from 0.06 to 1 mA delivered from the KeyPoint device stimulator.
- Descending fibers of the white matter of the spinal cord were stimulated by a stream of the magnetic field released from the electromagnetic coil over the spine and recorded using the MEP technique (motor evoked potentials induced by a magnetic field). They aimed to evaluate the total efferent neural conduction from lumbar spinal centers to the distal parts of nerves and effectors. Standard single pulses of the magnetic field were used for oververtebral stimulation to induce motor-evoked potentials (MEPs). They were induced from the MagPro R30 (Medtronic A/S, Skovlunde, Denmark) using a 50 mm diameter circular coil placed bilaterally over the descending fibers of white matter at the L3–L5 spinal cord level, and recordings were performed using the MEP technique 10 mm from the peripheral graft. The optimal site for stimulation was defined with tracking stimuli delivered at 1 Hz from 5–60% of maximal output stimulus strength (1.5 T—Tesla), while the maximal amplitude of MEPs was recorded from the nerve. The maximal stimulus output at less than 60% was the highest intensity stimulus applied. The recordings were performed at an amplification of 50–5000 µV and a time base of 2–5 ms. All MEPs recordings were made at 0.5 Hz low-pass filter settings, while the upper-pass filter of KeyPoint was set at 2 kHz. The outcome measures were the parameters of amplitudes (in µV) and latencies (in ms) of MEPs.
4.5. Histomorphometric Evaluation
- number of nerve fibers in the field;
- diameter of nerve fibers without myelin sheath;
- nerve fiber surface area without myelin sheath.
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czarnecki, P.; Górecki, M.; Romanowski, L. Factors affecting the final outcomes after reconstruction of the median and ulnar nerve at the level of the forearm: Analysis of 41 patients. Injury 2020, 51, 2910–2915. [Google Scholar] [CrossRef]
- Myckatyn, T.M.; Mackinnon, S.E. A review of research endeavors to optimize peripheral nerve reconstruction. Neurol. Res. 2004, 26, 124–138. [Google Scholar] [CrossRef]
- Yapici, A.K.; Bayram, Y.; Akgun, H.; Gumus, R.; Zor, F. The effect of in vivo created vascularized neurotube on peripheric nerve regeneration. Injury 2017, 48, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Regas, I.; Loisel, F.; Haight, H.; Menu, G.; Obert, L.; Pluvy, I. Functionalized nerve conduits for peripheral nerve regeneration: A literature review. Hand Surg. Rehabil. 2020, 39, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.; Goldberg, J.A. The use of vein grafts in upper extremity nerve surgery. E J. Plastic Surg. 1999, 22, 255–259. [Google Scholar] [CrossRef]
- Kornfeld, T.; Borger, A.; Radtke, C. Reconstruction of Critical Nerve Defects Using Allogenic Nerve Tissue: A Review of Current Approaches. Intern. J. Mol. Sci. 2021, 22, 3515. [Google Scholar] [CrossRef]
- Fox, I.K.; Mackinnon, S.E. Experience with Nerve Allograft Transplantation. Semin. Plast. Surg. 2007, 21, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Terzis, J.K.; Kostopoulos, V.K. Vascularized Nerve Grafts and Vascularized Fascia for Upper Extremity Nerve Reconstruction. Hand 2010, 5, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Toia, F.; Matta, D.; De Michele, F.; Pirrello, R.; Cordova, A. Animal models of vascularized nerve grafts: A systematic review. Neur. Regen. Res. 2023, 18, 2615. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Colbert, S.H. Nerve transfers in the hand and upper extremity surgery. Tech. Hand Up. Extrem. Surg. 2008, 12, 20–33. [Google Scholar] [CrossRef]
- Haninec, P.; Kaiser, R.; Dubový, P. A Comparison of collateral sprouting of sensory and motor axons after end-to-side neurorrhaphy with and without the perineurial window. Plast. Reconstr. Surg. 2012, 130, 609–614. [Google Scholar] [CrossRef]
- Tos, P.; Colzani, G.; Ciclamini, D.; Titolo, P.; Pugliese, P.; Artiaco, S. Clinical applications of end-to-side neurorrhaphy: An update. Biomed. Res. Int. 2014, 2014, 646128. [Google Scholar] [CrossRef] [Green Version]
- McCallister, W.V.; Tang, P.; Smith, J.; Trumble, T.E. Axonal regeneration stimulated by the combination of nerve growth factor and ciliary neurotrophic factor in an end-to-side model. J. Hand Surg. Am. 2001, 26, 478–488. [Google Scholar] [CrossRef]
- Konofaos, P.; Bassilios Habre, S.; Wallace, R.D. End-to-Side Nerve Repair: Current Concepts and Future Perspectives. Ann. Plast. Surg. 2018, 81, 736–740. [Google Scholar] [CrossRef]
- Dvali, L.T.; Myckatyn, T.M. End-to-side nerve repair: Review of the literature and clinical indications. Hand Clin. 2008, 24, 455–460, vii. [Google Scholar] [CrossRef]
- Chen, S.H.; Mao, S.H.; Lan, C.Y.; Huang, R.W.; Lee, C.H.; Hsu, C.C.; Lin, C.H.; Lin, Y.T.; Chuang, D.C. End-to-Side Anterior Interosseous Nerve Transfer: A Valuable Alternative for Traumatic High Ulnar Nerve Palsy. Ann. Plast. Surg. 2021, 86, S102–S107. [Google Scholar] [CrossRef]
- Bertelli, J.A.; Ghizoni, M.F. Nerve repair by end-to-side coaptation or fascicular transfer: A clinical study. J. Reconstr. Microsurg. 2003, 19, 313–318. [Google Scholar]
- Papalia, I.; Lacroix, C.; Brunelli, F.; d’Alcontres, F.S. Direct muscle neurotization after end-to-side neurorrhaphy. J. Reconstr. Microsurg. 2001, 17, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.R.; Mackinnon, S.E.; Hunter, D.A. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989, 83, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, F.; Firrell, J.C.; Breidenbach, W.C. Sciatic function index, nerve conduction tests, muscle contraction, and axon morphometry as indicators of regeneration. Plast. Reconstr. Surg. 1996, 98, 1264–1271; discussion 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- De Medinaceli, L.; Freed, W.J.; Wyatt, R.J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 1982, 77, 634–643. [Google Scholar] [CrossRef]
- De Medinaceli, L. Use of sciatic function index and walking track assessment. Microsurgery 1990, 11, 191–192. [Google Scholar] [PubMed]
- Viterbo, F.; Brock, R.S.; Maciel, F.; Ayestaray, B.; Garbino, J.A.; Rodrigues, C.P. End-to-side versus end-to-end neurorrhaphy at the peroneal nerve in rats. Acta Cir. Bras. 2017, 32, 697–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sá, J.M.R.; Mazzer, N.; Barbieri, C.H.; Barreira, A.A. The end-to-side peripheral nerve repair. Functional and morphometric study using the peroneal nerve of rats. J. Neurosci. Methods 2004, 136, 45–53. [Google Scholar] [CrossRef]
- Monte-Raso, V.V.; Barbieri, C.H.; Mazzer, N.; Yamasita, A.C.; Barbieri, G. Is the Sciatic Functional Index always reliable and reproducible? J. Neurosci. Methods 2008, 170, 255–261. [Google Scholar] [CrossRef]
- Kerns, J.M.; Sladek, E.H.; Malushte, T.S.; Bach, H.; El‐hassan, B.; Kitidumrongsook, P.; Kroin, J.S.; Shott, S.; Gantsoudes, G.; Gonzalez, M.H.; et al. End-to-side nerve grafting of the tibial nerve to bridge a neuroma-in-continuity. Microsurgery 2005, 25, 155–164; discussion 164–166. [Google Scholar] [CrossRef]
- Nichols, C.M.; Myckatyn, T.M.; Rickman, S.R.; Fox, I.K.; Hadlock, T.; Mackinnon, S.E. Choosing the correct functional assay: A comprehensive assessment of functional tests in the rat. Behav. Brain Res. 2005, 163, 143–158. [Google Scholar] [CrossRef]
- Rosen, J.M.; Jewett, D.L. Nerve repair. In Physiologic Methods of Evaluating Experimental Nerve Repairs; Mosby: St. Louis, MO, USA, 1980; pp. 15–161. [Google Scholar]
- Rupp, A.; Dornseifer, U.; Fischer, A.; Schmahl, W.; Rodenacker, K.; Jütting, U.; Gais, P.; Biemer, E.; Papadopulos, N.; Matiasek, V. Electrophysiologic assessment of sciatic nerve regeneration in the rat: Surrounding limb muscles feature strongly in recordings from the gastrocnemius muscle. J. Neurosci. Methods 2007, 166, 266–277. [Google Scholar] [CrossRef]
- Vleggeert-Lankamp, C.L.A.M.; van den Berg, R.J.; Feirabend, H.K.P.; Lakke, E.A.J.F.; Malessy, M.J.A.; Thomeer, R.T.W.M. Electrophysiology and morphometry of the Aalpha- and Abeta-fiber populations in the normal and regenerating rat sciatic nerve. Exp. Neurol. 2004, 187, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ohnishi, K.; Kiyotani, T.; Sekine, T.; Ueda, H.; Nakamura, T.; Endo, K.; Shimizu, Y. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: A histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000, 868, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Rovak, J.M.; Cederna, P.S.; Macionis, V.; Urbanchek, M.S.; Van Der Meulen, J.H.; Kuzon, W.M. Termino-lateral neurorrhaphy: The functional axonal anatomy. Microsurgery 2000, 20, 6–14. [Google Scholar] [CrossRef]
- Kovacic, U.; Tomsic, M.; Sketelj, J.; Bajrović, F.F. Collateral sprouting of sensory axons after end-to-side nerve coaptation--a longitudinal study in the rat. Exp. Neurol. 2007, 203, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Shields, L.B.E.; Zhang, Y.; Pei, J.; Xu, X.M.; Hoskins, R.; Cai, J.; Qiu, M.S.; Magnuson, D.S.K.; Burke, D.A.; et al. Use of magnetic stimulation to elicit motor evoked potentials, somatosensory evoked potentials, and H-reflexes in non-sedated rodents. J. Neurosci. Methods 2007, 165, 9–17. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Hudson, A.R.; Hunter, D.A. Histologic assessment of nerve regeneration in the rat. Plast. Reconstr. Surg. 1985, 75, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.D.; Lowe, A.S.; Garrett, A.R.; Woodward, R.; Bennett, W.; Canty, A.J.; Garry, M.I.; Hinder, M.R.; Summers, J.J.; Gersner, R.; et al. Construction and Evaluation of Rodent-Specific rTMS Coils. Front. Neural. Circuits 2016, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houlden, D.A.; Schwartz, M.L.; Tator, C.H.; Ashby, P.; MacKay, W.A. Spinal cord-evoked potentials and muscle responses evoked by transcranial magnetic stimulation in 10 awake human subjects. J. Neurosci. 1999, 19, 1855–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnecki, P.; Huber, J.; Szukała, A.; Górecki, M.; Romanowski, L. The Usefulness of Motor Potentials Evoked Transvertebrally at Lumbar Levels for the Evaluation of Peroneal Nerve Regeneration after Experimental Repair in Rats. J. Personal. Med. 2023, 13, 438. [Google Scholar] [CrossRef]
- Urso-Baiarda, F.; Grobbelaar, A.O. Practical nerve morphometry. J. Neurosci. Methods. 2006, 156, 333–341. [Google Scholar] [CrossRef]
- Ghalib, N.; Houst’ava, L.; Haninec, P.; Dubový, P. Morphometric analysis of early regeneration of motor axons through motor and cutaneous nerve grafts. Ann. Anat. 2001, 183, 363–368. [Google Scholar] [CrossRef]
- Lindemuth, R.; Mink, D.; Ernzerhof, C.; Schimrigk, K. Automated and Representative Fascicle Selection for Computer-Assisted Morphometry of Myelinated Nerve Fibres in Peripheral Nerves. J. Periph. Nerv. Syst. 2002, 7, 211. [Google Scholar] [CrossRef]
- Da Silva, A.P.D.; Jordão, C.E.R.; Fazan, V.P.S. Peripheral nerve morphometry: Comparison between manual and semi-automated methods in the analysis of a small nerve. J. Neurosci. Methods 2007, 159, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.; Zhao, Q.; Kanje, M.; Danielsen, N.; Kerns, J.M. Can sensory and motor collateral sprouting be induced from intact peripheral nerve by end-to-side anastomosis? J. Hand Surg. Br. 1994, 19, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.S.; Glaus, S.W.; Yee, A.; Nicoson, M.C.; Hunter, D.A.; Mackinnon, S.E.; Johnson, P.J. Reverse end-to-side nerve transfer: From animal model to clinical use. J. Hand Surg. Am. 2011, 36, 1631–1639.e2. [Google Scholar] [CrossRef]
- Zhang, Z.; Soucacos, P.N.; Beris, A.E.; Bo, J.; Ioachim, E.; Johnson, E.O. Long-term evaluation of rat peripheral nerve repair with end-to-side neurorrhaphy. J. Reconstr. Microsurg. 2000, 16, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Geuna, S.; Papalia, I.; Ronchi, G.; d’Alcontres, F.S.; Natsis, K.; Papadopulos, N.A.; Colonna, M.R. The reasons for end-to-side coaptation: How does lateral axon sprouting work? Neural. Regen Res. 2017, 12, 529–533. [Google Scholar] [PubMed]
- Brzezicki, G.; Jankowski, R.; Blok, T.; Klimczak, A.; Szymas, J.; Huber, J.; Szukala, A.; Siemionow, M.; Nowak, S. Postlaminectomy Osteopontin Expression and Associated Neurophysiological Findings in Rat Peridural Scar Model. Spine 2011, 36, 378. [Google Scholar] [CrossRef]
- Hayashi, A.; Pannucci, C.; Moradzadeh, A.; Kawamura, D.; Magill, C.; Hunter, D.A.; Tong, A.Y.; Parsadanian, A.; Mackinnon, S.E.; Myckatyn, T.M. Axotomy or compression is required for axonal sprouting following end-to-side neurorrhaphy. Exp. Neurol. 2008, 211, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Sotereanos, D.G.; Seaber, A.V.; Urbaniak, J.R.; Spiegel, D.A.; Sotereanos, D.; Anthony, D.C. Reversing nerve-graft polarity in a rat model: The effect on function. J. Reconstr. Microsurg. 1992, 8, 303–307. [Google Scholar] [CrossRef]






| Significance Level LSD Test | Correlation between PFI, TFI, and SFI and Follow-Up Time | Correlation between PFI and TFI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tested Site | Group A | Group B | Group C | r | p-Value | r | p-Value | ||
| Peroneal nerve (PFI) | Group A (N = 15) | 0.66 | 0.67 | 0.49 | 0.0001 | 0.74 | <0.05 | Tibial nerve (TFI) | |
| Group B (N = 15) | 0.66 | 0.38 | 0.39 | 0.003 | 0.85 | <0.05 | |||
| Group C (N = 15) | 0.67 | 0.38 | 0.23 | 0.0001 | 0.55 | <0.05 | |||
| Tibial nerve (TFI) | Group A (N = 15) | 0.78 | 0.23 | 0.43 | 0.0001 | ||||
| Group B (N = 15) | 0.78 | 0.33 | 0.44 | 0.0001 | |||||
| Group C (N = 15) | 0.23 | 0.33 | 0.31 | 0.0001 | |||||
| Sciatic nerve (SFI) | Group A (N = 15) | 0.73 | 0.63 | 0.43 | 0.0001 | ||||
| Group B (N = 15) | 0.73 | 0.89 | 0.43 | 0.0001 | |||||
| Group C (N = 15) | 0.63 | 0.89 | 0.28 | 0.0001 | |||||
| Type of Test Recording Site | Measured Parameter | Non-Operated Side Control | Operated Side | Non-Operated vs. Operated p-Value | ANOVA Variance Analysis between Operated Groups p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A (N = 14) | Group B (N = 12) | Group C (N = 14) | Group A (N = 14) | Group B (N = 12) | Group C (N = 14) | Group A | Group B | Group C | ||||
| Peroneal nerve | ENG | Amplitude (µV) | 8000 ± 4459 | 10,818 ± 6856 | 7078 ± 5847 | 7043 ± 3521 | 6908 ± 2524 | 6414 ± 4311 | 0.54 | 0.08 | 0.73 | 0.89 |
| Latency (ms) | 0.89 ± 0.20 | 0.88 ± 0.13 | 1.01 ± 0.38 | 1.02 ± 0.27 | 0.92 ± 0.27 | 0.96 ± 0.25 | 0.18 | 0.62 | 0.70 | 0.67 | ||
| MEP | Amplitude (µV) | 9238 ± 6243 | 11,992 ± 7939 | 8013 ± 5614 | 7354 ± 4824 | 9958 ± 6325 | 10,514 ± 5540 | 0.40 | 0.50 | 0.24 | 0.31 | |
| Latency (ms) | 1.59 ± 0.36 | 1.57 ± 0.19 | 1.70 ± 0.29 | 1.56 ± 0.65 | 1.51 ± 0.32 | 1.57 ± 0.34 | 0.89 | 0.61 | 0.28 | 0.94 | ||
| Tibial nerve | ENG | Amplitude (µV) | 8639 ± 4318 | 12,845 ± 7257 | 9354 ± 5661 | 5657 ± 2913 | 5915 ± 4923 | 6141 ± 5199 | 0.04 | 0.01 | 0.25 | 0.96 |
| Latency (ms) | 1.00 ± 0.31 | 0.92 ± 0.27 | 0.98 ± 0.40 | 1.14 ± 0.40 | 1.20 ± 0.49 | 1.04 ± 0.27 | 0.35 | 0.11 | 0.37 | 0.60 | ||
| MEP | Amplitude (µV) | 9508 ± 6161 | 12,583 ± 6395 | 7408 ± 4645 | 6485 ± 4435 | 7133 ± 2927 | 7454 ± 3331 | 0.17 | 0.01 | 0.96 | 0.83 | |
| Latency (ms) | 1.71 ± 0.31 | 1.60 ± 0.40 | 1.66 ± 0.29 | 1.51 ± 0.35 | 1.65 ± 0.28 | 1.52 ± 0.22 | 0.14 | 0.75 | 0.15 | 0.40 | ||
| Analyzed Morphometric Parameters | Number of Fibers | Diameter (um) | Surface Area (um2) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variance Analysis between Groups | Variance Analysis between Groups | Variance Analysis between Groups | |||||||||||||||
| Tested Site | Mean SD | Control | Group A | Group B | Group C | Mean SD | Control | Group A | Group B | Group C | Mean SD | Control | Group A | Group B | Group C | ||
| (n = 15) | (n = 8) | (n = 9) | (n = 13) | (n = 15) | (n = 8) | (n = 9) | (n = 13) | (n = 15) | (n = 8) | (n = 9) | (n = 13) | ||||||
| Peroneal nerve | Control | 353 ± 149 | 0.06 | 0.27 | 0.17 | 30 ± 7 | 0.004 | 0.0004 | 0.003 | 483 ± 234 | 0.0004 | 0.0006 | 0.006 | ||||
| Group A | 245 ± 119 | 0.06 | 0.45 | 0.5 | 22 ± 5 | 0.004 | 1 | 1 | 226 ± 123 | 0.004 | 1 | 1 | |||||
| Group B | 293 ± 138 | 0.27 | 0.45 | 0.88 | 21 ± 2 | 0.0004 | 1 | 1 | 199 ± 27 | 0.0006 | 1 | 1 | |||||
| Group C | 284 ± 103 | 0.17 | 0.5 | 0.88 | 22 ± 3 | 0.003 | 1 | 1 | 240 ± 69 | 0.004 | 1 | 1 | |||||
| Tibial nerve | Control | 399 ± 158 | 0.98 | 0.34 | 0.56 | 31 ± 8 | 0.51 | 0.15 | 0.84 | 477 ± 202 | 0.59 | 0.29 | 0.49 | ||||
| Group A | 397 ± 191 | 0.98 | 0.4 | 0.58 | 33 ± 5 | 0.51 | 0.47 | 0.72 | 518 ± 128 | 0.59 | 0.63 | 0.28 | |||||
| Group B | 329 ± 174 | 0.34 | 0.4 | 0.19 | 36 ± 10 | 0.15 | 0.47 | 0.31 | 559 ± 231 | 0.29 | 0.63 | 0.13 | |||||
| Group C | 446 ± 199 | 0.56 | 0.58 | 0.19 | 32 ± 8 | 0.84 | 0.72 | 0.31 | 418 ± 136 | 0.49 | 0.28 | 0.13 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnecki, P.; Huber, J.; Szymankiewicz-Szukała, A.; Górecki, M.; Romanowski, L. End-to-Side vs. Free Graft Nerve Reconstruction—Experimental Study on Rats. Int. J. Mol. Sci. 2023, 24, 10428. https://doi.org/10.3390/ijms241310428
Czarnecki P, Huber J, Szymankiewicz-Szukała A, Górecki M, Romanowski L. End-to-Side vs. Free Graft Nerve Reconstruction—Experimental Study on Rats. International Journal of Molecular Sciences. 2023; 24(13):10428. https://doi.org/10.3390/ijms241310428
Chicago/Turabian StyleCzarnecki, Piotr, Juliusz Huber, Agnieszka Szymankiewicz-Szukała, Michał Górecki, and Leszek Romanowski. 2023. "End-to-Side vs. Free Graft Nerve Reconstruction—Experimental Study on Rats" International Journal of Molecular Sciences 24, no. 13: 10428. https://doi.org/10.3390/ijms241310428
APA StyleCzarnecki, P., Huber, J., Szymankiewicz-Szukała, A., Górecki, M., & Romanowski, L. (2023). End-to-Side vs. Free Graft Nerve Reconstruction—Experimental Study on Rats. International Journal of Molecular Sciences, 24(13), 10428. https://doi.org/10.3390/ijms241310428






