Succinyl-CoA Synthetase Dysfunction as a Mechanism of Mitochondrial Encephalomyopathy: More than Just an Oxidative Energy Deficit
Abstract
1. Introduction
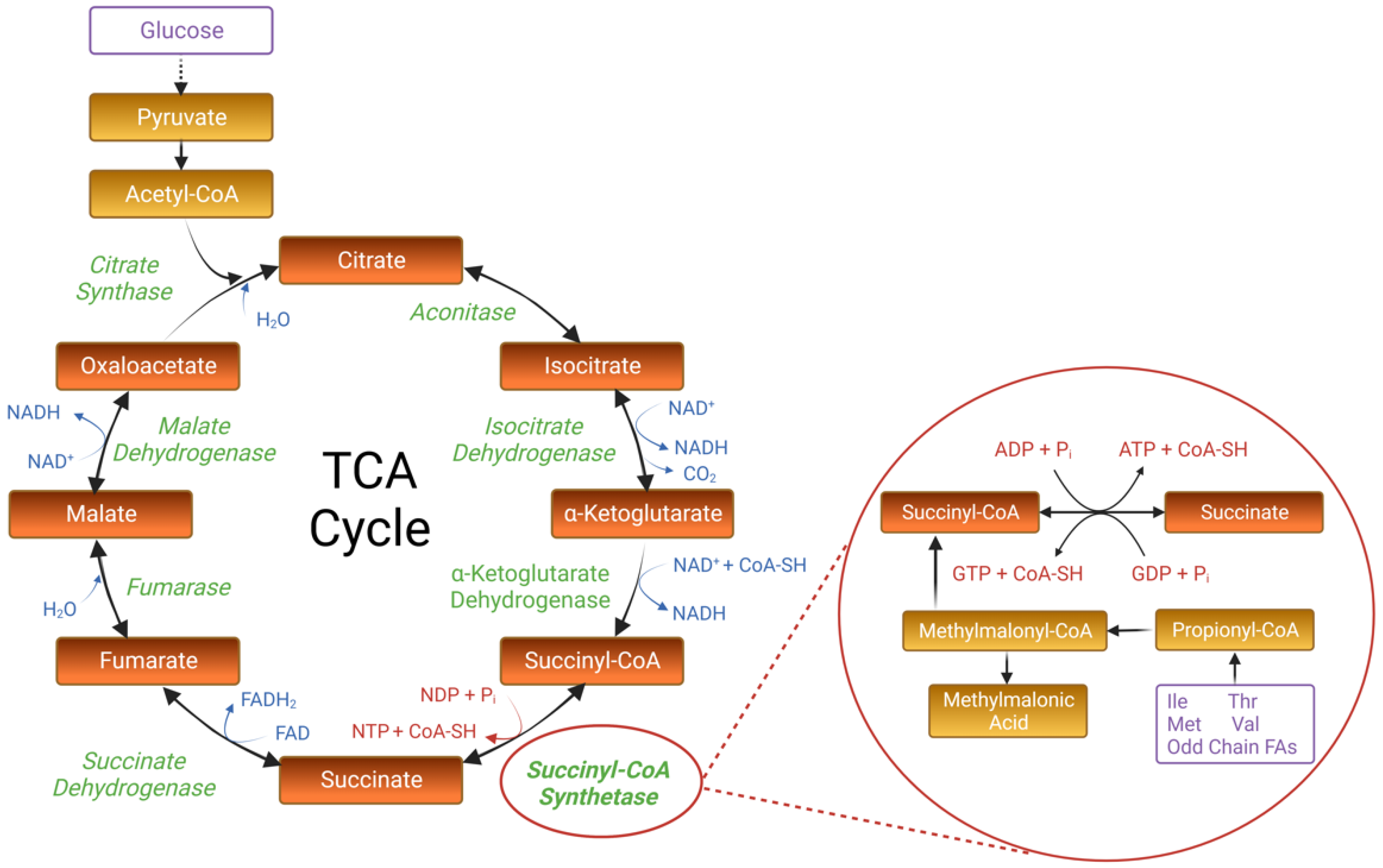
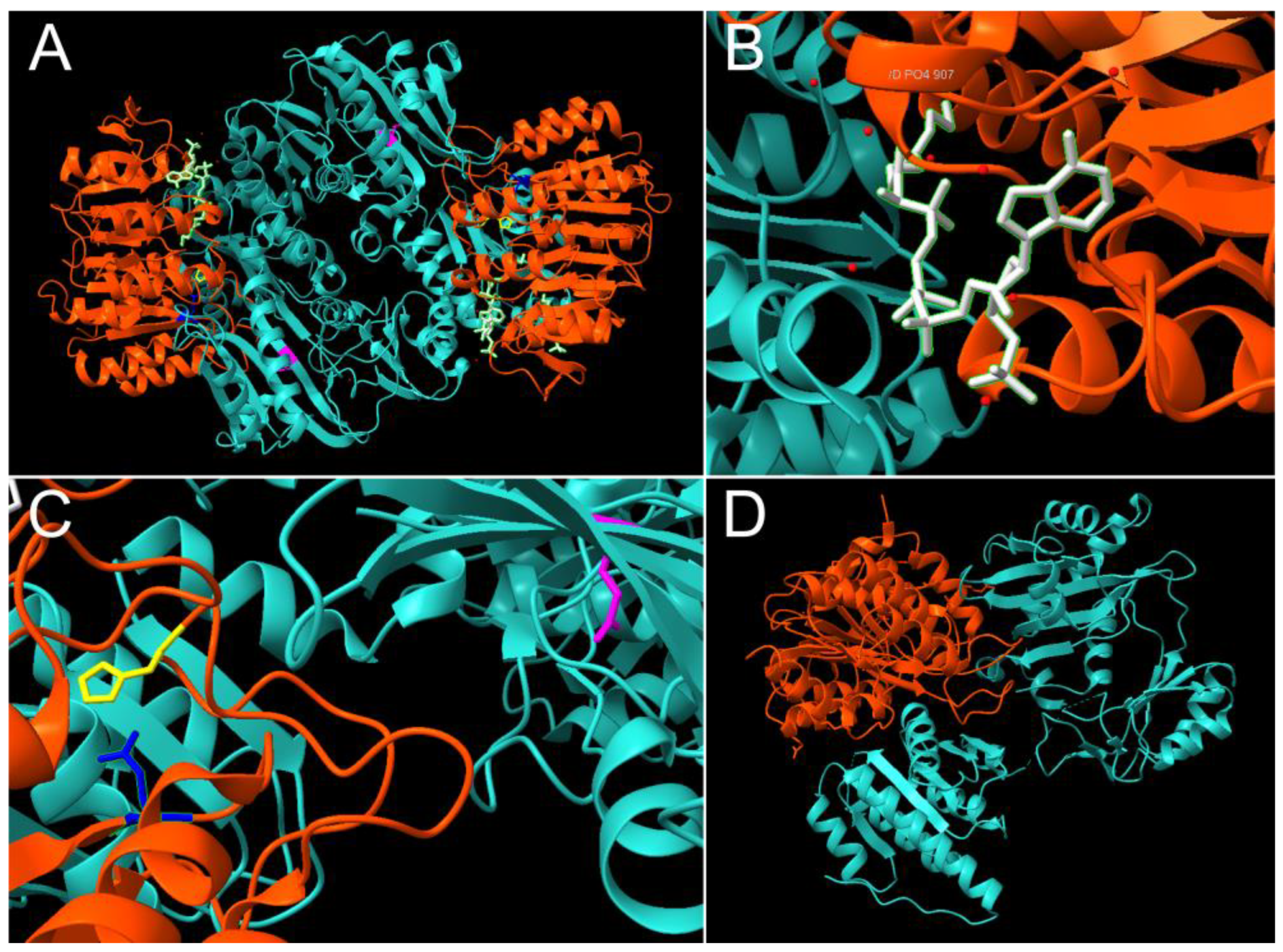
2. The Canonical Structure and Function of Succinyl-CoA Synthetase
2.1. Mechanistic Insights from Prokaryotic Research
2.2. SCS in Eukaryotic Organisms: The Presence of Two SCS Isoforms
2.3. The Biological Significance of SCS: Beyond the TCA Cycle
3. The Genetic and Phenotypic Spectrum of Human SCS Deficiency
3.1. Human SCS Variants
3.2. The Clinical Characteristics and Diagnosis of SCS-Deficient Encephalomyopathy
4. Research Models of SCS-Related Encephalomyopathy
4.1. Cellular Models of SCS-Deficiency
4.2. Animal Models of SCS-Deficiency
5. Pathogenic Mechanisms Requiring Further Exploration
5.1. Mechanisms of SCS-Related MtDNA Maintenance
5.2. Protein Acylation as a Novel Mechanism in SCS-Deficiency
6. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaefer, A.; Lim, A.; Gorman, G. Epidemiology of Mitochondrial Disease. In Diagnosis and Management of Mitochondrial Disorders; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–79. [Google Scholar]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
- Molnar, M.J.; Kovacs, G.G. Mitochondrial diseases. Handb. Clin. Neurol. 2017, 145, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Borst, P. Mitochondrial nucleic acids. Annu. Rev. Biochem. 1972, 41, 333–376. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Turnbull, D.M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005, 6, 389–402. [Google Scholar] [CrossRef]
- Stenton, S.L.; Prokisch, H. Genetics of mitochondrial diseases: Identifying mutations to help diagnosis. EBioMedicine 2020, 56, 102784. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.-J.C. (Ed.) . Mitochondrial Disorders Caused by Nuclear Genes, 1st ed.; Springer Publishing: New York, NY, USA, 2013. [Google Scholar]
- Carrozzo, R.; Verrigni, D.; Rasmussen, M.; de Coo, R.; Amartino, H.; Bianchi, M.; Buhas, D.; Mesli, S.; Naess, K.; Born, A.P.; et al. Succinate-CoA ligase deficiency due to mutations in SUCLA2 and SUCLG1: Phenotype and genotype correlations in 71 patients. J. Inherit. Metab. Dis. 2016, 39, 243–252. [Google Scholar] [CrossRef]
- Elpeleg, O.; Miller, C.; Hershkovitz, E.; Bitner-Glindzicz, M.; Bondi-Rubinstein, G.; Rahman, S.; Pagnamenta, A.; Eshhar, S.; Saada, A. Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am. J. Hum. Genet. 2005, 76, 1081–1086. [Google Scholar] [CrossRef]
- Bridger, W.A. Succinyl-CoA Synthetase. Enzymes 1974, 10, 581–606. [Google Scholar] [CrossRef]
- Nishimura, J.S. Succinyl-CoA synthetase structure-function relationships and other considerations. Adv. Enzymol. Relat. Areas. Mol. Biol. 1986, 58, 141–172. [Google Scholar] [CrossRef]
- Wolodko, W.T.; Fraser, M.E.; James, M.N.; Bridger, W.A. The crystal structure of succinyl-CoA synthetase from Escherichia coli at 2.5-A resolution. J. Biol. Chem. 1994, 269, 10883–10890. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.E.; James, M.N.; Bridger, W.A.; Wolodko, W.T. A detailed structural description of Escherichia coli succinyl-CoA synthetase. J. Mol. Biol. 1999, 285, 1633–1653. [Google Scholar] [CrossRef] [PubMed]
- Joyce, M.A.; Fraser, M.E.; James, M.N.; Bridger, W.A.; Wolodko, W.T. Crystal Structure of the Complex of ADP and MG2+ with Dephosphorylated E. coli Succinyl-CoA Synthetase. 1999. Available online: https://www.rcsb.org/structure/1CQI (accessed on 23 June 2023). [CrossRef]
- Buck, D.; Spencer, M.E.; Guest, J.R. Primary structure of the succinyl-CoA synthetase of Escherichia coli. Biochemistry 1985, 24, 6245–6252. [Google Scholar] [CrossRef]
- Joyce, M.A.; Fraser, M.E.; Brownie, E.R.; James, M.N.; Bridger, W.A.; Wolodko, W.T. Probing the nucleotide-binding site of Escherichia coli succinyl-CoA synthetase. Biochemistry 1999, 38, 7273–7283. [Google Scholar] [CrossRef] [PubMed]
- Joyce, M.A.; Fraser, M.E.; James, M.N.; Bridger, W.A.; Wolodko, W.T. ADP-binding site of Escherichia coli succinyl-CoA synthetase revealed by x-ray crystallography. Biochemistry 2000, 39, 17–25. [Google Scholar] [CrossRef]
- Huang, J.; Fraser, M.E. Structural basis for the binding of succinate to succinyl-CoA synthetase. Acta Crystallogr. D Struct. Biol. 2016, 72, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, R.; Guest, J.R.; Bridger, W.A. Functional consequences of substitution of the active site (phospho)histidine residue of Escherichia coli succinyl-CoA synthetase. Biochim. Biophys. Acta 1991, 1076, 86–90. [Google Scholar] [CrossRef]
- Fraser, M.E.; Joyce, M.A.; Ryan, D.G.; Wolodko, W.T. Two glutamate residues, Glu 208 alpha and Glu 197 beta, are crucial for phosphorylation and dephosphorylation of the active-site histidine residue in succinyl-CoA synthetase. Biochemistry 2002, 41, 537–546. [Google Scholar] [CrossRef]
- Bailey, H.J.; Shrestha, L.; Rembeza, E.; Sorrell, F.J.; Newman, J.; Strain-Damerell, C.; Burgess-Brown, N.; von Delft, F.; Arrowsmith, C.; Edwards, A.; et al. Structure of human ADP-forming succinyl-CoA ligase complex SUCLG1-SUCLA2. 2018. Available online: https://www.rcsb.org/structure/6g4q (accessed on 23 June 2023). [CrossRef]
- Weitzman, P.D.; Kinghorn, H.A. Occurrence of ‘large’ or ‘small’ forms of succinate thiokinase in diverse organisms. FEBS Lett. 1978, 88, 255–258. [Google Scholar] [CrossRef]
- Bridger, W.A.; Wolodko, W.T.; Henning, W.; Upton, C.; Majumdar, R.; Williams, S.P. The subunits of succinyl-coenzyme A synthetase--function and assembly. Biochem. Soc. Symp. 1987, 54, 103–111. [Google Scholar]
- Bailey, D.L.; Wolodko, W.T.; Bridger, W.A. Cloning, characterization, and expression of the beta subunit of pig heart succinyl-CoA synthetase. Protein Sci. 1993, 2, 1255–1262. [Google Scholar] [CrossRef]
- Sanadi, D.R.; Gibson, M.; Ayengar, P. Guanosine triphosphate, the primary product of phosphorylation coupled to the breakdown of succinyl coenzyme A. Biochim. Biophys. Acta 1954, 14, 434–436. [Google Scholar] [CrossRef]
- Hansford, R.G. An adenine nucleotide-linked succinic thiokinase of animal origin. FEBS Lett. 1973, 31, 317–320. [Google Scholar] [CrossRef]
- Johnson, J.D.; Muhonen, W.W.; Lambeth, D.O. Characterization of the ATP- and GTP-specific succinyl-CoA synthetases in pigeon. The enzymes incorporate the same alpha-subunit. J. Biol. Chem. 1998, 273, 27573–27579. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Mehus, J.G.; Tews, K.; Milavetz, B.I.; Lambeth, D.O. Genetic evidence for the expression of ATP- and GTP-specific succinyl-CoA synthetases in multicellular eucaryotes. J. Biol. Chem. 1998, 273, 27580–27586. [Google Scholar] [CrossRef]
- Lambeth, D.O.; Tews, K.N.; Adkins, S.; Frohlich, D.; Milavetz, B.I. Expression of two succinyl-CoA synthetases with different nucleotide specificities in mammalian tissues. J. Biol. Chem. 2004, 279, 36621–36624. [Google Scholar] [CrossRef]
- Dobolyi, A.; Ostergaard, E.; Bagó, A.G.; Dóczi, T.; Palkovits, M.; Gál, A.; Molnár, M.J.; Adam-Vizi, V.; Chinopoulos, C. Exclusive neuronal expression of SUCLA2 in the human brain. Brain Struct. Funct. 2015, 220, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A.; Johnson, W.A. Metabolism of ketonic acids in animal tissues. Biochem. J. 1937, 31, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Shane, B. Vitamins: Folate and Vitamin B12 Function. In Encyclopedia of Biological Chemistry, 3rd ed.; Jez, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1103–1108. [Google Scholar]
- Patel, K.; Master, S.R. Newborn screening and inborn errors of metabolism. In Contemporary Practice in Clinical Chemistry, 4th ed.; Clarke, W., Marzinke, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 865–883. [Google Scholar]
- Cantrell, C.B.; Mohiuddin, S.S. Biochemistry, Ketone Metabolism. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rouault, T.A. Heme, whence come thy carbon building blocks? Blood 2018, 132, 981–982. [Google Scholar] [CrossRef]
- Liu, X.; Si, W.; He, L.; Yang, J.; Peng, Y.; Ren, J.; Jin, T.; Yu, H.; Zhang, Z.; Cheng, X.; et al. The existence of a nonclassical TCA cycle in the nucleus that wires the metabolic-epigenetic circuitry. Signal Transduct. Target. Ther. 2021, 6, 375. [Google Scholar] [CrossRef]
- Yang, Y.; Gibson, G.E. Succinylation Links Metabolism to Protein Functions. Neurochem. Res. 2019, 44, 2346–2359. [Google Scholar] [CrossRef]
- Ostergaard, E.; Hansen, F.J.; Sorensen, N.; Duno, M.; Vissing, J.; Larsen, P.L.; Faeroe, O.; Thorgrimsson, S.; Wibrand, F.; Christensen, E.; et al. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain 2007, 130, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, E.; Chitsazian, F.; Ali Shahidi, G.; Rohani, M.; Sina, F.; Safari, I.; Malakouti Nejad, M.; Houshmand, M.; Klotzle, B.; Elahi, E. The novel mutation p.Asp251Asn in the β-subunit of succinate-CoA ligase causes encephalomyopathy and elevated succinylcarnitine. J. Hum. Genet. 2013, 58, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, S.; Isohanni, P.; Euro, L.; Lönnqvist, T.; Pihko, H.; Kivelä, T.; Knuutila, S.; Suomalainen, A. Mitochondrial encephalomyopathy and retinoblastoma explained by compound heterozygosity of SUCLA2 point mutation and 13q14 deletion. Eur. J. Hum. Genet. 2015, 23, 325–330. [Google Scholar] [CrossRef]
- Ostergaard, E.; Christensen, E.; Kristensen, E.; Mogensen, B.; Duno, M.; Shoubridge, E.A.; Wibrand, F. Deficiency of the alpha subunit of succinate-coenzyme A ligase causes fatal infantile lactic acidosis with mitochondrial DNA depletion. Am. J. Hum. Genet. 2007, 81, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Carrozzo, R.; Dionisi-Vici, C.; Steuerwalkd, U.; Lucioli, S.; Deodato, F.; Di Giandomenico, S.; Bertini, E.; Franke, B.; Kluijtmans, L.A.; Meschini, M.C.; et al. SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain 2007, 130, 862–874. [Google Scholar] [CrossRef]
- Lamperti, C.; Fang, M.; Invernizzi, F.; Liu, X.; Wang, H.; Zhang, Q.; Carrara, F.; Moroni, I.; Zeviani, M.; Zhang, J.; et al. A novel homozygous mutation in SUCLA2 gene identified by exome sequencing. Mol. Genet. Metab. 2012, 107, 403–408. [Google Scholar] [CrossRef]
- Navarro-Sastre, A.; Tort, F.; Garcia-Villoria, J.; Pons, M.R.; Nascimento, A.; Colomer, J.; Campistol, J.; Yoldi, M.E.; López-Gallardo, E.; Montoya, J.; et al. Mitochondrial DNA depletion syndrome: New descriptions and the use of citrate synthase as a helpful tool to better characterise the patients. Mol. Genet. Metab. 2012, 107, 409–415. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, F.; Ding, C.; Wu, H.; Lyu, J.; Wu, Y. SUCLA2-related encephalomyopathic mitochondrial DNA depletion syndrome: A case report and review of literature. Zhonghua Er Ke Za Zhi 2014, 52, 817–821. [Google Scholar] [CrossRef]
- Nogueira, C.; Meschini, M.C.; Nesti, C.; Garcia, P.; Diogo, L.; Valongo, C.; Costa, R.; Videira, A.; Vilarinho, L.; Santorelli, F.M. A novel SUCLA2 mutation in a Portuguese child associated with “mild” methylmalonic aciduria. J. Child Neurol. 2015, 30, 228–232. [Google Scholar] [CrossRef]
- Alkhater, R.A.; Ahonen, S.; Minassian, B.A. SUCLA2 Arg407Trp mutation can cause a nonprogressive movement disorder—Deafness syndrome. Ann. Clin. Transl. Neurol. 2021, 8, 252–258. [Google Scholar] [CrossRef]
- Güngör, O.; Özkaya, A.K.; Güngör, G.; Karaer, K.; Dilber, C.; Aydin, K. Novel mutation in SUCLA2 identified on sequencing analysis. Pediatr. Int. 2016, 58, 659–661. [Google Scholar] [CrossRef]
- Garone, C.; Gurgel-Giannetti, J.; Sanna-Cherchi, S.; Krishna, S.; Naini, A.; Quinzii, C.M.; Hirano, M. A Novel SUCLA2 Mutation Presenting as a Complex Childhood Movement Disorder. J. Child Neurol. 2017, 32, 246–250. [Google Scholar] [CrossRef]
- Huang, X.; Bedoyan, J.K.; Demirbas, D.; Harris, D.J.; Miron, A.; Edelheit, S.; Grahame, G.; DeBrosse, S.D.; Wong, L.J.; Hoppel, C.L.; et al. Succinyl-CoA synthetase (SUCLA2) deficiency in two siblings with impaired activity of other mitochondrial oxidative enzymes in skeletal muscle without mitochondrial DNA depletion. Mol. Genet. Metab. 2017, 120, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.F.; Fang, F.; Liu, Z.M.; Shen, D.M.; Ding, C.H.; Li, J.W.; Ren, X.T.; Wu, H.S. Phenotype and genotype of twelve Chinese children with mitochondrial DNA depletion syndromes. Zhonghua Er Ke Za Zhi 2019, 57, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, E.; Schwartz, M.; Batbayli, M.; Christensen, E.; Hjalmarson, O.; Kollberg, G.; Holme, E. A novel missense mutation in SUCLG1 associated with mitochondrial DNA depletion, encephalomyopathic form, with methylmalonic aciduria. Eur. J. Pediatr. 2010, 169, 201–205. [Google Scholar] [CrossRef]
- Van Hove, J.L.; Saenz, M.S.; Thomas, J.A.; Gallagher, R.C.; Lovell, M.A.; Fenton, L.Z.; Shanske, S.; Myers, S.M.; Wanders, R.J.; Ruiter, J.; et al. Succinyl-CoA ligase deficiency: A mitochondrial hepatoencephalomyopathy. Pediatr. Res. 2010, 68, 159–164. [Google Scholar] [CrossRef]
- Rouzier, C.; Le Guédard-Méreuze, S.; Fragaki, K.; Serre, V.; Miro, J.; Tuffery-Giraud, S.; Chaussenot, A.; Bannwarth, S.; Caruba, C.; Ostergaard, E.; et al. The severity of phenotype linked to SUCLG1 mutations could be correlated with residual amount of SUCLG1 protein. J. Med. Genet. 2010, 47, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Valayannopoulos, V.; Haudry, C.; Serre, V.; Barth, M.; Boddaert, N.; Arnoux, J.B.; Cormier-Daire, V.; Rio, M.; Rabier, D.; Vassault, A.; et al. New SUCLG1 patients expanding the phenotypic spectrum of this rare cause of mild methylmalonic aciduria. Mitochondrion 2010, 10, 335–341. [Google Scholar] [CrossRef]
- Rivera, H.; Merinero, B.; Martinez-Pardo, M.; Arroyo, I.; Ruiz-Sala, P.; Bornstein, B.; Serra-Suhe, C.; Gallardo, E.; Marti, R.; Moran, M.J.; et al. Marked mitochondrial DNA depletion associated with a novel SUCLG1 gene mutation resulting in lethal neonatal acidosis, multi-organ failure, and interrupted aortic arch. Mitochondrion 2010, 10, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Chinopoulos, C.; Batzios, S.; van den Heuvel, L.P.; Rodenburg, R.; Smeets, R.; Waterham, H.R.; Turkenburg, M.; Ruiter, J.P.; Wanders, R.J.A.; Doczi, J.; et al. Mutated SUCLG1 causes mislocalization of SUCLG2 protein, morphological alterations of mitochondria and an early-onset severe neurometabolic disorder. Mol. Genet. Metab. 2019, 126, 43–52. [Google Scholar] [CrossRef]
- Randolph, L.M.; Jackson, H.A.; Wang, J.; Shimada, H.; Sanchez-Lara, P.A.; Wong, D.A.; Wong, L.J.; Boles, R.G. Fatal infantile lactic acidosis and a novel homozygous mutation in the SUCLG1 gene: A mitochondrial DNA depletion disorder. Mol. Genet. Metab. 2011, 102, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, O.; Ohura, T.; Murayama, K.; Ohtake, A.; Harashima, H.; Abukawa, D.; Takeyama, J.; Haginoya, K.; Miyabayashi, S.; Kure, S. Neonatal lactic acidosis with methylmalonic aciduria due to novel mutations in the SUCLG1 gene. Pediatr. Int. 2011, 53, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Landsverk, M.L.; Zhang, V.W.; Wong, L.C.; Andersson, H.C. A SUCLG1 mutation in a patient with mitochondrial DNA depletion and congenital anomalies. Mol. Genet. Metab. Rep. 2014, 1, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Donti, T.R.; Masand, R.; Scott, D.A.; Craigen, W.J.; Graham, B.H. Expanding the phenotypic spectrum of Succinyl-CoA ligase deficiency through functional validation of a new SUCLG1 variant. Mol. Genet. Metab. 2016, 119, 68–74. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Wang, Q.; Ding, Y.; Song, J.; Yang, Y. Five novel SUCLG1 mutations in three Chinese patients with succinate-CoA ligase deficiency noticed by mild methylmalonic aciduria. Brain Dev. 2016, 38, 61–67. [Google Scholar] [CrossRef]
- Maalej, M.; Tej, A.; Bouguila, J.; Tilouche, S.; Majdoub, S.; Khabou, B.; Tabbebi, M.; Felhi, R.; Ammar, M.; Mkaouar-Rebai, E.; et al. Clinical, Molecular, and Computational Analysis in two cases with mitochondrial encephalomyopathy associated with SUCLG1 mutation in a consanguineous family. Biochem. Biophys. Res. Commun. 2018, 495, 1730–1737. [Google Scholar] [CrossRef]
- Demirbas, D.; Harris, D.J.; Arn, P.H.; Huang, X.; Waisbren, S.E.; Anselm, I.; Lerner-Ellis, J.P.; Wong, L.J.; Levy, H.L.; Berry, G.T. Phenotypic variability in deficiency of the α subunit of succinate-CoA ligase. JIMD Rep. 2019, 46, 63–69. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, N.; Li, W. Neonatal succinate-CoA ligase deficiency presented as mild methylmalonic aciduria: A case report. Chin. J. Perinat. Med. 2020, 12, 484–488. [Google Scholar]
- Chen, Y.M.; Chen, W.; Xu, Y.; Lu, C.S.; Zhu, M.M.; Sun, R.Y.; Wang, Y.; Chen, Y.; Shi, J.; Wang, D. Novel compound heterozygous SUCLG1 variants may contribute to mitochondria DNA depletion syndrome-9. Mol. Genet. Genom. Med. 2022, 10, e2010. [Google Scholar] [CrossRef]
- Florio, T.M.; Scarnati, E.; Rosa, I.; Di Censo, D.; Ranieri, B.; Cimini, A.; Galante, A.; Alecci, M. The Basal Ganglia: More than just a switching device. CNS Neurosci. Ther. 2018, 24, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Matilainen, S.; Meyer, J.G.; Pällijeff, P.; Richard, J.; Carroll, C.J.; Euro, L.; Jackson, C.B.; Isohanni, P.; Minassian, B.A.; et al. SUCLA2 mutations cause global protein succinylation contributing to the pathomechanism of a hereditary mitochondrial disease. Nat. Commun. 2020, 11, 5927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, J.; Sui, S.; Yuan, X.; Chen, H.; Qu, C.; Du, Y.; Guo, L.; Du, H. Loss of succinyl-CoA synthase ADP-forming β subunit disrupts mtDNA stability and mitochondrial dynamics in neurons. Sci. Rep. 2017, 7, 7169. [Google Scholar] [CrossRef] [PubMed]
- Donti, T.R.; Stromberger, C.; Ge, M.; Eldin, K.W.; Craigen, W.J.; Graham, B.H. Screen for abnormal mitochondrial phenotypes in mouse embryonic stem cells identifies a model for succinyl-CoA ligase deficiency and mtDNA depletion. Dis. Model. Mech. 2014, 7, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kacso, G.; Ravasz, D.; Doczi, J.; Németh, B.; Madgar, O.; Saada, A.; Ilin, P.; Miller, C.; Ostergaard, E.; Iordanov, I.; et al. Two transgenic mouse models for β-subunit components of succinate-CoA ligase yielding pleiotropic metabolic alterations. Biochem. J. 2016, 473, 3463–3485. [Google Scholar] [CrossRef]
- Anderson, M.S.; Doud, E.H.; Gao, H.; Chen, D.; Simpson, E.; Gillespie, P.J.; Chu, X.; Miller, M.J.; Wang, Y.; Liu, Y.; et al. Succinyl-CoA Synthetase Deficiency in Mouse Forebrain Results in Hyper-Succinylation with Perturbed Neuronal Transcriptional Regulation and Metabolism. Cell Press Sneak Peek 2022. Preprint. [Google Scholar] [CrossRef]
- Miller, C.; Wang, L.; Ostergaard, E.; Dan, P.; Saada, A. The interplay between SUCLA2, SUCLG2, and mitochondrial DNA depletion. Biochim. Biophys. Acta 2011, 1812, 625–629. [Google Scholar] [CrossRef]
- Xu, J.; Huang, B.; Jiang, H.; Li, X. Study progress of mitochondrial DNA depletion syndromes. Chin. J. Appl. Clin. Pediatr. 2019, 24, 314–317. [Google Scholar]
- Fasullo, M.; Endres, L. Nucleotide salvage deficiencies, DNA damage and neurodegeneration. Int. J. Mol. Sci. 2015, 16, 9431–9449. [Google Scholar] [CrossRef]
- Lacombe, M.L.; Tokarska-Schlattner, M.; Boissan, M.; Schlattner, U. The mitochondrial nucleoside diphosphate kinase (NDPK-D/NME4), a moonlighting protein for cell homeostasis. Lab. Investig. 2018, 98, 582–588. [Google Scholar] [CrossRef]
- Kowluru, A.; Tannous, M.; Chen, H.Q. Localization and characterization of the mitochondrial isoform of the nucleoside diphosphate kinase in the pancreatic beta cell: Evidence for its complexation with mitochondrial succinyl-CoA synthetase. Arch. Biochem. Biophys. 2002, 398, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, Z.; Bao, L. An Expanding Repertoire of Protein Acylations. Mol. Cell Proteom. 2022, 21, 100193. [Google Scholar] [CrossRef] [PubMed]
- Carrico, C.; Meyer, J.G.; He, W.; Gibson, B.W.; Verdin, E. The Mitochondrial Acylome Emerges: Proteomics, Regulation by Sirtuins, and Metabolic and Disease Implications. Cell Metab. 2018, 27, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.; Wiese, E.K.; Hitosugi, T. Enzymatic and metabolic regulation of lysine succinylation. Genes Dis. 2020, 7, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.H.; Zhang, X.X.; Tao, C.Y.; Liang, Y.J.; Yuan, J.; Yang, S.H.; Yang, Y.R.; Xiong, X.Y. Succinylation profiles of brain injury after intracerebral hemorrhage. PLoS ONE 2021, 16, e0259798. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Gao, S.; Han, T.; Mao, M.; Zhan, G.; Wang, Y.; Li, X. Sirtuin 5 aggravates microglia-induced neuroinflammation following ischaemic stroke by modulating the desuccinylation of Annexin-A1. J. Neuroinflamm. 2022, 19, 301. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Lv, T.; Hou, P.P.; Manaenko, A.; Liu, Y.; Jin, Y.; Gao, L.; Jia, F.; Tian, Y.; Li, P.; et al. Sirtuin 5-Mediated Lysine Desuccinylation Protects Mitochondrial Metabolism Following Subarachnoid Hemorrhage in Mice. Stroke 2021, 52, 4043–4053. [Google Scholar] [CrossRef]
- Yang, Y.; Tapias, V.; Acosta, D.; Xu, H.; Chen, H.; Bhawal, R.; Anderson, E.T.; Ivanova, E.; Lin, H.; Sagdullaev, B.T.; et al. Altered succinylation of mitochondrial proteins, APP and tau in Alzheimer’s disease. Nat. Commun. 2022, 13, 159. [Google Scholar] [CrossRef]
- Lee, S.; Annes, J.P. Mitochondrial Dysfunction Promotes Diabetes via A Previously Unrecognized Mechanism: Protein Succinylation. FASEB J. 2020, 34, 04969. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Cai, X.; Long, D.; Wang, X.; Liu, C.; Wu, Q. Sirt5-mediated desuccinylation of OPTN protects retinal ganglion cells from autophagic flux blockade in diabetic retinopathy. Cell Death Discov. 2022, 8, 63. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Cheng, Z.; Gao, P.; Sun, J.; Chen, X.; Chen, C.; Wang, Y.; Wang, Z. Quantitative global proteome and lysine succinylome analyses provide insights into metabolic regulation and lymph node metastasis in gastric cancer. Sci. Rep. 2017, 7, 42053. [Google Scholar] [CrossRef]
- Bringman-Rodenbarger, L.R.; Guo, A.H.; Lyssiotis, C.A.; Lombard, D.B. Emerging Roles for SIRT5 in Metabolism and Cancer. Antioxid. Redox Signal. 2018, 28, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Ma, Z.; Lu, C.; Wang, H.; Cheng, X.; Tuo, B.; Fan, Y.; Liu, X.; Li, T. Role of succinylation modification in thyroid cancer and breast cancer. Am. J. Cancer Res. 2021, 11, 4683–4699. [Google Scholar] [PubMed]
- Trefely, S.; Lovell, C.D.; Snyder, N.W.; Wellen, K.E. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol. Metab. 2020, 38, 100941. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shangguan, Y.; Tang, D.; Dai, Y. Histone succinylation and its function on the nucleosome. J. Cell. Mol. Med. 2021, 25, 7101–7109. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Ding, D.; Tian, G.; Kwan, K.C.J.; Liu, Z.; Ishibashi, T.; Li, X.D. Semisynthesis of site-specifically succinylated histone reveals that succinylation regulates nucleosome unwrapping rate and DNA accessibility. Nucleic Acids Res. 2020, 48, 9538–9549. [Google Scholar] [CrossRef]
- Chinopoulos, C. The Mystery of Extramitochondrial Proteins Lysine Succinylation. Int. J. Mol. Sci. 2021, 22, 6085. [Google Scholar] [CrossRef]
| Genotype | Genomic Rearrangement with deletion of 43nt + c.789_802+29delinsATAAA | Homozygous IVS4+1G>A | Homozygous c.850C>T | c.850C>T + c.352G>A | Homozygous c.534+1G>A * | Homozygous c.308C>A | c.1048G>A + c.1049G>T | Homozygous c.751G>A | Homozygous c.998A>G | c.998A<G + 1.54Mb Deletion in 13q14 | Homozygous c.970G>A | Homozygous c.985A>G | |
| Gene Effect | Skip Exon 6, Frameshift | Skip Exon 4 (null) | p.Arg284Cys | p.Arg284Cys + p.Gly118Arg | Multiple Exon Skipping (null) | p.Ala103Asp | p.Gly350Ser + p.Gly350Val | p.Asp251Asn | p.Asp333Gly | p.Asp333Gly + 7 Gene Deletion | p.Gly324Ser | p.Met329Val | |
| N | 4 | 12 | 2 | 1 | 12 | 2 | 2 | 2 | 5 | 1 | 1 | 1 | |
| Clinical Characteristics | |||||||||||||
| Cerebral Atrophy | + (1/2) | + (7/8) | + (2/2) | + | + (8/8) | + | + (2/2) | + (3/4) | + | + | + | ||
| Basal Ganglia Lesions | + (2/2) | + (4/8) | + (2/2) | + | + (7/7) | + (2/2) | + (2/2) | + (2/4) | + | + | |||
| Delayed/Demyelination | + (1/8) | + (2/11) | - | ||||||||||
| Ataxia | + (2/2) | ||||||||||||
| Chorea | + (2/5) | + | |||||||||||
| Sensorineural Hearing Impairment | + (2/2) | + (10/12) | + (2/2) | + (12/12) | + (2/2) | + (2/2) | + (2/2) | + (2/5) | + | + | + | ||
| Seizures | + (2/2) | + (1/12) | + (1/111) | - | - | + | |||||||
| Dystonia | + (1/2) | + (12/12) | + (2/2) | + | + (10/11) | + (2/2) | + (1/2) | + (2/2) | + (3/5) | + | + | ||
| Hyoptonia | + (2/2) | + (12/12) | + (2/2) | + | + (12/12) | + (2/2) | + (2/2) | + | + (5/5) | + | + | + | |
| Muscular Atrophy | + (12/12) | - (2/2) | + (1/2) | + (1/5) | + | + | + | ||||||
| Histochemical Myopathy | - (2/2) | + (7/7) | + (2/5) | + | |||||||||
| Delayed Psychomotor Development | + (2/2) | + (12/12) | + (2/2) | + | + (11/11) | + (2/2) | + (2/2) | + | + (5/5) | + | + | + | |
| Growth Deficit | + | + | + (2/2) | + (1/2) | + (4/5) | - | + | + | |||||
| Feeding Difficulties | + | + (2/2) | + | + (11/12) | + (2/2) | + (1/2) | + (2/2) | + (4/5) | + | + | |||
| Vomiting | + (2/2) | + | |||||||||||
| Headaches | |||||||||||||
| Failure to Thrive | + (1/2) | + | + (10/11) | + | + (2/2) | + | + (4/5) | + | + | ||||
| Ptosis | + (1/2) | + (2/12) | - | - | + (10/11) | + (2/2) | - | + (1/5) | + | ||||
| Gastroesophageal Reflux | + (1/2) | ||||||||||||
| Gastrointetinal Issues | + (2/2) | + | + (10/11) | + | + | ||||||||
| Respiratory Distress | + (2/2) | + | + | + (4/5) | + | ||||||||
| Cardiomyopathy | + (1/11) | + | - | ||||||||||
| Hepatopathy | - | ||||||||||||
| Renal Symptoms | + (1/11) | + | - | ||||||||||
| Anemia | + (2/2) | ||||||||||||
| Metabolic Profile | |||||||||||||
| Lactic Acidosis | + (2/2) | + (7/8) | + (2/2) | + | + (7/9) | + (2/2) | + (2/2) | + (1/1) | + (2/5) | + | + | + | |
| Mild Methylmalonic Aciduria | + (10/10) | + (1/1) | + | + (4/5) | - (1/1) | + (2/2) | - (1/1) | Mild to Normal | + | + | |||
| Elevated C3 Acylcarnitine | + (1/1) | + | + (3/3) | + (1/1) | + | ||||||||
| Elevated C4DC Acylcarnitine | + (2/2) | + | + (3/3) | + (1/1) | + (1/1) | + | + | ||||||
| MtDNA Content | Depleted in Muscle Normal in Fibroblasts | Depleted in Muscle (3/3) | Depletion in Muscle (1/1) Depletion in Fibroblasts (1/1) | Depletion in Muscle Depletion in Fibroblasts | Normal in Fibroblasts (1/1) | Depletion in Muscle (2/2) | Depleted in Muscle (1/1) | Normal in Muscle (1/2) Mild Depletion in Muscle (1/2) | + | Moderate Depletion in Fibroblasts Not Measured in Muscle | |||
| Respiratory Chain Defects | + (2/2) | + | + (2/2) | + (1/1) | + (2/5) | + | |||||||
| Reference | [10] | [39] | [43] | [43] | [9,43] | [44] | [9,45] | [40] | [9,41] | [41] | [46] | [47] | |
| Genotype | Homozygous c.1219C>T | Homozygous 258kb Deletion | c.1106dupA + 46kb deletion | Homozygous c.920C>T | Homozygous c.750C>A | c.160_161insAGA + c.850C>T | c.1204delA + c.308C>T | Homozygous c.1271delG | c.534+1G>A + c.985A>G | Homozygous c.83delC | Homozygous c.1276C>T | c.985A>G + c.920C>T | Homozygous Mutations c.851G>A; c.971G>A |
| Gene Effect | p.Arg407Trp | Whole Gene Deletion | p.Val370Glyfs*18 + Deletion of Exons 1-5 (null) | p.Ala307Val | p.Tyr250* | p.Ser54* + p.Arg284Cys | p.Ile402Tyrfs*18 + p.Ala103Asp | p.Gly424Aspfs*18 | Multiple Exon Skipping (null) + p.Met329Val | p.Ala28Valfs*32 | p.Arg407Trp | p.Met329Val + p.Ala307Val | p.Arg284His; p.Gly324Asp |
| N | 6 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 3 * | 1 | 1 | 2 | 2 |
| Clinical Characteristics | |||||||||||||
| Cerebral Atrophy | + (2/3) | + | + (2/2) | + | + | + | + | + | + | + (1/1) | |||
| Basal Ganglia Lesions | + (4/4) | + | + (2/2) | + | + | + | + | + (2/2) | + | + (1/1) | |||
| Delayed/Demyelination | |||||||||||||
| Ataxia | + | + | |||||||||||
| Chorea | + (6/6) | + | + | + (2/2) | |||||||||
| Sensorineural Hearing Impairment | + (6/6) | + (2/2) | + | + | + | + | + | + (3/3) | + | + | + (2/2) | + | |
| Seizures | + (1/3) | + (1/2) | + | + | |||||||||
| Dystonia | + (6/6) | + | + | + | + | + | + (1/2) | + | + (2/2) | + | |||
| Hyoptonia | + (3/3) | + | + (2/2) | + | + | + | + | + | + (2/3) | + | + | + (2/2) | + |
| Muscular Atrophy | + | + (2/2) | + | + (1/2) | |||||||||
| Histochemical Myopathy | - (4/4) | + (2/2) | + | + | + | + (2/2) | |||||||
| Delayed Psychomotor Development | + (3/3) | + | + (2/2) | + | + | + | + | + | + (3/3) | + | + | + (2/2) | + |
| Growth Deficit | + (2/2) | + | + | + (2/2) | + | ||||||||
| Feeding Difficulties | + (1/3) | + | + (2/2) | + | + | + | + | + | + (2/3) | + (2/2) | + | ||
| Vomiting | + (1/3) | + (1/2) | + | + | + | + (1/2) | |||||||
| Headaches | + | + (1/2) | |||||||||||
| Failure to Thrive | + (1/3) | + | + | + | + | + (1/2) | + | + | |||||
| Ptosis | + (1/3) | + | + | + (1/2) | + (1/2) | + | |||||||
| Gastroesophageal Reflux | + (1/3) | + (1/2) | + | + | + (1/2) | ||||||||
| Gastrointetinal Issues | + | + | + (2/2) | ||||||||||
| Respiratory Distress | + | + | + | + (1/3) | + (1/2) | ||||||||
| Cardiomyopathy | |||||||||||||
| Hepatopathy | + | ||||||||||||
| Renal Symptoms | |||||||||||||
| Anemia | + (1/2) | ||||||||||||
| Metabolic Profile | |||||||||||||
| Lactic Acidosis | + (6/6) | + | + (2/2) | + | + | - | + | + | + (3/3) | + | + | + (2/2) | + |
| Mild Methylmalonic Aciduria | + (5/6) | + | + (2/2) | + | + | + | + | + | + (3/3) | + | + (2/2) | ||
| Elevated C3 Acylcarnitine | + (3/3) | + (2/2) | |||||||||||
| Elevated C4DC Acylcarnitine | + (3/3) | + (2/2) | |||||||||||
| MtDNA Content | Depletion in Muscle (1/1) Normal in Muscle (3/3) | Mild Depletion in Muscle | Depletion in Muscle | Normal to Elevated in Muscle | |||||||||
| Respiratory Chain Defects | - (4/4) | + | + (1/1) | + | + | + | + | - | + | + (2/2) | |||
| Reference | [9,48] | [9] | [9] | [9] | [9] | [9] | [9] | [9] | [9] | [49] | [50] | [51] | [52] |
| Genotype | Homozygous c.113_114delAT | Homozygous c.215G>C (Also reported as c.254G>C) | Homozygous c.40A>T | c.509C>G + c.97+3G>C | c.448C>T + Unidentified Variant | Homozygous c.626C>A | c.309_310delTG + c.428T>G | Homozygous c.137C>T | c.626C>A * | Homozygous c.280-1G>A | c.41T>C + c599C>T | Homozygous c.749A>G | ||
| Gene Effect | Deletion in Exon 2 | p.Gly72Ala (Also reported as p.Gly85Ala) | p.Met14Leu | p.Pro170Arg + Skip Exon 1 | p.Gln150* | p.Ala209Glu | p.Thr103fs + p.Ile143Ser | p.Ser46Phe | p.Ala209Glu | Splice Variant | p.Met14Thr + p.Ser200Phe | p.Glu263Gly | ||
| N | 3 | 3 | 1 | 1 | 1 | 4 | 1 | 2 | 1 | 1 | 1 | 1 | ||
| Clinical Characteristics | ||||||||||||||
| Cerebral Atrophy | + (3/3) | + (2/3) | + | + | + (2/2) | + | + | + | + | + | ||||
| Basal Ganglia Lesions | + (3/3) | + | + | + | + (2/2) | + | + (2/2) | + | + | |||||
| Delayed/Demyelination | + (1/3) | |||||||||||||
| Ataxia | + (1/3) | + (1/4) | + | |||||||||||
| Chorea | ||||||||||||||
| Sensorineural Hearing Impairment | + (2/3) | + | + (1/2) | - | + (1/2) | + | + | |||||||
| Seizures | + (1/4) | |||||||||||||
| Dystonia | + (1/3) | + | + (2/4) | + | + (2/2) | + | ||||||||
| Hyoptonia | + (2/3) | + (3/3) | + | + | + | + (4/4) | + | + (2/2) | + | + | ||||
| Muscular Atrophy | + (2/3) | + | + | + (1/1) | + (2/2) | |||||||||
| Histochemical Myopathy | - (1/1) | + | + | + | + (1/1) | + | - | + | ||||||
| Delayed Psychomotor Development | + (3/3) | + | + | + (2/4) | + | + (2/2) | + | |||||||
| Growth Deficit | + (3/3) | + (2/3) | + (2/4) | + | + | + | ||||||||
| Feeding Difficulties | + (2/3) | + | + | + (1/3) | + | + (2/2) | + | |||||||
| Vomiting | + (1/3) | + | ||||||||||||
| Headaches | ||||||||||||||
| Failure to Thrive | + (2/3) | + | ||||||||||||
| Ptosis | + (1/3) | - (2/2) | ||||||||||||
| Gastroesophageal Reflux | + (1/3) | + (1/2) | ||||||||||||
| Gastrointetinal Issues | + (1/2) | |||||||||||||
| Respiratory Distress | + (3/3) | + | + | + | + (4/4) | + (2/2) | + | + | + | |||||
| Cardiomyopathy | + (3/4) | - (2/2) | + | + | + | + | ||||||||
| Hepatopathy | + (1/3) | + | + (3/3) | + | + (1/2) | + | + | |||||||
| Renal Symptoms | + (2/3) | - (1/2) | + | + | ||||||||||
| Anemia | ||||||||||||||
| Metabolic Profile | ||||||||||||||
| Lactic Acidosis | + (3/3) | + (2/3) | + | + | + | + (4/4) | + | + (2/2) | + | + | + | + | ||
| Mild Methylmalonic Aciduria | + (2/3) | + | + (3/3) | + | + (2/2) | + | + | + | ||||||
| Elevated C3 Acylcarnitine | + | + | + (2/3) | + | + (1/1) | + | + | + | ||||||
| Elevated C4DC Acylcarnitine | + | + | + (3/3) | + | + (1/1) | + | + | |||||||
| MtDNA Content | Depletion in Liver (1/3) Depletion in Muscle (1/3) | Mild Depletion in Muscle (1/1) | Depletion in Liver | Depletion in Muscle | Depletion in Muscle | Depletion in Muscle (4/4) Depletion in Liver (3/3) | Depletion in Muscle | Elevated in Muscle | + | Depleted in Muscle | Normal in Muscle Normal in Fibroblasts | Depleted in Muscle | ||
| Respiratory Chain Defects | + | + (2/3) | + | + | + | + | + (1/2) | + | + | + | ||||
| Reference | [42] | [9,53] | [54] | [55] | [55] | [56,57,58] | [56] | [9,56] | [58] | [59] | [60] | [61] | ||
| Genotype | Homozygous c.212A>G | c.787G>A + c.626C>A | c.40A>T + c.635A>G | c.826-2A>G + c.809A>C | c.826-2A>G + c.550G>A | c.961C>G + c.751C>T | Homozygous c.41T>C | Homozygous c.512A>G | c.40A>G + Deletion | Homozygous Mutations c.916G>T; c.619T>C; c.980dupT | c.601A>G + c.871G>C | |||
| Gene Effect | p.His71Arg | p.Glu263Lys + p.Ala209Glu | p.Met14Leu + Gln212Arg | Splice Variant + p.Lys207Trp | Splice Variant + p.Gly184Ser | p.Ala32Pro + p.Gly251Ser | p.Met14Thr | p.Asn171Ser | p.Met14Val + Deletion Exons 6-9 | p.Gly306Ter; p.Tyr207His; p.Met327Ilefs*15 | p.Arg201Gly + p.Ala291Pro | |||
| N | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 3 | 1 | |||
| Clinical Characteristics | ||||||||||||||
| Cerebral Atrophy | - | + | + (1/1) | + | + | |||||||||
| Basal Ganglia Lesions | + | - | + | + | + (1/1) | + | ||||||||
| Delayed/Demyelination | ||||||||||||||
| Ataxia | + | + | + | |||||||||||
| Chorea | + | + | + | + | + | |||||||||
| Sensorineural Hearing Impairment | + | + | + (2/2) | + | ||||||||||
| Seizures | + | - (2/2) | + | |||||||||||
| Dystonia | + | + | + | + | - (2/2) | + | + | |||||||
| Hyoptonia | + | + | + | + | + | + (2/2) | + | + | + | |||||
| Muscular Atrophy | + | + (2/2) | ||||||||||||
| Histochemical Myopathy | + | + | ||||||||||||
| Delayed Psychomotor Development | + | + | + | + | + | + (2/2) | + | + | ||||||
| Growth Deficit | + | + (2/2) | + | + | ||||||||||
| Feeding Difficulties | + | + | + | + | + | + (2/2) | + | |||||||
| Vomiting | + | - (2/2) | + | + | + | |||||||||
| Headaches | ||||||||||||||
| Failure to Thrive | + | + | + | + | + (2/2) | + | ||||||||
| Ptosis | - | + (2/2) | + | |||||||||||
| Gastroesophageal Reflux | + | |||||||||||||
| Gastrointetinal Issues | + | |||||||||||||
| Respiratory Distress | + | + (2/2) | + | + | ||||||||||
| Cardiomyopathy | - | - | - (2/2) | + | + | |||||||||
| Hepatopathy | + (2/2) | + | ||||||||||||
| Renal Symptoms | - (2/2) | + | ||||||||||||
| Anemia | ||||||||||||||
| Metabolic Profile | ||||||||||||||
| Lactic Acidosis | + | + | - | + | + | + | + (2/2) | + | + | + | + | |||
| Mild Methylmalonic Aciduria | + | + | + | + | + | + | + (2/2) | + | + | + | ||||
| Elevated C3 Acylcarnitine | + | + | + | + (1/1) | + | + | ||||||||
| Elevated C4DC Acylcarnitine | + | + | + | + (1/1) | + | + | ||||||||
| MtDNA Content | Normal in Muscle Elevated to Depleted in Fibroblasts | Normal in Leukocytes | Normal in Leukocytes | Normal in Leukocytes | Depletion in Leukocytes | Normal in Muscle | Depleted in Leukocytes | |||||||
| Respiratory Chain Defects | - | + | + | + | + | + | ||||||||
| Reference | [9] | [9] | [62] | [63] | [63] | [63] | [64] | [65] | [66] | [52] | [67] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lancaster, M.S.; Graham, B.H. Succinyl-CoA Synthetase Dysfunction as a Mechanism of Mitochondrial Encephalomyopathy: More than Just an Oxidative Energy Deficit. Int. J. Mol. Sci. 2023, 24, 10725. https://doi.org/10.3390/ijms241310725
Lancaster MS, Graham BH. Succinyl-CoA Synthetase Dysfunction as a Mechanism of Mitochondrial Encephalomyopathy: More than Just an Oxidative Energy Deficit. International Journal of Molecular Sciences. 2023; 24(13):10725. https://doi.org/10.3390/ijms241310725
Chicago/Turabian StyleLancaster, Makayla S., and Brett H. Graham. 2023. "Succinyl-CoA Synthetase Dysfunction as a Mechanism of Mitochondrial Encephalomyopathy: More than Just an Oxidative Energy Deficit" International Journal of Molecular Sciences 24, no. 13: 10725. https://doi.org/10.3390/ijms241310725
APA StyleLancaster, M. S., & Graham, B. H. (2023). Succinyl-CoA Synthetase Dysfunction as a Mechanism of Mitochondrial Encephalomyopathy: More than Just an Oxidative Energy Deficit. International Journal of Molecular Sciences, 24(13), 10725. https://doi.org/10.3390/ijms241310725






