Vomeronasal Receptors Associated with Circulating Estrogen Processing Chemosensory Cues in Semi-Aquatic Mammals
Abstract
:1. Introduction
2. Results
2.1. Morphological Observations and Histological Features of the VNO of Female Muskrats
2.2. Immunohistochemical Locations of V1R, V2R, ERα and ERβ in the VNO of Muskrats
2.3. The mRNA Expressions of v1r, v2r, erα and erβ in the VNO of the Muskrats
2.4. Concentration Profiles of 17β-Estradiol in the Female Muskrats
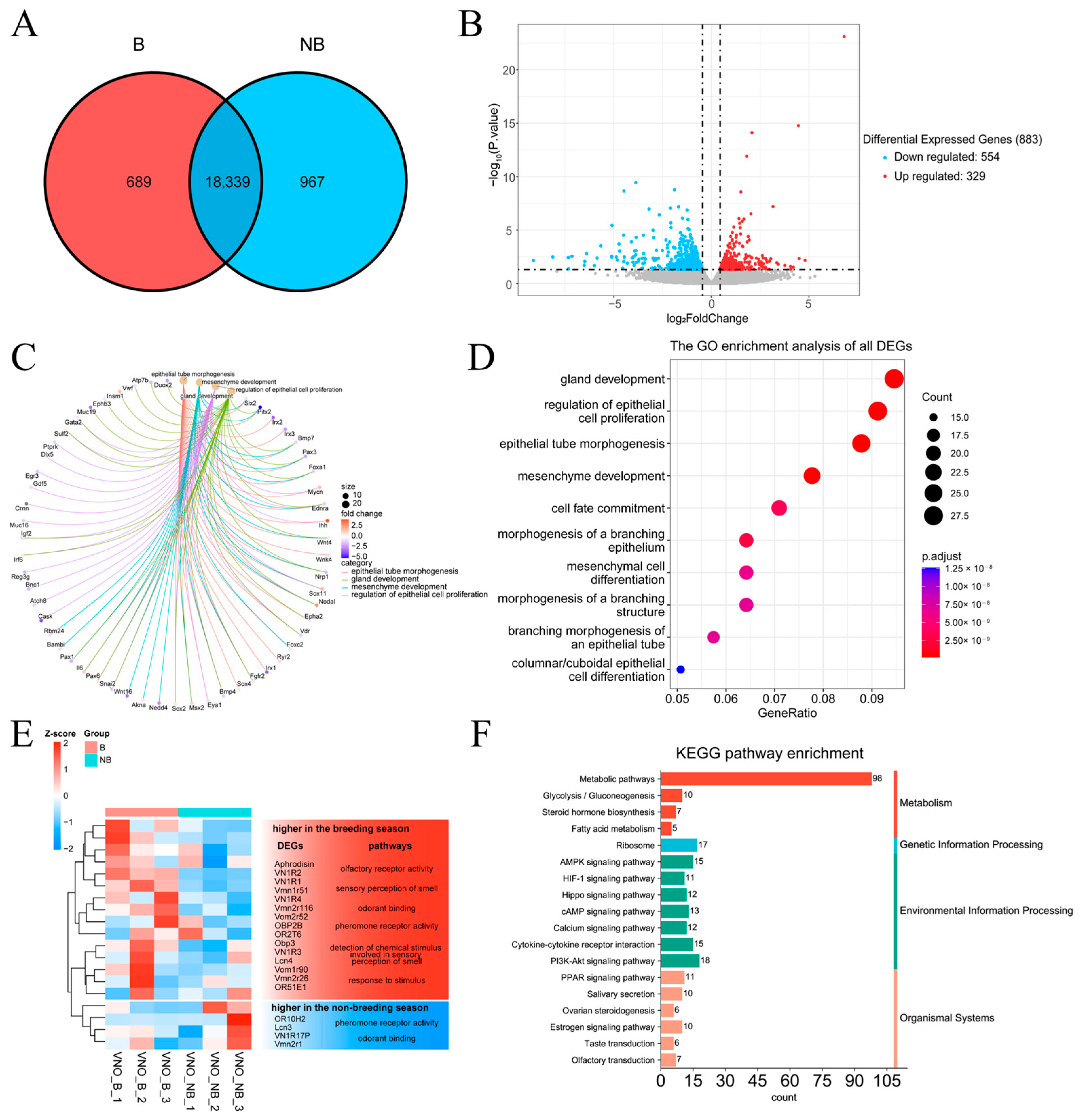
2.5. Transcriptome Data Characterization
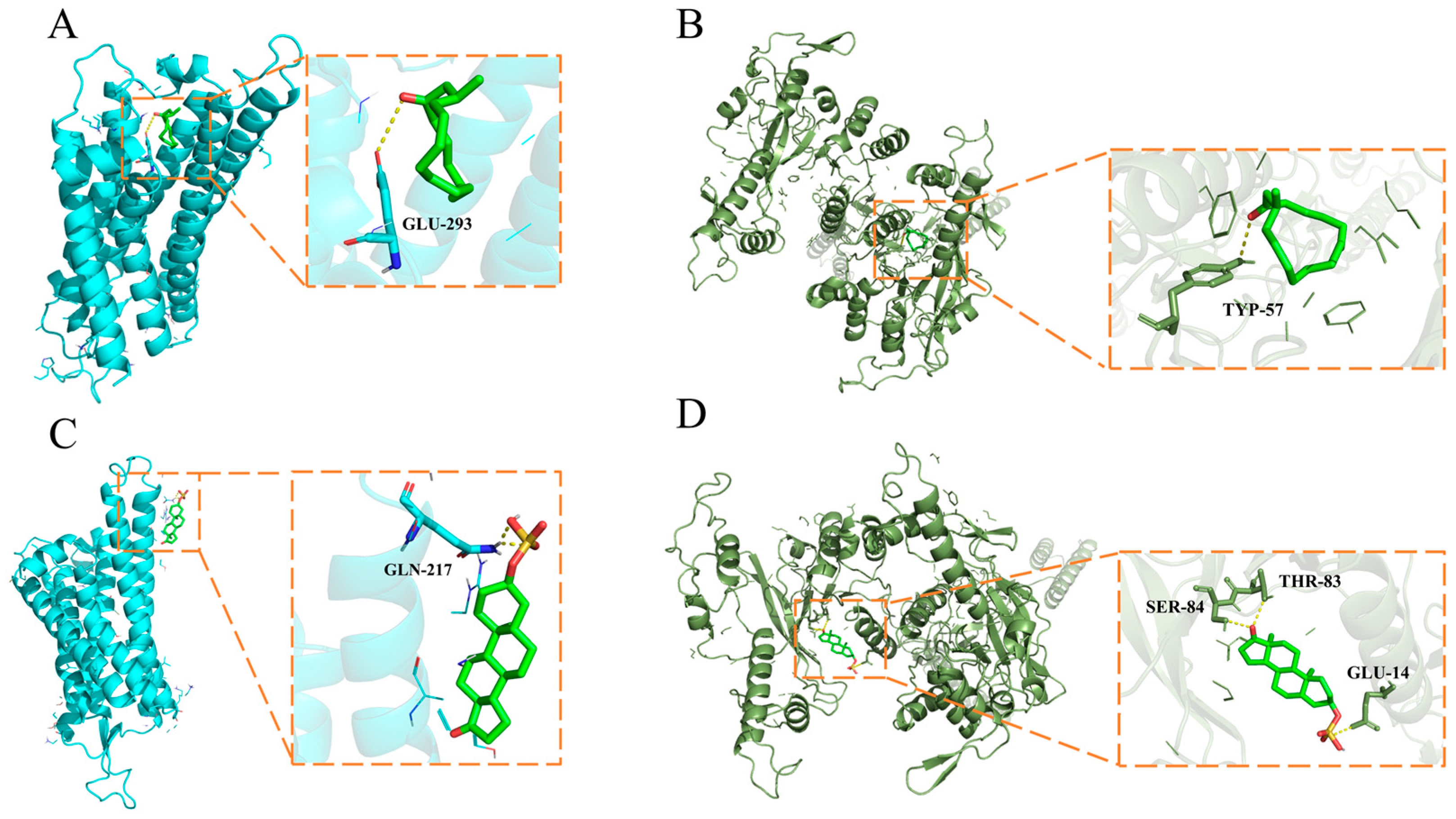
2.6. Molecular Docking Stimulation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Macroscopic Anatomy and Histological Process
4.3. Immunohistochemical Process
4.4. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.5. Hormone Assay
4.6. Library Preparation for Transcriptome Sequencing
4.7. Transcriptome Analysis
4.8. Protein-Ligand Interaction Analysis
4.9. Morphometry and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tirindelli, R.; Dibattista, M.; Pifferi, S.; Menini, A. From pheromones to behavior. Physiol. Rev. 2009, 89, 921–956. [Google Scholar] [CrossRef]
- Brennan, P.A.; Zufall, F. Pheromonal communication in vertebrates. Nature 2006, 444, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Dulac, C.; Torello, A.T. Molecular detection of pheromone signals in mammals: From genes to behaviour. Nat. Rev. Neurosci. 2003, 4, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Mukwaya, V.; Mann, S.; Dou, H. Chemical communication at the synthetic cell/living cell interface. Commun. Chem. 2021, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Dulac, C. Sensory coding of pheromone signals in mammals. Curr. Opin. Neurobiol. 2000, 10, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.A. The vomeronasal system. Cell. Mol. Life Sci. 2001, 58, 546–555. [Google Scholar] [CrossRef]
- Martin-Sanchez, A.; McLean, L.; Beynon, R.J.; Hurst, J.L.; Ayala, G.; Lanuza, E.; Martinez-Garcia, F. From sexual attraction to maternal aggression: When pheromones change their behavioural significance. Horm. Behav. 2015, 68, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Petrulis, A. Chemosignals, hormones and mammalian reproduction. Horm. Behav. 2013, 63, 723–741. [Google Scholar] [CrossRef] [Green Version]
- Kondo, Y.; Hayashi, H. Neural and hormonal basis of opposite-sex preference by chemosensory signals. Int. J. Mol. Sci. 2021, 22, 8311. [Google Scholar] [CrossRef]
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, J. Comparative genomic analysis identifies an evolutionary shift of vomeronasal receptor gene repertoires in the vertebrate transition from water to land. Genome Res. 2007, 17, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.; Antunes, A. Vomeronasal receptors in vertebrates and the evolution of pheromone detection. Annu. Rev. Anim. Biosci. 2017, 5, 353–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomiyasu, J.; Kondoh, D.; Sakamoto, H.; Matsumoto, N.; Sasaki, M.; Kitamura, N.; Haneda, S.; Matsui, M. Morphological and histological features of the vomeronasal organ in the brown bear. J. Anat. 2017, 231, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Kondoh, D.; Tanaka, Y.; Kawai, Y.K.; Mineshige, T.; Watanabe, K.; Kobayashi, Y. Morphological and histological features of the vomeronasal organ in African pygmy hedgehog (Atelerix albiventris). Animals 2021, 11, 1462. [Google Scholar] [CrossRef] [PubMed]
- Katreddi, R.R.; Forni, P.E. Mechanisms underlying pre- and postnatal development of the vomeronasal organ. Cell. Mol. Life Sci. 2021, 78, 5069–5082. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.R.; Sofer, Y.; Brumfeld, V.; Zilkha, N.; Kimchi, T. The nasopalatine ducts are required for proper pheromone signaling in mice. Front. Neurosci. 2020, 14, 585323. [Google Scholar] [CrossRef]
- Emes, R.D.; Beatson, S.A.; Ponting, C.P.; Goodstadt, L. Evolution and comparative genomics of odorant- and pheromone-associated genes in rodents. Genome Res. 2004, 14, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Shi, P.; Zhang, Y.P.; Zhang, J. Composition and evolution of the V2r vomeronasal receptor gene repertoire in mice and rats. Genomics 2005, 86, 306–315. [Google Scholar] [CrossRef]
- Hohenbrink, P.; Radespiel, U.; Mundy, N.I. Pervasive and ongoing positive selection in the vomeronasal-1 receptor (V1R) repertoire of mouse lemurs. Mol. Biol. Evol. 2012, 29, 3807–3816. [Google Scholar] [CrossRef] [Green Version]
- Muramoto, K.; Hashimoto, M.; Kaba, H. Target regulation of V2R expression and functional maturation in vomeronasal sensory cneurons in vitro. Eur. J. Neurosci. 2007, 26, 3382–3394. [Google Scholar] [CrossRef]
- Moriya-Ito, K.; Hayakawa, T.; Suzuki, H.; Hagino-Yamagishi, K.; Nikaido, M. Evolution of vomeronasal receptor 1 (V1R) genes in the common marmoset (Callithrix jacchus). Gene 2018, 642, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Kambere, M.; Trask, B.J.; Lane, R.P. Divergent V1R repertoires in five species: Amplification in rodents, decimation in primates, and a surprisingly small repertoire in dogs. Genome Res. 2005, 15, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heraud, C.; Hirschinger, T.; Baranek, E.; Larroquet, L.; Surget, A.; Sandres, F.; Lanuque, A.; Terrier, F.; Roy, J. Detection and modulation of olfactory sensing receptors in carnivorous rainbow trout (oncorhynchus mykiss) fed from first feeding with plant-based diet. Int. J. Mol. Sci. 2022, 23, 2123. [Google Scholar] [CrossRef] [PubMed]
- Bjarnadóttir, T.K.; Fredriksson, R.; Schiöth, H.B. The gene repertoire and the common evolutionary history of glutamate, pheromone (V2R), taste(1) and other related G protein-coupled receptors. Gene 2005, 362, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Wai Lam, Y.; McDaniels, I.; Struziak, M.; Delay, R.J. Estradiol rapidly modulates odor responses in mouse vomeronasal sensory neurons. Neuroscience 2014, 269, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Rodewald, A.; Mills, D.; Gebhart, V.M.; Jirikowski, G.F. Steroidal pheromones and their potential target sites in the vomeronasal organ. Steroids 2019, 142, 14–20. [Google Scholar] [CrossRef]
- Berglund, H.; Lindström, P.; Savic, I. Brain response to putative pheromones in lesbian women. Proc. Natl. Acad. Sci. USA 2006, 103, 8269–8274. [Google Scholar] [CrossRef] [Green Version]
- Celsi, F.; D’Errico, A.; Menini, A. Responses to sulfated steroids of female mouse vomeronasal sensory neurons. Chem. Senses 2012, 37, 849–858. [Google Scholar] [CrossRef] [Green Version]
- Caras, R.A. North American Mammals: Fur-Bearing Animals of the United States and Canada; Meredith Press: Minneapolis, MN, USA, 1967. [Google Scholar]
- Van Loon, E.E.; Bos, D.; van Hellenberg Hubar, C.J.; Ydenberg, R.C. A historical perspective on the effects of trapping and controlling the muskrat (Ondatra zibethicus) in the Netherlands. Pest Manag. Sci. 2017, 73, 305–312. [Google Scholar] [CrossRef]
- Xie, W.; Liu, H.; Liu, Q.; Gao, Q.; Gao, F.; Han, Y.; Yuan, Z.; Zhang, H.; Weng, Q. Seasonal expressions of prolactin, prolactin receptor and STAT5 in the scented glands of the male muskrats (Ondatra zibethicus). Eur. J. Histochem. 2019, 63, 2991. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Gao, Q.; Wei, J.; Xie, W.; Zhang, H.; Yuan, Z.; Han, Y.; Weng, Q. Seasonal changes in endoplasmic reticulum stress and ovarian steroidogenesis in the muskrats (Ondatra zibethicus). Front. Endocrinol. 2023, 14, 1123699. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Hattori, T.; Sato, T.; Sato, K.; Matsuda, S.; Kobayakawa, R.; Sakano, H.; Yoshihara, Y.; Kikusui, T.; Touhara, K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature 2010, 466, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Mu, S.; Zhong, J.; Zhang, C.; Zhang, H.; Wang, X.; Weng, Q. Mass spectrometry imaging of lipids in the scent glands of muskrat (Ondatra zibethicus) in different reproductive statuses. Cells 2022, 11, 2228. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, Z.; Liang, D.; Yu, H.; Zheng, X.; Huang, Y. GC-MS analysis of the differences in the chemical components of muskrat and musk. Nat. Prod. Res. Dev. 2021, 10, 1643–1648. (In Chinese) [Google Scholar]
- Liu, K.; Li, Z.; Zhang, H.; Yu, Y.; Liu, D. Study on chemical constituents and pharmacological action of Muskrat. J. Changchun Univ. Tradit. Chin. Med. 2008, 2, 157. (In Chinese) [Google Scholar]
- Chen, J.; Liu, Z.; Song, F.; Liu, S. Analysis of chemical constitutes in lipid from musk-rat musk by gas chromatography/mass spectrometry. Chin. J. Anal. Chem. 1998, 9, 1142–1145. (In Chinese) [Google Scholar]
- Salazar, I.; Sanchez-Quinteiro, P.; Aleman, N.; Prieto, D. Anatomical, immnunohistochemical and physiological characteristics of the vomeronasal vessels in cows and their possible role in vomeronasal reception. J. Anat. 2008, 212, 686–696. [Google Scholar] [CrossRef]
- Elgayar, S.A.M.; Saad-Eldin, H.M.; Haussein, O.A. Morphology of cat vomeronasal organ non-sensory epithelium during postnatal development. Anat. Cell Biol. 2017, 50, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Kondoh, D.; Nakamura, K.G.; Ono, Y.S.; Yuhara, K.; Bando, G.; Watanabe, K.; Horiuchi, N.; Kobayashi, Y.; Sasaki, M.; Kitamura, N. Histological features of the vomeronasal organ in the giraffe, Giraffa camelopardalis. Microsc. Res. Tech. 2017, 80, 652–656. [Google Scholar] [CrossRef]
- Villamayor, P.R.; Robledo, D.; Fernandez, C.; Gullon, J.; Quintela, L.; Sanchez-Quinteiro, P.; Martinez, P. Analysis of the vomeronasal organ transcriptome reveals variable gene expression depending on age and function in rabbits. Genomics 2021, 113, 2240–2252. [Google Scholar] [CrossRef]
- Segovia, S.; Guillamón, A. Sexual dimorphism in the vomeronasal pathway and sex differences in reproductive behaviors. Brain Res. Brain Res. Rev. 1993, 18, 51–74. [Google Scholar] [CrossRef]
- Surov, A.V.; Maltsev, A.N. Analysis of chemical communication in mammals: Zoological and ecological aspects. Biol. Bull. Russ. Acad. Sci. 2017, 43, 1175–1183. [Google Scholar] [CrossRef]
- Tomiyasu, J.; Korzekwa, A.; Kawai, Y.K.; Robstad, C.A.; Rosell, F.; Kondoh, D. The vomeronasal system in semiaquatic beavers. J. Anat. 2022, 241, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Wang, P.; Guo, X.; Wu, Y.; Zhang, J.X.; Huang, L. Two preputial gland-secreted pheromones evoke sexually dimorphic neural pathways in the mouse vomeronasal system. Front. Cell. Neurosci. 2019, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Tirindelli, R. Coding of pheromones by vomeronasal receptors. Cell Tissue Res. 2021, 383, 367–386. [Google Scholar] [CrossRef]
- Kishida, T.; Go, Y.; Tatsumoto, S.; Tatsumi, K.; Kuraku, S.; Toda, M. Loss of olfaction in sea snakes provides new perspectives on the aquatic adaptation of amniotes. Proc. R. Soc. B 2019, 286, 20191828. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Marcucci, F.; Firestein, S. High-throughput microarray detection of vomeronasal receptor gene expression in rodents. Front. Neurosci. 2010, 4, 164. [Google Scholar] [CrossRef] [Green Version]
- Dawley, E.M.; Fingerlin, A.; Hwang, D.; John, S.S.; Stankiewicz, C.A. Seasonal cell proliferation in the chemosensory epithelium and brain of red-backed salamanders, Plethodon cinereus. Brain Behav. Evol. 2000, 56, 1–13. [Google Scholar] [CrossRef]
- Brykczynska, U.; Tzika, A.C.; Rodriguez, I.; Milinkovitch, M.C. Contrasted evolution of the vomeronasal receptor repertoires in mammals and squamate reptiles. Genome Biol. Evol. 2013, 5, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Liberles, S.D. Mammalian pheromones. Annu. Rev. Physiol. 2014, 76, 151–175. [Google Scholar] [CrossRef] [Green Version]
- Ploß, V.M.; Gebhart, V.M.; Gisder, D.; Dölz, W.; Jirikowski, G.F. Localization of sex hormone binding globulin in the rat vomeronasal organ. J. Chem. Neuroanat. 2014, 61–62, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.N.; Houck, L.D.; Feldhoff, P.W.; Feldhoff, R.C.; Woodley, S.K. Effects of androgens on behavioral and vomeronasal responses to chemosensory cues in male terrestrial salamanders (Plethodon shermani). Horm. Behav. 2006, 50, 469–476. [Google Scholar] [CrossRef]
- McCarthy, E.A.; Naik, A.S.; Coyne, A.F.; Cherry, J.A.; Baum, M.J. Effect of ovarian hormones and mating experience on the preference of female mice to investigate male urinary pheromones. Chem. Senses 2018, 43, 97–104. [Google Scholar] [CrossRef]
- Wnuk, A.; Przepiórska, K.; Pietrzak, B.A.; Kajta, M. Emerging Evidence on Membrane Estrogen Receptors as Novel Therapeutic Targets for Central Nervous System Pathologies. Int. J. Mol. Sci. 2023, 24, 4043. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhao, X.; Guo, L.; Han, Y.; Yuan, Z.; Zhang, H.; Weng, Q. Seasonal expressions of ERalpha, ERbeta, EGF, EGFR, PI3K and Akt in the scent glands of the muskrats (Ondatra zibethicus). J. Steroid. Biochem. Mol. Biol. 2021, 213, 105961. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Delano, W.L. PyMOL: An Open-Source Molecular Graphics Tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]

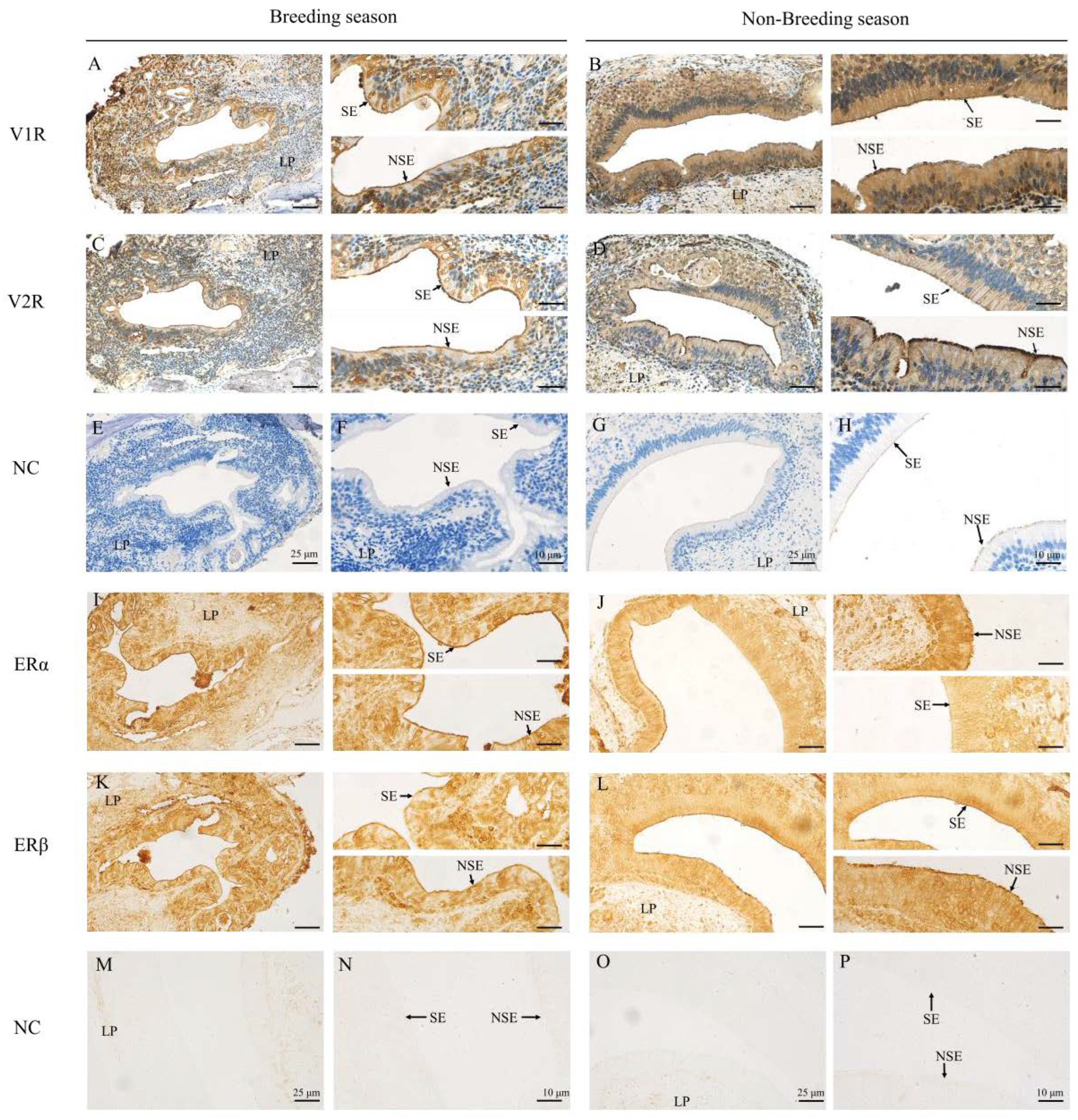

| Antibodies | Breeding Season | Non-Breeding Season | ||
|---|---|---|---|---|
| SE | NSE | SE | NSE | |
| V1R | ++ | ++ | +++ | +++ |
| V2R | +++ | +++ | ++ | ++ |
| ERα | +++ | +++ | + | ++ |
| ERβ | ++ | ++ | +++ | +++ |
| Metabolite Compound | MS Platform | References |
|---|---|---|
| Muscone | GC-MS | [36,37] |
| Cyclopentadecanone | GC-MS | [34,35,37] |
| Cyclopentadecanol | GC-MS | [34,35,37] |
| Palmitoleic acid | GC-MS | [35] |
| Z-7-Hexadecenoic acid | GC-MS | [35] |
| PC (16:0/16:0) | LC-MS | [35] |
| Phosphatidylethanolamine | LC-MS | [35] |
| LysoPC (16:0) | LC-MS | [35] |
| Androsterone sulfate | LC-MS | [35] |
| Dopamine | LC-MS | [35] |
| Compound | Protein Name | Docking Score (KJ/mol) | Interacting Residues |
|---|---|---|---|
| Muscone | V1R | −5.5 | GLU-394 |
| Androsterone sulfate | V1R | −6.6 | GLN-217 |
| Muscone | V2R | −7.3 | TYP-57 |
| SER-84 | |||
| Androsterone sulfate | V2R | −8.1 | THR-83 |
| GLU-14 |
| Gene | Primer Sequence | Product Length (bp) | Accession Numbers |
|---|---|---|---|
| v1r | F: 5′-ATGCAGCACATCCTCACTCC-3′ | 205 | NM_001167146.1 |
| R: 5′-AGATACTGGGGAAGACCGCA-3′ | |||
| v2r | F: 5′-AAGGAGCCAGTTCTCACTGC-3′ | 285 | NM_001385059.1 |
| R: 5′-TACATTCCGCACTGCACACA-3′ | |||
| erα | F: 5′-CGACTATGCCTCTGGCTACC-2′ | 157 | NM_012689.1 |
| R: 5′-TCATCATGCCCACTTCGTAA-2′ | |||
| erβ | F: 5′-CCTTGGTGTGAAGCAAGATC-3′ | 286 | NM_012754.3 |
| R: 5′-GGATCCACACTTGACCATTC-3′ | |||
| actb | F: 5′-GACTCGTCGTACTCCTGCTT-3′ | 223 | NM_007393.5 |
| R: 5′-AAGACCTCTATGCCAACACC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Chen, M.; Shen, Y.; Liu, Y.; Zhang, H.; Weng, Q. Vomeronasal Receptors Associated with Circulating Estrogen Processing Chemosensory Cues in Semi-Aquatic Mammals. Int. J. Mol. Sci. 2023, 24, 10724. https://doi.org/10.3390/ijms241310724
Xie W, Chen M, Shen Y, Liu Y, Zhang H, Weng Q. Vomeronasal Receptors Associated with Circulating Estrogen Processing Chemosensory Cues in Semi-Aquatic Mammals. International Journal of Molecular Sciences. 2023; 24(13):10724. https://doi.org/10.3390/ijms241310724
Chicago/Turabian StyleXie, Wenqian, Meiqi Chen, Yuyao Shen, Yuning Liu, Haolin Zhang, and Qiang Weng. 2023. "Vomeronasal Receptors Associated with Circulating Estrogen Processing Chemosensory Cues in Semi-Aquatic Mammals" International Journal of Molecular Sciences 24, no. 13: 10724. https://doi.org/10.3390/ijms241310724





